Frailty Index and Cardiovascular Disease among Middle-Aged and Older Chinese Adults: A Nationally Representative Cross-Sectional and Follow-Up Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measurement
2.2.1. Frailty Index
2.2.2. Outcomes
2.2.3. Covariates
2.3. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Association between the Frailty Index and CVD in Cross-Sectional Analysis
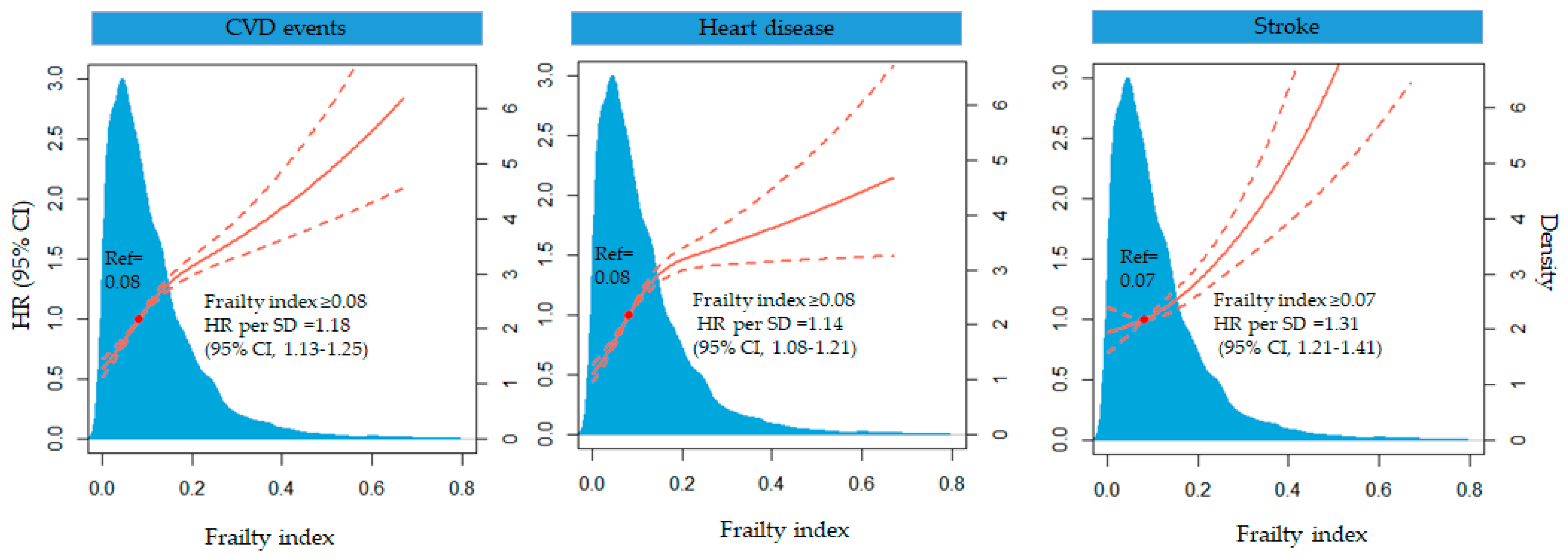
3.3. Association between the Frailty Index and CVD Events in Cohort Analysis
3.4. Subgroup Analysis
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.; Li, Y.; Zeng, X.; Wang, H.; Yin, P.; Wang, L.; Liu, Y.; Liu, J.; Qi, J.; Ran, S.; et al. Burden of Cardiovascular Diseases in China, 1990-2016: Findings From the 2016 Global Burden of Disease Study. JAMA Cardiol. 2019, 4, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Y.; Liu, J.; Yin, P.; Wang, L.; Qi, J.; You, J.; Lin, L.; Meng, S.; Wang, F.; et al. Mortality and years of life lost of cardiovascular diseases in China, 2005-2020: Empirical evidence from national mortality surveillance system. Int. J. Cardiol. 2021, 340, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yu, C.; Guo, Y.; Bian, Z.; Sun, Z.; Yang, L.; Chen, Y.; Du, H.; Li, Z.; Lei, Y.; et al. Frailty index and all-cause and cause-specific mortality in Chinese adults: A prospective cohort study. Lancet Public Health 2020, 5, e650–e660. [Google Scholar] [CrossRef]
- Kim, S.; Myers, L.; Wyckoff, J.; Cherry, K.E.; Jazwinski, S.M. The frailty index outperforms DNA methylation age and its derivatives as an indicator of biological age. Geroscience 2017, 39, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Hanlon, P.; Nicholl, B.I.; Jani, B.D.; Lee, D.; McQueenie, R.; Mair, F.S. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: A prospective analysis of 493 737 UK Biobank participants. Lancet Public Health 2018, 3, e323–e332. [Google Scholar] [CrossRef]
- Boyd, C.M.; Xue, Q.L.; Simpson, C.F.; Guralnik, J.M.; Fried, L.P. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am. J. Med. 2005, 118, 1225–1231. [Google Scholar] [CrossRef]
- Song, Y.; Deng, Y.; Li, J.; Hao, B.; Cai, Y.; Chen, J.; Shi, H.; Xu, W. Associations of falls and severe falls with blood pressure and frailty among Chinese community-dwelling oldest olds: The Chinese Longitudinal Health and Longevity Study. Aging (Albany N. Y.) 2021, 13, 16527–16540. [Google Scholar] [CrossRef]
- Li, G.; Prior, J.C.; Leslie, W.D.; Thabane, L.; Papaioannou, A.; Josse, R.G.; Kaiser, S.M.; Kovacs, C.S.; Anastassiades, T.; Towheed, T.; et al. Frailty and Risk of Fractures in Patients With Type 2 Diabetes. Diabetes Care 2019, 42, 507–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooqi, M.A.M.; Gerstein, H.; Yusuf, S.; Leong, D.P. Accumulation of Deficits as a Key Risk Factor for Cardiovascular Morbidity and Mortality: A Pooled Analysis of 154,000 Individuals. J. Am. Heart Assoc. 2020, 9, e014686. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.M.; Theou, O.; Kirkland, S.A.; Rockwood, M.R.; Davidson, K.W.; Shimbo, D.; Rockwood, K. Accumulation of non-traditional risk factors for coronary heart disease is associated with incident coronary heart disease hospitalization and death. PLoS ONE 2014, 9, e90475. [Google Scholar] [CrossRef] [Green Version]
- Aguayo, G.A.; Vaillant, M.T.; Donneau, A.F.; Schritz, A.; Stranges, S.; Malisoux, L.; Chioti, A.; Guillaume, M.; Muller, M.; Witte, D.R. Comparative analysis of the association between 35 frailty scores and cardiovascular events, cancer, and total mortality in an elderly general population in England: An observational study. PLoS Med. 2018, 15, e1002543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Hu, Y.; Smith, J.P.; Strauss, J.; Yang, G. Cohort profile: The China Health and Retirement Longitudinal Study (CHARLS). Int. J. Epidemiol. 2014, 43, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Dupre, M.E.; Gu, D.; Warner, D.F.; Yi, Z. Frailty and type of death among older adults in China: Prospective cohort study. BMJ 2009, 338, b1175. [Google Scholar] [CrossRef] [Green Version]
- Bennett, S.; Song, X.; Mitnitski, A.; Rockwood, K. A limit to frailty in very old, community-dwelling people: A secondary analysis of the Chinese longitudinal health and longevity study. Age Ageing 2013, 42, 372–377. [Google Scholar] [CrossRef] [Green Version]
- Barker, F.J.; Davies, J.I.; Gomez-Olive, F.X.; Kahn, K.; Matthews, F.E.; Payne, C.F.; Salomon, J.A.; Tollman, S.M.; Wade, A.N.; Walker, R.W.; et al. Developing and evaluating a frailty index for older South Africans-findings from the HAALSI study. Age Ageing 2021, 50, 2167–2173. [Google Scholar] [CrossRef]
- Oude Voshaar, R.C.; Jeuring, H.W.; Borges, M.K.; van den Brink, R.H.S.; Marijnissen, R.M.; Hoogendijk, E.O.; van Munster, B.; Aprahamian, I. Course of frailty stratified by physical and mental multimorbidity patterns: A 5-year follow-up of 92,640 participants of the LifeLines cohort study. BMC Med. 2021, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Yan, L.; Zhong, B.; Yang, Z.; Xie, W. Progression of cognitive decline before and after incident stroke. Neurology 2019, 93, e20–e28. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zheng, D.; Li, Z.; Wu, Z.; Feng, W.; Cao, X.; Wang, J.; Gao, Q.; Li, X.; Wang, W.; et al. Association of Depressive Symptoms With Incident Cardiovascular Diseases in Middle-Aged and Older Chinese Adults. JAMA Netw. Open 2019, 2, e1916591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, K.; Cao, L.F.; Ma, W.Z.; Gao, Y.J.; Luo, M.S.; Zhu, J.; Li, T.; Zhou, D. Association between sarcopenia and cardiovascular disease among middle-aged and older adults: Findings from the China health and retirement longitudinal study. EClinicalMedicine 2022, 44, 101264. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Chen, X.; Crimmins, E.; Hu, P.P.; Kim, J.K.; Meng, Q.; Strauss, J.; Wang, Y.; Zeng, J.; Zhang, Y.; Zhao, Y. Venous Blood-Based Biomarkers in the China Health and Retirement Longitudinal Study: Rationale, Design, and Results From the 2015 Wave. Am. J. Epidemiol. 2019, 188, 1871–1877. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care 2010, 33 (Suppl. 1), S11–S61. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Qian, F.; Hou, C.; Li, X.; Gao, Q.; Luo, Y.; Tao, L.; Yang, X.; Wang, W.; Zheng, D.; et al. Longitudinal Changes in Depressive Symptoms and Risks of Cardiovascular Disease and All-Cause Mortality: A Nationwide Population-Based Cohort Study. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2200–2206. [Google Scholar] [CrossRef]
- Yao, S.S.; Cao, G.Y.; Han, L.; Chen, Z.S.; Huang, Z.T.; Gong, P.; Hu, Y.; Xu, B. Prevalence and Patterns of Multimorbidity in a Nationally Representative Sample of Older Chinese: Results From the China Health and Retirement Longitudinal Study. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1974–1980. [Google Scholar] [CrossRef]
- Zhao, Y.; Atun, R.; Oldenburg, B.; McPake, B.; Tang, S.; Mercer, S.W.; Cowling, T.E.; Sum, G.; Qin, V.M.; Lee, J.T. Physical multimorbidity, health service use, and catastrophic health expenditure by socioeconomic groups in China: An analysis of population-based panel data. Lancet Glob. Health 2020, 8, e840–e849. [Google Scholar] [CrossRef]
- Veronese, N.; Sigeirsdottir, K.; Eiriksdottir, G.; Marques, E.A.; Chalhoub, D.; Phillips, C.L.; Launer, L.J.; Maggi, S.; Gudnason, V.; Harris, T.B. Frailty and Risk of Cardiovascular Diseases in Older Persons: The Age, Gene/Environment Susceptibility-Reykjavik Study. Rejuvenation Res. 2017, 20, 517–524. [Google Scholar] [CrossRef] [Green Version]
- Marinus, N.; Vigorito, C.; Giallauria, F.; Haenen, L.; Jansegers, T.; Dendale, P.; Feys, P.; Meesen, R.; Timmermans, A.; Spildooren, J.; et al. Frailty is highly prevalent in specific cardiovascular diseases and females, but significantly worsens prognosis in all affected patients: A systematic review. Ageing Res. Rev. 2021, 66, 101233. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Gambassi, G.; van Kan, G.A.; Vellas, B. The frailty phenotype and the frailty index: Different instruments for different purposes. Age Ageing 2014, 43, 10–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damluji, A.A.; Chung, S.E.; Xue, Q.L.; Hasan, R.K.; Moscucci, M.; Forman, D.E.; Bandeen-Roche, K.; Batchelor, W.; Walston, J.D.; Resar, J.R.; et al. Frailty and cardiovascular outcomes in the National Health and Aging Trends Study. Eur. Heart J. 2021, 42, 3856–3865. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Xu, C.; Lu, Q.; Zhang, Y.; Cao, Z.; Li, S.; Yang, H.; Sun, L.; Cao, X.; Zhao, Y.; et al. Associations of frailty with cardiovascular disease and life expectancy: A prospective cohort study. Arch. Gerontol. Geriatr. 2022, 99, 104598. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Soysal, P.; Arik, F.; Smith, L.; Jackson, S.E.; Isik, A.T. Inflammation, Frailty and Cardiovascular Disease. Adv. Exp. Med. Biol. 2020, 1216, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Stewart, R. Cardiovascular Disease and Frailty: What Are the Mechanistic Links? Clin. Chem. 2019, 65, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-I.; Chan, D.-C.; Kuo, K.-N.; Hsiung, C.A.; Chen, C.-Y. Prevalence and Correlates of Geriatric Frailty in a Northern Taiwan Community. J. Formosan Med. Assoc. 2011, 110, 247–257. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Guan, S.; Ding, H.; Wang, Z.; Zhang, J.; Zhao, J.; Ma, J.; Chan, P. Prevalence and Incidence of Frailty in Community-Dwelling Older People: Beijing Longitudinal Study of Aging II. J. Am. Geriatr. Soc. 2016, 64, 1281–1286. [Google Scholar] [CrossRef]
- Wu, C.; Smit, E.; Xue, Q.L.; Odden, M.C. Prevalence and Correlates of Frailty Among Community-Dwelling Chinese Older Adults: The China Health and Retirement Longitudinal Study. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 102–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Tang, Z.; Zhang, L.; Sun, F.; Li, Y.; Chan, P. Prevalence of Frailty and Associated Factors in the Community-Dwelling Population of China. J. Am. Geriatr. Soc. 2018, 66, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Gao, J.; Fu, H. Associations between lifestyle, physical and social environments and frailty among Chinese older people: A multilevel analysis. BMC Geriatr. 2018, 18, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, Y.; Guo, Y.; Kowal, P.; Lu, Y.; Liu, C.; Sun, S.; Huang, Z.; Zheng, Y.; Wang, W.; Li, G.; et al. Association between anemia and frailty in 13,175 community-dwelling adults aged 50 years and older in China. BMC Geriatr. 2019, 19, 327. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xu, L.; Sun, L.; Li, J.; Qin, W. Gender difference in the association of frailty and health care utilization among Chinese older adults: Results from a population-based study. Aging Clin. Exp. Res. 2020, 32, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Lv, X.; Du, J.; Kong, G.; Zhang, L. Age- and Gender-Specific Prevalence of Frailty and Its Outcomes in the Longevous Population: The Chinese Longitudinal Healthy Longevity Study. Front. Med. 2021, 8, 719806. [Google Scholar] [CrossRef]
- Orkaby, A.R. Moving beyond chronological age: Frailty as an important risk factor for cardiovascular disease. Eur. Heart J. 2021, 42, 3866–3868. [Google Scholar] [CrossRef]
- Afilalo, J.; Alexander, K.P.; Mack, M.J.; Maurer, M.S.; Green, P.; Allen, L.A.; Popma, J.J.; Ferrucci, L.; Forman, D.E. Frailty assessment in the cardiovascular care of older adults. J. Am. Coll. Cardiol. 2014, 63, 747–762. [Google Scholar] [CrossRef] [Green Version]
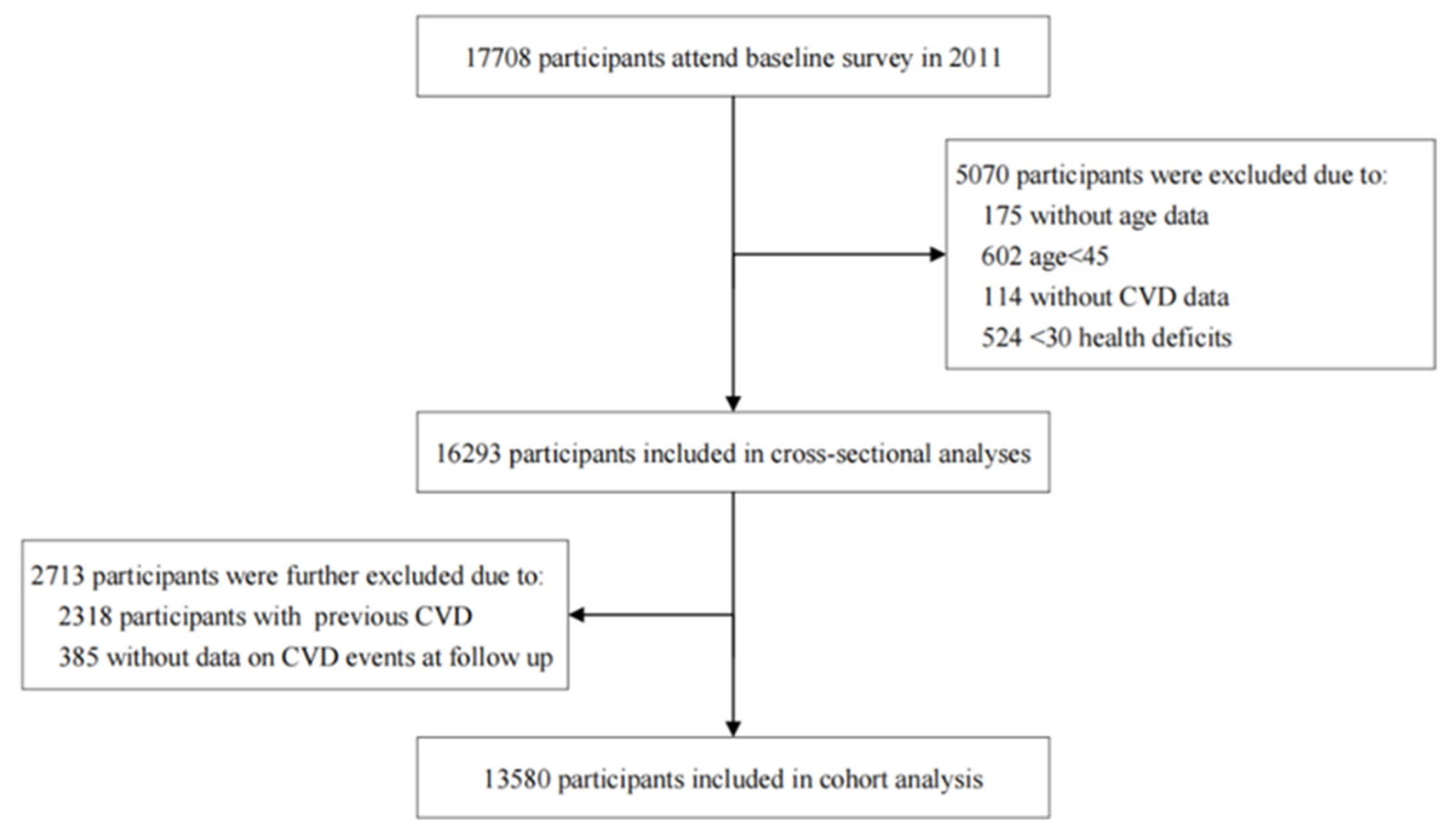

| Characteristic | Total (N = 13,580) | Robust (N = 7917) | Pre-Frailty (N = 4583) | Frailty (N = 1080) | p Value |
|---|---|---|---|---|---|
| Age (mean ± SD) | 58.43 ± 9.54 | 56.53 ± 8.62 | 60.01 ± 9.61 | 65.65 ± 10.86 | <0.0001 |
| Sex, Male, n (%) | 6787 (49.98) | 3489 (44.07) | 2628 (57.34) * | 670 (62.04) *,† | <0.0001 |
| Education level, n (%) | <0.0001 | ||||
| No formal education (Illiterate) | 3713 (27.34) | 1555 (19.64) | 1594 (34.78) * | 564 (52.22) *,† | |
| Elementary school | 5273 (38.83) | 2914 (36.81) | 1975 (43.09) * | 384 (35.56) *,† | |
| Junior high school or above | 4594 (33.83) | 3448 (43.55) | 1014 (22.13) * | 132 (12.22) *,† | |
| Married, n (%) | 11,002 (81.02) | 6586 (83.19) | 3639 (79.40) * | 777 (71.94) *,† | <0.0001 |
| Residence, rural, n (%) | 8322 (61.28) | 4355 (55.01) | 3166 (69.08) * | 801 (74.17) *,† | <0.0001 |
| Current smoker, n (%) a | 4339 (32.14) | 2755 (35.08) | 1337 (29.25) * | 247 (23.00) *,† | <0.0001 |
| Drinker, n (%) | 4773 (35.15) | 3180 (40.17) | 1371 (29.91) * | 222 (20.56) *,† | <0.0001 |
| Comorbidities a | |||||
| Hypertension, n (%) | 2917 (21.52) | 1468 (18.56) | 1113 (24.34) * | 336 (31.26) *,† | <0.0001 |
| Diabetes mellitus, n (%) | 663 (4.89) | 323 (4.08) | 254 (5.56) * | 86 (8.01) *,† | <0.0001 |
| Dyslipidemia, n (%) | 1033 (7.65) | 558 (7.07) | 381 (8.36) * | 94 (8.88) *,† | 0.0039 |
| History of medication use, n (%) a | |||||
| Antihypertensive medications | 2114 (15.60) | 1029 (13.02) | 817 (17.87) * | 268 (24.98) *,† | <0.0001 |
| Antidiabetic medications | 430 (3.17) | 196 (2.48) | 168 (3.68) * | 66 (6.16) *,† | <0.0001 |
| Lipid-lowering therapy | 470 (3.48) | 231 (2.93) | 185 (4.06) * | 54 (5.10) *,† | <0.0001 |
| Height, m (mean ± SD) | 1.58 ± 0.09 | 1.60 ± 0.08 | 1.57 ± 0.08 * | 1.54 ± 0.09 *,† | <0.0001 |
| Weight, kg (mean ± SD) | 58.46 ± 11.67 | 60.26 ± 11.63 | 56.70 ± 11.18 * | 53.78 ± 11.67 *,† | <0.0001 |
| BMI, Kg/m2 (mean ± SD) a | 23.30 ± 3.86 | 23.58 ± 3.79 | 23.03 ± 3.92 * | 22.56 ± 3.95 *,† | <0.0001 |
| SBP (mean ± SD) a | 129.00 ± 21.13 | 128.43 ± 20.26 | 128.94 ± 21.64 | 133.01 ± 24.02 *,† | <0.0001 |
| DBP (mean ± SD) a | 76.67 ± 12.78 | 77.16 ± 12.74 | 75.85 ± 12.67 * | 77.01 ± 13.31 | <0.0001 |
| Biomarkers b,c | |||||
| FBG, mg/dL | 102.24 (94.32, 112.86) | 102.24 (94.32, 113.04) | 101.70 (93.78, 112.14) | 102.60 (94.68, 116.10) † | 0.0174 |
| HbA1c, % | 5.1 (4.9, 5.4) | 5.1 (4.9, 5.4) | 5.1 (4.9, 5.4) | 5.2 (4.9, 5.5) | 0.0093 |
| TC mg/dL | 192.63 ± 37.73 | 192.54 ± 38.11 | 193.16 ± 37.03 | 190.98 ± 38.06 | 0.3595 |
| TG, mg/dL | 104.43 (74.34, 152.22) | 104.43 (74.34, 153.88) | 103.55 (74.34, 147.80) | 106.20 (77.00, 157.53) | 0.2979 |
| LDL-c, mg/dL | 115.88 ± 34.62 | 115.88 ± 35.107 | 116.38 ± 34.06 | 113.38 ± 33.87 | 0.1055 |
| HDL-c, mg/dL | 51.43 ± 15.32 | 50.76 ± 15.09 | 52.45 ± 15.38 * | 51.61 ± 16.36 | <0.0001 |
| eGFR, mL/min/1.73 m2 | 90.16 (71.50, 102.34) | 91.88 (75.45, 103.68) | 88.40 (68.67, 101.11) * | 84.28 (61.33, 98.44) *,† | <0.0001 |
| Frailty index, mean (IQR) | 0.08 (0.04, 0.15) | 0.05 (0.02, 0.07) | 0.15 (0.12, 0.19) * | 0.32 (0.28, 0.39) *,† | <0.0001 |
| Outcome | Case/N | Incidence per 1000 Person-Years | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 3 HR (95% CI) |
|---|---|---|---|---|---|
| CVD events | |||||
| Robust | 954/7917 | 22.47 | Reference | Reference | Reference |
| Pre-frailty | 890/4583 | 36.52 | 1.53 (1.39, 1.68) *** | 1.51 (1.37, 1.66) *** | 1.53 (1.39, 1.68) *** |
| Frailty | 278/1080 | 62.21 | 2.19 (1.91, 2.52) *** | 2.13 (1.85, 2.46) *** | 2.17 (1.88, 2.50) *** |
| Per 0.1 increment | 1.30 (1.25, 1.35) *** | 1.28 (1.23, 1.33) *** | 1.29 (1.24, 1.34) *** | ||
| Heart disease | |||||
| Robust | 707/7917 | 16.43 | Reference | Reference | Reference |
| Pre-frailty | 701/4583 | 28.30 | 1.61 (1.44, 1.79) *** | 1.60 (1.44, 1.79) *** | 1.62 (1.45, 1.81) *** |
| Frailty | 209/1080 | 41.60 | 2.14 (1.82, 2.52) *** | 2.19 (1.80, 2.50) *** | 2.16 (1.83, 2.56) *** |
| Per 0.1 increment | 1.28 (1.23, 1.34) *** | 1.27 (1.21, 1.33) *** | 1.28 (1.22, 1.34) *** | ||
| Stroke | |||||
| Robust | 322/7917 | 7.25 | Reference | Reference | Reference |
| Pre-frailty | 256/4583 | 9.71 | 1.23 (1.07, 1.50) ** | 1.20 (1.01, 1.43) * | 1.23 (1.03, 1.46) * |
| Frailty | 102/1080 | 18.38 | 2.26 (1.79, 2.86) *** | 2.01 (1.59, 2.53) *** | 2.06 (1.62, 2.63) *** |
| Per 0.1 increment | 1.31 (1.23, 1.41) *** | 1.27 (1.18, 1.36) *** | 1.28 (1.20, 1.37) *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Dai, G.; He, Q.; Ma, H.; Hu, H. Frailty Index and Cardiovascular Disease among Middle-Aged and Older Chinese Adults: A Nationally Representative Cross-Sectional and Follow-Up Study. J. Cardiovasc. Dev. Dis. 2022, 9, 228. https://doi.org/10.3390/jcdd9070228
Liu X, Dai G, He Q, Ma H, Hu H. Frailty Index and Cardiovascular Disease among Middle-Aged and Older Chinese Adults: A Nationally Representative Cross-Sectional and Follow-Up Study. Journal of Cardiovascular Development and Disease. 2022; 9(7):228. https://doi.org/10.3390/jcdd9070228
Chicago/Turabian StyleLiu, Xinyao, Guolin Dai, Qile He, Hao Ma, and Hongpu Hu. 2022. "Frailty Index and Cardiovascular Disease among Middle-Aged and Older Chinese Adults: A Nationally Representative Cross-Sectional and Follow-Up Study" Journal of Cardiovascular Development and Disease 9, no. 7: 228. https://doi.org/10.3390/jcdd9070228
APA StyleLiu, X., Dai, G., He, Q., Ma, H., & Hu, H. (2022). Frailty Index and Cardiovascular Disease among Middle-Aged and Older Chinese Adults: A Nationally Representative Cross-Sectional and Follow-Up Study. Journal of Cardiovascular Development and Disease, 9(7), 228. https://doi.org/10.3390/jcdd9070228





