Preparation and Performance Evaluation of CO2 Foam Gel Fracturing Fluid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Construction of CO2 Foam Gel Fracturing Fluid
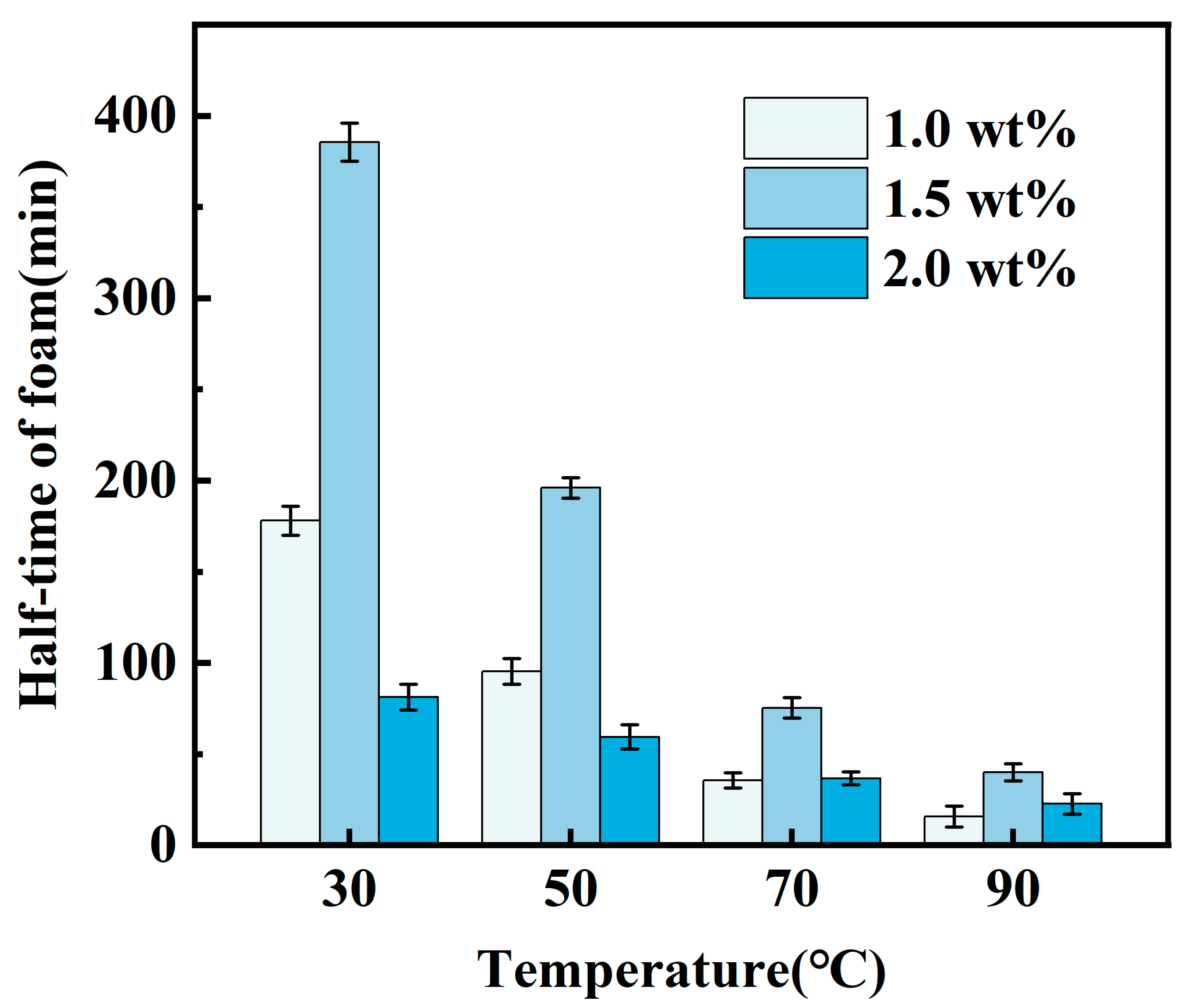
2.1.1. Determination of the Amount of Foaming Agent
2.1.2. Determination of the Amount of Thickening Agent
2.2. Performance Evaluation of the CO2 Foam Gel Fracturing Fluid
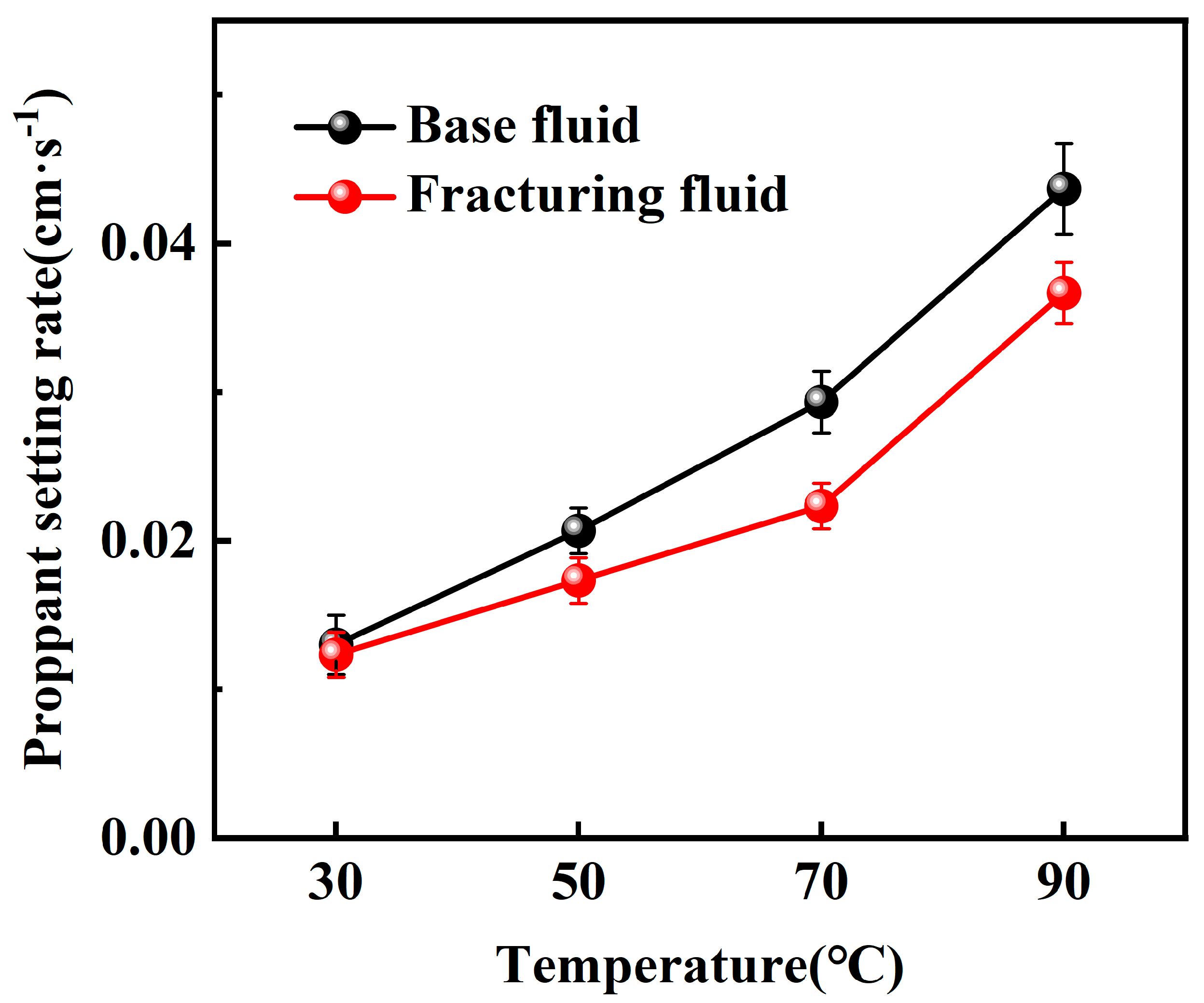
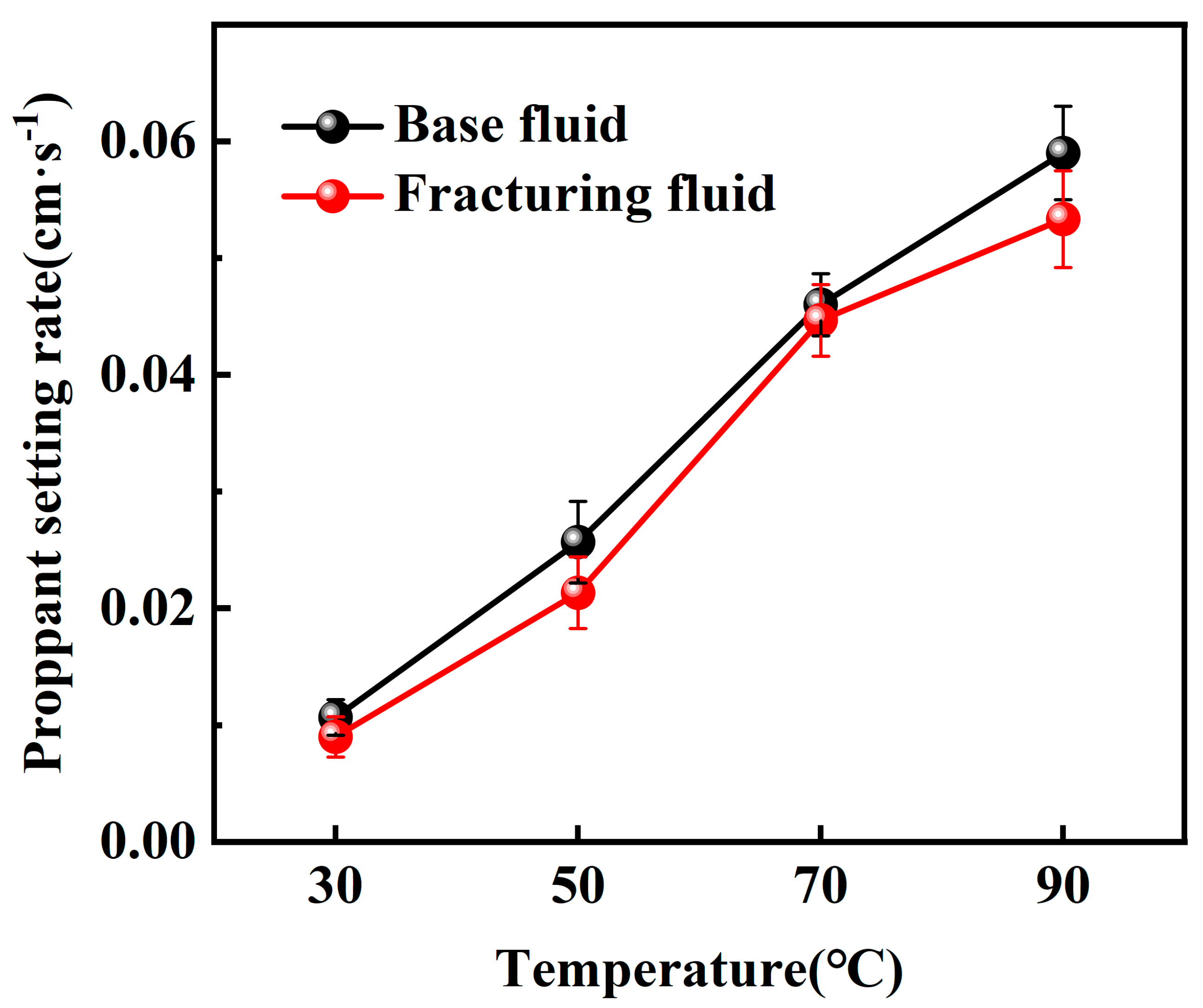
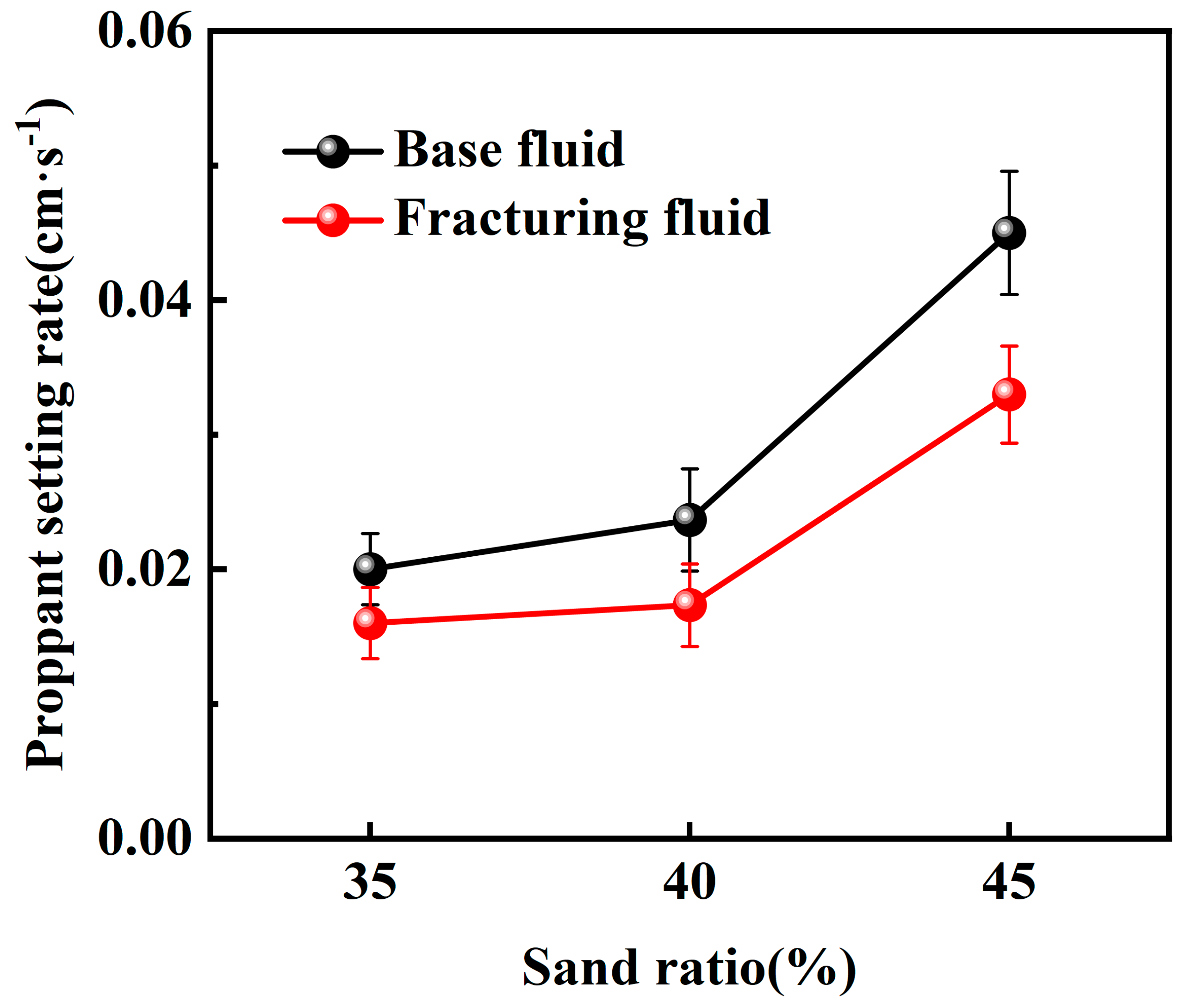
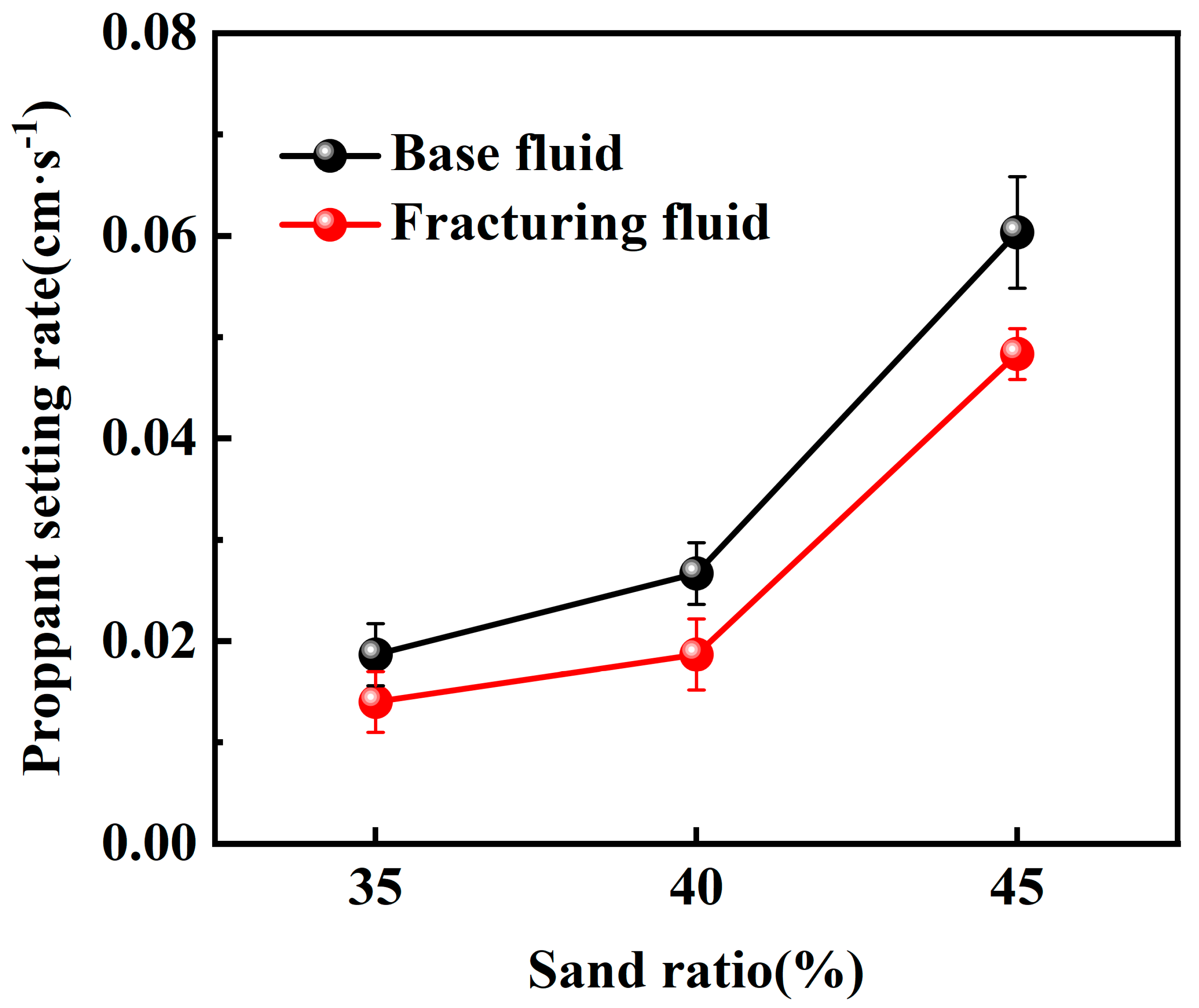
2.2.1. Static Sand-Carrying Performance
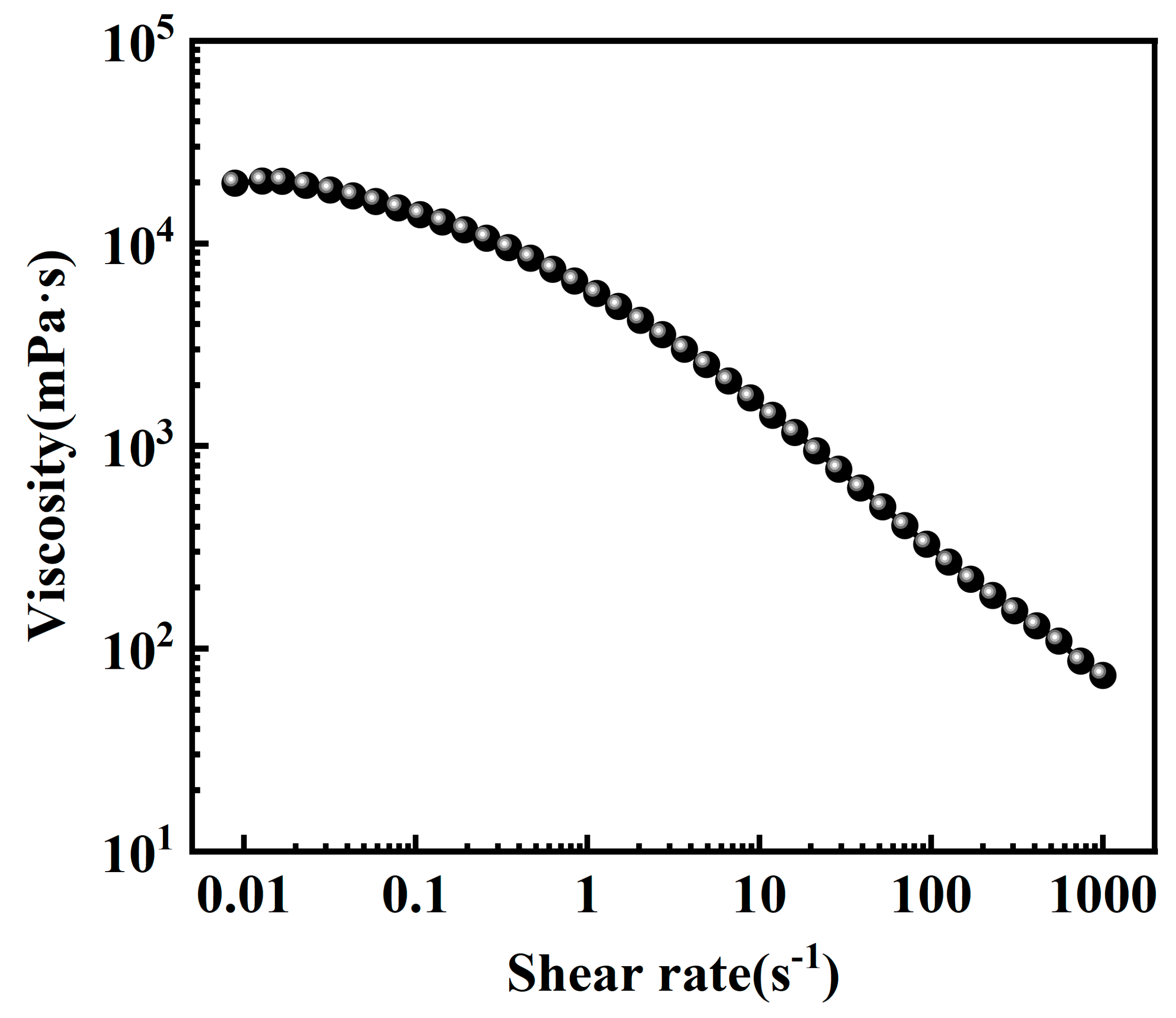
2.2.2. Steady Shear Performance
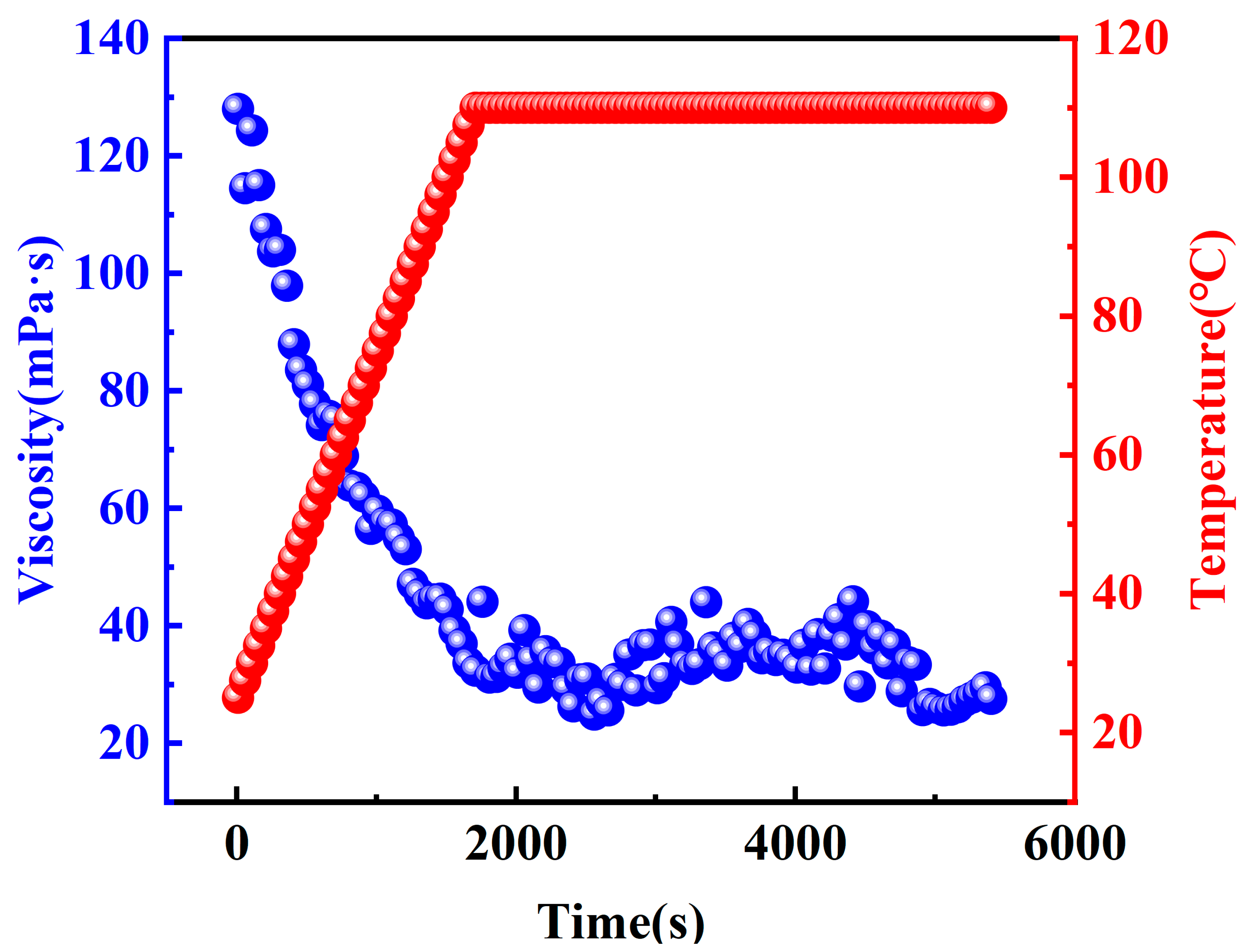
2.2.3. Temperature and Shear Resistant Performance
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the CO2 Foam Gel Fracturing Fluid
4.3. Sand-Carrying Performance Test Experiment
4.3.1. High-Temperature Visualization Static Sand-Carrying Experiment
4.3.2. Static Sand-Carrying Measuring Cylinder Test
4.4. Temperature and Shear Resistance Test Experiments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Tan, J.; Wood, D.A.; Zhao, Z.; Becker, D.; Lyu, Q.; Shu, B.; Chen, H. A review of the current status of induced seismicity monitoring for hydraulic fracturing in unconventional tight oil and gas reservoirs. Fuel 2019, 242, 195–210. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Wang, B.; Chen, Z.; Nie, D. The status quo review and suggested policies for shale gas development in China. Renew. Sustain. Energy Rev. 2016, 59, 420–428. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, S.; Yang, R.; Wu, X.; Li, R.; Zhang, H.; Hung, P. A review of liquid nitrogen fracturing technology. Fuel 2020, 266, 117040. [Google Scholar] [CrossRef]
- Li, J.; Wei, J.; Chen, Y.; Wang, A.; Zhou, X. Analysis of asynchronous/inter-fracture injection-production mechanism under the condition of five-spot vertical and horizontal well combination in low permeability oil reservoirs. Energy 2024, 308, 132923. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, M.; Yang, Z.; Wang, X.; Dai, C. Development and gelation mechanism of ultra-high-temperature-resistant polymer gel. Gels 2023, 9, 726. [Google Scholar] [CrossRef]
- Li, Q.; Xing, H.; Liu, J.; Liu, X. A review on hydraulic fracturing of unconventional reservoir. Petroleum 2015, 1, 8–15. [Google Scholar] [CrossRef]
- Guo, C.; Xu, J.; Wei, M.; Jiang, R. Experimental study and numerical simulation of hydraulic fracturing tight sandstone reservoirs. Fuel 2015, 159, 334–344. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, Z.; Dai, C.; Wu, W.; Sun, Y.; Song, X.; Cheng, Y.; Wang, X. Preparation and performance evaluation of the slickwater using novel polymeric drag reducing agent with high temperature and shear resistance ability. Pet. Sci. 2024, 21, 1113–1121. [Google Scholar] [CrossRef]
- Zhou, J.; Ranjith, P.G.; Wanniarachchi, W.A.M. Different strategies of foam stabilization in the use of foam as a fracturing fluid. Adv. Colloid Interface Sci 2020, 276, 102104. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, H.; Zhang, W.; Fan, J.; Zhang, C.; Zhao, J. Dissymmetric beauty: A novel design of heterogemini viscoelastic surfactant for the clean fracturing fluid. J. Ind. Eng. Chem. 2018, 60, 133–142. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, J.; Yang, X.; Zhang, H.; Zhang, Z.; Yang, B.; Zhang, Y.; Zhao, J. Study of a novel gemini viscoelastic surfactant with high performance in clean fracturing fluid application. Polymers 2018, 10, 1215. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Ma, X.; Jing, Z. Dynamic proppant-carrying performance of VES-CO2 foam fracturing fluid in the pipeline and the fracture. J. Petrol. Sci. Eng. 2022, 210, 110034. [Google Scholar] [CrossRef]
- Kong, B.; Wang, S.; Chen, S. Minimize formation damage in water-sensitive montney formation with energized fracturing fluid. SPE Res. Eval. Eng. 2017, 20, 562–571. [Google Scholar] [CrossRef]
- Gao, C.; Cheng, S.; Wang, M.; Wu, W.; Gao, Z.; Li, S.; Meng, X. Optimization of carbon dioxide foam fracturing technology for shale gas reservoir. Geofluids 2023, 1, 6187764. [Google Scholar] [CrossRef]
- Gupta, D.V.S.; Jackson, T.L.; Hlabinka, G.J.; Evans, J.B.; Le, H.V.; Batrashkin, A.; Shaefer, M.T. Development and field application of a low-pH, efficient fracturing fluid for tight gas fields in the greater green giver basin, wyoming. SPE Prod. Oper. 2009, 24, 602–610. [Google Scholar] [CrossRef]
- Cong, Z.; Li, Y.; Pan, Y.; Liu, B.; Shi, Y.; Wei, J.; Li, W. Study on CO2 foam fracturing model and fracture propagation simulation. Energy 2022, 238, 121778. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhai, C.; Sun, Y.; Cong, Y.; Tang, W.; Yu, X.; Xu, J.; Chen, A.; Xu, H.; Wu, X. Effect of reservoir temperatures on the stabilization and flowback of CO2 foam fracturing fluid containing nano-SiO2 particles: An experimental study. Geoenergy Sci. Eng. 2023, 228, 212048. [Google Scholar] [CrossRef]
- Bakhsh, A.; Zhang, L.; Wei, H.; Shaikh, A.; Khan, N.; Khan, Z.; Shaoran, R. Development of CO2-sensitive viscoelastic fracturing fluid for low permeability reservoirs: A review. Processes 2022, 10, 885. [Google Scholar] [CrossRef]
- Gao, J.; Wang, H.; Sharma, M. Research progress and prospects of CO2 fracturing for developing unconventional energy sources. Geoenergy Sci. Eng. 2024, 241, 213137. [Google Scholar] [CrossRef]
- Guo, J.; Ma, L.; Lu, C. Progress and development directions of fracturing flooding technology for tight reservoirs in China. Acta Pet. Sin. 2022, 43, 1788–1797. [Google Scholar] [CrossRef]
- Tong, S.; Singh, R.; Mohanty, K.K. A visualization study of proppant transport in foam fracturing fluids. J. Nat. Gas Sci. Eng. 2018, 52, 235–247. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Eftekhari, A.A.; Dafnomilis, G.; Lake, L.W.; Bruining, J. On the sustainability of CO2 storage through CO2-enhanced oil recovery. Appl. Energy 2020, 261, 114467. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Chang, B.; DiCarlo, D.; Prodanović, M. CO2 foam rheology in rough shale and sandstone fractures at elevated temperatures. Fuel 2024, 366, 131373. [Google Scholar] [CrossRef]
- Wang, G. Carbon dioxide capture, enhanced-oil recovery and storage technology and engineering practice in Jilin Oilfield, NE China. Pet. Explor. Dev. 2023, 50, 245–254. [Google Scholar] [CrossRef]
- Li, X.; Peng, B.; Liu, Q.; Liu, J.; Shang, L. Micro and nanobubbles technologies as a new horizon for CO2-EOR and CO2 geological storage techniques: A review. Fuel 2023, 341, 127661. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Zhang, J.; Meng, S.; Duan, Y. Fracturing with carbon dioxide: Application status and development trend. Pet. Explor. Dev. 2014, 41, 513–519. [Google Scholar] [CrossRef]
- Zhang, H. Study on microscale stress sensitivity of CO2 foam fracturing in tight reservoirs. Energy 2024, 294, 130766. [Google Scholar] [CrossRef]
- Khan, J.A.; Padmanabhan, E.; Haq, I.U.; Yekeen, N.; Mumtaz, M.; Prajapati, S. Experimental assessment of fracture initiation and wettability in low and high brittle shales by CO2 foam fracturing fluids. Energy Fuels 2022, 36, 8288–8300. [Google Scholar] [CrossRef]
- Yang, H.; Lv, Z.; Wang, L.; Feng, C.; Wang, J.; Xu, Z.; Huang, Y.; Li, Z.; Kang, W. Stability mechanism of controlled acid-resistant hydrophobic polymer nanospheres on CO2 foam. Fuel 2023, 346, 128332. [Google Scholar] [CrossRef]
- Kang, W.; Yan, L.; Ding, F.; Xu, Z. Effect of polysaccharide polymers on the surface and foam properties of aqueous film-forming foam. Colloid Interface Sci. Commun. 2021, 45, 100540. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Lu, Y. Application of CO2 foam fracturing technology in low permeability and low-pressure gas reservoir. Acta Pet. Sin. 2004, 25, 66–70. [Google Scholar] [CrossRef]
- Abdelaal, A.; Aljawad, M.S.; Alyousef, Z.; Almajid, M.M. A review of foam-based fracturing fluids applications: From lab studies to field implementations. J. Nat. Gas Sci. Eng. 2021, 95, 104236. [Google Scholar] [CrossRef]
- Bhakta, A.; Ruckenstein, E. Drainage and coalescence in standing foams. J. Colloid Interface Sci. 1997, 191, 184–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Nie, F.; Deng, X.; Wang, Y.; Hou, B. Evaluation of an Oxygen-Reduced Air Flooding Plugging System and Its Injection Method in the Honghe Oilfield. Energy Fuels 2022, 36, 12524–12532. [Google Scholar] [CrossRef]






| YFP-1 (wt%) | Foaming Volume (mL) | Half-Life of Foam (min) | Foam Composite Index (mL·min) |
|---|---|---|---|
| 0.3 | 120 | 196 | 23,520 |
| 0.5 | 148 | 230 | 34,040 |
| 0.7 | 173 | 394 | 68,162 |
| 0.9 | 140 | 197 | 27,580 |
| 1.5 | 195 | 119 | 23,205 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Yang, J.; Li, Z.; Ma, Z.; Xu, X.; Liu, R.; Li, X.; Zhang, L.; Zhao, M. Preparation and Performance Evaluation of CO2 Foam Gel Fracturing Fluid. Gels 2024, 10, 804. https://doi.org/10.3390/gels10120804
Gao Y, Yang J, Li Z, Ma Z, Xu X, Liu R, Li X, Zhang L, Zhao M. Preparation and Performance Evaluation of CO2 Foam Gel Fracturing Fluid. Gels. 2024; 10(12):804. https://doi.org/10.3390/gels10120804
Chicago/Turabian StyleGao, Yan, Jiahui Yang, Zefeng Li, Zhenfeng Ma, Xinjie Xu, Ruiqiong Liu, Xin Li, Lixiao Zhang, and Mingwei Zhao. 2024. "Preparation and Performance Evaluation of CO2 Foam Gel Fracturing Fluid" Gels 10, no. 12: 804. https://doi.org/10.3390/gels10120804
APA StyleGao, Y., Yang, J., Li, Z., Ma, Z., Xu, X., Liu, R., Li, X., Zhang, L., & Zhao, M. (2024). Preparation and Performance Evaluation of CO2 Foam Gel Fracturing Fluid. Gels, 10(12), 804. https://doi.org/10.3390/gels10120804








