“From Waste to Wonder”: Comparative Evaluation of Chinese Cabbage Waste and Banana Peel Derived Hydrogels on Soil Water Retention Performance
Abstract
1. Introduction
2. Results and Discussion
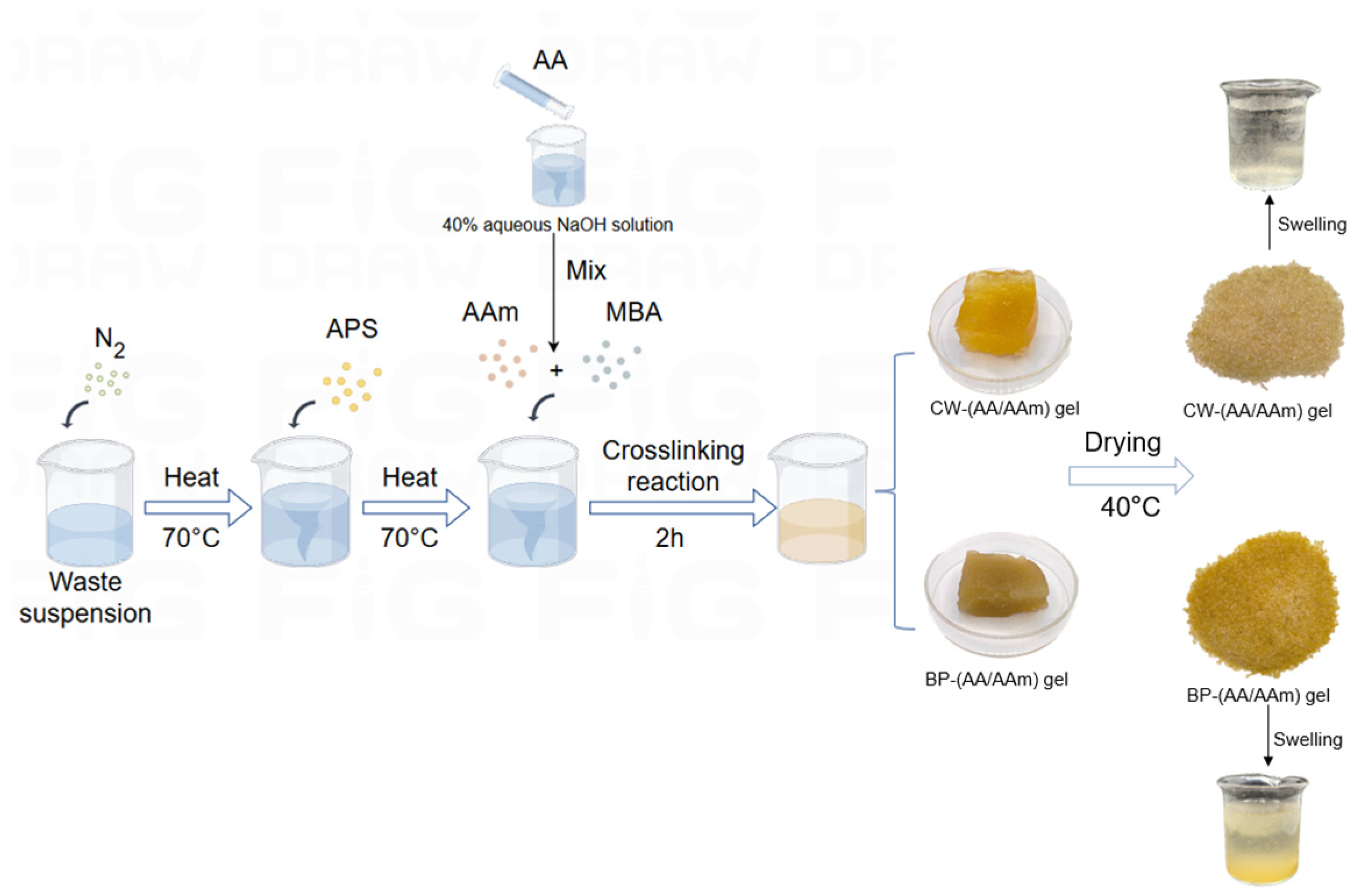
2.1. Synthesis of (AA-AAm) Gels
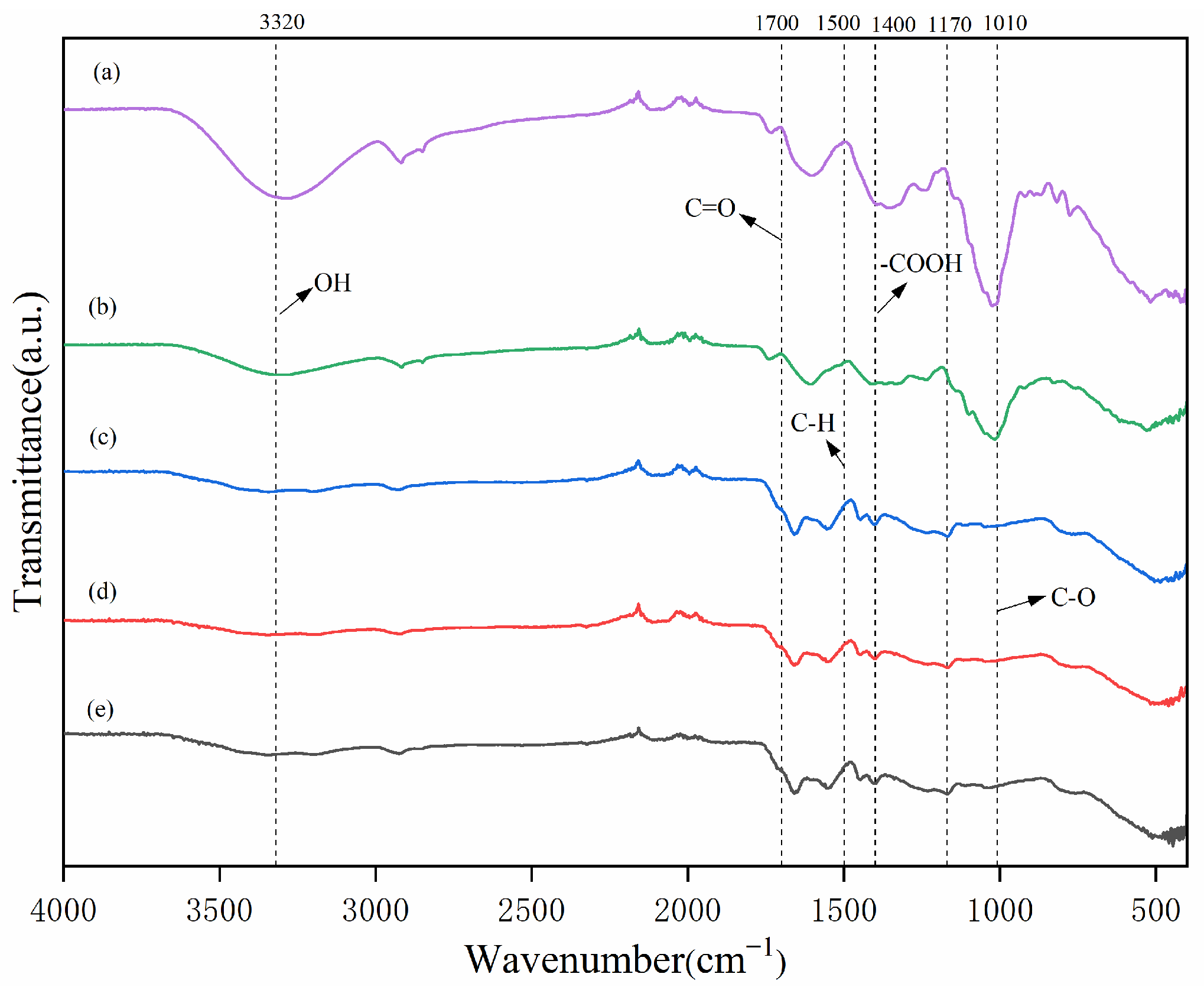
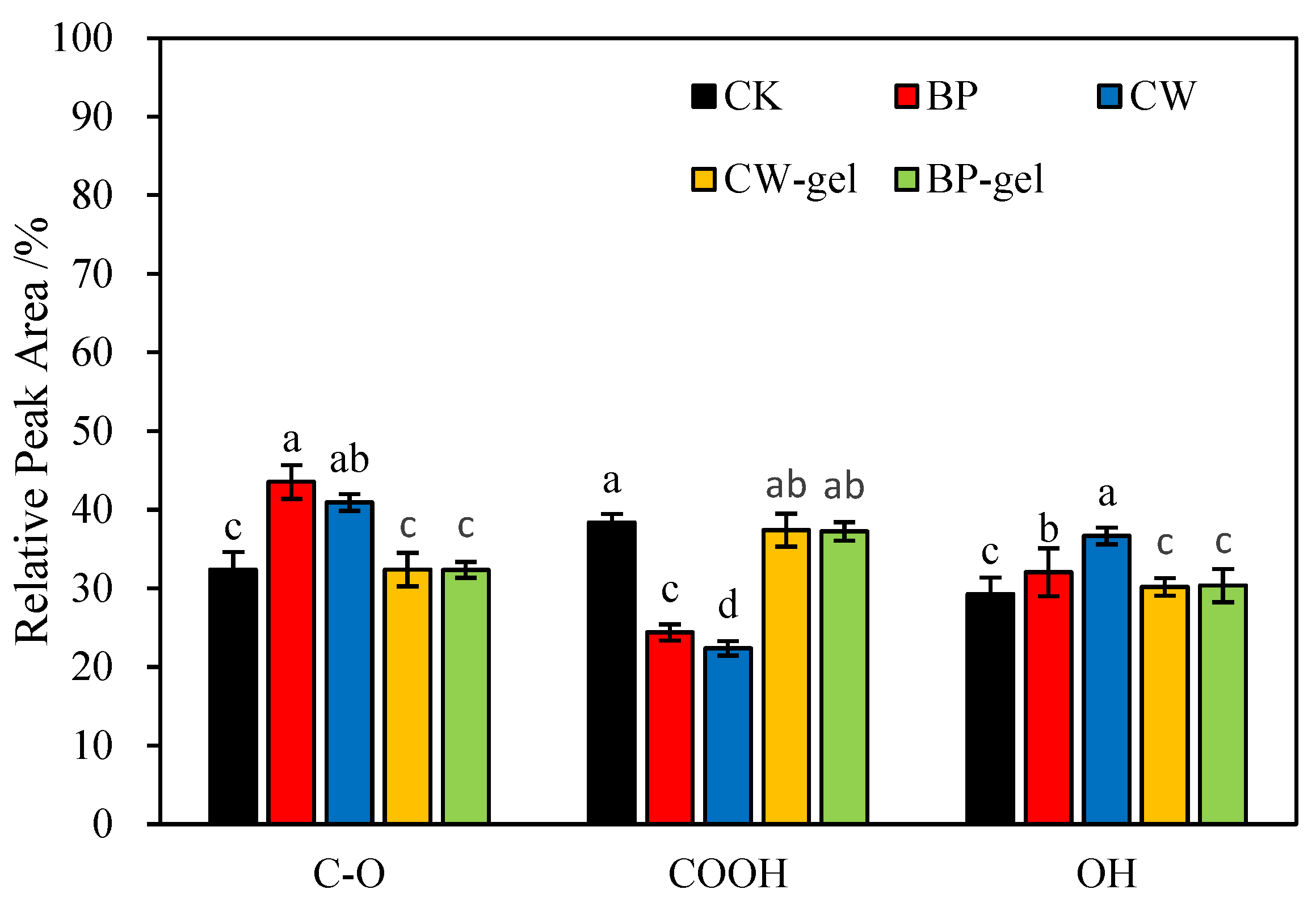
2.2. FTIR Spectra Characterization Analysis
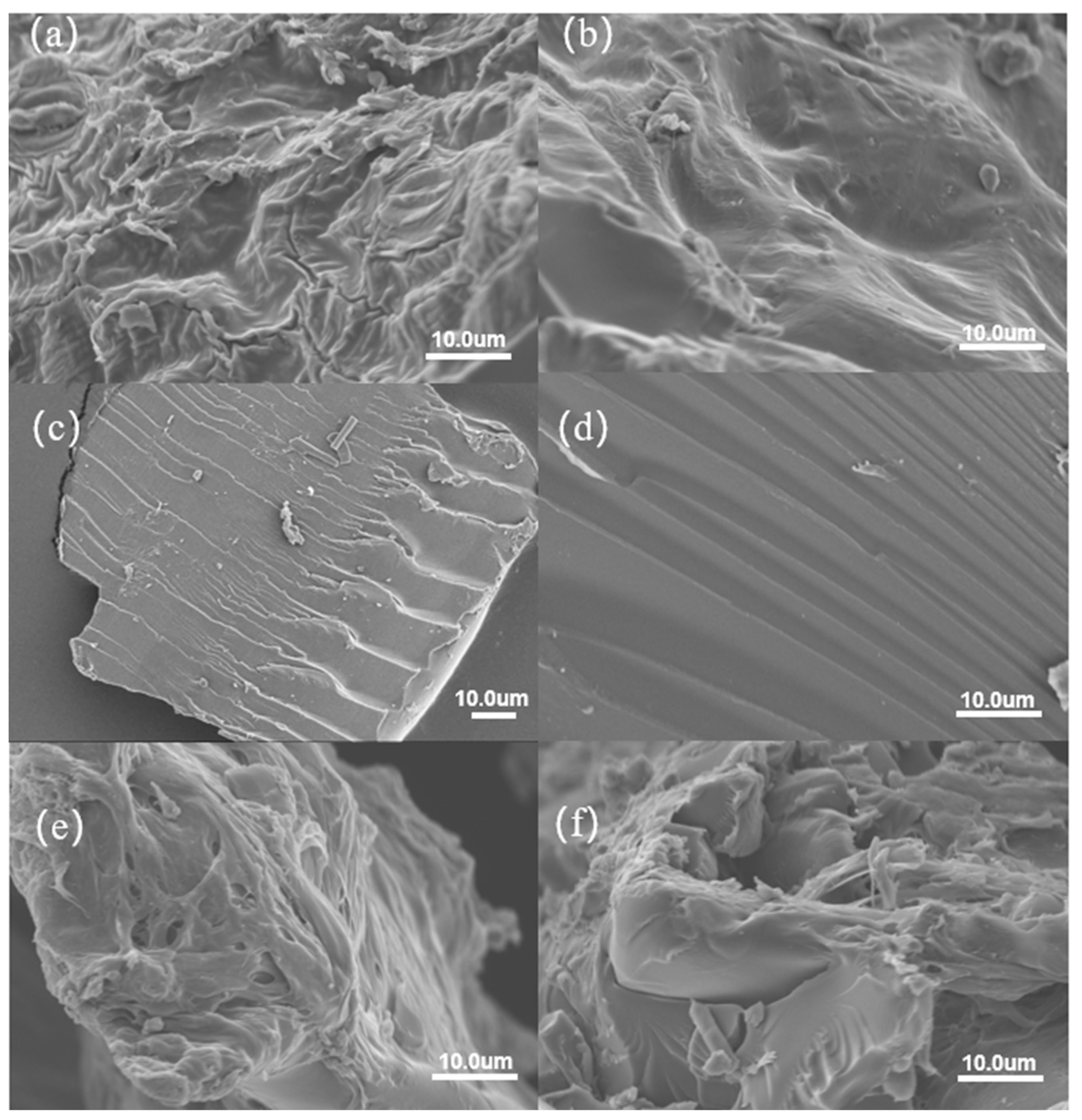
2.3. SEM Characterization Analysis
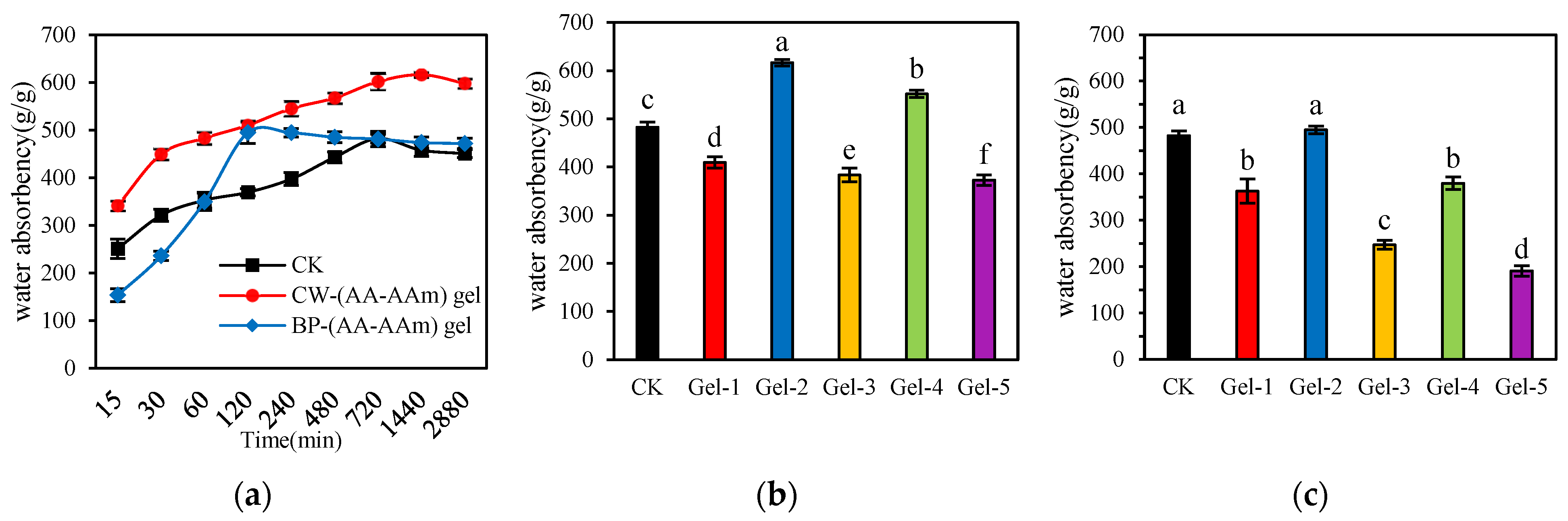
2.4. Analysis of Water Absorption of Hydrogel
2.5. Analysis of Swelling Capacity of Hydrogels at Different pH
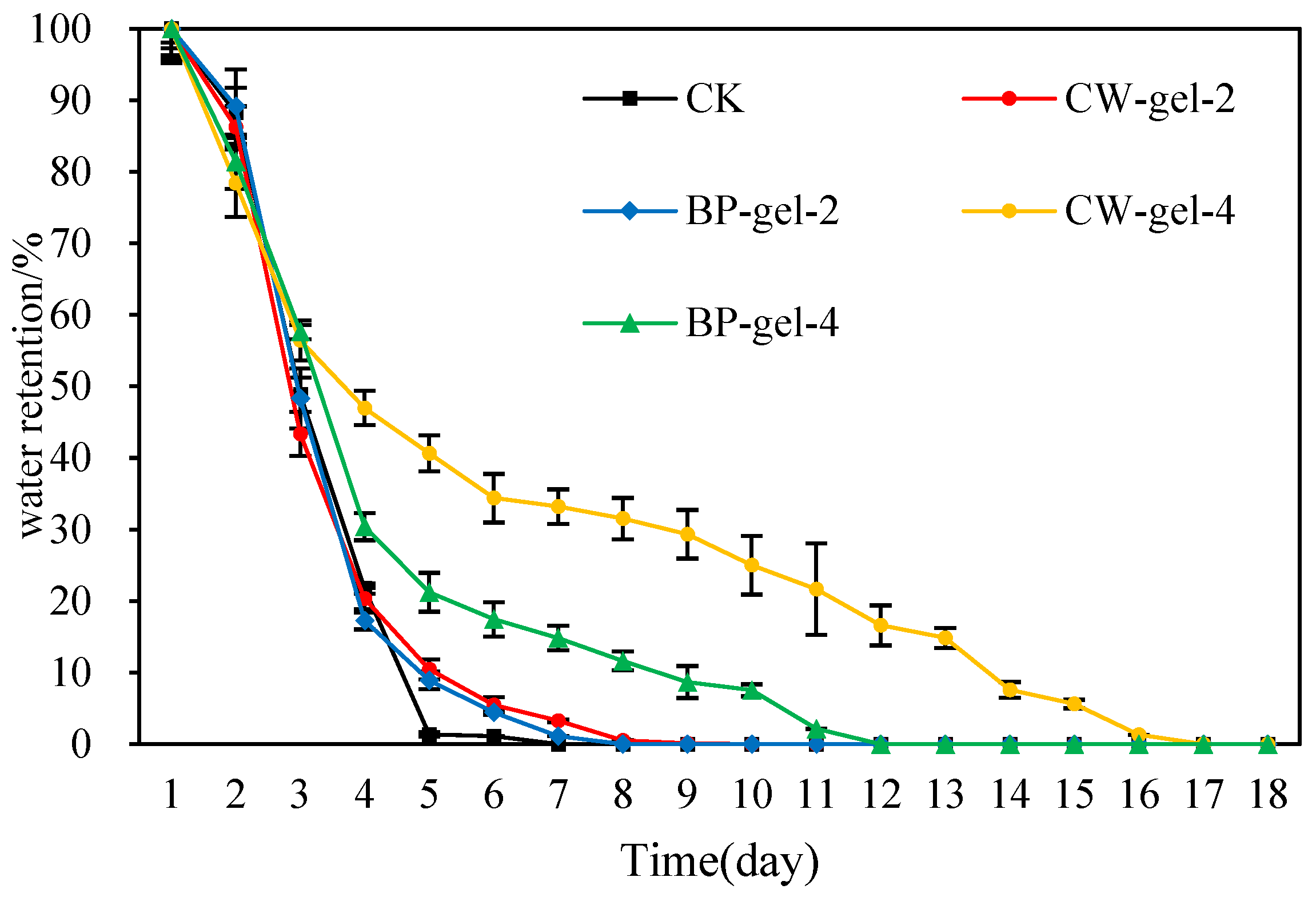
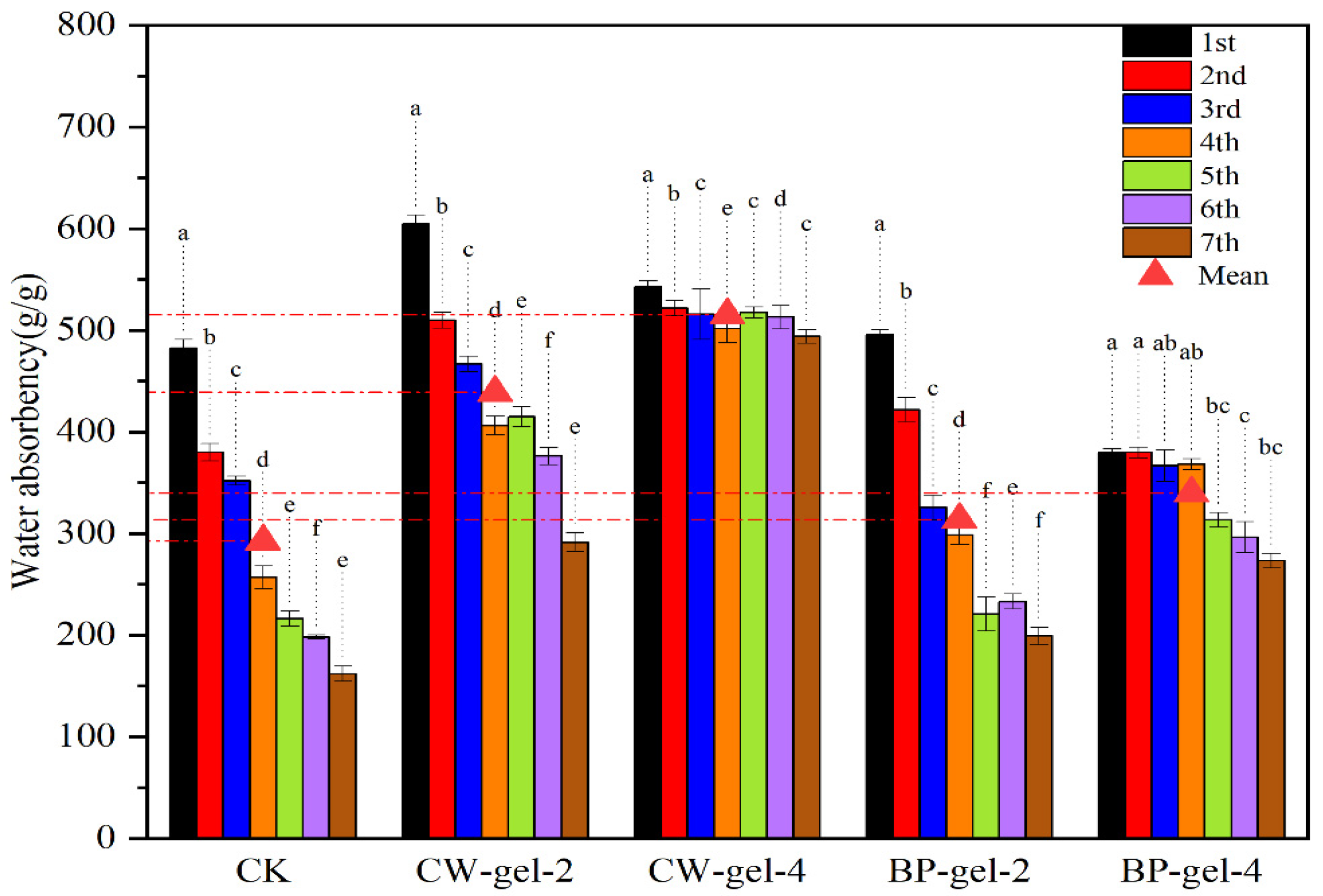
2.6. Water Retention and Reuse Properties
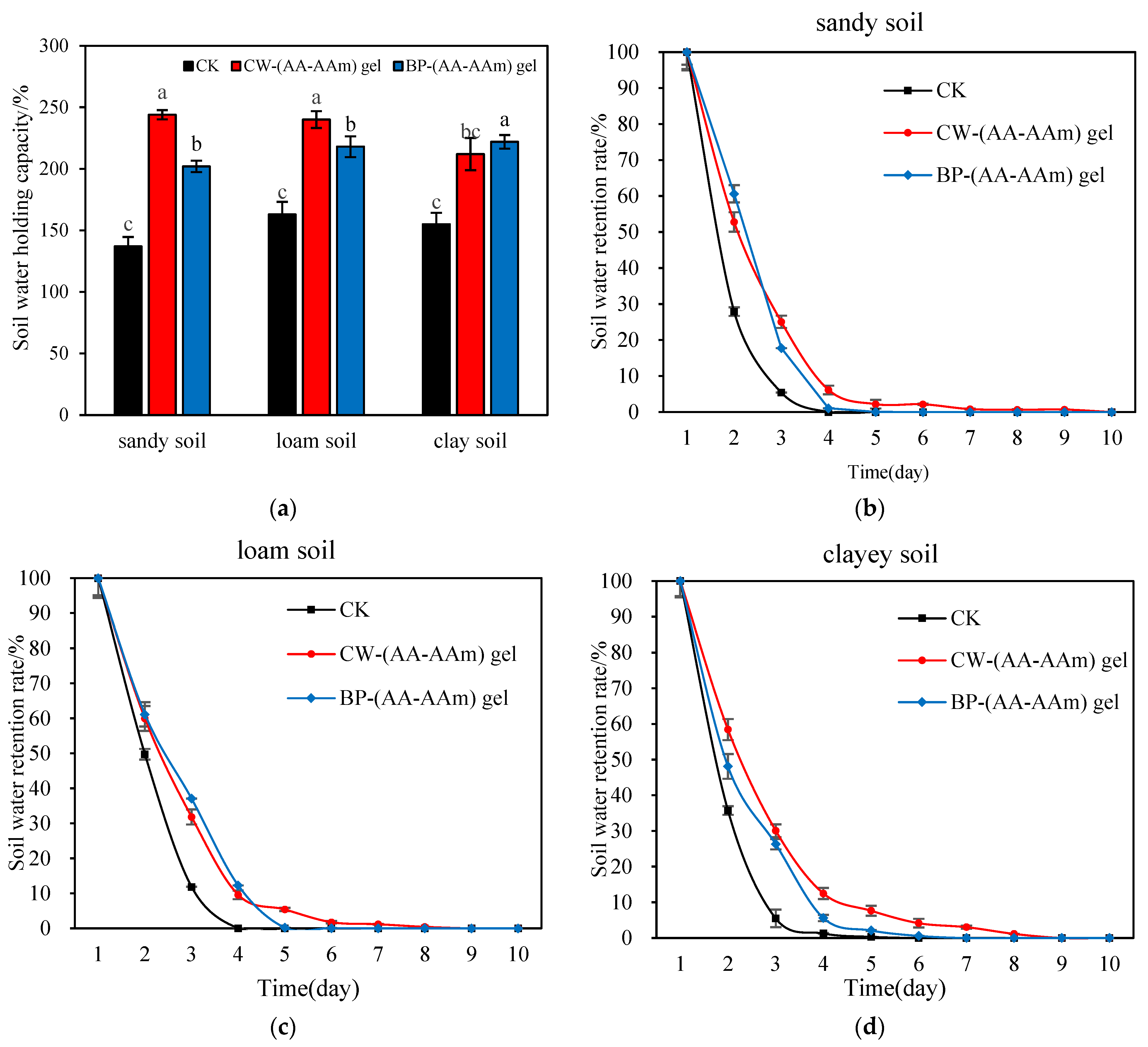
2.7. Water Retention and Water Holding Capacity of (AA-AAm) Gels in Different Soils
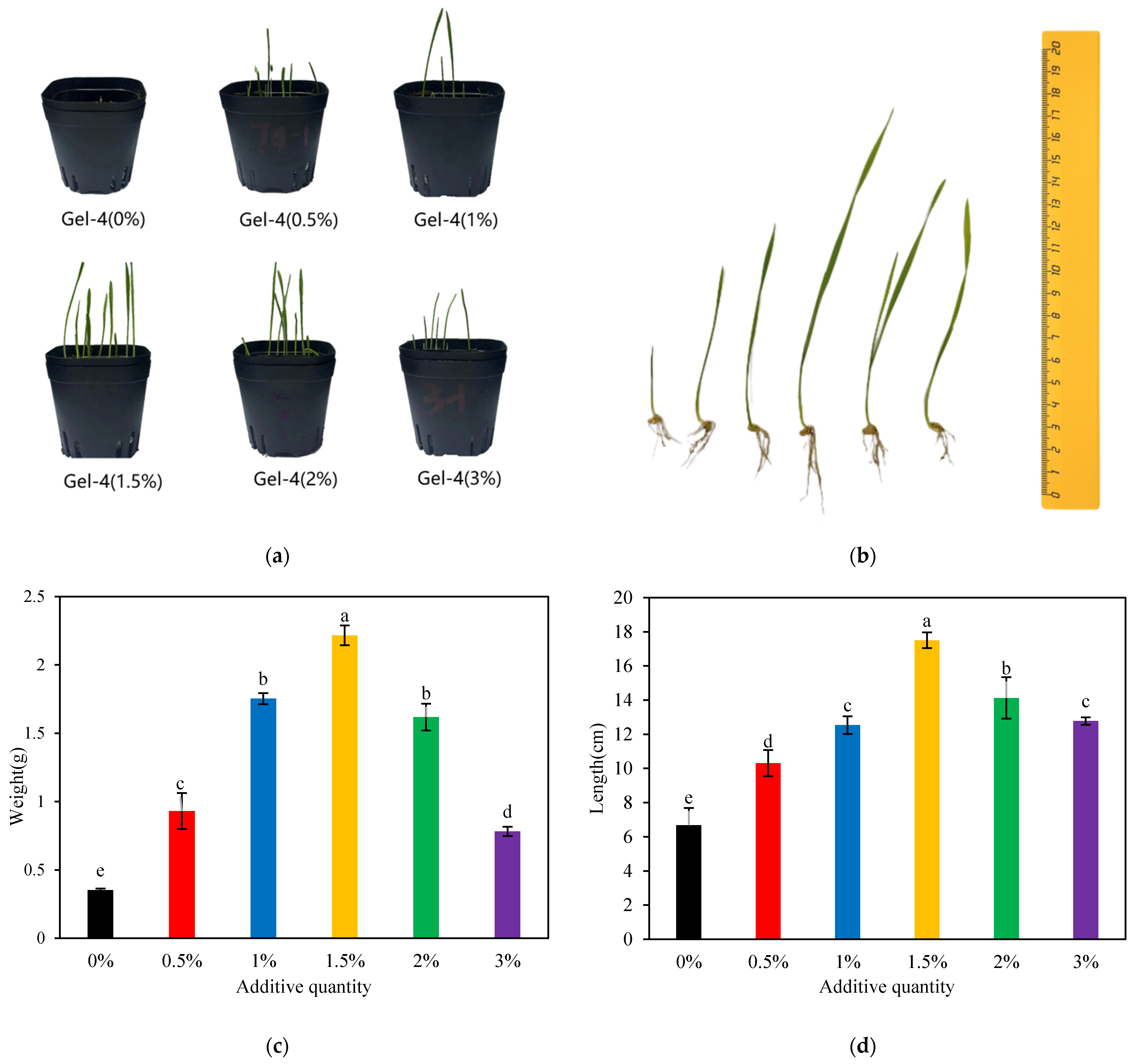
2.8. Evaluation of CW-(AA-AAm) Gel for Plant Growth Performance
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of (AA-AAm) Gels
4.3. Methods of Characterization
4.3.1. FTIR Spectroscopy
4.3.2. Morphological Characterization
4.3.3. Swelling Study in Water
4.3.4. Swelling Study in Different pH
4.3.5. Repeated Swelling Performance
4.3.6. Water Holding and Water Retention Studies
4.3.7. Potting Trials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nie, M.; Huang, S.; Zeng, X.M.; Peng, J.; Bai, G. Spatiotemporal desynchronization in the propagation from meteorological to soil moisture drought in the Loess Plateau, China. J. Hydrol. Reg. Stud. 2024, 56, 102025. [Google Scholar] [CrossRef]
- Ji, C.; Xu, Y.; Yang, M.; Shi, Y. The use of water retention agent in saline-alkali soil promotes the expression of nutrient transporter genes in wheat and increases grain yield. Plant Growth Regul. 2024, 104, 1047–1057. [Google Scholar] [CrossRef]
- Xiong, H.; Peng, H.; Kong, Y.; Wang, N.; Yang, F.; Meni, B.H.; Lei, Z. High salt tolerance hydrogel prepared of hydroxyethyl starch and its ability to increase soil water holding capacity and decrease water evaporation. Soil Tillage Res. 2022, 222, 105427. [Google Scholar] [CrossRef]
- Hastuti, N.; Agustini, L.; Amin, Y.; Indrawan, D.A.; Efiyanti, L. A novel transparent hydrogel made of nanocellulose derived from oil palm residue: Evaluation of its water retention and biodegradation properties. Bull. Mater. Sci. 2023, 46, 214. [Google Scholar] [CrossRef]
- Shan, B.H.; Wu, F.G. Hydrogel-based growth factor delivery platforms: Strategies and recent advances. Adv. Mater. 2024, 36, 2210707. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ma, L.; Duan, Q.; Xie, H.; Dong, X.; Zhang, H.; Yu, L. Development of Slow-Release Fertilizers with Function of Water Retention Using Eco-Friendly Starch Hydrogels. Molecules 2024, 29, 4835. [Google Scholar] [CrossRef] [PubMed]
- Luligo-Montealegre, W.E.; Prado-Alzate, S.; Ayala-Aponte, A.; Tirado, D.F.; Serna-Cock, L. Aloe vera Cuticle: A Promising Organic Water-Retaining Agent for Agricultural Use. Horticulturae 2024, 10, 797. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Agricultural waste-derived superabsorbent hydrogels: Preparation, performance, and socioeconomic impacts. J. Clean. Prod. 2020, 251, 119669. [Google Scholar] [CrossRef]
- Miljković, V.; Gajić, I.; Nikolić, L. Waste materials as a resource for production of cmc superabsorbent hydrogel for sustainable agriculture. Polymers 2021, 13, 4115. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Q.; Guo, L.; Wang, P.; Liu, S.; Chen, J.; Lei, Z. White Cabbage (Brassica oleracea L.) waste, as biowaste for the preparation of novel superabsorbent polymer gel. J. Environ. Chem. Eng. 2021, 9, 106689. [Google Scholar] [CrossRef]
- Zgallai, H.; Zoghlami, R.I.; Annabi, M.; Zarrouk, O.; Jellali, S.; Hamdi, H. Mitigating soil water deficit using organic waste compost and commercial water retainer: A comparative study under semiarid conditions. Euro-Mediterr. J. Environ. Integr. 2024, 9, 377–391. [Google Scholar] [CrossRef]
- Ma, D.; Li, J.; Liu, J.; Wang, R.; Meng, Q.; Li, J.; Zhang, S.; Shan, A. The gain effect of microbial consortia induced by adaptive domestication for efficient conversion of Chinese cabbage waste by anaerobic fermentation. Sci. Total Environ. 2024, 922, 171313. [Google Scholar] [CrossRef]
- Yao, Z.; Miao, J.; Wang, B.; Xu, W.; Wang, Y.; Lu, Q.; Zhang, J. Comparative analysis of crop rotation systems: The impact of ginger (Zingiber officinale) and sponge gourd (Luffa aegyptiaca) residues on growth of Chinese cabbage (Brassica rapa var. chinensis). Front. Plant Sci. 2024, 15, 1428943. [Google Scholar] [CrossRef]
- Ariyanti, D.; Swantomo, D.; Mustari AP, A.; Permana, S. One Stage Method–Activated Carbon Modified by Surfactant and Irra-diation to Highly Improve Adsorption of Lead (II) Ion Wastewater Simulation (Utilization of Banana Peel Biomass). J. Ecol. Eng. 2024, 25, 70–82. [Google Scholar] [CrossRef]
- Bernardino, C.A.R.; Mahler, C.F.; Silva, F.W.L.; Fernandes, J.O.; Braz, B.F.; Borges, R.C.; Veloso, M.C.C.; Archanjo, B.S.; Santelli, R.E.; Romeiro, G.A.; et al. Banana peel biochar from pyrolysis for the removal of nitrofurantoin in wastewater. Biomass Convers. Biorefin. 2024, 1–12. [Google Scholar] [CrossRef]
- Moumakwa, N.L.; Mohammed, A.S.; Olakanmi, E.O.; Bader, T.; Gessesse, A. Sustainable surface modification of sorghum residue-based fiber reinforced polymer composites: Properties and adhesion mechanism. Clean. Mater. 2023, 8, 100189. [Google Scholar] [CrossRef]
- Hua, B.; Wei, H.; Hu, C.; Zhang, Y.; Yang, S.; Wang, G.; Guo, T.; Li, J. Preparation of pH/temperature-responsive semi-IPN hydrogels based on sodium alginate and humic acid as slow-release and water-retention fertilizers. Polym. Bull. 2024, 81, 4175–4198. [Google Scholar] [CrossRef]
- Atassi, Y.; Said, M.; Tally, M.; Kouba, L. Synthesis and characterization of chitosan-g-poly (AMPS-co-AA-co-AM)/ground basalt composite hydrogel: Antibacterial activity. Polym. Bull. 2020, 77, 5281–5302. [Google Scholar] [CrossRef]
- Madramootoo, C.A.; Jain, A.; Oliva, C.; Wang, Y.; Abbasi, N.A. Growth and yield of tomato on soil amended with waste paper based hydrogels. Sci. Hortic. 2023, 310, 111752. [Google Scholar] [CrossRef]
- Pettinelli, N.; Sabando, C.; Rodríguez-Llamazares, S.; Bouza, R.; Castaño, J.; Valverde, J.C.; Rubilar, R.; Frizzo, M.; Recio-Sánchez, G. Sodium alginate-g-polyacrylamide hydrogel for water retention and plant growth promotion in water-deficient soils. Ind. Crops Prod. 2024, 222, 119759. [Google Scholar] [CrossRef]
- Salleh, K.M.; Zakaria, S.; Sajab, M.S.; Gan, S.; Chia, C.H.; Jaafar, S.N.S.; Amran, U.A. Chemically crosslinked hydrogel and its driving force towards superabsorbent behaviour. Int. J. Biol. Macromol. 2018, 118, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Teng, B.; Zhong, Y.; Wu, J.; Zhu, J.; Cai, L.; Qi, P.; Luo, Z. Transforming watermelon (Citrullus lanatus) rind into durable superabsorbent hydrogels for enhanced soil water retention properties and adsorbs dye in water. Heliyon 2024, 10, e38656. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, J.; Wang, Y.; Fang, B.; Ge, W.; Wang, X.; Zou, J.; Ji, R. Transcriptome Analysis of Chinese Cabbage Infected with Plasmodiophora Brassicae in the Primary Stage. Sci. Rep. 2024, 14, 26180. [Google Scholar] [CrossRef] [PubMed]
- Ritonga, F.N.; Gong, Z.; Zhang, Y.; Wang, F.; Gao, J.; Li, C.; Li, J. Exploiting Brassica rapa L. subsp. pekinensis Genome Research. Plants 2024, 13, 2823. [Google Scholar] [CrossRef]
- Chhetry, G.; Pattader, P.S.G. Effect of patterns on Polyacrylamide hydrogel surface towards enhancement of water retention. Polymer 2024, 308, 127362. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Liu, A.; Chen, X.; Xu, W.; Duan, X.; Shi, J.; Li, X. Preparation and properties of oriented and hydrophobic aerogels from corn stover. Ind. Crops Prod. 2023, 205, 117414. [Google Scholar] [CrossRef]
- Liu, C.; Tang, M.; Zhang, F.; Lei, F.; Li, P.; Wang, K.; Zeng, H.; Jiang, J. Facile Access to Gleditsia microphylla Galactomannan Hydrogel with Rapid Self-Repair Capacity and Multicyclic Water-Retaining Performance of Sandy Soil. Polymers 2022, 14, 5430. [Google Scholar] [CrossRef]
- Atassi, Y.; Said, M.; Tally, M.; Kouba, L. Ultrahigh water-retention cellulose hydrogels as soil amendments for early seed germination under harsh conditions. J. Clean. Prod. 2022, 370, 133602. [Google Scholar]
- Ribeiro, M.G.S.; Miranda, I.P.; Kriven, W.M.; Ozer, A.; Ribeiro, R.A.S. High strength and low water absorption of bamboo fiber-reinforced geopolymer composites. Constr. Build. Mater. 2024, 411, 134179. [Google Scholar] [CrossRef]
- Marcuello, C.; Foulon, L.; Chabbert, B.; Aguié-Béghin, V.; Molinari, M. Atomic force microscopy reveals how relative humidity impacts the Young’s modulus of lignocellulosic polymers and their adhesion with cellulose nanocrystals at the nanoscale. Int. J. Biol. Macromol. 2020, 147, 1064–1075. [Google Scholar] [CrossRef]
- Alam, M.N.; Islam, M.S.; Christopher, L.P. Sustainable production of cellulose-based hydrogels with superb absorbing potential in physiological saline. ACS Omega 2019, 4, 9419–9426. [Google Scholar] [CrossRef]
- Lang, Z.; Yan, S.; Zhu, Q. Water retention and sustained release of magnesium-based biochar modified hydrogel composite materials. J. Environ. Chem. Eng. 2023, 11, 111380. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, P.; Liu, S.; Chen, J.; Chen, R.; He, X.; Ma, G.; Lei, Z. Factors affecting the properties of superabsorbent polymer hydrogels and methods to improve their performance: A review. J. Mater. Sci. 2021, 56, 16223–16242. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Z.; Wen, Y.; Song, X.; Tan, W.K.; Ong, C.N.; Li, J. Recent Advances in Superabsorbent Hydrogels Derived from Agro Waste Materials for Sustainable Agriculture: A Review. J. Agric. Food Chem. 2024, 72, 22399–22419. [Google Scholar] [CrossRef]
- Abedi-Koupai, J.; Sohrab, F.; Swarbrick, G. Evaluation of hydrogel application on soil water retention characteristics. J. Plant Nutr. 2008, 31, 317–331. [Google Scholar] [CrossRef]
- Abdallah, A.M. The effect of hydrogel particle size on water retention properties and availability under water stress. Int. Soil Water Conserv. Res. 2019, 7, 275–285. [Google Scholar] [CrossRef]
- Lv, Q.; Wu, M.; Shen, Y. Enhanced swelling ratio and water retention capacity for novel super-absorbent hydrogel. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123972. [Google Scholar] [CrossRef]
- Tang, J.; Xing, T.; Chen, S.; Feng, J. A Shape Memory Hydrogel with Excellent Mechanical Properties, Water Retention Capacity, and Tunable Fluorescence for Dual Encryption. Small 2024, 20, 2305928. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhao, Y.; Li, X.; Lin, H.; Song, S.; Li, X.; Dong, Y. A novel strategy to construct hydrogels with anti-swelling and water-retention abilities by covalent surface modification. Soft Matter 2024, 20, 6215–6220. [Google Scholar] [CrossRef]
- Zhu, T.; Jiang, C.; Wang, M.; Zhu, C.; Zhao, N.; Xu, J. Skin-inspired double-hydrophobic-coating encapsulated hydrogels with enhanced water retention capacity. Adv. Funct. Mater. 2021, 31, 2102433. [Google Scholar] [CrossRef]
- El-Rehim, H.A.A.; Hegazy, E.S.A.; El-Mohdy, H.L.A. Radiation synthesis of hydrogels to enhance sandy soils water retention and increase plant performance. J. Appl. Polym. Sci. 2004, 93, 1360–1371. [Google Scholar] [CrossRef]
- Liu, T.Y.; Chen, S.Y.; Lin, Y.L.; Liu, D.M. Synthesis and characterization of amphiphatic carboxymethyl-hexanoyl chitosan hydrogel: Water-retention ability and drug encapsulation. Langmuir 2006, 22, 9740–9745. [Google Scholar] [CrossRef] [PubMed]
- Kawate, T.; Wang, Y.; Chan, K.; Shibata, N.; Doi, Y.; Masubuchi, Y.; Zinchenko, A. Polyion Hydrogels of Polymeric and Nanofibrous Carboxymethyl Cellulose and Chitosan: Mechanical Characteristics and Potential Use in Environmental Remediation. Gels 2024, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Jahandideh, A.; Moini, N.; Kabiri, K.; Zohuriaan-Mehr, M.J. A green strategy to endow superabsorbents with stretchability and self-healability. Chem. Eng. J. 2019, 370, 274–286. [Google Scholar] [CrossRef]
- Li, Y.; Hu, C.; Lan, J.; Yan, B.; Zhang, Y.; Shi, L.; Ran, R. Hydrogel-based temperature sensor with water retention, frost resistance and remoldability. Polymer 2020, 186, 122027. [Google Scholar] [CrossRef]
- Chavda, H.V.; Patel, C.N. Effect of crosslinker concentration on characteristics of superporous hydrogel. Int. J. Pharm. Investig. 2011, 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Rui, D.; Wang, S.; Du, Y. Water retention and heat storage characteristics of phase change hydrogel in cooling pavement. Constr. Build. Mater. 2024, 448, 138267. [Google Scholar] [CrossRef]
- Rawls, W.J.; Pachepsky, Y.A.; Ritchie, J.C.; Sobecki, T.M.; Bloodworth, H. Effect of soil organic carbon on soil water retention. Geoderma 2003, 116, 61–76. [Google Scholar] [CrossRef]
- Omer, A.M.; Tamer, T.M.; Hassan, M.E.; Khalifa, R.E.; Abd El-Monaem, E.M.; Eltaweil, A.S.; Mohy Eldin, M.S. Fabrication of grafted carboxymethyl cellulose superabsorbent hydrogel for water retention and sustained release of ethephon in sandy soil. Arab. J. Sci. Eng. 2023, 48, 561–572. [Google Scholar] [CrossRef]
- Dengxiao, Z.; Hongbin, J.; Wenjing, Z.; Qingsong, Y.; Zhihang, M.; Haizhong, W.; Wei, R.; Daichang, W. Combined biochar and water-retaining agent application increased soil water retention capacity and maize seedling drought resistance in Fluvisols. Sci. Total Environ. 2024, 907, 167885. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, E.; Saghir, S.; Xiao, Z. Novel superabsorbent polymer composite embedded with sodium alginate and diatomite for excellent water absorbency, water retention capacity, and high thermal stability. J. Mol. Struct. 2024, 1300, 137244. [Google Scholar] [CrossRef]
- Garg, S.; Garg, A.; Vishwavidyalaya, R.D. Hydrogel: Classification, properties, preparation and technical features. Asian J. Biomater. Res. 2016, 2, 163–170. [Google Scholar]
- Oyen, M.L. Mechanical characterisation of hydrogel materials. Int. Mater. Rev. 2014, 59, 44–59. [Google Scholar] [CrossRef]
- Zhu, J.; Tan, W.K.; Song, X.; Gao, Z.; Wen, Y.; Ong, C.N.; Loh, C.S.; Swarup, S.; Li, J. Converting Okara to superabsorbent hydrogels as soil supplements for enhancing the growth of Choy Sum (Brassica sp.) under water-limited conditions. ACS Sustain. Chem. Eng. 2020, 8, 9425–9433. [Google Scholar] [CrossRef]
- Candry, P.; Godfrey, B.J.; Winkler MK, H. Microbe-cellulose hydrogels as a model system for particulate carbon degradation in soil aggregates. ISME Commun. 2024, 4, ycae068. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.I.H.; Kadri, H.J.; Ahmed, F.; Rahman, M.H. Synthesis and characterization of starch-g-polyacrylamide-co-polylactic acid hydrogel for the potential wound dressing application. J. Polym. Environ. 2024. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Mori, T.; Hashimoto, T.; Sakai, Y. Evaluating the tea bag method as a potential tool for detecting the effects of added nutrients and their interactions with climate on litter decomposition. bioRxiv 2021. bioRxiv:2021.01.28.428520. [Google Scholar]
- Radwan, M.A.; Al-Sweasy, O.H.; Elazab, H.A. Preparation of hydrogel based on acryl amide and investigation of different factors affecting rate and amount of absorbed water. Agric. Sci. 2017, 8, 161. [Google Scholar] [CrossRef][Green Version]
- Karchoubi, F.; Ghotli, R.A.; Pahlevani, H.; Salehi, M.B. New insights into nanocomposite hydrogels; a review on recent advances in characteristics and applications. Adv. Ind. Eng. Polym. Res. 2024, 7, 54–78. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Chen, H.; Cheng, D. Environmentally friendly hydrogel: A review of classification, preparation and application in agriculture. Sci. Total Environ. 2022, 846, 157303. [Google Scholar] [CrossRef] [PubMed]
- Hackl, H.; Mistele, B.; Hu, Y.; Schmidhalter, U. Spectral assessments of wheat plants grown in pots and containers under saline conditions. Funct. Plant Biol. 2013, 40, 409–424. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, S.; Sun, Q.; Guo, B.; Zhang, Y.; Li, M.; Zhang, R. GC-IMS determination of volatile organic compounds as potential indicators of wheat germination rate. Food Biosci. 2024, 57, 103535. [Google Scholar] [CrossRef]
- Alvarez, A.L.; Weyers, S.L.; Gardner, R.D. Cyanobacteria-based soil amendments in the soil-plant system: Effects of inoculations on soil nutrient and microbial dynamics under spring wheat growth. Algal Res. 2024, 77, 103326. [Google Scholar] [CrossRef]


| Number | P (g) | AA (g) | Aam (g) | MBA (g) | APS (g) | NaOH (g) | Water (g) |
|---|---|---|---|---|---|---|---|
| CK | 0.000 | 40.320 | 17.280 | 0.144 | 1.152 | 8.960 | 124.144 |
| Gel-1 | 28.800 | 40.320 | 17.280 | 0.288 | 1.152 | 8.960 | 124.000 |
| Gel-2 | 28.800 | 40.320 | 17.280 | 0.144 | 1.152 | 8.960 | 124.144 |
| Gel-3 | 28.800 | 40.320 | 17.280 | 0.576 | 1.152 | 8.960 | 123.712 |
| Gel-4 | 28.800 | 28.800 | 28.800 | 0.288 | 1.152 | 6.400 | 126.560 |
| Gel-5 | 28.800 | 17.280 | 40.320 | 0.288 | 1.152 | 3.840 | 129.120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Zhong, Y.; Wu, J.; Fang, S.; Cai, L.; Li, M.; Cao, J.; Zhao, H.; Dong, B. “From Waste to Wonder”: Comparative Evaluation of Chinese Cabbage Waste and Banana Peel Derived Hydrogels on Soil Water Retention Performance. Gels 2024, 10, 833. https://doi.org/10.3390/gels10120833
Xie Y, Zhong Y, Wu J, Fang S, Cai L, Li M, Cao J, Zhao H, Dong B. “From Waste to Wonder”: Comparative Evaluation of Chinese Cabbage Waste and Banana Peel Derived Hydrogels on Soil Water Retention Performance. Gels. 2024; 10(12):833. https://doi.org/10.3390/gels10120833
Chicago/Turabian StyleXie, Yufan, Yuan Zhong, Jun Wu, Shiwei Fang, Liqun Cai, Minjun Li, Jun Cao, Hejie Zhao, and Bo Dong. 2024. "“From Waste to Wonder”: Comparative Evaluation of Chinese Cabbage Waste and Banana Peel Derived Hydrogels on Soil Water Retention Performance" Gels 10, no. 12: 833. https://doi.org/10.3390/gels10120833
APA StyleXie, Y., Zhong, Y., Wu, J., Fang, S., Cai, L., Li, M., Cao, J., Zhao, H., & Dong, B. (2024). “From Waste to Wonder”: Comparative Evaluation of Chinese Cabbage Waste and Banana Peel Derived Hydrogels on Soil Water Retention Performance. Gels, 10(12), 833. https://doi.org/10.3390/gels10120833






