Effect of Early Initiation of Evolocumab on Lipoprotein(a) in Patients with Acute Myocardial Infarction: Sub-Analysis of a Randomized Controlled Trial
Abstract
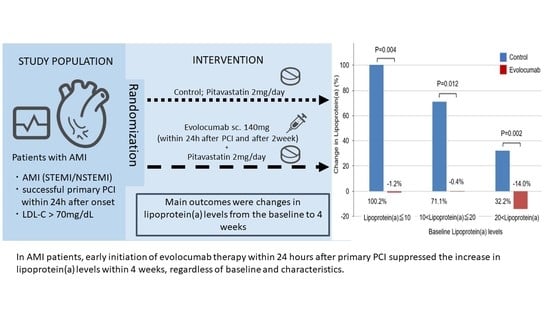
:1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Lipoprotein(a)
3. Discussion
Limitations
4. Materials and Methods
4.1. Study Design and Participants
4.2. PCI Procedure
4.3. Outcomes
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsimikas, S.; Hall, J.L. Lipoprotein(a) as a potential causal genetic risk factor of cardiovascular disease: A rationale for increased efforts to understand its pathophysiology and develop targeted therapies. J. Am. Coll. Cardiol. 2012, 60, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Noureen, A.; Kronenberg, F.; Utermann, G. Structure, function, and genetics of lipoprotein(a). J. Lipid. Res. 2016, 57, 1339–1359. [Google Scholar] [CrossRef] [PubMed]
- Erqou, S.; Kaptoge, S.; Perry, P.L.; Di Angelantonio, E.; Thompson, A.; White, I.R.; Marcovina, S.M.; Collins, R.; Thompson, S.G.; Danesh, J. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 2009, 302, 412–423. [Google Scholar] [PubMed]
- Clarke, R.; Peden, J.F.; Hopewell, J.C.; Kyriakou, T.; Goel, A.; Heath, S.C.; Parish, S.; Barlera, S.; Franzosi, M.G.; Rust, S.; et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 2009, 361, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Kamstrup, P.R.; Tybjaerg-Hansen, A.; Steffensen, R.; Nordestgaard, B.G. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA 2009, 301, 2331–2339. [Google Scholar] [CrossRef]
- Kotani, K.; Serban, M.C.; Penson, P.; Lippi, G.; Banach, M. Evidence-based assessment of lipoprotein(a) as a risk biomarker for cardiovascular diseases—Some answers and still many questions. Crit. Rev. Clin. Lab. Sci. 2016, 53, 370–378. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol 2019, 73, e285–e350. [Google Scholar] [CrossRef]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Desai, N.R.; Kohli, P.; Rogers, W.J.; Somaratne, R.; Huang, F.; Liu, T.; Mohanavelu, S.; Hoffman, E.B.; McDonald, S.T.; et al. LAPLACE-TIMI 57 Investigators. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): A randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet 2012, 380, 2007–2017. [Google Scholar] [PubMed]
- Raal, F.J.; Stein, E.A.; Dufour, R.; Turner, T.; Civeira, F.; Burgess, L.; Langslet, G.; Scott, R.; Olsson, A.G.; Sullivan, D.; et al. RUTHERFORD-2 Investigators. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): A randomised, double-blind, placebo-controlled trial. Lancet 2015, 385, 331–340. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Wiviott, S.D.; Raal, F.J.; Blom, D.J.; Robinson, J.; Ballantyne, C.M.; Somaratne, R.; Legg, J.; Wasserman, S.M.; et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015, 372, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Doi, M.; Miyoshi, T.; Nosaka, K.; Taya, S.; Yamamoto, K.; Sakamoto, A.; Ugawa, S.; Tsushima, R.; Ito, H. Early initiation of evolocumab markedly reduces low-density lipoprotein cholesterol levels after myocardial infarction. JACC Cardiovasc. Interv. 2020, 13, 2944–2946. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D. Third universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2012, 60, 1581–1598. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, K.; Miyoshi, T.; Iwamoto, M.; Kajiya, M.; Okawa, K.; Tsukuda, S.; Yokohama, F.; Sogo, M.; Nishibe, T.; Matsuo, N.; et al. Early initiation of eicosapentaenoic acid and statin treatment is associated with better clinical outcomes than statin alone in patients with acute coronary syndromes: 1-year outcomes of a randomized controlled study. Int. J. Cardiol. 2017, 228, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Group JCSJW. Guidelines for Secondary Prevention of Myocardial Infarction (JCS 2011). Circ. J. 2013, 77, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Bittner, V.A.; Szarek, M.; Aylward, P.E.; Bhatt, D.L.; Diaz, R.; Edelberg, J.M.; Fras, Z.; Goodman, S.G.; Halvorsen, S.; Hanotin, C.; et al. ODYSSEY OUTCOMES Committees and Investigators. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J. Am. Coll. Cardiol. 2020, 75, 133–144. [Google Scholar] [CrossRef]
- Koskinas, K.C.; Windecker, S.; Pedrazzini, G.; Mueller, C.; Cook, S.; Matter, C.M.; Muller, O.; Häner, J.; Gencer, B.; Crljenica, C.; et al. Evolocumab for early reduction of LDL cholesterol levels in patients with acute coronary syndromes (EVOPACS). J. Am. Coll. Cardiol. 2019, 74, 2452–2462. [Google Scholar] [CrossRef]
- Furtado, R.H.M.; Fagundes, A.A., Jr.; Oyama, K.; Zelniker, T.A.; Tang, M.; Kuder, J.F.; Murphy, S.A.; Hamer, A.; Wang, H.; Keech, A.C.; et al. Effect of Evolocumab in patients with prior percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2022, 15, e011382. [Google Scholar] [CrossRef]
- Toth, P.P.; Dwyer, J.P.; Cannon, C.P.; Colhoun, H.M.; Rader, D.J.; Upadhyay, A.; Louie, M.J.; Koren, A.; Letierce, A.; Mandel, J.; et al. Efficacy and safety of lipid lowering by alirocumab in chronic kidney disease. Kidney Int. 2018, 93, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Gordts, P.; Nora, C.; Yeang, C.; Witztum, J.L. Statin therapy increases lipoprotein(a) levels. Eur. Heart J. 2019, 41, 2275–2284. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Kanazawa, M.; Kagaya, Y.; Kondo, M.; Sato, K.; Endo, H.; Nozaki, E. Plasma kinetics of mature PCSK9, furin-cleaved PCSK9, and Lp(a) with or without administration of PCSK9 inhibitors in acute myocardial infarction. J. Cardiol. 2020, 76, 395–401. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, M.L.; Fazio, S.; Giugliano, R.P.; Stroes, E.G.S.; Kanevsky, E.; Gouni-Berthold, J.; Im, K.; Pineda, A.L.; Wasserman, S.M.; Češka, R.; et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation 2019, 139, 1483–1492. [Google Scholar] [CrossRef]
- Madsen, C.M.; Kamstrup, P.R.; Langsted, A.; Varbo, A.; Nordestgaard, B.G. Lipoprotein(a)-lowering by 50 mg/dL (105 nmol/L) may be needed to reduce cardiovascular disease 20% in secondary prevention: A population-based study. Arter. Thromb. Vasc. Biol. 2020, 40, 255–266. [Google Scholar] [CrossRef]
- Kaiser, Y.; Daghem, M.; Tzolos, E.; Meah, M.N.; Doris, M.; Moss, A.J.; Kwiecinski, J.; Kroon, J.; Nurmohamed, N.S.; van der Harst, P.; et al. Association of lipoprotein(a) with atherosclerotic plaque progression. J. Am. Coll. Cardiol. 2022, 79, 223–233. [Google Scholar] [CrossRef]
- Waldmann, E.; Wu, L.; Busygina, K.; Altenhofer, J.; Henze, K.; Folwaczny, A.; Parhofer, K.G. Effect of PCSK9 inhibition with evolocumab on lipoprotein subfractions in familial dysbetalipoproteinemia (type III hyperlipidemia). PLoS ONE 2022, 17, e0265838. [Google Scholar] [CrossRef]



| Characteristics | Evolocumab (n = 52) | Control (n = 50) | p-Value |
|---|---|---|---|
| Age (years) | 66.4 ± 13.1 | 63.4 ± 14.0 | 0.34 |
| Male sex | 43 (82) | 47 (94) | 0.12 |
| Body mass index, kg/m2 | 24.4 ± 4.4 | 25.2 ± 4.5 | 0.35 |
| Diabetes mellitus | 17 (32) | 14 (28) | 0.66 |
| Hypertension | 31 (59) | 33 (66) | 0.54 |
| Smoker | 25 (48) | 18 (36) | 0.23 |
| Previous myocardial infarction | 1 (1.9) | 5 (10) | 0.10 |
| Previous PCI | 4 (7.6) | 10 (20) | 0.08 |
| Previous CABG | 0 | 0 | 1.00 |
| Peripheral artery disease | 1 (1.9) | 0 | 1.00 |
| Stroke | 0 | 3 (6.0) | 0.11 |
| Atrial fibrillation | 3 (5.7) | 3 (6.0) | 1.00 |
| eGFR, mL−1min−1 1.73 m2 | 69.1 ± 25.1 | 70.0 ± 22.3 | 0.92 |
| Statin treatment at baseline | 0.82 | ||
| Any statin | 14 (26) | 12 (24) | |
| No statin | 38 (73) | 38 (76) | |
| Index AMI event | 0.14 | ||
| STEMI | 48 (92) | 41 (82) | |
| NSTEMI | 4 (7.6) | 9 (18) | |
| Initial TIMI flow grade | 0.51 | ||
| Grade 0 | 39 (75) | 35 (70) | |
| Grade 1 | 5 (9.6) | 5 (10) | |
| Grade 2 | 5 (9.6) | 7 (14) | |
| Grade 3 | 3 (5.7) | 3 (6) | |
| Final TIMI flow grade | 1.00 | ||
| Grade 2 | 1 (2) | 0 (0) | |
| Grade 3 | 51 (98) | 50(100) | |
| Access site for PCI | 0.73 | ||
| Transfemoral approach | 4 (7.6) | 5 (10) | |
| Transradial approach | 48 (92) | 45 (90) | |
| Target coronary artery | 0.47 | ||
| Left anterior descending | 25 (48) | 23 (46) | |
| Left circumflex | 7 (13) | 5 (10) | |
| Right | 20 (38) | 22 (44) | |
| Lipoprotein(a) | Evolocumab | Control | Mean Difference | p-Value |
|---|---|---|---|---|
| (95% CI) * | ||||
| Baseline, mg/dL | 14.9 ± 15.5 | 14.2 ± 15.1 | 0.8 (−6.3 to 8.0) | 0.82 |
| At week 4, mg/dL | 14.2 ± 15.4 | 21.8 ± 20.7 | −7.6 (−16.2 to 0.9) | 0.08 |
| Absolute change from baseline, mg/dL | −0.6 ± 6.6 | 7.5 ± 8.9 | −8.4 (−12.2 to −4.7) | <0.001 |
| % change from baseline, % | −2.7 ± 48.6 | 82.0 ± 135.9 | −86.3 (−134.5 to −38.0) | 0.01 |
| Lipoprotein(a) ≤ 10 | (n = 24) | (n = 29) | ||
| Baseline, mg/dL | 5.4 ± 3.0 | 5.7 ± 2.9 | −0.3 (−1.9 to 1.3) | 0.719 |
| At week 4, mg/dL | 5.5 ± 4.7 | 10.0 ± 7.1 | −4.5 (−7.8 to −1.2) | 0.008 |
| Absolute change from baseline, mg/dL | 0.04 ± 3.6 | 4.2 ± 6.3 | −4.2 (−7.0 to −1.4) | 0.004 |
| % change from baseline, % | −1.2 ± 55.5 | 100.2 ± 167.5 | −101.4 (−168.6 to −34.2) | 0.004 |
| 10 < lipoprotein(a) ≤ 20 | (n = 18) | (n = 12) | ||
| Baseline, mg/dL | 15.1 ± 2.9 | 15.0 ± 2.9 | 0.1 (−2.0 to 2.4) | 0.88 |
| At week 4, mg/dL | 15.6 ± 8.5 | 26.3 ± 13.7 | −10.6 (−20.0 to −1.2) | 0.02 |
| Absolute change from baseline, mg/dL | 0.5 ± 6.9 | 11.3 ± 12.2 | −10.8 (−19.0 to −2.5) | 0.013 |
| % change from baseline, % | −0.4 ± 46.9 | 71.1 ± 78.7 | −71.5 (−125.1 to −18.0) | 0.012 |
| Lipoprotein (a) > 20 | (n = 7) | (n = 8) | ||
| Baseline, mg/dL | 46.7 ± 18.3 | 43.8 ± 15.1 | 2.8 (−16.3 to 21.9) | 0.752 |
| At week 4, mg/dL | 40.8 ± 21.6 | 57.7 ± 18.8 | −16.8 (−39.8 to 6.1) | 0.136 |
| Absolute change from baseline, mg/dL | −5.8 ± 11.2 | 13.8 ± 6.2 | −19.7 (−30.5 to −8.8) | 0.003 |
| % change from baseline, % | −14.0 ± 25.5 | 32.2 ± 15.7 | −46.3 (−71.2 to −21.3) | 0.002 |
| Lipoprotein(a) | Evolocumab | Control | Mean Difference | p-Value |
|---|---|---|---|---|
| (95% CI) * | ||||
| Patients with diabetes | ||||
| n | 16 | 14 | ||
| Baseline, mg/dL | 17.3 ± 20.9 | 10.2 ± 8.6 | 7.1 (−4.3 to 19.0) | 0.22 |
| At week 4, mg/dL | 15.5 ± 19.8 | 20.0 ± 16.1 | −4.4 (−17.9 to 9.0) | 0.50 |
| Absolute change from baseline, mg/dL | −1.8 ± 6.1 | 9.7 ± 12.3 | −11.5 (−19.2 to −3.9) | 0.005 |
| % Change from baseline, % | −15.8 ± 45.5 | 168.2 ± 222.1 | −184.1 (−313.8 to −54.4) | 0.009 |
| Patients without diabetes | ||||
| n | 35 | 35 | ||
| Baseline, mg/dL | 13.2 ± 12.1 | 15.8 ± 16.9 | −2.6 (−9.6 to 4.4) | 0.46 |
| At week 4, mg/dL | 13.4 ± 12.6 | 22.5 ± 22.4 | −9.0 (−17.8 to −0.3) | 0.04 |
| Absolute change from baseline, mg/dL | 0.2 ± 6.7 | 6.6 ± 7.2 | −6.4 (−9.8 to −3.1) | <0.001 |
| % Change from baseline, % | 8.5 ± 52.8 | 47.5 ± 54.1 | −38.9 (−64.4 to −13.4) | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okada, T.; Miyoshi, T.; Doi, M.; Nosaka, K.; Tsushima, R.; Ugawa, S.; Takagi, W.; Sogo, M.; Takahashi, M.; Ito, H. Effect of Early Initiation of Evolocumab on Lipoprotein(a) in Patients with Acute Myocardial Infarction: Sub-Analysis of a Randomized Controlled Trial. J. Cardiovasc. Dev. Dis. 2022, 9, 153. https://doi.org/10.3390/jcdd9050153
Okada T, Miyoshi T, Doi M, Nosaka K, Tsushima R, Ugawa S, Takagi W, Sogo M, Takahashi M, Ito H. Effect of Early Initiation of Evolocumab on Lipoprotein(a) in Patients with Acute Myocardial Infarction: Sub-Analysis of a Randomized Controlled Trial. Journal of Cardiovascular Development and Disease. 2022; 9(5):153. https://doi.org/10.3390/jcdd9050153
Chicago/Turabian StyleOkada, Tomoaki, Toru Miyoshi, Masayuki Doi, Kazumasa Nosaka, Ryu Tsushima, Satoko Ugawa, Wataru Takagi, Masahiro Sogo, Masahiko Takahashi, and Hiroshi Ito. 2022. "Effect of Early Initiation of Evolocumab on Lipoprotein(a) in Patients with Acute Myocardial Infarction: Sub-Analysis of a Randomized Controlled Trial" Journal of Cardiovascular Development and Disease 9, no. 5: 153. https://doi.org/10.3390/jcdd9050153
APA StyleOkada, T., Miyoshi, T., Doi, M., Nosaka, K., Tsushima, R., Ugawa, S., Takagi, W., Sogo, M., Takahashi, M., & Ito, H. (2022). Effect of Early Initiation of Evolocumab on Lipoprotein(a) in Patients with Acute Myocardial Infarction: Sub-Analysis of a Randomized Controlled Trial. Journal of Cardiovascular Development and Disease, 9(5), 153. https://doi.org/10.3390/jcdd9050153