Practical Approaches to Build and Sustain a Cardio-Oncology Clinic
Abstract
:1. Introduction
2. Cardio-Oncology Team Members
3. Services of a Cardio-Oncology Program
- i.
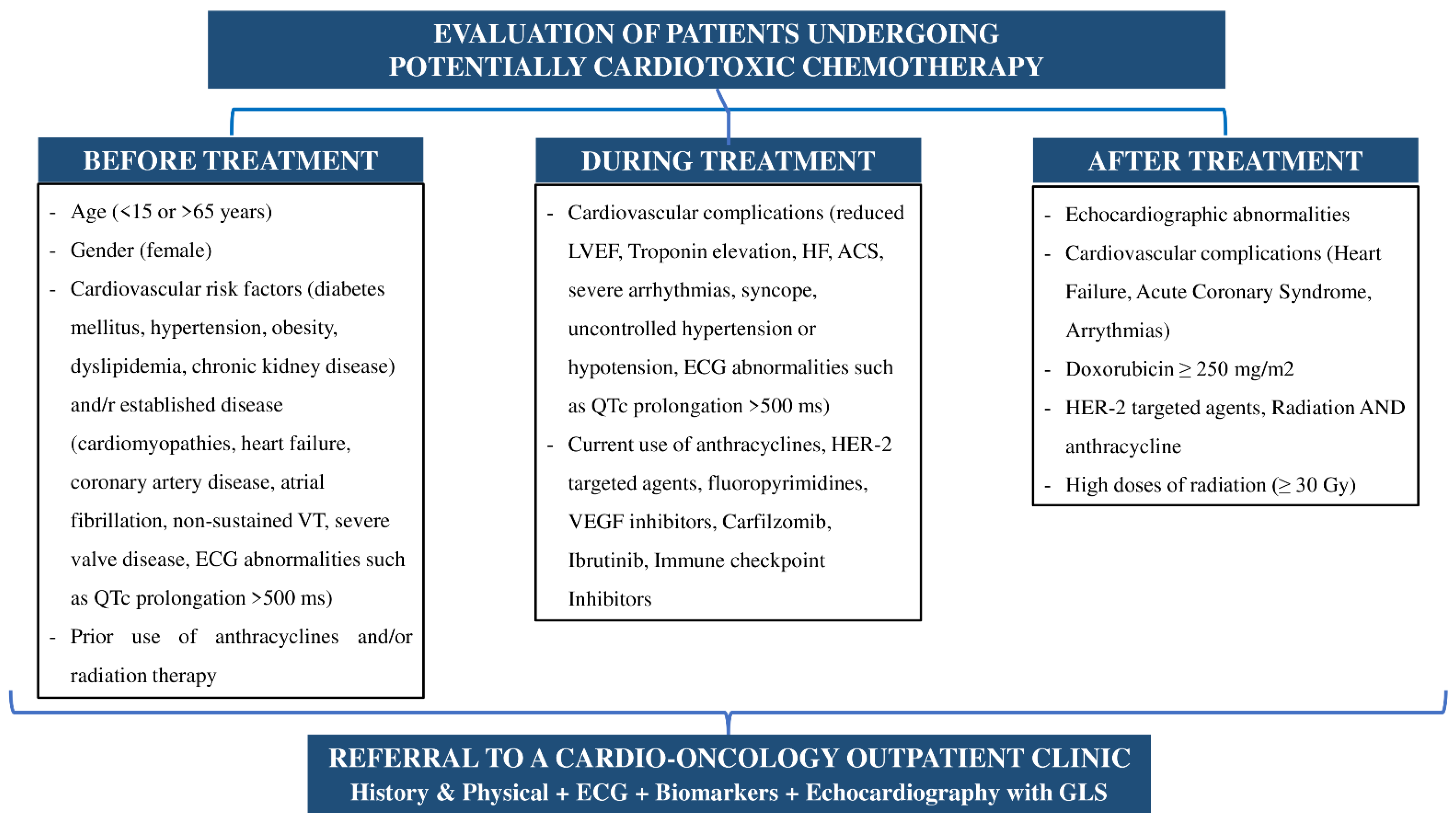
- The first time point is immediately after the diagnosis of the disease and before any treatment is administered. The purpose of this stage is the initial risk stratification for cardiotoxicity and the possibility of a potential cardiac monitoring and/or treatment [21].
- ii.
- The second time point is during chemotherapy at regular intervals depending on the type of treatment and especially when heart failure symptoms or signs appear [21].
- iii.
- The last, but not least important timepoint is after the end of treatment with chemotherapeutics and/or radiation, especially in patients who have experienced cardiotoxicity or are a candidate to develop long-term complications of the cardiovascular system [21]. The evaluation of patients with cancer and steps of the development of the cardio-oncology clinic are summarized in Figure 1 and Figure 2.
4. Patient Referral Criteria
- i.
- Medical history of established cardiovascular disease or multiple (two or more) cardiovascular risk factors.
- ii.
- History of previous cardiotoxicity, receiving a potentially cardiotoxic chemotherapy, or history of previous radiotherapy in the chest area.
- iii.
- Onset of symptoms or signs of heart failure before, during, or after cancer treatment.
- iv.
- Special priority should be given to a pre-operative evaluation of patients with solid tumor cancer who are candidates to undergo surgery or patients with types of cancer that directly affect the cardiovascular system (amyloidosis and cardiac tumors).
5. Follow-Up Protocols
6. Prevention Strategies
7. Long-Term Oncology Patient Follow-Up
8. Limitations
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cardinale, D.; Colombo, A.; Lamantia, G.; Colombo, N.; Civelli, M.; De Giacomi, G.; Rubino, M.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Anthracycline-induced cardiomyopathy: Clinical relevance and response to pharmacologic therapy. JACC 2010, 55, 213–220. [Google Scholar] [CrossRef] [Green Version]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Curigliano, G.; Cardinale, D.; Dent, S.; Criscitiello, C.; Aseyev, O.; Lenihan, D.; Cipolla, C.M. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J. Clin. 2016, 66, 309–325. [Google Scholar] [CrossRef] [Green Version]
- Leemasawat, K.; Phrommintikul, A.; Chattipakorn, S.C.; Chattipakorn, N. Mechanisms and potential interventions associated with the cardiotoxicity of ErbB2-targeted drugs: Insights from in vitro, in vivo, and clinical studies in breast cancer patients. Cell. Mol. Life Sci. 2020, 77, 1571–1589. [Google Scholar] [CrossRef]
- Deidda, M.; Madonna, R.; Mango, R.; Pagliaro, P.; Bassareo, P.P.; Cugusi, L.; Romano, S.; Penco, M.; Romeo, F.; Mercuro, G. Novel insights in pathophysiology of antiblastic drugs-induced cardiotoxicity and cardioprotection. J. Cardiovasc. Med. 2016, 17 (Suppl. 1), e76–e83. [Google Scholar] [CrossRef] [Green Version]
- Briasoulis, A.; Chasouraki, A.; Sianis, A.; Panagiotou, N.; Kourek, C.; Ntalianis, A.; Paraskevaidis, I. Cardiotoxicity of Non-Anthracycline Cancer Chemotherapy Agents. J. Cardiovasc. Dev. Dis. 2022, 9, 66. [Google Scholar] [CrossRef]
- van Ramshorst, M.S.; van Werkhoven, E.; Honkoop, A.H.; Dezentjé, V.O.; Oving, I.O.; Mandjes, I.A.; Kemper, I.; Smorenburg, C.H.; Stouthard, J.A.; Linn, S.C. Dutch Breast Cancer Research Group (BOOG). Toxicity of dual HER2-blockade with pertuzumab added to anthracycline versus non-anthracycline containing chemotherapy as neoadjuvant treatment in HER2-positive breast cancer: The TRAIN-2 study. Breast 2016, 29, 153–159. [Google Scholar] [CrossRef]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar]
- Sadler, D.; Arnold, A.; Herrmann, J.; Daniele, A.; Silva, C.M.P.D.C.; Ghosh, A.K.; Szmit, S.; Khan, R.I.; Raez, L.; Blaes, A. Reaching Across the Aisle: Cardio-Oncology Advocacy and Program Building. Curr. Oncol. Rep. 2021, 23, 64. [Google Scholar] [CrossRef]
- Sundlöf, D.W.; Patel, B.D.; Schadler, K.C.; Biggs, R.G.; Silverstei-Fadlon, C.A.; Corotto, P.S.; Tolay, S.; Nadeem, A.J.; Gupta, R.; Ahmad, N.V. Development of a Cardio-Oncology Program in a Community Hospital. JACC Cardio Oncol. 2019, 1, 310–313. [Google Scholar] [CrossRef]
- Lancellotti, P.; Sutter, T.; López-Fernández, T.; Galderisi, M.; Lyon, A.R.; Van der Meer, P.; Cohen Solal, A.; Zamorano, J.L.; Jerusalem, G.; Moonen, M.; et al. Cardio-Oncology Services: Rationale, organization, and implementation: A report from the ESC Cardio-Oncology council. Eur. Heart J. 2019, 40, 1756–1763. [Google Scholar] [CrossRef] [PubMed]
- Barros-Gomes, S.; Herrmann, J.; Mulvagh, S.L.; Lerman, A.; Lin, G.; Villarraga, H.R. Rationale for setting up a cardio-oncology unit: Our experience at Mayo Clinic. Cardio Oncol. 2016, 2, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canale, M.L.; Camerini, A.; Magnacca, M.; Casolo, G.; Associazione Nazionale Medici Cardiologi Ospedalieri (ANMCO) Tuscany. A cardio-oncology experience in Italy: Results of a Tuscany regional-based survey. J. Cardiovasc. Med. 2014, 15, 135–140. [Google Scholar] [CrossRef]
- Task force of the Hellenic Heart Failure Clinics Network. How to develop a national heart failure clinics network: A consensus document of the Hellenic Heart Failure Association. ESC Heart Fail. 2020, 7, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ades, F.; Zardavas, D.; Pinto, A.C.; Criscitiello, C.; Aftimos, P.; de Azambuja, E. Cardiotoxicity of systemic agents used in breast cancer. Breast 2014, 23, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Chargari, C.; Guy, J.B.; Falk, A.T.; Schouver, E.D.; Trone, J.C.; Moncharmont, C.; Védrine, L.; Magné, N. Cardiotoxicity research in breast cancer patients: Past and future. Am. J. Cardiol. 2014, 113, 1447–1448. [Google Scholar] [CrossRef]
- Chargari, C.; Kirov, K.M.; Bollet, M.A.; Magné, N.; Védrine, L.; Cremades, S.; Beuzeboc, P.; Fourquet, A.; Kirova, Y.M. Cardiac toxicity in breast cancer patients: From a fractional point of view to a global assessment. Cancer Treat. Rev. 2011, 37, 321–330. [Google Scholar] [CrossRef]
- Daher, I.; Daigle, T.; Bhatia, N.; Durand, J. The prevention of cardiovascular disease in cancer survivors. Tex. Heart Inst. J. 2012, 39, 190–198. [Google Scholar]
- Jensen, S.A.; Hasbak, P.; Mortensen, J.; Sørensen, J.B. Fluorouracil induces myocardial ischemia with increases of plasma brain natriuretic peptide and lactic acid but without dysfunction of left ventricle. J. Clin. Oncol. 2010, 28, 5280–5286. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; et al.; ESC Scientific Document Group 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart. J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Herrmann, J.; Lerman, A.; Sandhu, N.P.; Villarraga, H.R.; Mulvagh, S.L.; Kohli, M. Evaluation and management of patients with heart disease and cancer: Cardio-oncology. Mayo Clin. Proc. 2014, 89, 1287–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taskforce of the Hellenic Heart Failure Clinics Network. Distribution, infrastructure, and expertise of heart failure and cardio-oncology clinics in a developing network: Temporal evolution and challenges during the coronavirus disease 2019 pandemic. ESC Heart Fail. 2020, 7, 3408–3413. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.B.; Ewer, M.; et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 893–911. [Google Scholar] [CrossRef] [PubMed]
- Groarke, J.; Nguyen, P.; Nohria, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Cardiovascular complications of radiation therapy for thoracic malignancies: The role for non-invasive imaging for detection of cardiovascular disease. Eur. Heart J. 2014, 35, 612–623. [Google Scholar] [CrossRef] [Green Version]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.; et al. Expert Consensus for Multimodality Imaging Evaluation of Adult Patients during and after Cancer Therapy: A Report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2014, 27, 911–939. [Google Scholar] [CrossRef] [Green Version]
- Cardinale, D.; Colombo, A.; Torrisi, R.; Sandri, M.T.; Civelli, M.; Salvatici, M.; Lamantia, G.; Colombo, N.; Cortinovis, S.; Dessanai, M.A.; et al. Trastuzumab-Induced Cardiotoxicity: Clinical and Prognostic Implications of Troponin I Evaluation. J. Clin. Oncol. 2010, 28, 3910–3916. [Google Scholar] [CrossRef]
- Cardinale, D.; Sandri, M.T.; Colombo, A.; Colombo, N.; Boeri, M.; Lamantia, G.; Civelli, M.; Peccatori, F.; Martinelli, G.; Fiorentini, C.; et al. Prognostic Value of Troponin I in Cardiac Risk Stratification of Cancer Patients Undergoing High-Dose Chemotherapy. Circulation 2004, 109, 2749–2754. [Google Scholar] [CrossRef] [Green Version]
- Curigliano, C.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef] [Green Version]
- Guglin, M.; Krischer, J.; Tamura, R.; Fink, A.; Bello-Matricaria, L.; McCaskill-Stevens, W.; Munster, P.N. Randomized Trial of Lisinopril vs. Carvedilol to Prevent Trastuzumab Cardiotoxicity in Patients with Breast Cancer. J. Am. Coll. Cardiol. 2019, 73, 2859–2868. [Google Scholar] [CrossRef]
- Bosch, X.; Rovira, M.; Sitges, M.; Domènech, A.; Ortiz-Pérez, J.T.; de Caralt, T.M.; Morales-Ruiz, M.; Perea, R.J.; Monzó, M.; Esteve, J. Enalapril and Carvedilol for Preventing Chemotherapy-Induced Left Ventricular Systolic Dysfunction in Patients with Malignant Hemopathies: The OVERCOME Trial (prevention of left ventricular dysfunction with Enalapril and carvedilol in patients submitted to intensive chemotherapy for the treatment of malignant hemopathies). J. Am. Coll. Cardiol. 2013, 23, 2355–2362. [Google Scholar]
- Cardinale, D.; Colombo, A.; Sandri, M.; Lamantia, G.; Colombo, N.; Civelli, M.; Martinelli, G.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Prevention of High-Dose Chemotherapy–Induced Cardiotoxicity in High-Risk Patients by Angiotensin-Converting Enzyme Inhibition. Circulation 2006, 114, 2474–2481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewinter, C.; Nielsen, T.H.; Edfords, L.R.; Linde, C.; Bland, J.M.; LeWinter, M.; Cleland, J.G.F.; Køber, L.; Braunschweig, F.; Mansson-Broberg, A.A. Systematic review and meta-analysis of beta-blockers and renin–angiotensin system inhibitors for preventing left ventricular dysfunction due to anthracyclines or trastuzumab in patients with breast cancer. Eur. Heart J. 2021, ehab843. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Hirji, S.A.; Qamar, A.; Bajaj, N.; Gupta, A.; Zaha, V.; Chandra, A.; Haykowsky, M.; Ky, B.; Moslehi, J.; et al. Efficacy of Neurohormonal Therapies in Preventing Cardiotoxicity in Patients with Cancer Undergoing Chemotherapy. JACC Cardio Oncol. 2019, 1, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.L.; Barlow, M.; Barrett-Lee, P.J.; Canney, P.A.; Gilmour, I.M.; Robb, S.D.; Plummer, C.J.; Wardley, A.M.; Verrill, M.W. Management of cardiac health in trastuzumab-treated patients with breast cancer: Updated United Kingdom National Cancer Research Institute recommendations for monitoring. Br. J. Cancer 2009, 100, 684–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chasouraki, A.; Kourek, C.; Sianis, A.; Loritis, K.; Kostakou, P.; Tsougos, E.; Paraskevaidis, I.; Ntalianis, A.; Briasoulis, A. Practical Approaches to Build and Sustain a Cardio-Oncology Clinic. J. Cardiovasc. Dev. Dis. 2022, 9, 158. https://doi.org/10.3390/jcdd9050158
Chasouraki A, Kourek C, Sianis A, Loritis K, Kostakou P, Tsougos E, Paraskevaidis I, Ntalianis A, Briasoulis A. Practical Approaches to Build and Sustain a Cardio-Oncology Clinic. Journal of Cardiovascular Development and Disease. 2022; 9(5):158. https://doi.org/10.3390/jcdd9050158
Chicago/Turabian StyleChasouraki, Angeliki, Christos Kourek, Alexandros Sianis, Konstantinos Loritis, Peggy Kostakou, Elias Tsougos, Ioannis Paraskevaidis, Argyrios Ntalianis, and Alexandros Briasoulis. 2022. "Practical Approaches to Build and Sustain a Cardio-Oncology Clinic" Journal of Cardiovascular Development and Disease 9, no. 5: 158. https://doi.org/10.3390/jcdd9050158
APA StyleChasouraki, A., Kourek, C., Sianis, A., Loritis, K., Kostakou, P., Tsougos, E., Paraskevaidis, I., Ntalianis, A., & Briasoulis, A. (2022). Practical Approaches to Build and Sustain a Cardio-Oncology Clinic. Journal of Cardiovascular Development and Disease, 9(5), 158. https://doi.org/10.3390/jcdd9050158







