Shear-Thinning Extrudable Hydrogels Based on Star Polypeptides with Antimicrobial Properties
Abstract
1. Introduction
2. Results and Discussion
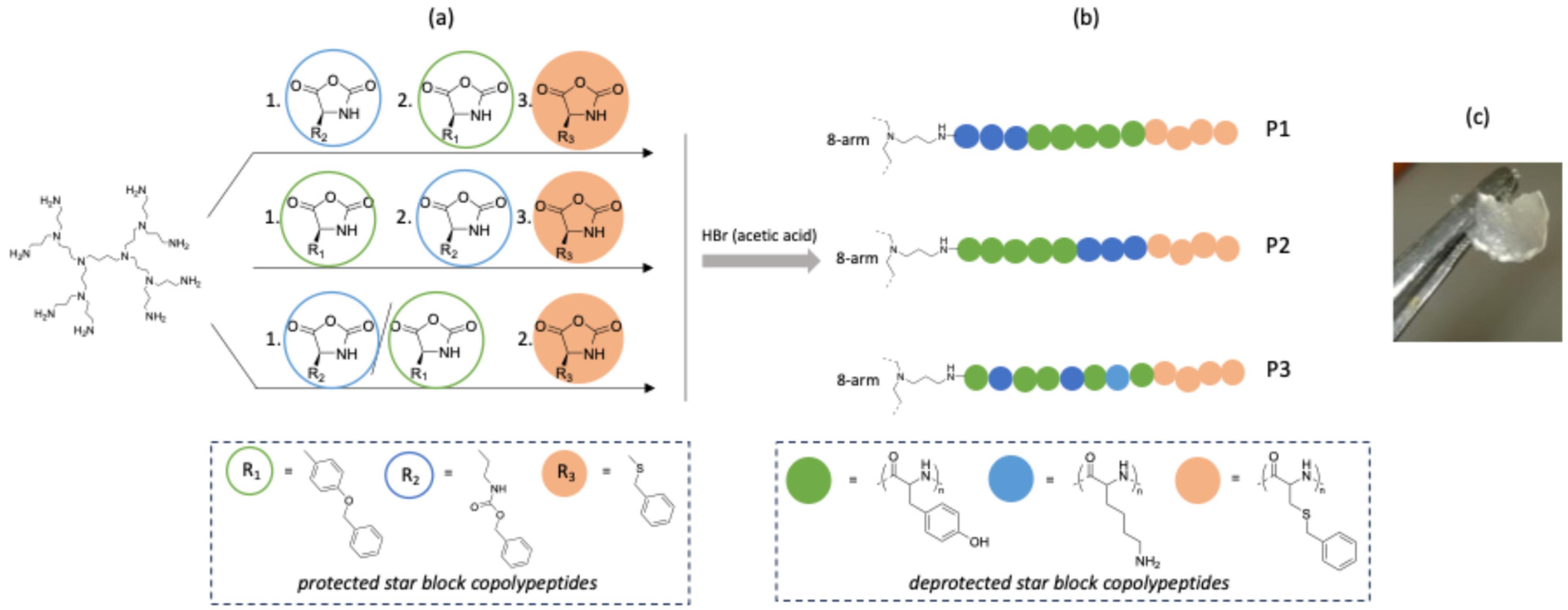
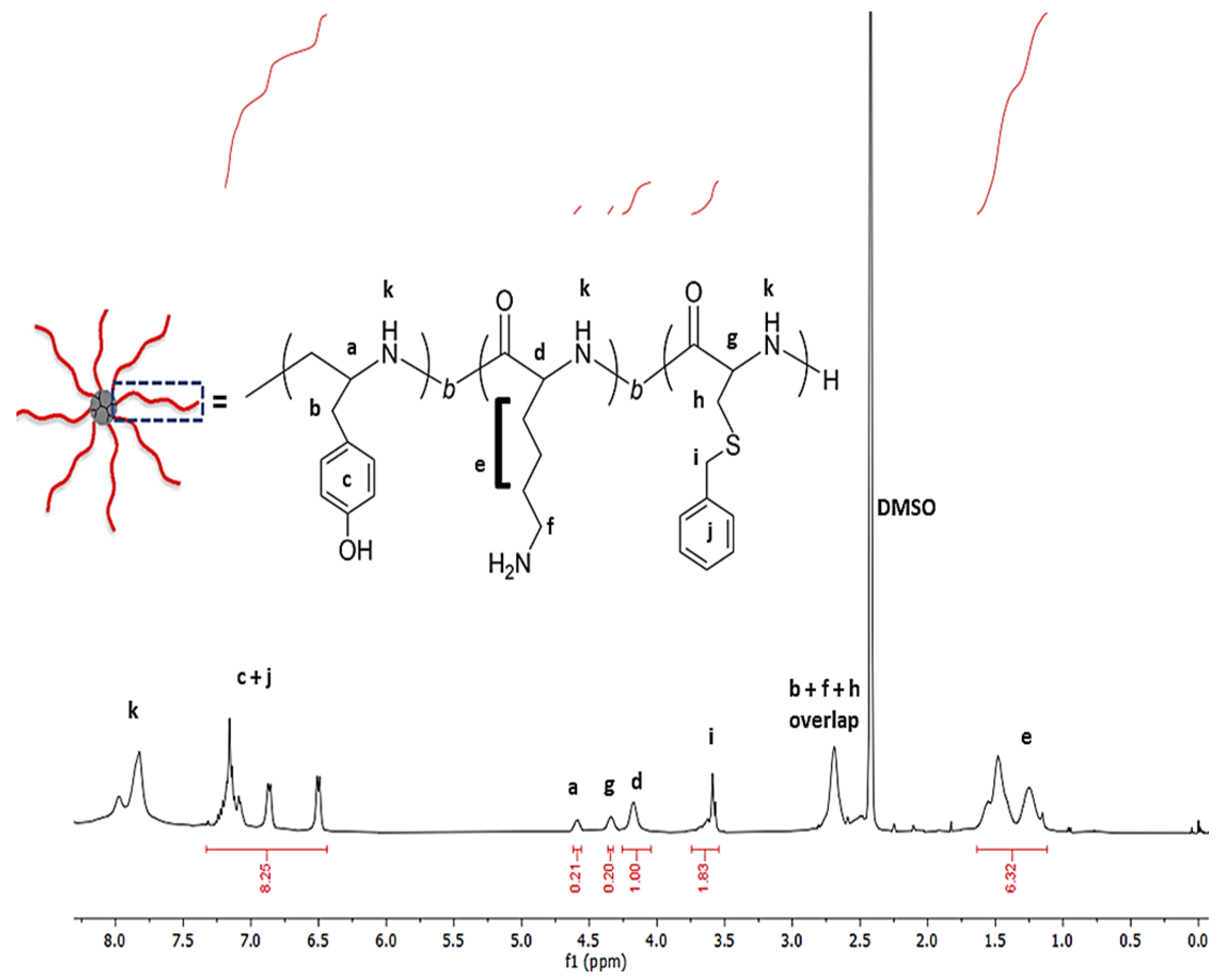
2.1. Design, Synthesis and Characterization of Star Polypeptides
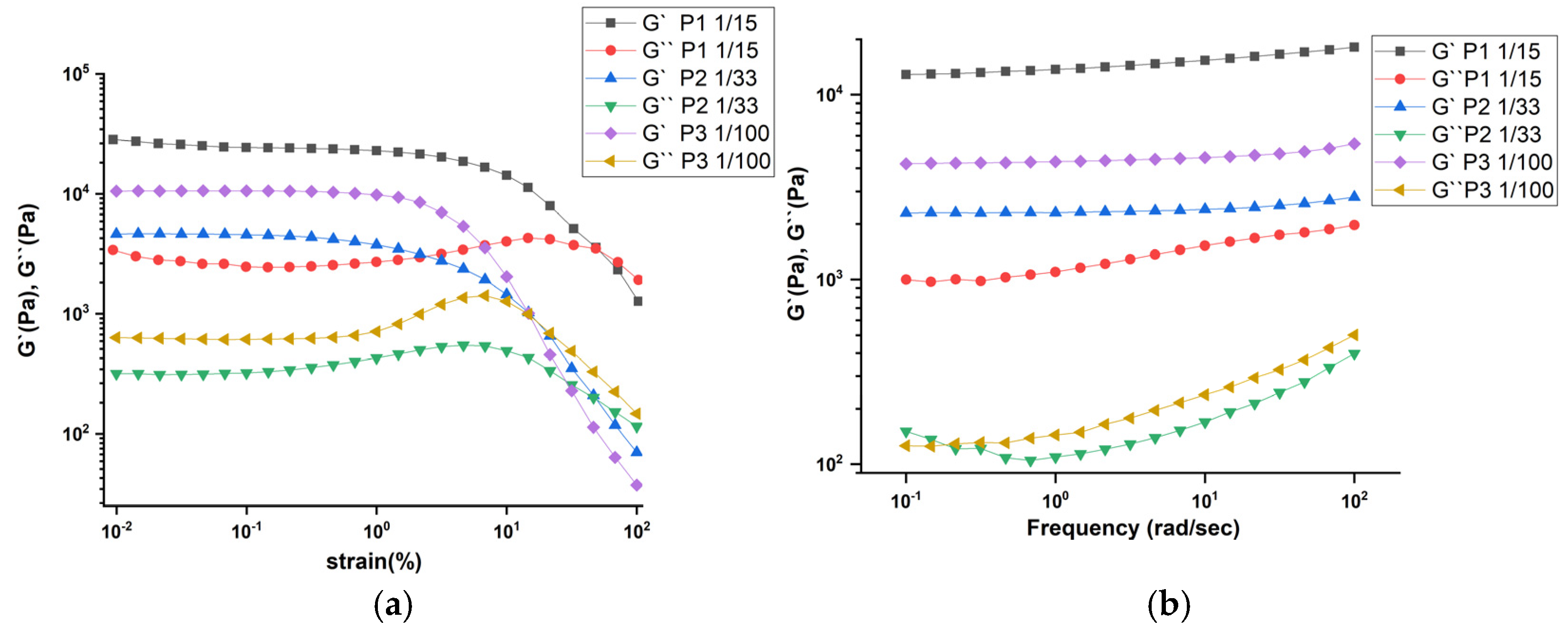
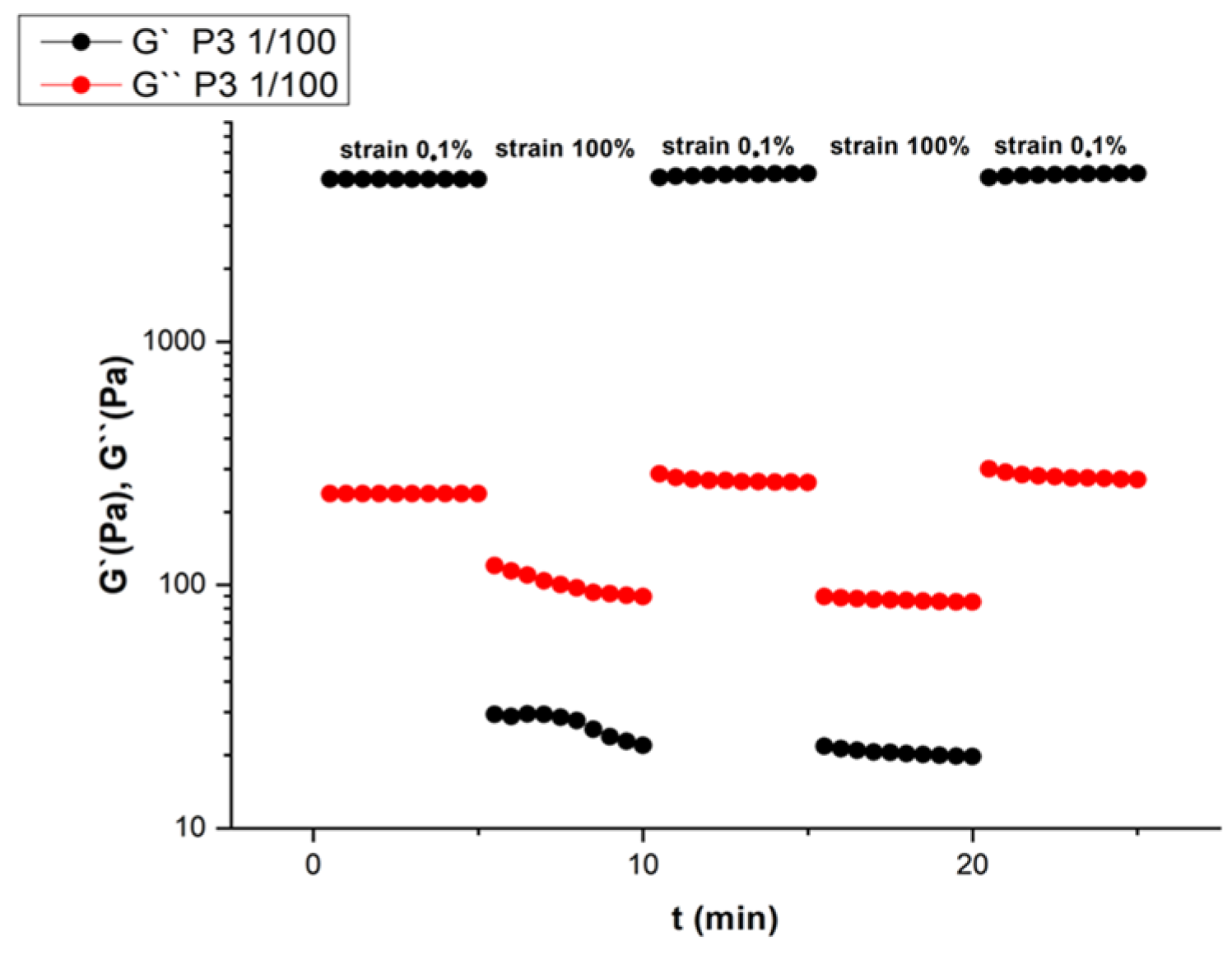
2.2. Rheological Investigation and Hydrogelation
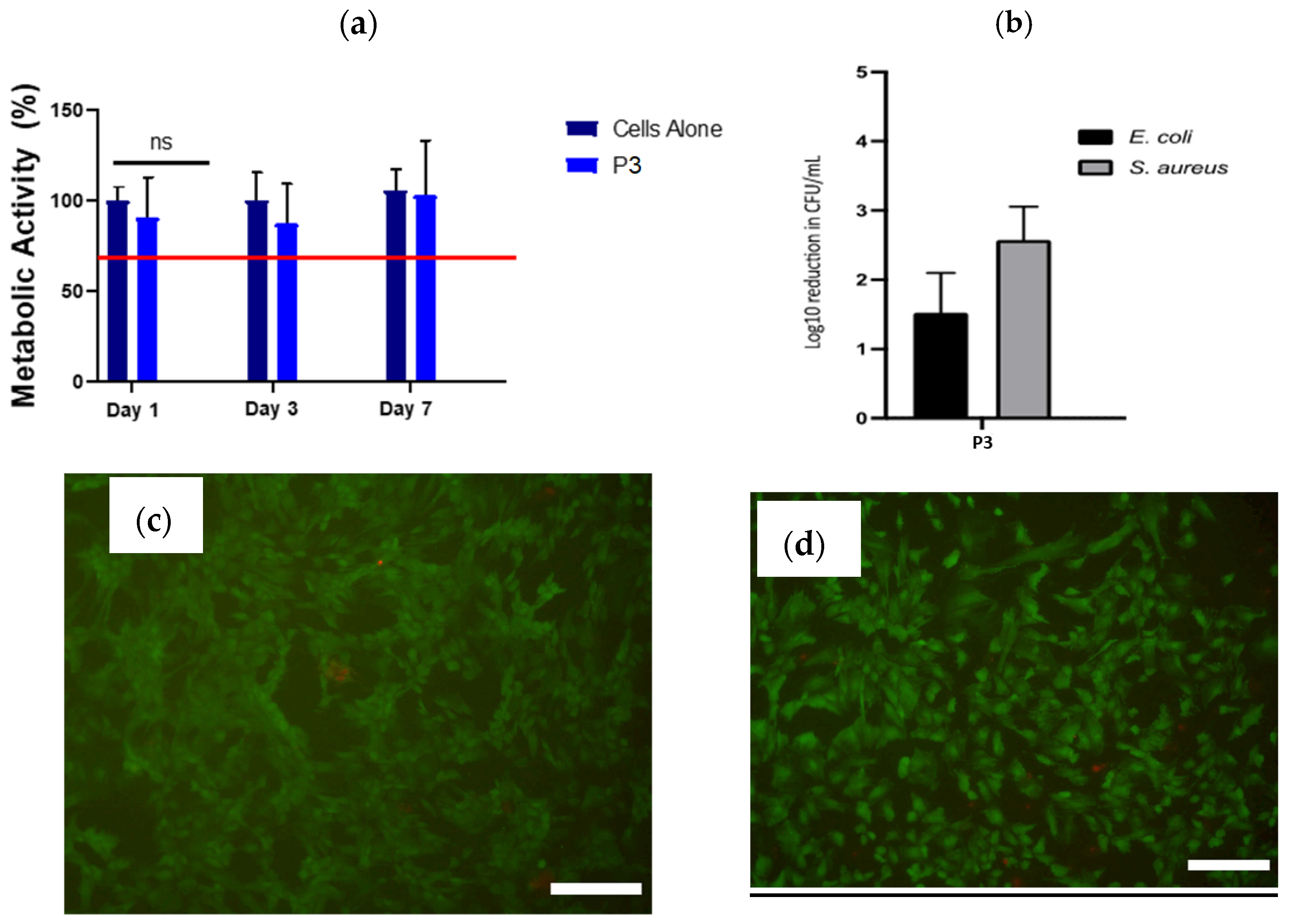
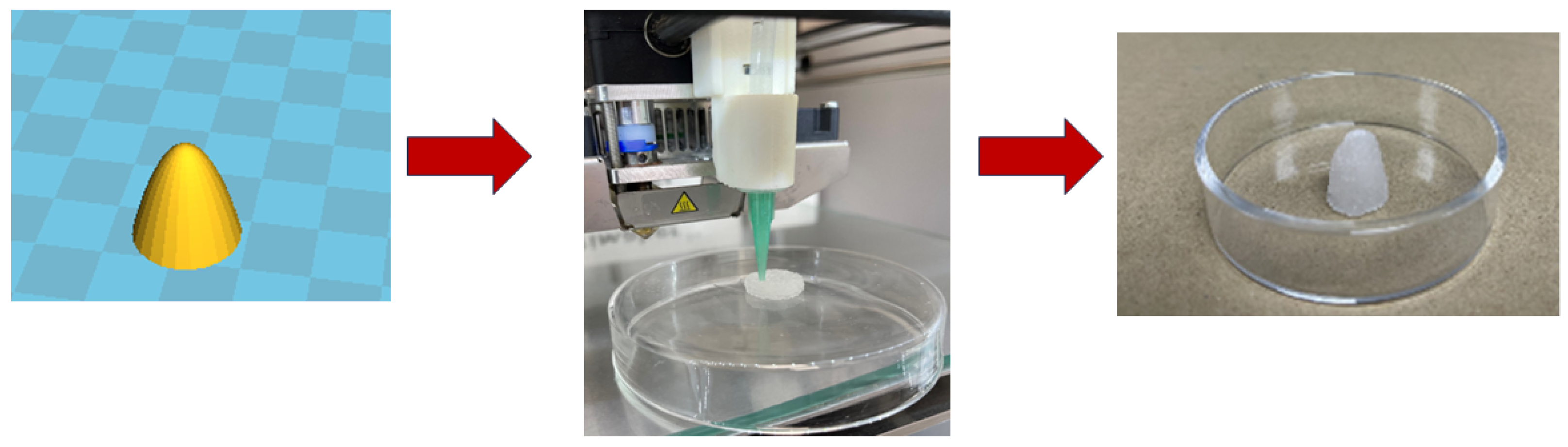
2.3. In Vitro Cytotoxicity, Antimicrobial Potency and 3D Printing
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rice, L.B. Progress and challenges in implementing the research on ESKAPE pathogens. Infect. Control Hosp. Epidemiol. 2010, 31 (Suppl. S1), S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Belanger, C.R.; Hancock, R.E.W. Testing physiologically relevant conditions in minimal inhibitory concentration assays. Nat. Protoc. 2021, 16, 3761–3774. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, R.; LaBauve, A.E.; Akoolo, L.; Patel, S.; Alqarzaee, A.A.; Fok Lung, T.W.; Poorey, K.; Stinear, T.P.; Thomas, V.C.; Meagher, R.J.; et al. Dual gene expression analysis identifies factors associated with Staphylococcus aureus virulence in diabetic mice. Infect. Immun. 2019, 87, e00163-19. [Google Scholar] [CrossRef] [PubMed]
- Geisinger, E.; Isberg, R.R. Interplay between antibiotic resistance and virulence during Disease promoted by multidrug-resistant bacteria. J. Infect. Dis. 2017, 215, S9–S17. [Google Scholar] [CrossRef]
- de Macedo, G.H.R.V.; Costa, G.D.E.; Oliveira, E.R.; Damasceno, G.V.; Mendonça, J.S.P.; Silva, L.S.; Chagas, V.L.; Bazán, J.M.N.; Aliança, A.S.S.; de Miranda, R.C.M.; et al. Interplay between eskape pathogens and immunity in skin infections: An overview of the major determinants of virulence and antibiotic resistance. Pathogens 2021, 10, 148. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 668632. [Google Scholar] [CrossRef]
- Seo, M.D.; Won, H.S.; Kim, J.H.; Mishig-Ochir, T.; Lee, B.J. Antimicrobial peptides for therapeutic applications: A review. Molecules 2012, 17, 12276–12286. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Gao, Q.; Yu, M.; Su, Y.; Xie, M.; Zhao, X.; Li, P.; Ma, P.X. Rationally designed dual functional block copolymers for bottlebrush-like coatings: In vitro and in vivo antimicrobial, antibiofilm, and antifouling properties. Acta Biomater. 2017, 51, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Qi, X.; Li, P.; Chen, W.N.; Mouad, L.; Chang, M.W.; Leong, S.S.J.; Chan-Park, M.B. High potency and broad-spectrum antimicrobial peptides synthesized via ring-opening polymerization of α-Aminoacid-N-carboxyanhydrides. Biomacromolecules 2010, 11, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Salas-Ambrosio, P.; Tronnet, A.; Verhaeghe, P.; Bonduelle, C. Synthetic Polypeptide Polymers as Simplified Analogues of Antimicrobial Peptides. Biomacromolecules 2021, 22, 57–75. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Zeimaran, E.; Fauzi, M.B. Antibacterial polylysine-containing hydrogels for hemostatic and wound healing applications: Preparation methods, current advances and future perspectives. Biomater. Sci. 2024, 12, 3293–3320. [Google Scholar] [CrossRef]
- Su, X.; Zhou, X.; Tan, Z.; Zhou, C. Highly efficient antibacterial diblock copolypeptides based on lysine and phenylalanine. Biopolymers 2017, 107, 16162. [Google Scholar] [CrossRef]
- Lam, S.J.; O’Brien-Simpson, N.M.; Pantarat, N.; Sulistio, A.; Wong, E.H.H.; Chen, Y.Y.; Lenzo, J.C.; Holden, J.A.; Blencowe, A.; Reynolds, E.C.; et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol. 2016, 1, 16162. [Google Scholar] [CrossRef]
- Yang, C.; Krishnamurthy, S.; Liu, J.; Liu, S.; Lu, X.; Coady, D.J.; Cheng, W.; De Libero, G.; Singhal, A.; Hedrick, J.L.; et al. Broad-Spectrum Antimicrobial Star Polycarbonates Functionalized with Mannose for Targeting Bacteria Residing inside Immune Cells. Adv. Healthc. Mater. 2016, 5, 1272–1281. [Google Scholar] [CrossRef]
- Pu, Y.; Hou, Z.; Khin, M.M.; Zamudio-Vázquez, R.; Poon, K.L.; Duan, H.; Chan-Park, M.B. Synthesis and Antibacterial Study of Sulfobetaine/Quaternary Ammonium-Modified Star-Shaped Poly[2-(dimethylamino)ethyl methacrylate]-Based Copolymers with an Inorganic Core. Biomacromolecules 2017, 18, 44–55. [Google Scholar] [CrossRef]
- Doherty, A.; Fitzgerald-Hughes, D.; Fitzpatrick, F.; Heise, A.; Murphy, R. 81 Progressing the clinical applications of novel star-shaped antimicrobial polypeptides for recalcitrant infections. Clin. Infect. Pract. 2023, 20, 100273. [Google Scholar] [CrossRef]
- Murphy, R.; Walsh, D.P.; Hamilton, C.A.; Cryan, S.A.; In Het Panhuis, M.; Heise, A. Degradable 3D-Printed Hydrogels Based on Star-Shaped Copolypeptides. Biomacromolecules 2018, 19, 2691–2699. [Google Scholar] [CrossRef] [PubMed]
- Kimmins, S.D.; Hanay, S.B.; Murphy, R.; O’Dwyer, J.; Ramalho, J.; Ryan, E.J.; Kearney, C.J.; O’Brien, F.J.; Cryan, S.-A.; Fitzgerald-Hughes, D.; et al. Antimicrobial and degradable triazolinedione (TAD) crosslinked polypeptide hydrogels. J. Mater. Chem. B 2021, 9, 5456–5464. [Google Scholar] [CrossRef] [PubMed]
- Murali Mohan, Y.; Vimala, K.; Thomas, V.; Varaprasad, K.; Sreedhar, B.; Bajpai, S.K.; Mohana Raju, K. Controlling of silver nanoparticles structure by hydrogel networks. J. Colloid Interface Sci. 2010, 342, 73–82. [Google Scholar] [CrossRef]
- Manju, S.; Antony, M.; Sreenivasan, K. Synthesis and evaluation of a hydrogel that binds glucose and releases ciprofloxacin. J. Mater. Sci. 2010, 45, 4006–4012. [Google Scholar] [CrossRef]
- Sa, Y.; Wang, M.; Deng, H.; Wang, Y.; Jiang, T. Beneficial effects of biomimetic nano-sized hydroxyapatite/antibiotic gentamicin enriched chitosan–glycerophosphate hydrogel on the performance of injectable polymethylmethacrylate. RSC Adv. 2015, 5, 91082–91092. [Google Scholar] [CrossRef]
- Chen, Q.; He, Y.; Li, Q.; Yang, K.; Sun, L.; Xu, H.; Wang, R. Intelligent design and medical applications of antimicrobial hydrogels. Colloid Interface Sci. Commun. 2023, 53, 100696. [Google Scholar] [CrossRef]
- Kopecki, Z. Development of next-generation antimicrobial hydrogel dressing to combat burn wound infection. Biosci. Rep. 2021, 41, BSR20203404. [Google Scholar] [CrossRef]
- Murphy, R.; Kordbacheh, S.; Skoulas, D.; Ng, S.; Suthiwanich, K.; Kasko, A.M.; Cryan, S.A.; Fitzgerald-Hughes, D.; Khademhosseini, A.; Sheikhi, A.; et al. Three-dimensionally printable shear-thinning triblock copolypeptide hydrogels with antimicrobial potency. Biomater. Sci. 2021, 9, 5144–5149. [Google Scholar] [CrossRef]
- Phan, T.H.M.; Huang, C.C.; Tsai, Y.J.; Hu, J.J.; Jan, J.S. Polypeptide composition and topology affect hydrogelation of star-shaped poly(l-lysine)-based amphiphilic copolypeptides. Gels 2021, 7, 131. [Google Scholar] [CrossRef]
- Chen, Y.F.; Lai, Y.D.; Chang, C.H.; Tsai, Y.C.; Tang, C.C.; Jan, J.S. Star-shaped polypeptides exhibit potent antibacterial activities. Nanoscale 2019, 11, 11696–11708. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.C.; Zhang, S.H.; My Phan, T.H.; Tseng, Y.C.; Jan, J.S. Block length and topology affect self-assembly and gelation of poly(L-lysine)-block-poly(S-benzyl-L-cysteine) block copolypeptides. Polymer 2021, 228, 123891. [Google Scholar] [CrossRef]
- Hou, S.S.; Fan, N.S.; Tseng, Y.C.; Jan, J.S. Self-Assembly and Hydrogelation of Coil-Sheet Poly(l -lysine)- block-poly(l -threonine) Block Copolypeptides. Macromolecules 2018, 51, 8054–8063. [Google Scholar] [CrossRef]
- Huang, J.; Hastings, C.L.; Duffy, G.P.; Kelly, H.M.; Raeburn, J.; Adams, D.J.; Heise, A. Supramolecular hydrogels with reverse thermal gelation properties from (Oligo)tyrosine containing block copolymers. Biomacromolecules 2013, 14, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.S.; Lee, J.H.; Park, Y.S.; Kim, Y.O.; Park, J.; Yang, T.Y.; Jin, K.; Lee, J.; Park, S.; You, J.M.; et al. Tyrosine-mediated two-dimensional peptide assembly and its role as a bio-inspired catalytic scaffold. Nat. Commun. 2014, 5, 3665. [Google Scholar] [CrossRef]
- Shirbin, S.J.; Insua, I.; Holden, J.A.; Lenzo, J.C.; Reynolds, E.C.; O’Brien-Simpson, N.M.; Qiao, G.G. Architectural Effects of Star-Shaped “Structurally Nanoengineered Antimicrobial Peptide Polymers” (SNAPPs) on Their Biological Activity. Adv. Healthc. Mater. 2018, 7, e1800627. [Google Scholar] [CrossRef]
- Murphy, R.D.; in het Panhuis, M.; Cryan, S.-A.; Heise, A. Disulphide crosslinked star block copolypeptide hydrogels: Influence of block sequence order on hydrogel properties. Polym. Chem. 2018, 9, 3908–3916. [Google Scholar] [CrossRef]
- Bass, M.B.; Hopkins, D.F.; Jaquysh, W.A.N.; Ornstein, R.L. A method for determining the positions of polar hydrogens added to a protein structure that maximizes protein hydrogen bonding. Proteins Struct. 1992, 12, 266–277. [Google Scholar] [CrossRef]
- Smith, J.S.; Scholtz, J.M. Energetics of Polar Side-Chain Interactions in Helical Peptides: Salt Effects on Ion Pairs and Hydrogen Bonds. Biochemistry 1998, 37, 33–40. [Google Scholar] [CrossRef]
- Zandarashvili, L.; Iwahara, J. Temperature Dependence of Internal Motions of Protein Side-Chain NH3+ Groups: Insight into Energy Barriers for Transient Breakage of Hydrogen Bonds. Biochemistry 2015, 54, 538–545. [Google Scholar] [CrossRef]
- Donald, J.E.; Kulp, D.W.; DeGrado, W.F. Salt bridges: Geometrically specific, designable interactions. Proteins 2011, 79, 898–915. [Google Scholar] [CrossRef] [PubMed]
- Skoulas, D.; Mangiapia, G.; Parisi, D.; Kasimatis, M.; Glynos, E.; Stratikos, E.; Vlassopoulos, D.; Frielinghaus, H.; Iatrou, H. Tunable Hydrogels with Improved Viscoelastic Properties from Hybrid Polypeptides. Macromolecules 2021, 54, 10786–10800. [Google Scholar] [CrossRef]
- Tierney, E.G.; Duffy, G.P.; Hibbitts, A.J.; Cryan, S.-A.; O’Brien, F.J. The development of non-viral gene-activated matrices for bone regeneration using polyethyleneimine (PEI) and collagen-based scaffolds. J. Control. Release 2012, 158, 304–311. [Google Scholar] [CrossRef] [PubMed]

| Sample | Polymer | DP a) Lys theor. | DP a) Tyr theor. | DP a) Cys(Bz) theor. | Mnb) 1st Block [kg mol−1] | Mnb) 2nd Block [kg mol−1] | Ð c) |
|---|---|---|---|---|---|---|---|
| P1 | PPI-p(Tyr-b-Lys-b-Cys(Bz)) | 400 | 80 | 80 | 24 | 106 | 1.27 |
| P2 | PPI-p(Lys-b-Tyr-b-Cys(Bz)) | 400 | 80 | 80 | 84 | 104 | 1.28 |
| P3 | PPI-p(Lys-co-Tyr-b-Cys(Bz)) | 400 | 80 | 80 | 74 | - | 1.16 |
| Sample | Maximum Ratio of Polymer Mass/Hydrogel Mass a) | G′ (Pa) b) | Tan(delta) b) |
|---|---|---|---|
| P1 | 1/15 | 18,119 | 0.109 |
| P2 | 1/33 | 2796 | 0.142 |
| P3 | 1/100 | 5439 | 0.092 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skoulas, D.; Fallon, M.; Genoud, K.J.; O’Brien, F.J.; Hughes, D.F.; Heise, A. Shear-Thinning Extrudable Hydrogels Based on Star Polypeptides with Antimicrobial Properties. Gels 2024, 10, 652. https://doi.org/10.3390/gels10100652
Skoulas D, Fallon M, Genoud KJ, O’Brien FJ, Hughes DF, Heise A. Shear-Thinning Extrudable Hydrogels Based on Star Polypeptides with Antimicrobial Properties. Gels. 2024; 10(10):652. https://doi.org/10.3390/gels10100652
Chicago/Turabian StyleSkoulas, Dimitrios, Muireann Fallon, Katelyn J. Genoud, Fergal J. O’Brien, Deirdre Fitzgerald Hughes, and Andreas Heise. 2024. "Shear-Thinning Extrudable Hydrogels Based on Star Polypeptides with Antimicrobial Properties" Gels 10, no. 10: 652. https://doi.org/10.3390/gels10100652
APA StyleSkoulas, D., Fallon, M., Genoud, K. J., O’Brien, F. J., Hughes, D. F., & Heise, A. (2024). Shear-Thinning Extrudable Hydrogels Based on Star Polypeptides with Antimicrobial Properties. Gels, 10(10), 652. https://doi.org/10.3390/gels10100652









