Advances in Hydrogels for Meniscus Tissue Engineering: A Focus on Biomaterials, Crosslinking, Therapeutic Additives
Abstract
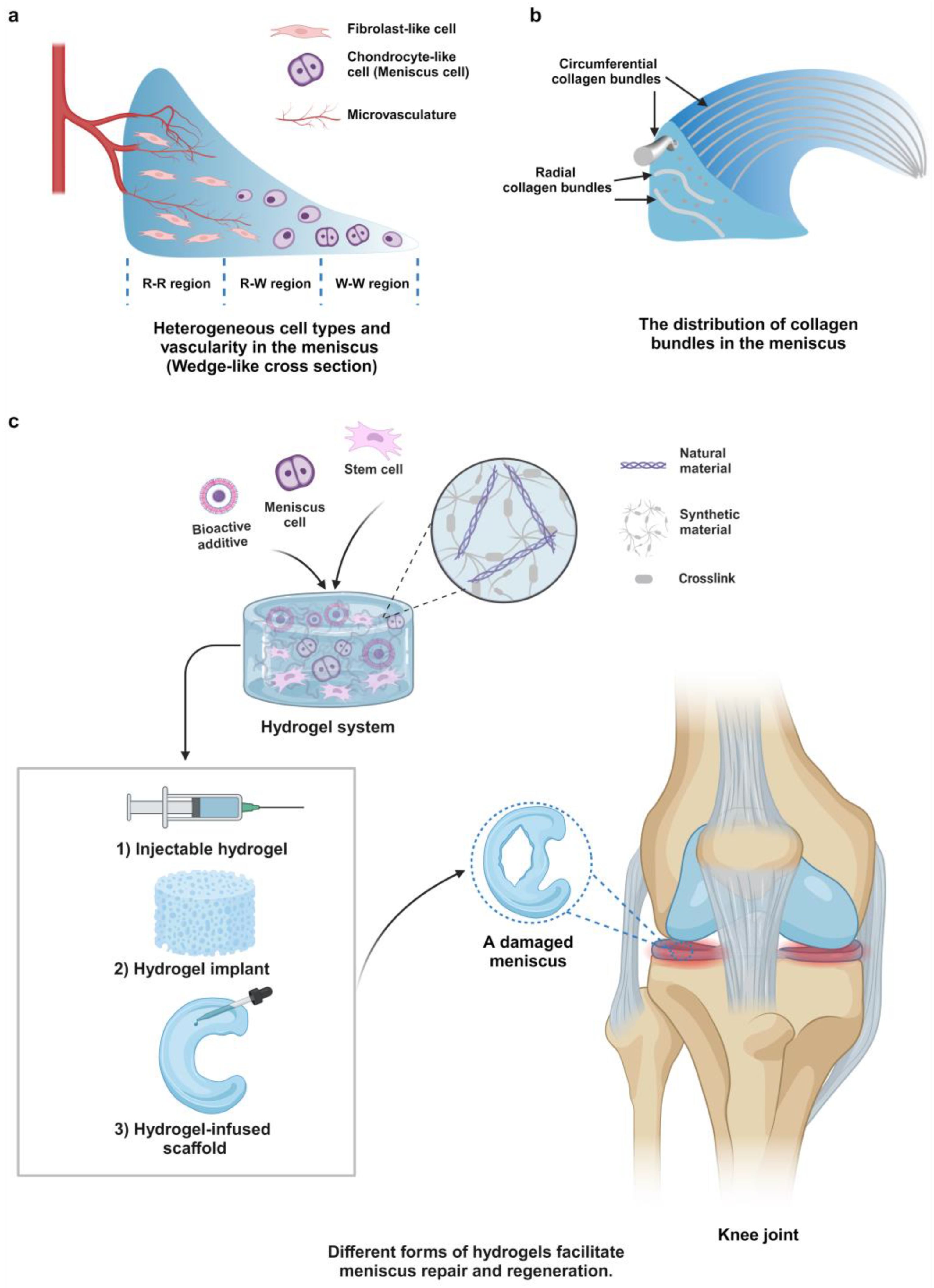
:1. Introduction
2. Biomaterials for Constructing Hydrogels
2.1. Natural Materials
2.1.1. Collagen Type I (COL I)
| Categories (Subtypes) | Names (Derivatives) | Functions | References |
|---|---|---|---|
| Natural materials (proteins) | Collagen type I | Stimulating cell proliferation | [40,47,48,49,50] |
| Promoting cell adhesion | |||
| Inducing fibrochondrogenic differentiation of cells | |||
| Gelatin (GelMA) | Stimulating cell proliferation | [24,43,51,52,53,54,55] | |
| Promoting cell adhesion | |||
| Inducing cells to acquire a fibrotic phenotype | |||
| Silk fibroin | Supporting cell growth and adhesion | [41] | |
| Fibrinogen | Supporting cell growth and adhesion | [38,56,57,58,59] | |
| Natural materials (polysaccharides) | Hyaluronic acid (TA-HA) | Promoting meniscus cell proliferation and migration Chondrogenesis Inhibiting meniscus cell apoptosis Inflammatory modulation | [47,50,60,75] |
| Alginate (ADA) | Supporting tissue regrowth | [51,61,62,63,64] | |
| Improvement in biocompatibility | |||
| Agarose | Supporting cell growth Chondrogenesis | [42,43,65] | |
| Cellulose | Improving hydrogel mechanical properties | [55,61,66] | |
| Chondroitin sulfate | Promoting cell proliferation | [67] | |
| Chondrogenesis | |||
| Chitosan | Supporting cell growth Promoting cell proliferation Potential chondrogenesis | [37,68,76] | |
| Natural materials (meniscus extracts) | m-dECMs | Supporting cell growth Promoting cell proliferation Sustaining meniscus cell phenotype Inducing fibrochondrogenic differentiation of cells | [23,53,62,63,69,70,71,72,77] |
| Synthetic materials | PEG (PEG-CHO, PEG-NHS, PEG-NH2, PEGDA) | Supporting cell growth Encapsulating cells and additives | [37,39,61,78] |
| PVA (PVA-g-GMA) | Supporting cell growth Improving hydrogel strength | [66] | |
| F-127 and PEO (F127DA) | Supporting cell growth Encapsulating cells and additives Improving hydrogel strength | [55,56,57] | |
| KI24RGDS | Supporting cell growth Stimulating cell proliferation Promoting cell adhesion | [79] |
2.1.2. Gelatin (Gel)
2.1.3. Fibrinogen (FB)
2.1.4. Silk Fibroin (SF)
2.1.5. Hyaluronic Acid (HA)
2.1.6. Alginate (Alg)
2.1.7. Agarose (Ag)
2.1.8. Cellulose
2.1.9. Chondroitin Sulfate (CS)
2.1.10. Chitosan (Chi)
2.1.11. Meniscus Decellularized Extracellular Matrices (m-dECMs)
2.2. Synthetic Materials
2.2.1. Polyethylene Glycol (PEG)
2.2.2. Poly (Vinyl Alcohol) (PVA)
2.2.3. Poloxamer (Pluronic® F-127) and Polyethylene Oxide (PEO)
2.2.4. Synthetic Peptide
2.3. Pros and Cons
3. Hydrogel Crosslinking Strategies
3.1. Covalent Crosslinking
3.1.1. Photo-Crosslinking

3.1.2. Schiff Base Reaction
3.1.3. Crosslinking Agents
3.1.4. Enzyme-Mediated Crosslinking
3.1.5. N-Hydroxysuccinimide-Ester (NHS-Ester) Chemistry
3.1.6. Radiation-Induced Crosslinking
3.2. Non-Covalent Crosslinking
3.2.1. Ionic Crosslinking
3.2.2. Electrostatic Interaction
3.2.3. Hydrogen Bonds
3.2.4. Physical Entanglement
3.3. Pros and Cons
4. Therapeutic Additives
4.1. Loading Methods
4.1.1. Physical Entrapment
4.1.2. Covalent Binding
4.1.3. Incorporation of Other Delivery Vehicles
4.2. Cells
4.2.1. Mesenchymal Stem Cells (MSCs)
4.2.2. Meniscus Fibrochondrocytes (MFCs) and Articular Chondrocytes (ACs)
4.3. Growth Factors
4.3.1. Transforming Growth Factor-β (TGF-β)
4.3.2. Connective Tissue Growth Factor (CTGF)
4.3.3. Platelet-Rich Plasma (PRP)
4.4. Proteins and Peptides
4.4.1. LTHPRWP Peptide (L7)
4.4.2. Wingless-Type MMTV Integration Site Family Member 5a (Wnt5a)
4.4.3. Annexin-1 Mimetic Peptide (Ac2-26)
4.4.4. Cluster of Differentiation 44 (CD44)
4.5. Drugs and Other Compounds
4.5.1. Simvastatin (SIM)
4.5.2. Diclofenac Sodium (DS)
4.5.3. Kartogenin (KGN)
4.5.4. Sodium Tanshinone IIA Sulfonate (STS)
4.5.5. Aptamer-Apt19S (Apt19S)
4.6. Pros and Cons
5. Key Issues in the Design of Hydrogel Systems for Application in MTE
5.1. Shear-Thinning Property and Toughness
5.2. Bio-Adhesive Property
5.3. Lubricating Property
5.4. Heterogeneity
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arner, J.W.; Ruzbarsky, J.J.; Vidal, A.F.; Frank, R.M. Meniscus Repair Part 1: Biology, Function, Tear Morphology, and Special Considerations. J. Am. Acad. Orthop. Surg. 2022, 30, e852–e858. [Google Scholar] [CrossRef]
- Gee, S.M.; Posner, M. Meniscus Anatomy and Basic Science. Sports Med. Arthrosc. Rev. 2021, 29, e18–e23. [Google Scholar] [CrossRef]
- Bilgen, B.; Jayasuriya, C.T.; Owens, B.D. Current Concepts in Meniscus Tissue Engineering and Repair. Adv. Healthc. Mater. 2018, 7, e1701407. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Hadidi, P.; Athanasiou, K.A. The knee meniscus: Structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 2011, 32, 7411–7431. [Google Scholar] [CrossRef]
- Murphy, C.A.; Garg, A.K.; Silva-Correia, J.; Reis, R.L.; Oliveira, J.M.; Collins, M.N. The Meniscus in Normal and Osteoarthritic Tissues: Facing the Structure Property Challenges and Current Treatment Trends. Annu. Rev. Biomed. Eng. 2019, 21, 495–521. [Google Scholar] [CrossRef] [PubMed]
- Abbadessa, A.; Crecente-Campo, J.; Alonso, M.J. Engineering Anisotropic Meniscus: Zonal Functionality and Spatiotemporal Drug Delivery. Tissue Eng. Part B Rev. 2021, 27, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qu, F.; Han, B.; Wang, C.; Li, H.; Mauck, R.L.; Han, L. Micromechanical anisotropy and heterogeneity of the meniscus extracellular matrix. Acta Biomater. 2017, 54, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.L.; Yi, S.J.; Ren, Y.; Zimmerman, T.A.; Zhang, L.Q. Tibiofemoral Contact Mechanics with Horizontal Cleavage Tear and Resection of the Medial Meniscus in the Human Knee. J. Bone Jt. Surg. Am. Vol. 2016, 98, 1829–1836. [Google Scholar] [CrossRef]
- Du, M.Z.; Dou, Y.; Ai, L.Y.; Su, T.; Zhang, Z.; Chen, Y.R.; Jiang, D. Meniscus heterogeneity and 3D-printed strategies for engineering anisotropic meniscus. Int. J. Bioprinting 2023, 9, 693. [Google Scholar] [CrossRef]
- Clark, C.R.; Ogden, J.A. Development of the menisci of the human knee joint. Morphological changes and their potential role in childhood meniscal injury. J. Bone Jt. Surg. Am. Vol. 1983, 65, 538–547. [Google Scholar] [CrossRef]
- Kwon, H.; Brown, W.E.; Lee, C.A.; Wang, D.; Paschos, N.; Hu, J.C.; Athanasiou, K.A. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 2019, 15, 550–570. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Kim, D.H.; Bansal, S.; Bae, Y.; Mauck, R.L.; Heo, S.J. Development of a decellularized meniscus matrix-based nanofibrous scaffold for meniscus tissue engineering. Acta Biomater. 2021, 128, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Matar, H.E.; Duckett, S.P.; Raut, V. Degenerative meniscal tears of the knee: Evaluation and management. Br. J. Hosp. Med. 2019, 80, 46–50. [Google Scholar] [CrossRef]
- Kim, S.; Bosque, J.; Meehan, J.P.; Jamali, A.; Marder, R. Increase in outpatient knee arthroscopy in the United States: A comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J. Bone Jt. Surg. Am. Vol. 2011, 93, 994–1000. [Google Scholar] [CrossRef]
- Novaretti, J.V.; Lian, J.; Patel, N.K.; Chan, C.K.; Cohen, M.; Musahl, V.; Debski, R.E. Partial Lateral Meniscectomy Affects Knee Stability Even in Anterior Cruciate Ligament-Intact Knees. J. Bone Jt. Surg. Am. Vol. 2020, 102, 567–573. [Google Scholar] [CrossRef]
- Liu, Z.; Zhuang, Y.; Fang, L.; Yuan, C.; Wang, X.; Lin, K. Breakthrough of extracellular vesicles in pathogenesis, diagnosis and treatment of osteoarthritis. Bioact. Mater. 2023, 22, 423–452. [Google Scholar] [CrossRef]
- Sihvonen, R.; Paavola, M.; Malmivaara, A.; Itälä, A.; Joukainen, A.; Kalske, J.; Nurmi, H.; Kumm, J.; Sillanpää, N.; Kiekara, T.; et al. Arthroscopic partial meniscectomy for a degenerative meniscus tear: A 5 year follow-up of the placebo-surgery controlled FIDELITY (Finnish Degenerative Meniscus Lesion Study) trial. Br. J. Sports Med. 2020, 54, 1332–1339. [Google Scholar] [CrossRef]
- Cavendish, P.A.; DiBartola, A.C.; Everhart, J.S.; Kuzma, S.; Kim, W.J.; Flanigan, D.C. Meniscal allograft transplantation: A review of indications, techniques, and outcomes. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2020, 28, 3539–3550. [Google Scholar] [CrossRef] [PubMed]
- Kaarre, J.; Herman, Z.J.; Zsidai, B.; Grassi, A.; Zaffagnini, S.; Samuelsson, K.; Musahl, V. Meniscus allograft transplantation for biologic knee preservation: Gold standard or dilemma? Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2023, 31, 3579–3581. [Google Scholar] [CrossRef] [PubMed]
- Winkler, P.W.; Faber, S.; Balke, M.; Metzlaff, S.; Niethammer, T.R.; Roessler, P.P.; Henkelmann, R.; Kurme, A.; Colcuc, S.; Zimmermann, G.; et al. Germany has a high demand in meniscal allograft transplantation but is subject to health economic and legal challenges: A survey of the German Knee Society. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2022, 30, 2352–2357. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Maimaitimin, M.; Wu, Y.; Fan, Y.; Ren, S.; Zhao, F.; Cao, C.; Hu, X.; Cheng, J.; Ao, Y. Meniscal fibrocartilage regeneration inspired by meniscal maturational and regenerative process. Sci. Adv. 2023, 9, eadg8138. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Chen, Y.R.; Wang, S.J.; Zhao, F.; Wang, X.G.; Yang, F.; Shi, J.J.; Ge, Z.G.; Ding, W.Y.; Yang, Y.C.; et al. Orchestrated biomechanical, structural, and biochemical stimuli for engineering anisotropic meniscus. Sci. Transl. Med. 2019, 11, eaao0750. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Yao, J.; Huang, X.; Luo, Y.; Wang, M.; Han, J.; Chen, F.; Yu, Y. Injectable ECM hydrogel for delivery of BMSCs enabled full-thickness meniscus repair in an orthotopic rat model. Bioact. Mater. 2020, 5, 871–879. [Google Scholar] [CrossRef]
- Liu, L.; Xian, Y.; Wang, W.; Huang, L.; Fan, J.; Ma, W.; Li, Y.; Liu, H.; Yu, J.K.; Wu, D. Meniscus-Inspired Self-Lubricating and Friction-Responsive Hydrogels for Protecting Articular Cartilage and Improving Exercise. ACS Nano 2023, 17, 24308–24319. [Google Scholar] [CrossRef]
- Gao, S.; Chen, M.; Wang, P.; Li, Y.; Yuan, Z.; Guo, W.; Zhang, Z.; Zhang, X.; Jing, X.; Li, X.; et al. An electrospun fiber reinforced scaffold promotes total meniscus regeneration in rabbit meniscectomy model. Acta Biomater. 2018, 73, 127–140. [Google Scholar] [CrossRef]
- Yan, R.; Chen, Y.; Gu, Y.; Tang, C.; Huang, J.; Hu, Y.; Zheng, Z.; Ran, J.; Heng, B.; Chen, X.; et al. A collagen-coated sponge silk scaffold for functional meniscus regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, J.; Rahimnejad, M.; Ur Rehman, M.R.; Beheshtizadeh, N.; Barati, A. Chapter 29—Development of 3D-printed biocompatible materials for meniscus substitution. In Cartilage Tissue and Knee Joint Biomechanics; Nochehdehi, A.R., Nemavhola, F., Thomas, S., Maria, H.J., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 487–506. [Google Scholar]
- Stocco, E.; Porzionato, A.; De Rose, E.; Barbon, S.; De Caro, R.; Macchi, V. Meniscus regeneration by 3D printing technologies: Current advances and future perspectives. J. Tissue Eng. 2022, 13, 20417314211065860. [Google Scholar] [CrossRef] [PubMed]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, Z.; Zhang, X.; Liu, C.; Yang, R.; Sun, Y.; Zhang, Y.; Liu, W. 3D Printed High-Strength Supramolecular Polymer Hydrogel-Cushioned Radially and Circumferentially Oriented Meniscus Substitute. Adv. Funct. Mater. 2022, 32, 202200360. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q. Enzyme-Laden Bioactive Hydrogel for Biocatalytic Monitoring and Regulation. Acc. Chem. Res. 2021, 54, 1274–1287. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Inda, M.E.; Lai, Y.; Lu, T.K.; Zhao, X. Engineered Living Hydrogels. Adv. Mater. 2022, 34, e2201326. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.M.; Carvalho, L.; Silva-Correia, J.; Vieira, S.; Majchrzak, M.; Lukomska, B.; Stanaszek, L.; Strymecka, P.; Malysz-Cymborska, I.; Golubczyk, D.; et al. Hydrogel-based scaffolds to support intrathecal stem cell transplantation as a gateway to the spinal cord: Clinical needs, biomaterials, and imaging technologies. NPJ Regen. Med. 2018, 3, 8. [Google Scholar] [CrossRef]
- Kharaziha, M.; Baidya, A.; Annabi, N. Rational Design of Immunomodulatory Hydrogels for Chronic Wound Healing. Adv. Mater. 2021, 33, e2100176. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Song, J.; Qiu, J.; Zhao, J. Repair of a Meniscal Defect in a Rabbit Model Through Use of a Thermosensitive, Injectable, In Situ Crosslinked Hydrogel with Encapsulated Bone Mesenchymal Stromal Cells and Transforming Growth Factor β1. Am. J. Sports Med. 2020, 48, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, S.; Ghataure, J.; Langford, D.; Brooke, R.; Kim, R.; Eyen, S.L.; Bensadoun, J.; Felix, J.T.; Cook, J.L.; Lee, C.H. Advanced bioactive glue tethering Lubricin/PRG4 to promote integrated healing of avascular meniscus tears. Bioact. Mater. 2023, 28, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Ye, J.; Fan, B.S.; Wang, X.; Zhang, J.Y.; Song, S.; Song, Y.; Jiang, W.B.; Wang, X.; Yu, J.K. Protein-spatiotemporal partition releasing gradient porous scaffolds and anti-inflammatory and antioxidant regulation remodel tissue engineered anisotropic meniscus. Bioact. Mater. 2023, 20, 194–207. [Google Scholar] [CrossRef]
- Stocco, T.D.; Moreira Silva, M.C.; Corat, M.A.F.; Gonçalves Lima, G.; Lobo, A.O. Towards Bioinspired Meniscus-Regenerative Scaffolds: Engineering a Novel 3D Bioprinted Patient-Specific Construct Reinforced by Biomimetically Aligned Nanofibers. Int. J. Nanomed. 2022, 17, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, N.; Cheng, J.; Sun, M.; Yang, P.; Zhao, F.; Zhang, J.; Duan, X.; Fu, X.; Zhang, J.; et al. Biomechanically, structurally and functionally meticulously tailored polycaprolactone/silk fibroin scaffold for meniscus regeneration. Theranostics 2020, 10, 5090–5106. [Google Scholar] [CrossRef]
- Bahcecioglu, G.; Hasirci, N.; Bilgen, B.; Hasirci, V. A 3D printed PCL/hydrogel construct with zone-specific biochemical composition mimicking that of the meniscus. Biofabrication 2019, 11, 025002. [Google Scholar] [CrossRef]
- Bahcecioglu, G.; Bilgen, B.; Hasirci, N.; Hasirci, V. Anatomical meniscus construct with zone specific biochemical composition and structural organization. Biomaterials 2019, 218, 119361. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Cucchiarini, M.; Madry, H. Hydrogels for precision meniscus tissue engineering: A comprehensive review. Connect Tissue Res 2017, 58, 317–328. [Google Scholar] [CrossRef]
- Jin, P.; Liu, L.; Chen, X.; Cheng, L.; Zhang, W.; Zhong, G. Applications and prospects of different functional hydrogels in meniscus repair. Front. Bioeng. Biotechnol. 2022, 10, 1082499. [Google Scholar] [CrossRef]
- Duan, W.L.; Zhang, L.N.; Bohara, R.; Martin-Saldaña, S.; Yang, F.; Zhao, Y.Y.; Xie, Y.; Bu, Y.Z.; Pandit, A. Adhesive hydrogels in osteoarthritis: From design to application. Mil. Med. Res. 2023, 10, 4. [Google Scholar] [CrossRef]
- Koh, R.H.; Jin, Y.; Kang, B.J.; Hwang, N.S. Chondrogenically primed tonsil-derived mesenchymal stem cells encapsulated in riboflavin-induced photocrosslinking collagen-hyaluronic acid hydrogel for meniscus tissue repairs. Acta Biomater. 2017, 53, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Abar, B.; Alonso-Calleja, A.; Kelly, A.; Kelly, C.; Gall, K.; West, J.L. 3D printing of high-strength, porous, elastomeric structures to promote tissue integration of implants. J. Biomed. Mater. Res. Part A 2021, 109, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Hagmeijer, M.H.; Vonk, L.A.; Fenu, M.; van Keep, Y.W.; Krych, A.J.; Saris, D.B. Meniscus regeneration combining meniscus and mesenchymal stromal cells in a degradable meniscus implant: An in vitro study. Eur. Cells Mater. 2019, 38, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Koh, R.H.; Shim, W.; Kim, H.D.; Yim, H.G.; Hwang, N.S. Riboflavin-induced photo-crosslinking of collagen hydrogel and its application in meniscus tissue engineering. Drug Deliv. Transl. Res. 2016, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Resmi, R.; Parvathy, J.; John, A.; Joseph, R. Injectable self-crosslinking hydrogels for meniscal repair: A study with oxidized alginate and gelatin. Carbohydr. Polym. 2020, 234, 115902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Matsushita, T.; Kuroda, R.; Nishida, K.; Matsuzaki, T.; Matsumoto, T.; Takayama, K.; Nagai, K.; Oka, S.; Tabata, Y.; et al. Local Administration of Simvastatin Stimulates Healing of an Avascular Meniscus in a Rabbit Model of a Meniscal Defect. Am. J. Sports Med. 2016, 44, 1735–1743. [Google Scholar] [CrossRef]
- Rothrauff, B.B.; Shimomura, K.; Gottardi, R.; Alexander, P.G.; Tuan, R.S. Anatomical region-dependent enhancement of 3-dimensional chondrogenic differentiation of human mesenchymal stem cells by soluble meniscus extracellular matrix. Acta Biomater. 2017, 49, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Rothrauff, B.B.; Alexander, P.G.; Lin, H.; Gottardi, R.; Fu, F.H.; Tuan, R.S. In Vitro Repair of Meniscal Radial Tear with Hydrogels Seeded With Adipose Stem Cells and TGF-β3. Am. J. Sports Med. 2018, 46, 2402–2413. [Google Scholar] [CrossRef]
- Jeencham, R.; Tawonsawatruk, T.; Numpaisal, P.O.; Ruksakulpiwat, Y. Reinforcement of Injectable Hydrogel for Meniscus Tissue Engineering by Using Cellulose Nanofiber from Cassava Pulp. Polymers 2023, 15, 2092. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-A.; An, Y.-H.; Yim, H.-G.; Han, W.-J.; Park, Y.-B.; Park, H.J.; Kim, M.Y.; Jang, J.; Koh, R.H.; Kim, S.-H.; et al. Injectable Fibrin/Polyethylene Oxide Semi-IPN Hydrogel for a Segmental Meniscal Defect Regeneration. Am. J. Sports Med. 2021, 49, 1538–1550. [Google Scholar] [CrossRef] [PubMed]
- An, Y.H.; Kim, J.A.; Yim, H.G.; Han, W.J.; Park, Y.B.; Jin Park, H.; Young Kim, M.; Jang, J.; Koh, R.H.; Kim, S.H.; et al. Meniscus regeneration with injectable Pluronic/PMMA-reinforced fibrin hydrogels in a rabbit segmental meniscectomy model. J. Tissue Eng. 2021, 12, 20417314211050141. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, S.; Gulko, J.; Kim, D.; Sim, K.H.; Gutman, S.; Yang, J.; Cook, J.L.; Lee, C.H. Effect of dose and release rate of CTGF and TGFβ3 on avascular meniscus healing. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2019, 37, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, S.; Gulko, J.; Sim, K.H.; Yang, J.; Cook, J.L.; Lee, C.H. Engineered Healing of Avascular Meniscus Tears by Stem Cell Recruitment. Sci. Rep. 2018, 8, 8150. [Google Scholar] [CrossRef]
- Kim, S.-H.; An, Y.-H.; Kim, H.D.; Kim, K.; Lee, S.-H.; Yim, H.-G.; Kim, B.-G.; Hwang, N.S. Enzyme-mediated tissue adhesive hydrogels for meniscus repair. Int. J. Biol. Macromol. 2018, 110, 479–487. [Google Scholar] [CrossRef]
- Karami, P.; Wyss, C.S.; Khoushabi, A.; Schmocker, A.; Broome, M.; Moser, C.; Bourban, P.E.; Pioletti, D.P. Composite Double-Network Hydrogels to Improve Adhesion on Biological Surfaces. ACS Appl. Mater. Interfaces 2018, 10, 38692–38699. [Google Scholar] [CrossRef]
- Li, M.; Yin, H.; Chen, M.; Deng, H.; Tian, G.; Guo, W.; Yi, G.; Guo, Q.; Chen, Z.; Liu, S. STS loaded PCL-MECM based hydrogel hybrid scaffolds promote meniscal regeneration via modulating macrophage phenotype polarization. Biomater. Sci. 2023, 11, 2759–2774. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Feng, Z.; Guo, W.; Yang, D.; Gao, S.; Li, Y.; Shen, S.; Yuan, Z.; Huang, B.; Zhang, Y.; et al. PCL-MECM-Based Hydrogel Hybrid Scaffolds and Meniscal Fibrochondrocytes Promote Whole Meniscus Regeneration in a Rabbit Meniscectomy Model. ACS Appl. Mater. Interfaces 2019, 11, 41626–41639. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Onodera, T.; Kondo, E.; Kawaguchi, Y.; Terkawi, M.A.; Baba, R.; Hontani, K.; Joutoku, Z.; Matsubara, S.; Homan, K.; et al. Effects of Ultra-Purified Alginate Gel Implantation on Meniscal Defects in Rabbits. Am. J. Sports Med. 2019, 47, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Bahcecioglu, G.; Hasirci, N.; Bilgen, B.; Hasirci, V. Hydrogels of agarose, and methacrylated gelatin and hyaluronic acid are more supportive for in vitro meniscus regeneration than three dimensional printed polycaprolactone scaffolds. Int. J. Biol. Macromol. 2019, 122, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Sinna, J.; Jeencham, R.; Mueangkhot, P.; Sophon, S.; Noralak, P.; Raksapakdee, R.; Numpaisal, P.O.; Ruksakulpiwat, Y. Development of Poly(vinyl alcohol) Grafted Glycidyl Methacrylate/Cellulose Nanofiber Injectable Hydrogels for Meniscus Tissue Engineering. Polymers 2023, 15, 4230. [Google Scholar] [CrossRef]
- Simson, J.A.; Strehin, I.A.; Allen, B.W.; Elisseeff, J.H. Bonding and fusion of meniscus fibrocartilage using a novel chondroitin sulfate bone marrow tissue adhesive. Tissue Eng. Part A 2013, 19, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Canciani, B.; Semeraro, F.; Herrera Millar, V.R.; Gervaso, F.; Polini, A.; Stanzione, A.; Peretti, G.M.; Di Giancamillo, A.; Mangiavini, L. In Vitro and In Vivo Biocompatibility Assessment of a Thermosensitive Injectable Chitosan-Based Hydrogel for Musculoskeletal Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 10446. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wei, Y.; Villasante, A.; Ng, J.J.D.; Arkonac, D.E.; Chao, P.-h.G.; Vunjak-Novakovic, G. Stem cell delivery in tissue-specific hydrogel enabled meniscal repair in an orthotopic rat model. Biomaterials 2017, 132, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ding, Q.; Dutta, A.; Wang, Y.; Huang, Y.H.; Weng, H.; Tang, L.; Hong, Y. An injectable extracellular matrix derived hydrogel for meniscus repair and regeneration. Acta Biomater. 2015, 16, 49–59. [Google Scholar] [CrossRef]
- Li, H.; Liao, Z.; Yang, Z.; Gao, C.; Fu, L.; Li, P.; Zhao, T.; Cao, F.; Chen, W.; Yuan, Z.; et al. 3D Printed Poly(ε-Caprolactone)/Meniscus Extracellular Matrix Composite Scaffold Functionalized with Kartogenin-Releasing PLGA Microspheres for Meniscus Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 662381. [Google Scholar] [CrossRef]
- Wu, J.; Xu, J.; Huang, Y.; Tang, L.; Hong, Y. Regional-specific meniscal extracellular matrix hydrogels and their effects on cell-matrix interactions of fibrochondrocytes. Biomed. Mater. 2021, 17, 014105. [Google Scholar] [CrossRef]
- Huang, D.; Li, Y.; Ma, Z.; Lin, H.; Zhu, X.; Xiao, Y.; Zhang, X. Collagen hydrogel viscoelasticity regulates MSC chondrogenesis in a ROCK-dependent manner. Sci. Adv. 2023, 9, eade9497. [Google Scholar] [CrossRef] [PubMed]
- Kremer, A.; Ribitsch, I.; Reboredo, J.; Dürr, J.; Egerbacher, M.; Jenner, F.; Walles, H. Three-Dimensional Coculture of Meniscal Cells and Mesenchymal Stem Cells in Collagen Type I Hydrogel on a Small Intestinal Matrix-A Pilot Study Toward Equine Meniscus Tissue Engineering. Tissue Eng. Part A 2017, 23, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Otsuki, S.; Okamoto, Y.; Nakagawa, K.; Wakama, H.; Okuno, N.; Neo, M. Hyaluronic acid promotes proliferation and migration of human meniscus cells via a CD44-dependent mechanism. Connect. Tissue Res. 2019, 60, 117–127. [Google Scholar] [CrossRef]
- Oryan, A.; Sahvieh, S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int. J. Biol. Macromol. 2017, 104, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, K.; Rothrauff, B.B.; Tuan, R.S. Region-Specific Effect of the Decellularized Meniscus Extracellular Matrix on Mesenchymal Stem Cell-Based Meniscus Tissue Engineering. Am. J. Sports Med. 2017, 45, 604–611. [Google Scholar] [CrossRef]
- Andress, B.; Kim, J.H.; Cutcliffe, H.C.; Amendola, A.; Goode, A.P.; Varghese, S.; DeFrate, L.E.; McNulty, A.L. Meniscus cell regional phenotypes: Dedifferentiation and reversal by biomaterial embedding. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2021, 39, 2177–2186. [Google Scholar] [CrossRef]
- Okuno, N.; Otsuki, S.; Aoyama, J.; Nakagawa, K.; Murakami, T.; Ikeda, K.; Hirose, Y.; Wakama, H.; Okayoshi, T.; Okamoto, Y.; et al. Feasibility of a self-assembling peptide hydrogel scaffold for meniscal defect: An in vivo study in a rabbit model. J. Orthop. Res. 2021, 39, 165–176. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Liu, Y.; Weng, R.; Wang, W.; Wei, X.; Li, J.; Chen, X.; Liu, Y.; Lu, F.; Li, Y. Tunable physical and mechanical properties of gelatin hydrogel after transglutaminase crosslinking on two gelatin types. Int. J. Biol. Macromol. 2020, 162, 405–413. [Google Scholar] [CrossRef]
- Yang, J.; He, H.; Li, D.; Zhang, Q.; Xu, L.; Ruan, C. Advanced strategies in the application of gelatin-based bioink for extrusion bioprinting. Bio-Des. Manuf. 2023, 6, 586–608. [Google Scholar] [CrossRef]
- Kurian, A.G.; Singh, R.K.; Patel, K.D.; Lee, J.H.; Kim, H.W. Multifunctional GelMA platforms with nanomaterials for advanced tissue therapeutics. Bioact. Mater. 2022, 8, 267–295. [Google Scholar] [CrossRef] [PubMed]
- Hense, D.; Büngeler, A.; Kollmann, F.; Hanke, M.; Orive, A.; Keller, A.; Grundmeier, G.; Huber, K.; Strube, O.I. Self-Assembled Fibrinogen Hydro- and Aerogels with Fibrin-like 3D Structures. Biomacromolecules 2021, 22, 4084–4094. [Google Scholar] [CrossRef] [PubMed]
- Nele, V.; Schutt, C.E.; Wojciechowski, J.P.; Kit-Anan, W.; Doutch, J.J.; Armstrong, J.P.K.; Stevens, M.M. Ultrasound-Triggered Enzymatic Gelation. Adv. Mater. 2020, 32, e1905914. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, M.T.; Lee, O.J.; Lee, J.S.; Yoon, S.I.; et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 2018, 9, 1620. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, M.; Yan, J.; Zhou, W.; Gao, S.; Liu, S.; Li, Q.; Zheng, Y.; Cheng, Y.; Guo, Q. Tannic acid/Sr(2+)-coated silk/graphene oxide-based meniscus scaffold with anti-inflammatory and anti-ROS functions for cartilage protection and delaying osteoarthritis. Acta Biomater. 2021, 126, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, M.; Zhou, W.; Gao, S.; Luo, X.; Peng, L.; Yan, J.; Wang, P.; Li, Q.; Zheng, Y.; et al. Cell-free 3D wet-electrospun PCL/silk fibroin/Sr(2+) scaffold promotes successful total meniscus regeneration in a rabbit model. Acta Biomater. 2020, 113, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Amorim, S.; Reis, C.A.; Reis, R.L.; Pires, R.A. Extracellular Matrix Mimics Using Hyaluronan-Based Biomaterials. Trends Biotechnol. 2021, 39, 90–104. [Google Scholar] [CrossRef]
- An, C.; Li, H.; Zhao, Y.; Zhang, S.; Zhao, Y.; Zhang, Y.; Yang, J.; Zhang, L.; Ren, C.; Zhang, Y.; et al. Hyaluronic acid-based multifunctional carriers for applications in regenerative medicine: A review. Int. J. Biol. Macromol. 2023, 231, 123307. [Google Scholar] [CrossRef]
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydrogel-Forming Algae Polysaccharides: From Seaweed to Biomedical Applications. Biomacromolecules 2021, 22, 1027–1052. [Google Scholar] [CrossRef]
- Xu, M.; Qin, M.; Cheng, Y.; Niu, X.; Kong, J.; Zhang, X.; Huang, D.; Wang, H. Alginate microgels as delivery vehicles for cell-based therapies in tissue engineering and regenerative medicine. Carbohydr. Polym. 2021, 266, 118128. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dutt, D.; Mishra, N.C. Cotton pulp for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2020, 31, 2094–2113. [Google Scholar] [CrossRef] [PubMed]
- Ganewatta, M.S.; Wang, Z.; Tang, C. Chemical syntheses of bioinspired and biomimetic polymers toward biobased materials. Nat. Rev. Chem. 2021, 5, 753–772. [Google Scholar] [CrossRef]
- Yang, J.; Shen, M.; Wen, H.; Luo, Y.; Huang, R.; Rong, L.; Xie, J. Recent advance in delivery system and tissue engineering applications of chondroitin sulfate. Carbohydr. Polym. 2020, 230, 115650. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef]
- Asgarpour, R.; Masaeli, E.; Kermani, S. Development of meniscus-inspired 3D-printed PCL scaffolds engineered with chitosan/extracellular matrix hydrogel. Polym. Adv. Technol. 2021, 32, 4721–4732. [Google Scholar] [CrossRef]
- Ke, X.; Li, M.; Wang, X.; Liang, J.; Wang, X.; Wu, S.; Long, M.; Hu, C. An injectable chitosan/dextran/β -glycerophosphate hydrogel as cell delivery carrier for therapy of myocardial infarction. Carbohydr. Polym. 2020, 229, 115516. [Google Scholar] [CrossRef]
- Xu, X.; Gu, Z.; Chen, X.; Shi, C.; Liu, C.; Liu, M.; Wang, L.; Sun, M.; Zhang, K.; Liu, Q.; et al. An injectable and thermosensitive hydrogel: Promoting periodontal regeneration by controlled-release of aspirin and erythropoietin. Acta Biomater. 2019, 86, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, D.; Yee, M.; Sheng, Z.L.J.; Amirul, A.; Naing, M.W. Decellularization systems and devices: State-of-the-art. Acta Biomater. 2020, 115, 51–59. [Google Scholar] [CrossRef]
- Lee, K.I.; Olmer, M.; Baek, J.; D’Lima, D.D.; Lotz, M.K. Platelet-derived growth factor-coated decellularized meniscus scaffold for integrative healing of meniscus tears. Acta Biomater. 2018, 76, 126–134. [Google Scholar] [CrossRef]
- Liang, Y.; Idrees, E.; Szojka, A.R.A.; Andrews, S.H.J.; Kunze, M.; Mulet-Sierra, A.; Jomha, N.M.; Adesida, A.B. Chondrogenic differentiation of synovial fluid mesenchymal stem cells on human meniscus-derived decellularized matrix requires exogenous growth factors. Acta Biomater. 2018, 80, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhao, H.; Li, Y.; Lee, A.L.; Li, Z.; Fu, M.; Li, C.; Yang, Y.Y.; Yuan, P. Synthetic peptide hydrogels as 3D scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2020, 160, 78–104. [Google Scholar] [CrossRef]
- Wu, X.; He, C.; Wu, Y.; Chen, X. Synergistic therapeutic effects of Schiff’s base cross-linked injectable hydrogels for local co-delivery of metformin and 5-fluorouracil in a mouse colon carcinoma model. Biomaterials 2016, 75, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.G. Production and Application of Biomaterials Based on Polyvinyl alcohol (PVA) as Wound Dressing. Chem. Asian J. 2022, 17, e202200595. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Huang, J.; Li, Y.; Lv, X.; Zhou, H.; Wang, H.; Xu, Y.; Wang, C.; Wang, J.; Liu, Z. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials 2020, 258, 120286. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef]
- Shriky, B.; Kelly, A.; Isreb, M.; Babenko, M.; Mahmoudi, N.; Rogers, S.; Shebanova, O.; Snow, T.; Gough, T. Pluronic F127 thermosensitive injectable smart hydrogels for controlled drug delivery system development. J. Colloid Interface Sci. 2020, 565, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, X.; Wu, Z.; Cao, L. Photocrosslinked methacrylated natural macromolecular hydrogels for tissue engineering: A review. Int. J. Biol. Macromol. 2023, 246, 125570. [Google Scholar] [CrossRef]
- Hung, B.P.; Harvestine, J.N.; Saiz, A.M.; Gonzalez-Fernandez, T.; Sahar, D.E.; Weiss, M.L.; Leach, J.K. Defining hydrogel properties to instruct lineage- and cell-specific mesenchymal differentiation. Biomaterials 2019, 189, 1–10. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.S.; Yue, K.; Khademhosseini, A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017, 57, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Ashammakhi, N.; Wu, X.Y.; Khademhosseini, A. Crosslinking Strategies for 3D Bioprinting of Polymeric Hydrogels. Small 2020, 16, e2002931. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Hsu, S.H. Hydrogels Based on Schiff Base Linkages for Biomedical Applications. Molecules 2019, 24, 3005. [Google Scholar] [CrossRef] [PubMed]
- Duceac, I.A.; Coseri, S. Chitosan Schiff-Base Hydrogels-A Critical Perspective Review. Gels 2022, 8, 779. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, H.; Luo, W.; Cai, T.; Li, Z.; Liu, Y.; Gao, W.; Wan, Q.; Wang, X.; Wang, J.; et al. Regeneration of skeletal system with genipin crosslinked biomaterials. J. Tissue Eng. 2020, 11, 2041731420974861. [Google Scholar] [CrossRef]
- Silva, M.; Faustino, H.; Coelho, J.A.S.; Pinto, M.V.; Fernandes, A.; Compañón, I.; Corzana, F.; Gasser, G.; Gois, P.M.P. Efficient Amino-Sulfhydryl Stapling on Peptides and Proteins Using Bifunctional NHS-Activated Acrylamides. Angew. Chem. (Int. Ed. Engl.) 2021, 60, 10850–10857. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.L.; Zhao, X.Y.; Xiong, W.; Ji, L.F.; Jia, M.X.; Liu, Y.Y.; Guo, H.T.; Qu, F.; Cui, W.; Gu, Q.; et al. Cartilage Lacuna-Inspired Microcarriers Drive Hyaline Neocartilage Regeneration. Adv. Mater. 2023, 35, e2212114. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kumar, A. Hydrogel formation by radiation induced crosslinked copolymerization of acrylamide onto moringa gum for use in drug delivery applications. Carbohydr. Polym. 2018, 200, 262–270. [Google Scholar] [CrossRef]
- Jeong, J.O.; Park, J.S.; Kim, E.J.; Jeong, S.I.; Lee, J.Y.; Lim, Y.M. Preparation of Radiation Cross-Linked Poly(Acrylic Acid) Hydrogel Containing Metronidazole with Enhanced Antibacterial Activity. Int. J. Mol. Sci. 2019, 21, 187. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-induced gelation of alginate: Mechanisms and applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Sathish, P.B.; Gayathri, S.; Priyanka, J.; Muthusamy, S.; Narmadha, R.; Shankar, K.G.; Selvakumar, R. Tricomposite gelatin-carboxymethylcellulose-alginate bioink for direct and indirect 3D printing of human knee meniscal scaffold. Int. J. Biol. Macromol. 2022, 195, 179–189. [Google Scholar] [CrossRef]
- Hoque, M.; Alam, M.; Wang, S.; Zaman, J.U.; Rahman, M.S.; Johir, M.A.H.; Tian, L.; Choi, J.-G.; Ahmed, M.B.; Yoon, M.-H. Interaction chemistry of functional groups for natural biopolymer-based hydrogel design. Mater. Sci. Eng. R Rep. 2023, 156, 100758. [Google Scholar] [CrossRef]
- Penner, R.C.; Andersen, E.S.; Jensen, J.L.; Kantcheva, A.K.; Bublitz, M.; Nissen, P.; Rasmussen, A.M.H.; Svane, K.L.; Hammer, B.; Rezazadegan, R.; et al. Hydrogen bond rotations as a uniform structural tool for analyzing protein architecture. Nat. Commun. 2014, 5, 5803. [Google Scholar] [CrossRef]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef]
- Puza, F.; Zheng, Y.; Han, L.; Xue, L.; Cui, J. Physical entanglement hydrogels: Ultrahigh water content but good toughness and stretchability. Polym. Chem. 2020, 11, 2339–2345. [Google Scholar] [CrossRef]
- Schoenmakers, D.C.; Rowan, A.E.; Kouwer, P.H.J. Crosslinking of fibrous hydrogels. Nat. Commun. 2018, 9, 2172. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Mooney, D.J. Chemical strategies to engineer hydrogels for cell culture. Nat. Rev. Chem. 2022, 6, 726–744. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xu, H.; Huang, H. A novel kartogenin-platelet-rich plasma gel enhances chondrogenesis of bone marrow mesenchymal stem cells in vitro and promotes wounded meniscus healing in vivo. Stem Cell Res. Ther. 2019, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Tang, R.; Shi, Z.; Feng, G.; Zhang, W. Wnt5a/Platelet-rich plasma synergistically inhibits IL-1β-induced inflammatory activity through NF-κB signaling pathway and prevents cartilage damage and promotes meniscus regeneration. J. Tissue Eng. Regen. Med. 2021, 15, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, T.; Cao, F.; Deng, H.; He, S.; Li, J.; Liu, S.; Yang, Z.; Yuan, Z.; Guo, Q. Integrated bioactive scaffold with aptamer-targeted stem cell recruitment and growth factor-induced pro-differentiation effects for anisotropic meniscal regeneration. Bioeng. Transl. Med. 2022, 7, e10302. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Shireman, P.K.; Hampton, B.; Burgess, W.H.; Greisler, H.P. Modulation of vascular cell growth kinetics by local cytokine delivery from fibrin glue suspensions. J. Vasc. Surg. 1999, 29, 852–861; discussion 862. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Volkova, E.; Blatchley, M.R.; Gerecht, S. Hydrogel vehicles for sequential delivery of protein drugs to promote vascular regeneration. Adv. Drug Deliv. Rev. 2019, 149–150, 95–106. [Google Scholar] [CrossRef] [PubMed]
- De Witte, T.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef]
- Salazar-Noratto, G.E.; Luo, G.; Denoeud, C.; Padrona, M.; Moya, A.; Bensidhoum, M.; Bizios, R.; Potier, E.; Logeart-Avramoglou, D.; Petite, H. Understanding and leveraging cell metabolism to enhance mesenchymal stem cell transplantation survival in tissue engineering and regenerative medicine applications. Stem Cells 2020, 38, 22–33. [Google Scholar] [CrossRef]
- McCorry, M.C.; Puetzer, J.L.; Bonassar, L.J. Characterization of mesenchymal stem cells and fibrochondrocytes in three-dimensional co-culture: Analysis of cell shape, matrix production, and mechanical performance. Stem Cell Res. Ther. 2016, 7, 39. [Google Scholar] [CrossRef]
- Cui, X.; Hasegawa, A.; Lotz, M.; D’Lima, D. Structured three-dimensional co-culture of mesenchymal stem cells with meniscus cells promotes meniscal phenotype without hypertrophy. Biotechnol. Bioeng. 2012, 109, 2369–2380. [Google Scholar] [CrossRef]
- Casiraghi, F.; Perico, N.; Cortinovis, M.; Remuzzi, G. Mesenchymal stromal cells in renal transplantation: Opportunities and challenges. Nat. Rev. Nephrol. 2016, 12, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, Y.; Song, J.; Li, X.; Zhang, X.; Zhou, Z.; Chen, D.; Ma, P.X.; Peng, W.; Wang, W.; et al. Cartilage regeneration using arthroscopic flushing fluid-derived mesenchymal stem cells encapsulated in a one-step rapid cross-linked hydrogel. Acta Biomater. 2018, 79, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Regmi, S.; Seo, Y.; Ahn, J.S.; Pathak, S.; Acharya, S.; Nguyen, T.T.; Yook, S.; Sung, J.H.; Park, J.B.; Kim, J.O.; et al. Heterospheroid formation improves therapeutic efficacy of mesenchymal stem cells in murine colitis through immunomodulation and epithelial regeneration. Biomaterials 2021, 271, 120752. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.K.; Dinnes, D.L.; Myers, P.T.; Cooper-White, J.J. Effects of biomimetic surfaces and oxygen tension on redifferentiation of passaged human fibrochondrocytes in 2D and 3D cultures. Biomaterials 2011, 32, 5600–5614. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Cai, L.; Xie, J.; Zhou, X. The role of TGF-beta3 in cartilage development and osteoarthritis. Bone Res. 2023, 11, 2. [Google Scholar] [CrossRef]
- Futrega, K.; Robey, P.G.; Klein, T.J.; Crawford, R.W.; Doran, M.R. A single day of TGF-β1 exposure activates chondrogenic and hypertrophic differentiation pathways in bone marrow-derived stromal cells. Commun. Biol. 2021, 4, 29. [Google Scholar] [CrossRef]
- Tuli, R.; Tuli, S.; Nandi, S.; Huang, X.; Manner, P.A.; Hozack, W.J.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J. Biol. Chem. 2003, 278, 41227–41236. [Google Scholar] [CrossRef]
- Kim, H.; Son, S.; Ko, Y.; Shin, I. CTGF regulates cell proliferation, migration, and glucose metabolism through activation of FAK signaling in triple-negative breast cancer. Oncogene 2021, 40, 2667–2681. [Google Scholar] [CrossRef]
- Jun, J.-I.; Lau, L.F. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 945–963. [Google Scholar] [CrossRef]
- Khattab, H.M.; Aoyama, E.; Kubota, S.; Takigawa, M. Physical interaction of CCN2 with diverse growth factors involved in chondrocyte differentiation during endochondral ossification. J. Cell Commun. Signal. 2015, 9, 247–254. [Google Scholar] [CrossRef]
- Brigstock, D.R. Connective tissue growth factor (CCN2, CTGF) and organ fibrosis: Lessons from transgenic animals. J. Cell Commun. Signal. 2010, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, X.; Jiang, W.; Zhu, Y.; Hu, Y.; Zhao, Y.; Song, X.; Zhao, J.; Zhang, W.; Peng, J.; et al. Platelet-Rich Plasma Therapy in the Treatment of Diseases Associated with Orthopedic Injuries. Tissue Eng. Part B Rev. 2020, 26, 571–585. [Google Scholar] [CrossRef]
- Riewruja, K.; Phakham, S.; Sompolpong, P.; Reantragoon, R.; Tanavalee, A.; Ngarmukos, S.; Udomsinprasert, W.; Suantawee, T.; Dechsupa, S.; Honsawek, S. Cytokine Profiling and Intra-Articular Injection of Autologous Platelet-Rich Plasma in Knee Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 890. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Sun, B.H.; Dai, Y.F.; Cui, T.Y.; Sun, J.L.; Shen, K.; Zhang, L.S.; Shi, C.X.; Wang, X.F. Healing of the Torn Anterior Horn of Rabbit Medial Meniscus to Bone after Transtibial Pull-Out Repair and Autologous Platelet-Rich Plasma Gel Injection. Orthop. Surg. 2023, 15, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Hagmeijer, M.H.; Korpershoek, J.V.; Crispim, J.F.; Chen, L.T.; Jonkheijm, P.; Krych, A.J.; Saris, D.B.F.; Vonk, L.A. The regenerative effect of different growth factors and platelet lysate on meniscus cells and mesenchymal stromal cells and proof of concept with a functionalized meniscus implant. J. Tissue Eng. Regen. Med. 2021, 15, 648–659. [Google Scholar] [CrossRef]
- Howard, D.; Shepherd, J.H.; Kew, S.J.; Hernandez, P.; Ghose, S.; Wardale, J.A.; Rushton, N. Release of growth factors from a reinforced collagen GAG matrix supplemented with platelet rich plasma: Influence on cultured human meniscal cells. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2014, 32, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Kurnaz, R.; Balta, O. Effect of platelet-rich plasma and platelet-rich fibrin matrix on healing of vertical meniscal tears in a rabbit model. Acta Orthop. Traumatol. Turc. 2020, 54, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.F.; Yang, Y.T.; Xie, W.Q.; He, M.; Liu, D.; Cai, Z.J.; Yu, D.J.; Li, Y.S.; Wei, L.C. Effects of Platelet-Rich Plasma and Bone Marrow Mesenchymal Stem Cells on Meniscal Repair in the White-White Zone of the Meniscus. Orthop. Surg. 2021, 13, 2423–2432. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Shon, O.J.; Park, S.I.; Kim, H.J.; Kim, S.; Ahn, M.W.; Do, S.H. Platelet-Rich Plasma Increases the Levels of Catabolic Molecules and Cellular Dedifferentiation in the Meniscus of a Rabbit Model. Int. J. Mol. Sci. 2016, 17, 120. [Google Scholar] [CrossRef]
- Shao, Z.; Zhang, X.; Pi, Y.; Wang, X.; Jia, Z.; Zhu, J.; Dai, L.; Chen, W.; Yin, L.; Chen, H.; et al. Polycaprolactone electrospun mesh conjugated with an MSC affinity peptide for MSC homing in vivo. Biomaterials 2012, 33, 3375–3387. [Google Scholar] [CrossRef]
- Shao, Z.; Zhang, X.; Pi, Y.; Yin, L.; Li, L.; Chen, H.; Zhou, C.; Ao, Y. Surface modification on polycaprolactone electrospun mesh and human decalcified bone scaffold with synovium-derived mesenchymal stem cells-affinity peptide for tissue engineering. J. Biomed. Mater. Res. Part A 2015, 103, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, X.; Li, R.; Li, Z.; Yang, B.; Shi, P.; Zhang, H.; Wang, C.; Wen, C.; Li, G.; et al. Biomaterial-mediated presentation of wnt5a mimetic ligands enhances chondrogenesis and metabolism of stem cells by activating non-canonical Wnt signaling. Biomaterials 2022, 281, 121316. [Google Scholar] [CrossRef] [PubMed]
- Farrera-Hernández, A.; Marín-Llera, J.C.; Chimal-Monroy, J. WNT5A-Ca(2+)-CaN-NFAT signalling plays a permissive role during cartilage differentiation in embryonic chick digit development. Dev. Biol. 2021, 469, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, Y.; Cheng, J.; Guo, J.; Zhang, Q.; Zhang, X.; Ren, J.; Wang, F.; Huang, J.; Hu, H.; et al. A Proresolving Peptide Nanotherapy for Site-Specific Treatment of Inflammatory Bowel Disease by Regulating Proinflammatory Microenvironment and Gut Microbiota. Adv. Sci. 2019, 6, 1900610. [Google Scholar] [CrossRef]
- Yuan, J.; Li, L.; Yang, Q.; Ran, H.; Wang, J.; Hu, K.; Pu, W.; Huang, J.; Wen, L.; Zhou, L.; et al. Targeted Treatment of Ischemic Stroke by Bioactive Nanoparticle-Derived Reactive Oxygen Species Responsive and Inflammation-Resolving Nanotherapies. ACS Nano 2021, 15, 16076–16094. [Google Scholar] [CrossRef]
- Weiß, E.; Kretschmer, D. Formyl-Peptide Receptors in Infection, Inflammation, and Cancer. Trends Immunol. 2018, 39, 815–829. [Google Scholar] [CrossRef]
- Ponta, H.; Sherman, L.; Herrlich, P.A. CD44: From adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003, 4, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Jay, G.D.; Waller, K.A. The biology of lubricin: Near frictionless joint motion. Matrix Biol. J. Int. Soc. Matrix Biol. 2014, 39, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, J.; Zhang, J.; Hua, Y. Simvastatin with PRP Promotes Chondrogenesis of Bone Marrow Stem Cells In Vitro and Wounded Rat Achilles Tendon-Bone Interface Healing In Vivo. Am. J. Sports Med. 2019, 47, 729–739. [Google Scholar] [CrossRef]
- Al-Baadani, M.A.; Xu, L.; Cai, K.; Yie, K.H.R.; Shen, Y.; Al-Bishari, A.M.; Al-Shaaobi, B.A.; Ma, P.; Shen, X.; Liu, J. Preparation of co-electrospinning membrane loaded with simvastatin and substance P to accelerate bone regeneration by promoting cell homing, angiogenesis and osteogenesis. Mater. Today. Bio 2023, 21, 100692. [Google Scholar] [CrossRef]
- da Costa, B.R.; Pereira, T.V.; Saadat, P.; Rudnicki, M.; Iskander, S.M.; Bodmer, N.S.; Bobos, P.; Gao, L.; Kiyomoto, H.D.; Montezuma, T.; et al. Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: Network meta-analysis. BMJ (Clin. Res. Ed.) 2021, 375, n2321. [Google Scholar] [CrossRef]
- Sufian, A.; Bhattacherjee, D.; Barman, P.; Srivastava, A.; Thummer, R.P.; Bhabak, K.P. Stimuli-responsive prodrug of non-steroidal anti-inflammatory drug diclofenac: Self-immolative drug release with turn-on near-infrared fluorescence. Chem. Commun. 2022, 58, 7833–7836. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Peng, L.; Li, L.; Huang, C.; Shi, K.; Meng, X.; Wang, P.; Wu, M.; Li, L.; Cao, H.; et al. 3D-bioprinted BMSC-laden biomimetic multiphasic scaffolds for efficient repair of osteochondral defects in an osteoarthritic rat model. Biomaterials 2021, 279, 121216. [Google Scholar] [CrossRef]
- Johnson, K.; Zhu, S.; Tremblay, M.S.; Payette, J.N.; Wang, J.; Bouchez, L.C.; Meeusen, S.; Althage, A.; Cho, C.Y.; Wu, X.; et al. A stem cell-based approach to cartilage repair. Science 2012, 336, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Chen, S.; Wu, Q.; Chen, T.; Li, S. A network pharmacology approach to investigate the anti-inflammatory mechanism of effective ingredients from Salvia miltiorrhiza. Int. Immunopharmacol. 2020, 81, 106040. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Y.; Li, H.; Dai, Y.; Zhou, G.; Zhou, Z.; Xia, H.; Liu, H. Tanshinone IIA Delivery Silk Fibroin Scaffolds Significantly Enhance Articular Cartilage Defect Repairing via Promoting Cartilage Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 21470–21480. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, Y.; Tan, Y.; Wang, J.; Liu, H.; Wang, Y.; Yang, S.; Shi, M.; Zhao, S.; Zhang, Y.; et al. A Difunctional Regeneration Scaffold for Knee Repair based on Aptamer-Directed Cell Recruitment. Adv. Mater. 2017, 29, 1605235. [Google Scholar] [CrossRef]
- Bashor, C.J.; Hilton, I.B.; Bandukwala, H.; Smith, D.M.; Veiseh, O. Engineering the next generation of cell-based therapeutics. Nat. Rev. Drug Discov. 2022, 21, 655–675. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]



| Hydrogels (Backbones) | Crosslinking Strategies | Cells | Additives (@Vehicles) | Models | Applications | References |
|---|---|---|---|---|---|---|
| Gelatin | Transglutaminase | None | KGN/DS@ NLPs | Cells*; rats | Injectable hydrogels | [24] |
| Gelatin; ADA | Schiff base reaction; | MFCs | None | Cells | Injectable hydrogels | [51] |
| Gelatin; TA-HA; | Tyrosinase | MFCs | None | Cells; in vitro explants# | Injectable hydrogels | [60] |
| FB; PEO | Thrombin; H-bonds | None | None | Rabbits | Injectable hydrogels | [56] |
| Alg | Ca2+ Crosslinking | None | None | Rabbits | Injectable hydrogels | [64] |
| GelMA; CNF; F127DA | Photo-crosslinking; H-bonds | None | None | Cells* | Injectable hydrogels | [54] |
| CS-NHS | NHS-ester chemistry | MFCs | BM | Cells; rats | Injectable hydrogels | [67] |
| Chitosan; β-GP | Electrostatic interaction | MSCs | None | Cells; nude mice | Injectable hydrogels | [68] |
| m-dECMs | H-bonds | MSCs; ACs | None | Cells; rats | Injectable hydrogels | [23,69,70] |
| 4-arm PEG-CHO; Chi | Schiff base reaction | MSCs | TGF-β1 | Cells; rabbits | Injectable hydrogels | [37] |
| Gelatin | Glutaraldehyde | None | SIM@Micelles | Cells*; rabbits | Hydrogel implants | [52] |
| FB | Thrombin | None | CTGF; TGF-β3@MPs | Cells*; in vitro explants# | Hydrogel implants | [58] |
| FB | Thrombin; genipin | None | CD44; TGF-β3@MPs; CTGF | Cells*; in vitro explants# | Hydrogel implants | [38] |
| PRP | Thrombin | MSCs | KGN | Cell; rabbits | Hydrogel implants | [132] |
| PRP | Thrombin/Ca2+ | None | Wnt5a | Cells*; rabbits | Hydrogel implants | [133] |
| Alg; m-dECMs (3DP-PCL) | Ca2+ Crosslinking | MFCs | None | Cells; rats; rabbits | Hydrogel-infused scaffolds | [63] |
| SF (3DP-PCL) | Radiation-induced crosslinking | None | L7 peptide | Cells*; rats; rabbits | Hydrogel-infused scaffolds | [41] |
| PEG-NHS; PEG-NH2 (3DP-PCL) | NHS-ester chemistry | MSCs | Ac2-26; TGF-β3; CTGF | Cells | Hydrogel-infused scaffolds | [39] |
| m-dECMs (3DP-PCL) | H-bonds | None | KGN@MPs | Cells*; rats; rabbits | Hydrogel-infused scaffolds | [71] |
| Alg; m-dECMs (3DP-PCL) | Ca2+ Crosslinking | MFCs | STS | Cells; rats; rabbits | Hydrogel-infused scaffolds | [62] |
| GelMA; Ag (3DP-PCL) | Photo-crosslinking; H-bonds | MFCs | None | Cells | Hydrogel-infused scaffolds | [43] |
| m-dECMs; GelMA (3DP-PCL) | Photo-crosslinking | None | Apt19s; TGF-β3@MPs; CTGF@NPs | Cells*; rats; rabbits | Hydrogel-infused scaffolds | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Wang, J.; Jiang, C.; Xu, K.; Xu, T.; Yu, X.; Fang, J.; Yang, Y.; Dai, X. Advances in Hydrogels for Meniscus Tissue Engineering: A Focus on Biomaterials, Crosslinking, Therapeutic Additives. Gels 2024, 10, 114. https://doi.org/10.3390/gels10020114
Zhou Z, Wang J, Jiang C, Xu K, Xu T, Yu X, Fang J, Yang Y, Dai X. Advances in Hydrogels for Meniscus Tissue Engineering: A Focus on Biomaterials, Crosslinking, Therapeutic Additives. Gels. 2024; 10(2):114. https://doi.org/10.3390/gels10020114
Chicago/Turabian StyleZhou, Zhuxing, Jiajie Wang, Chaoqian Jiang, Kaiwang Xu, Tengjing Xu, Xinning Yu, Jinghua Fang, Yanyu Yang, and Xuesong Dai. 2024. "Advances in Hydrogels for Meniscus Tissue Engineering: A Focus on Biomaterials, Crosslinking, Therapeutic Additives" Gels 10, no. 2: 114. https://doi.org/10.3390/gels10020114
APA StyleZhou, Z., Wang, J., Jiang, C., Xu, K., Xu, T., Yu, X., Fang, J., Yang, Y., & Dai, X. (2024). Advances in Hydrogels for Meniscus Tissue Engineering: A Focus on Biomaterials, Crosslinking, Therapeutic Additives. Gels, 10(2), 114. https://doi.org/10.3390/gels10020114






