Luminescence of Binary-Doped Silica Aerogel Powders: A Two-Step Sol-Gel Approach
Abstract
1. Introduction
2. Experimental Results
3. Discussion
3.1. Optical Properties
3.2. Structure and Thermal Conductivity
3.3. Preparation—Properties Relation
4. Conclusions
5. Materials and Methods
5.1. Sol-Gel Preparation
5.2. Optical Measurements
5.3. X-ray Diffraction and Thermal Properties
5.4. Color Coordinates
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. Aerogels Handbook; Springer: New York, NY, USA, 2011. [Google Scholar]
- Gutzov, S.; Danchova, N.; Kirilova, R.; Petrov, V.; Yordanova, S. Preparation and Luminescence of Silica Aerogel Composites Containing an Europium (III) Phenanthroline Nitrate Complex. J. Lumin. 2017, 193, 108–112. [Google Scholar] [CrossRef]
- Gutzov, S.; Shandurkov, D.; Danchova, N.; Petrov, V.; Spassov, T. Hybrid Composites Based on Aerogels: Preparation, Structure and Tunable Luminescence. J. Lumin. 2022, 251, 119171. [Google Scholar] [CrossRef]
- Shandurkov, D.; Ignatov, P.; Spassova, I.; Gutzov, S. Spectral and Texture Properties of Hydrophobic Aerogel Powders Obtained from Room Temperature Drying. Molecules 2021, 26, 1796. [Google Scholar] [CrossRef]
- Aguado, R.J.; Gomes, B.O.; Durães, L.; Valente, A.J.M. Luminescent Papers with Asymmetric Complexes of Eu(III) and Tb(III) in Polymeric Matrices and Suggested Combinations for Color Tuning. Molecules 2023, 28, 6164. [Google Scholar] [CrossRef]
- Hull, R.R.M.; Osgood, J.; Paris, J.; Warlimont, H. Spectroscopic Properties of Rare Earths in Optical Materials; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 3540238867. [Google Scholar]
- Koseva, I.; Tzvetkova, P.; Ivanov, P.; Yordanova, A.; Nikolova, V. Terbium and Europium Co-Doped NaAlSiO4 Nano Glass-Ceramics for LED Application. Optik 2017, 137, 45–50. [Google Scholar] [CrossRef]
- Koseva, I.; Tzvetkov, P.; Ivanov, P.; Yordanova, A.; Nikolov, V. Some Investigations on Tb3+ and Eu3+ Doped Na2SiO3 as a Material for LED Application. Optik 2018, 168, 376–383. [Google Scholar] [CrossRef]
- Pawlik, N.; Szpikowska-Sroka, B.; Goryczka, T.; Pisarska, J.; Pisarski, W.A. Structural and Photoluminescence Investigations of Tb3+/Eu3+ Co-Doped Silicate Sol-Gel Glass-Ceramics Containing CaF2, Nanocrystals. Materials 2021, 14, 754. [Google Scholar] [CrossRef]
- Han, S.; Tao, Y.; Du, Y.; Yan, S.; Chen, Y.; Chen, D. Luminescence Behavior of GdVO4: Tb Nanocrystals in Silica Glass-Ceramics. Crystals 2020, 10, 396. [Google Scholar] [CrossRef]
- Halubek-Gluchowska, K. Upconversion Luminescence of Silica–Calcia Nanoparticles Co-Doped with Tm3+ and Yb3+ Ions. Materials 2021, 14, 937. [Google Scholar] [CrossRef]
- Kameneva, S.V.; Yorov, K.E.; Kamilov, R.K.; Kottsov, S.Y.; Teplonogova, M.A.; Khamova, T.V.; Popkov, M.A.; Tronev, I.V.; Baranchikov, A.E.; Ivanov, V.K. Epoxide Synthesis of Binary Rare Earth Oxide Aerogels with High Molar Ratios (1:1) of Eu, Gd, and Yb. J. Sol-Gel Sci. Technol. 2023, 107, 586–597. [Google Scholar] [CrossRef]
- Zhang, Y. High-Entropy Materials: A Brief Introduction; Springer: Singapore, 2019; ISBN 978-981-13-8526-1. [Google Scholar]
- Workman, J.; Weyer, L. Practical Guide to Interpretive Near-Infrared Spectroscopy; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2008; ISBN 13: 978-1-57444-784-2. [Google Scholar]
- Mirochnik, A.G.; Bukvetskii, B.V.; Zhikhareva, P.A.; Karasev, V.E. Crystal Structure and Luminescence of the [Eu(Phen)2(NO3)3] Complex. The Role of the Ion-Coactivator. Russ. J. Coord. Chem. 2001, 27, 443–448. [Google Scholar] [CrossRef]
- Xie, T.; He, Y.-L.; Hu, Z.-J. Theoretical Study on Thermal Conductivities of Silica Aerogel Composite Insulating Material. Int. J. Heat Mass Transf. 2013, 58, 540–552. [Google Scholar] [CrossRef]
- Wei, D.-Y.; Lin, J.-L.; Zheng, Y.-Q. Note: The Crystal Structure of Tris(Nitrato-O,O′)Bis(1,10-Phenanthroline-N,N′)Terbium(III). J. Coord. Chem. 2002, 55, 1259–1262. [Google Scholar] [CrossRef]
- Zahariev, T.; Shandurkov, D.; Gutzov, S.; Trendafilova, N.; Enseling, D.; Jüstel, T.; Georgieva, I. Phenanthroline Chromophore as Efficient Antenna for Tb3+ Green Luminescence: A Theoretical Study. Dye. Pigment. 2020, 185, 108890. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of Europium(III) Spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Blasse, G.; Grabmaier, B.C. Luminescent Materials; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Fujishiro, F.; Murakami, M.; Hashimoto, T.; Takahashi, M. Orange Luminescence of Eu3+ Doped CuLaO2 Delafossite Oxide. J. Ceram. Soc. Japan 2010, 118, 1217–1220. [Google Scholar] [CrossRef][Green Version]
- Gomez-Romero, P.; Sanchez, C. Functional Hybrid Materials; Wiley-VCH: Hoboken, NJ, USA, 2003; ISBN 9781787284395. [Google Scholar]
- Dieke, G.H.; Crosswhite, H.M.; Crosswhite, H. Spectra and Energy Levels of Rare Earth Ions in Crystals; Interscience Publishers: New York, NY, USA, 1968; ISBN 0470213906. [Google Scholar]
- Singh, S.; Singh, D. Synthesis and Spectroscopic Investigations of Trivalent Europium-Doped M2SiO5 (M = Y and Gd) Nanophosphor for Display Applications. J. Mater. Sci. Mater. Electron. 2020, 31, 5165–5175. [Google Scholar] [CrossRef]
- Boruc, Z.; Fetlinski, B.; Kaczkan, M.; Turczynski, S.; Pawlak, D.A.; Malinowski, M. Temperature and Concentration Quenching of Tb3+ Emissions in Y4Al2O9 Crystals. J. Alloys Compd. 2012, 532, 92–97. [Google Scholar] [CrossRef]
- Culubrk, S.; Lojpur, V.; Antic, Z.; Drami, M.D. Structural and Optical Properties of Europium Doped Y2O3 Nanoparticles Prepared by Self-Propagation Room Temperature Reaction Method. J. Res. Phys. 2013, 37, 39–45. [Google Scholar] [CrossRef]
- Shandurkov, D.; Danchova, N.; Spassov, T.; Petrov, V.; Tsekov, R.; Gutzov, S. Silica Gels Doped with Gold Nanoparticles: Preparation, Structure and Optical Properties. Gels 2023, 9, 663. [Google Scholar] [CrossRef]
- Meldrum, F.C.; O’Shaughnessy, C. Crystallization in Confinement. Adv. Mater. 2020, 32, 2001068. [Google Scholar] [CrossRef] [PubMed]
- Brinker, J.C.; Scherer, G.W. The Physics and Chemistry of Sol-Gel Processing; Academic Press, INC: London, UK, 1990. [Google Scholar]
- Schmidt, H. Sol-Gel Production; Trans Tech Publications Ltd.: Uetikon-Zürich, Switzerland, 1998. [Google Scholar]
- Yadav, R.; Sardar, S.; Singh, M.; Mukherjee, R.K.; Anju; Singh, S.; Kushwaha, P.; Singh, S.P. Preparation of Holmium Oxide Solution as a Wavelength Calibration Standard for UV–Visible Spectrophotometer. Mapan J. Metrol. Soc. India 2021, 37, 579–584. [Google Scholar]
- Suzuki, K.; Kobayashi, A.; Kaneko, S.; Takehira, K.; Yoshihara, T.; Ishida, H.; Shiina, Y.; Oishi, S.; Tobita, S. Reevaluation of Absolute Luminescence Quantum Yields of Standard Solutions Using a Spectrometer with an Integrating Sphere and a Back-Thinned CCD Detector. Phys. Chem. Chem. Phys. 2009, 11, 9850–9860. [Google Scholar] [CrossRef] [PubMed]
- Wyman, C.; Sloan, P.-P.; Shirley, P. Simple Analytic Approximations to the CIE XYZ Color Matching Functions. J. Comput. Graph. Tech. 2013, 2, 11. [Google Scholar]
- Pharr, M.; Jakob, W.; Humphreys, G. Physically Based Rendering: From Theory to Implementation; The MIT Press: Cambridge, MA, USA, 2023; ISBN 978-0262048026. [Google Scholar]
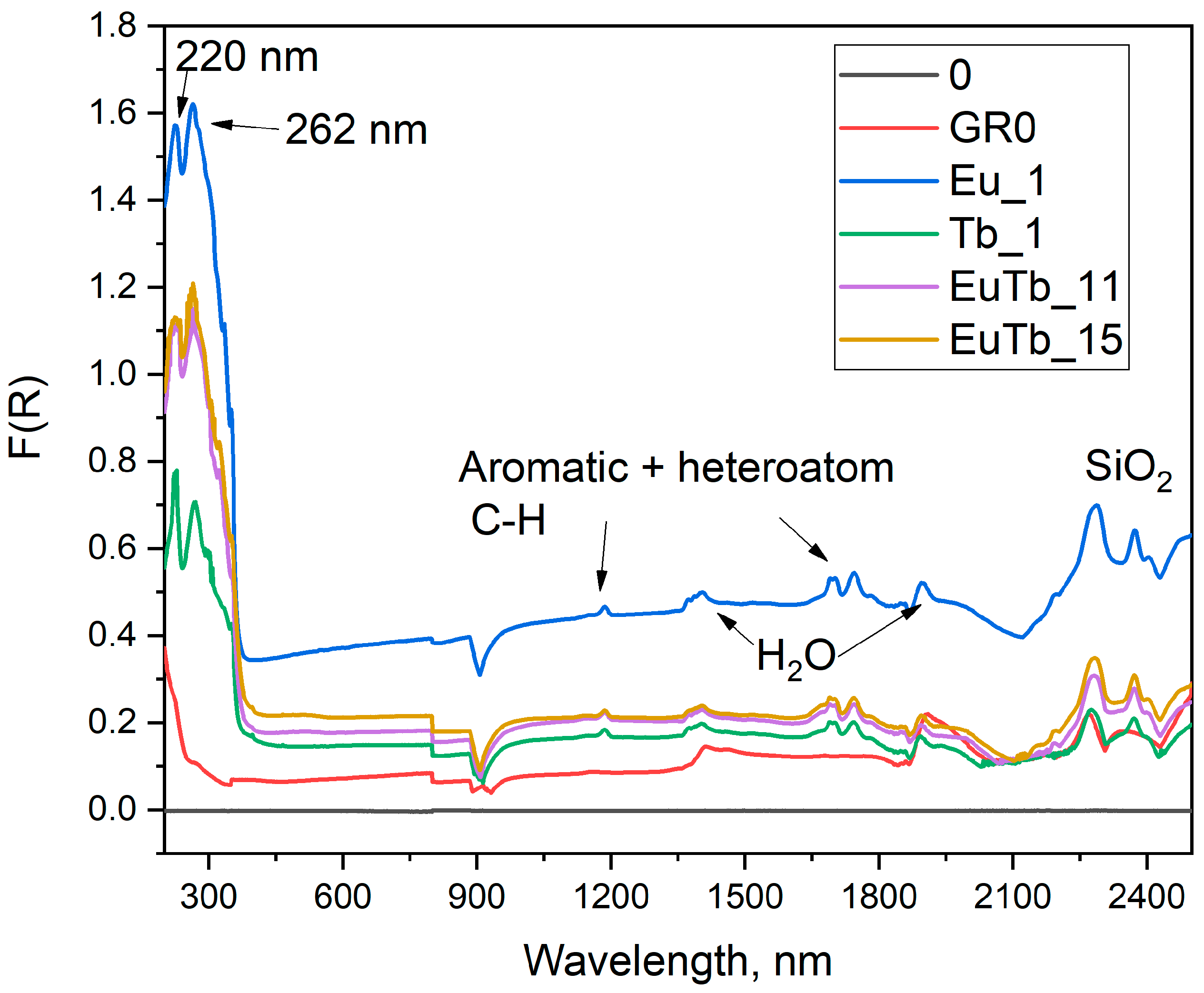
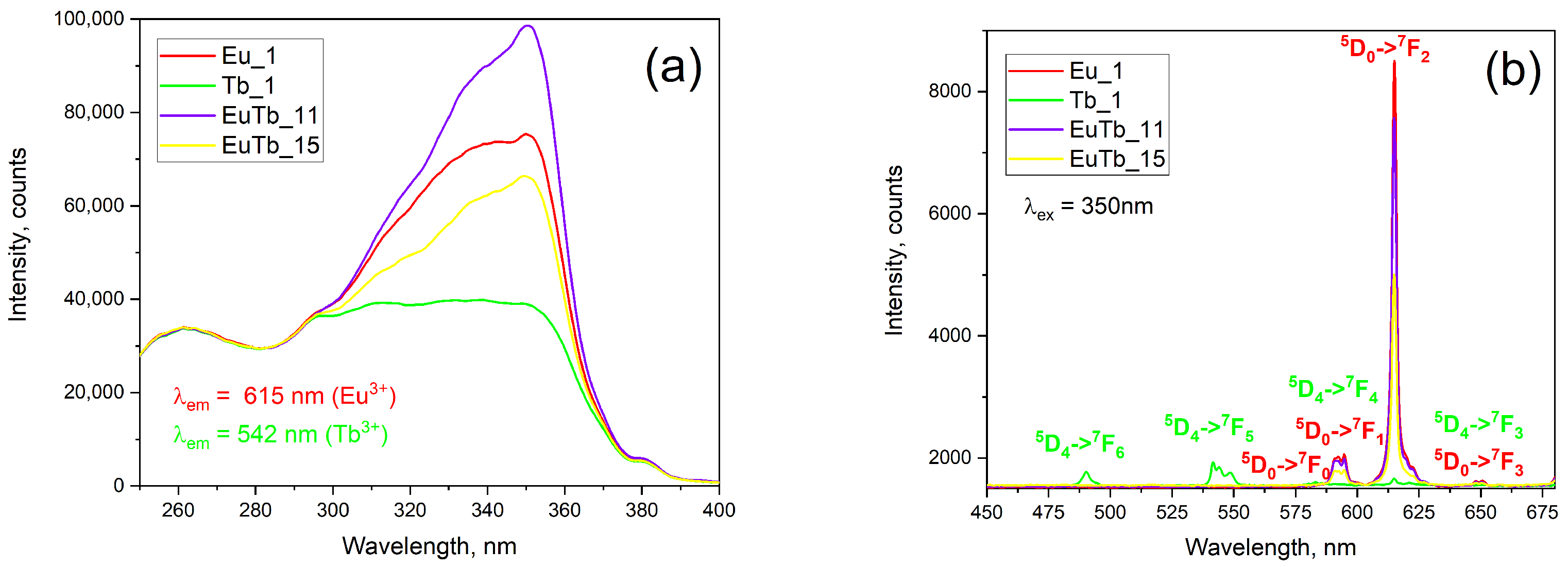
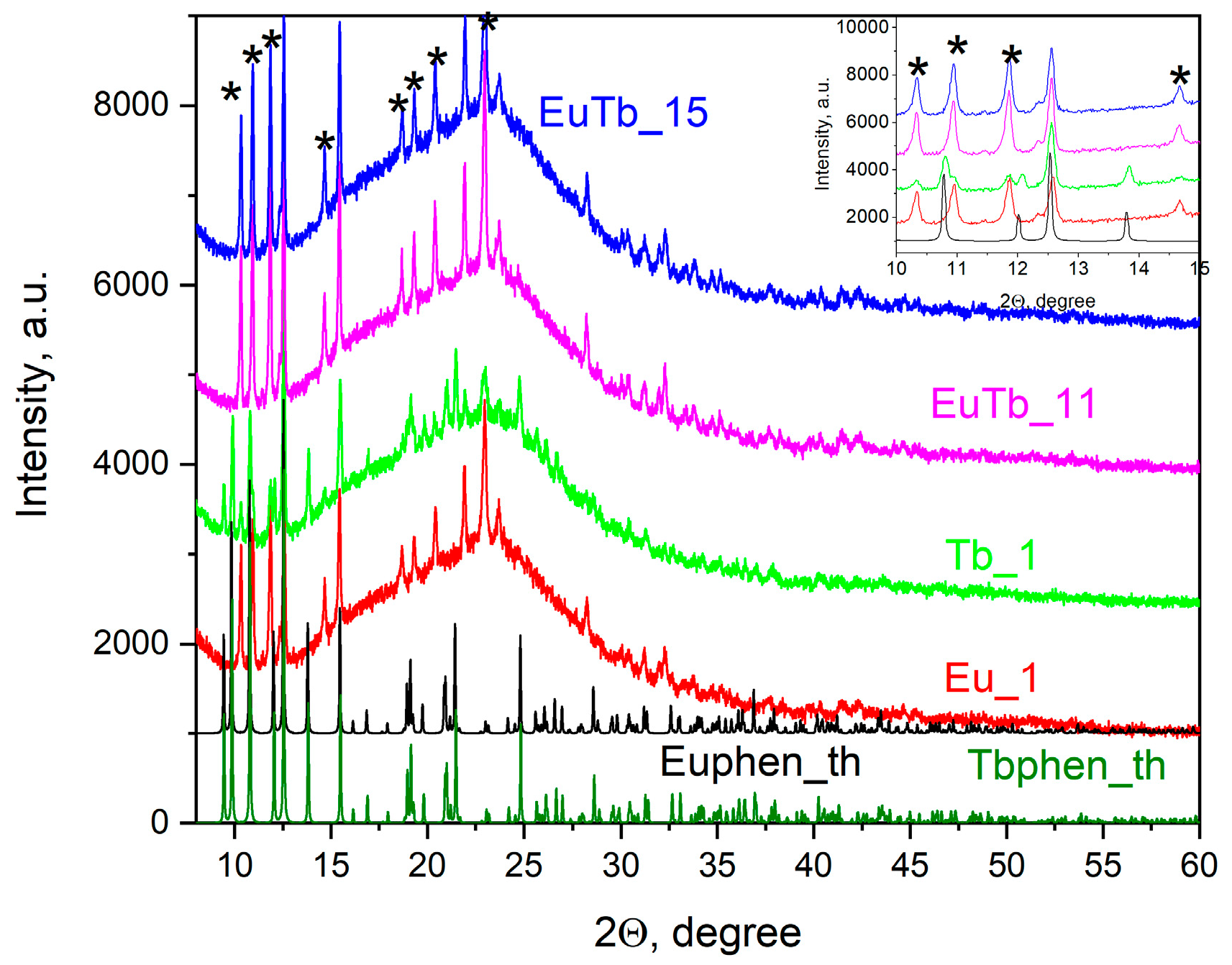
| Sample | α | λ W/m.K | Δλ W/m.K | e W.s1/2/m2.K | Δe W.s1/2/m2.K | ρ g/cm3 | nLn/nSiO2 |
|---|---|---|---|---|---|---|---|
| GR0 | 0 | 0.0640 | 0.0002 | 145.3896 | 0.8531 | 0.45 | 0 |
| Eu_1 | 1.1 | 0.0523 | 0.0001 | 95.6041 | 0.5516 | 0.23 | 0.01 |
| Tb_1 | 1.1 | 0.0467 | 0.0001 | 71.2591 | 0.5505 | 0.18 | 0.01 |
| EuTb_11 | 1.1 | 0.0510 | 0.0001 | 90.2086 | 0.4896 | 0.18 | 0.01 |
| EuTb_15 | 1.1 | 0.0523 | 0.0003 | 95.5750 | 1.1232 | 0.18 | 0.01 |
| Sample | Chemistry | QY, Red % | QY, Green % | IED/IMD | x | y | z |
|---|---|---|---|---|---|---|---|
| GR0 | SiO2 | - | - | - | - | - | - |
| Tb_1 | SiO2:0.01Tb | - | 6.3 | 0.39 | 0.631 | 0.340 | 0.03 |
| Eu_1 | SiO2:0.01Eu | 35 | - | 5.98 | 0.358 | 0.486 | 0.156 |
| EuTb1_1 | SiO2:0.005Eu;0.005Tb | 30 | - | 5.92 | 0.626 | 0.336 | 0.038 |
| EuTb1_5 | SiO2:0.0017Eu;0.0083Tb | 32 | - | 6.06 | 0.597 | 0.322 | 0.081 |
| Euphen | [Eu(phen)2](NO3)3 [2,3] | 35 | - | 7.25 | 0.309 | 0.602 | 0.089 |
| Tbphen | [Tb(phen)2](NO3)3 [2,3] | - | 13 | 0.37 | 0.665 | 0.333 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shandurkov, D.; Danchova, N.; Spassov, T.; Petrov, V.; Gutzov, S. Luminescence of Binary-Doped Silica Aerogel Powders: A Two-Step Sol-Gel Approach. Gels 2024, 10, 104. https://doi.org/10.3390/gels10020104
Shandurkov D, Danchova N, Spassov T, Petrov V, Gutzov S. Luminescence of Binary-Doped Silica Aerogel Powders: A Two-Step Sol-Gel Approach. Gels. 2024; 10(2):104. https://doi.org/10.3390/gels10020104
Chicago/Turabian StyleShandurkov, Dimitar, Nina Danchova, Tony Spassov, Vesselin Petrov, and Stoyan Gutzov. 2024. "Luminescence of Binary-Doped Silica Aerogel Powders: A Two-Step Sol-Gel Approach" Gels 10, no. 2: 104. https://doi.org/10.3390/gels10020104
APA StyleShandurkov, D., Danchova, N., Spassov, T., Petrov, V., & Gutzov, S. (2024). Luminescence of Binary-Doped Silica Aerogel Powders: A Two-Step Sol-Gel Approach. Gels, 10(2), 104. https://doi.org/10.3390/gels10020104









