A Case of T/NK-Cell Post-Transplantation Lymphoproliferative Disease 7 Years after Heart Transplantation
Abstract
:1. Introduction
2. Case Report
2.1. Heart Transplantation (7 Years Ago)
2.2. Adjustment of Immunosuppression Treatment
2.3. Fever and Diagnosis of Pneumonia (1 Month Ago)
2.4. On Admission
2.5. Diagnosis of EBV-Associated Hemophagocytic Syndrome
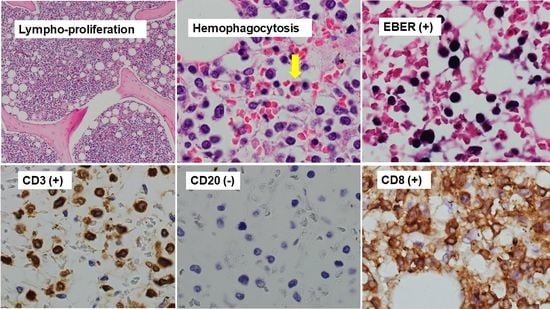
2.6. Diagnosis of T/NK-Cell PTLD on Autopsy
3. Discussion
3.1. T/NK-Cell PTLD
3.2. EBV Infections
3.3. EBV-Associated Hemophagocytic Syndrome and PTLD
3.4. Detection and Treatment of T/NK-Cell PTLD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Mansour, Z.; Nelson, B.P.; Evens, A.M. Post-transplant lymphoproliferative disease (PTLD): Risk factors, diagnosis, and current treatment strategies. Curr. Hematol. Malig. Rep. 2013, 8, 173–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohta, H.; Fukushima, N.; Ozono, K. Pediatric post-transplant lymphoproliferative disorder after cardiac transplantation. Int. J. Hematol. 2009, 90, 127–136. [Google Scholar] [CrossRef]
- Swerdlow, S.H. T-cell and NK-cell posttransplantation lymphoproliferative disorders. Am. J. Clin. Pathol. 2007, 127, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Draoua, H.Y.; Tsao, L.; Mancini, D.M.; Addonizio, L.J.; Bhagat, G.; Alobeid, B. T-cell post-transplantation lymphoproliferative disorders after cardiac transplantation: A single institutional experience. Br. J. Haematol. 2004, 127, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syrykh, C.; Péricart, S.; Lamaison, C.; Escudié, F.; Brousset, P.; Laurent, C. Epstein-Barr Virus-Associated T- and NK-Cell Lymphoproliferative Diseases: A Review of Clinical and Pathological Features. Cancers 2021, 13, 3315. [Google Scholar] [CrossRef] [PubMed]
- Henter, J.I.; Horne, A.; Aricó, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Kwong, Y.L. EBV Viral Loads in Diagnosis, Monitoring, and Response Assessment. Front. Oncol. 2019, 9, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, M.; Imamura, T.; Takagi, K.; Hori, M.; Tanaka, S.; Imura, J.; Kinugawa, K. A Case of T/NK-Cell Post-Transplantation Lymphoproliferative Disease 7 Years after Heart Transplantation. J. Cardiovasc. Dev. Dis. 2022, 9, 38. https://doi.org/10.3390/jcdd9020038
Nakamura M, Imamura T, Takagi K, Hori M, Tanaka S, Imura J, Kinugawa K. A Case of T/NK-Cell Post-Transplantation Lymphoproliferative Disease 7 Years after Heart Transplantation. Journal of Cardiovascular Development and Disease. 2022; 9(2):38. https://doi.org/10.3390/jcdd9020038
Chicago/Turabian StyleNakamura, Makiko, Teruhiko Imamura, Kohji Takagi, Masakazu Hori, Shinichi Tanaka, Joji Imura, and Koichiro Kinugawa. 2022. "A Case of T/NK-Cell Post-Transplantation Lymphoproliferative Disease 7 Years after Heart Transplantation" Journal of Cardiovascular Development and Disease 9, no. 2: 38. https://doi.org/10.3390/jcdd9020038
APA StyleNakamura, M., Imamura, T., Takagi, K., Hori, M., Tanaka, S., Imura, J., & Kinugawa, K. (2022). A Case of T/NK-Cell Post-Transplantation Lymphoproliferative Disease 7 Years after Heart Transplantation. Journal of Cardiovascular Development and Disease, 9(2), 38. https://doi.org/10.3390/jcdd9020038