3D-Printable Sustainable Bioplastics from Gluten and Keratin
Abstract
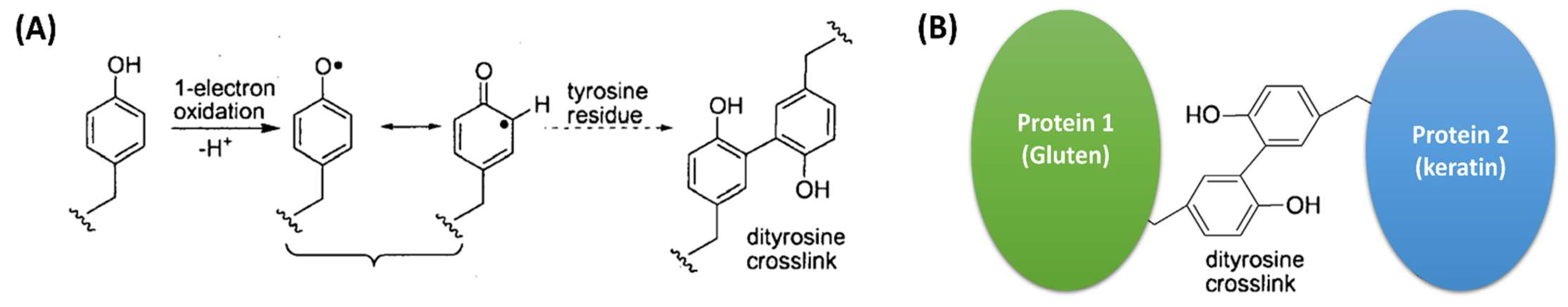
1. Introduction
2. Results and Discussion
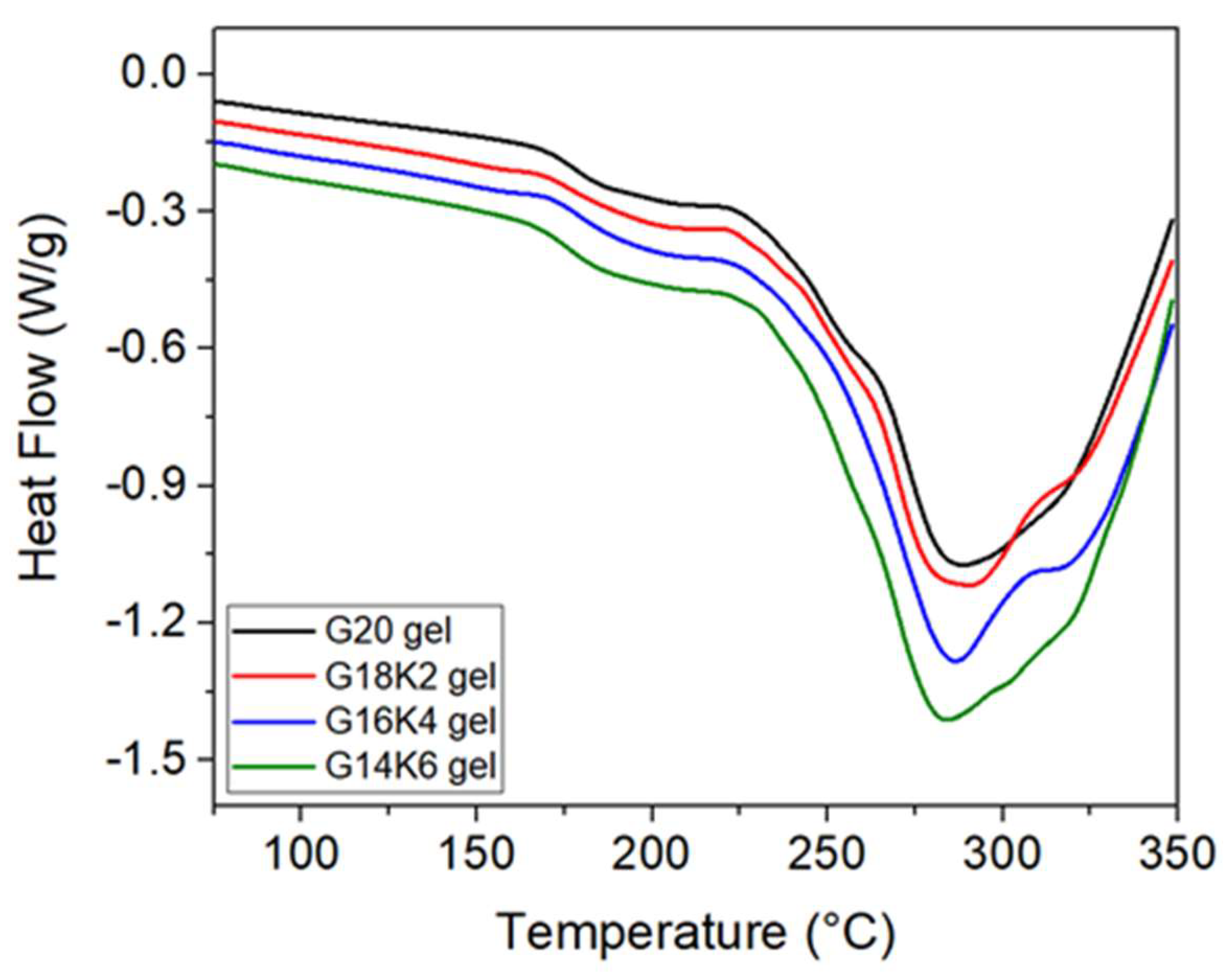
2.1. Secondary Structure and Molecular Chain Mobility of Gluten/Keratin Hybrid Film
2.2. Water Uptake Behavior and Morphology of Gluten/Keratin Hybrid Film
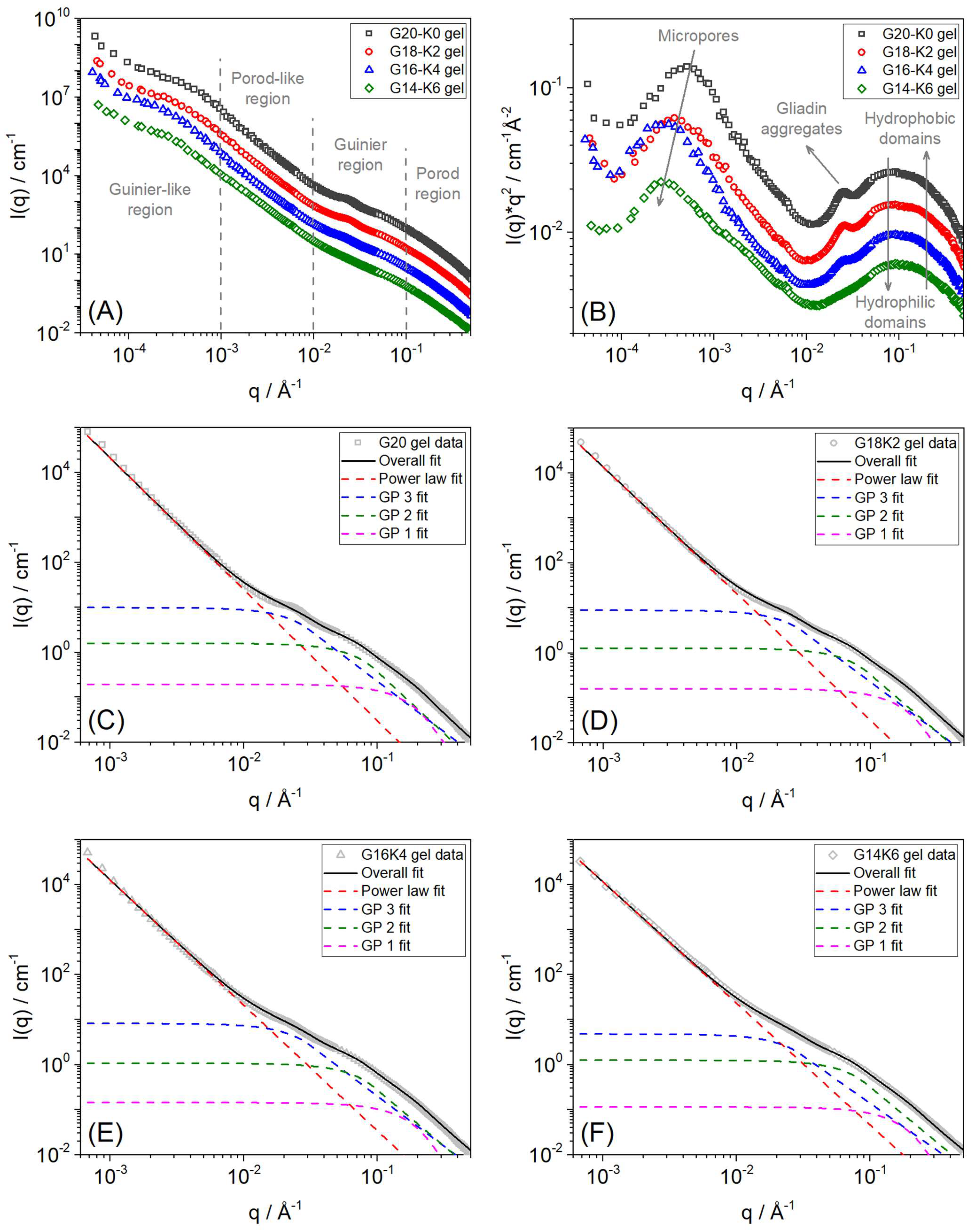
2.3. Hierarchical Structure of Equilibrium Water Swollen Gluten/Keratin Hybrid Film
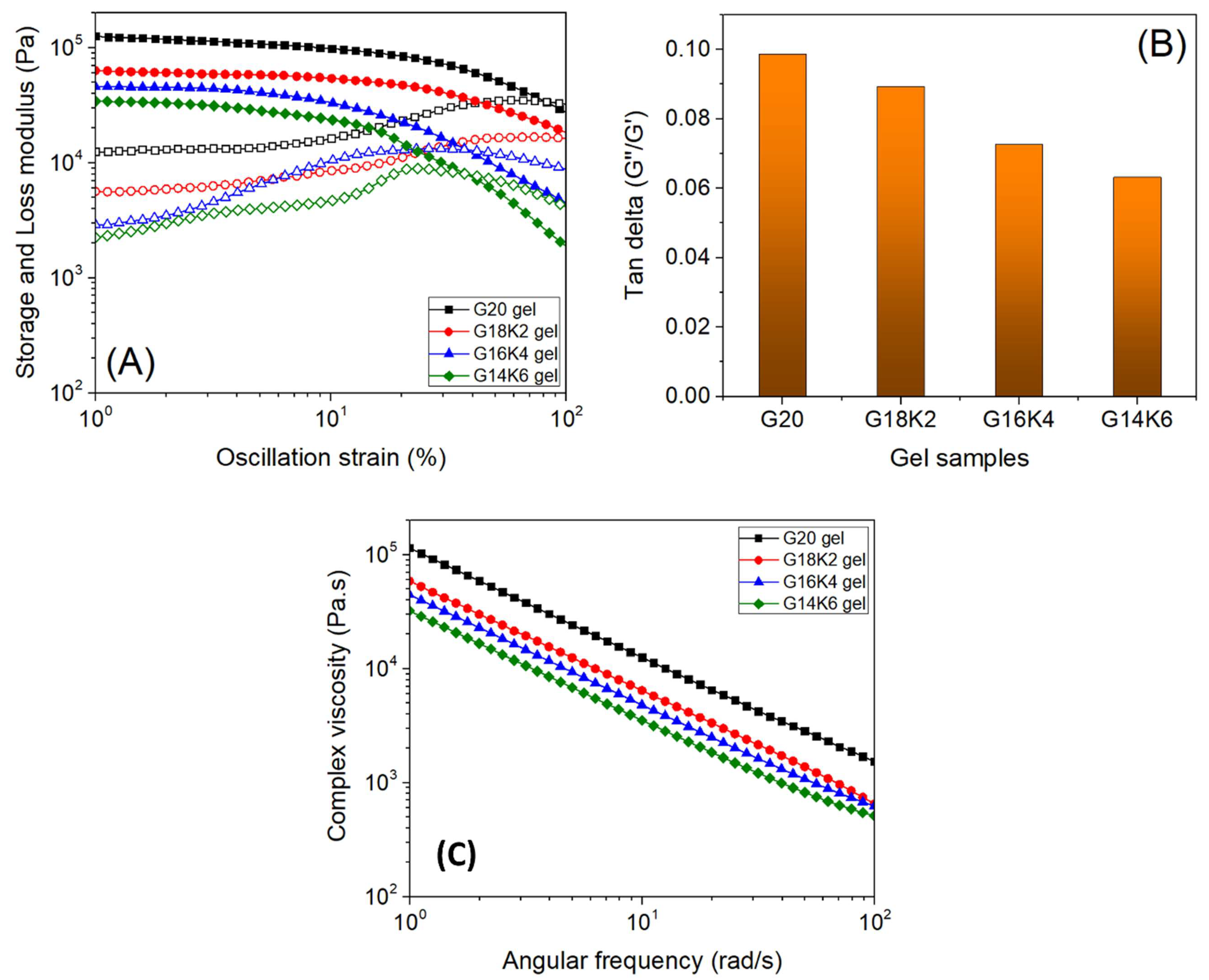
2.4. Viscoelastic Properties of Gluten/Keratin Hybrid Film
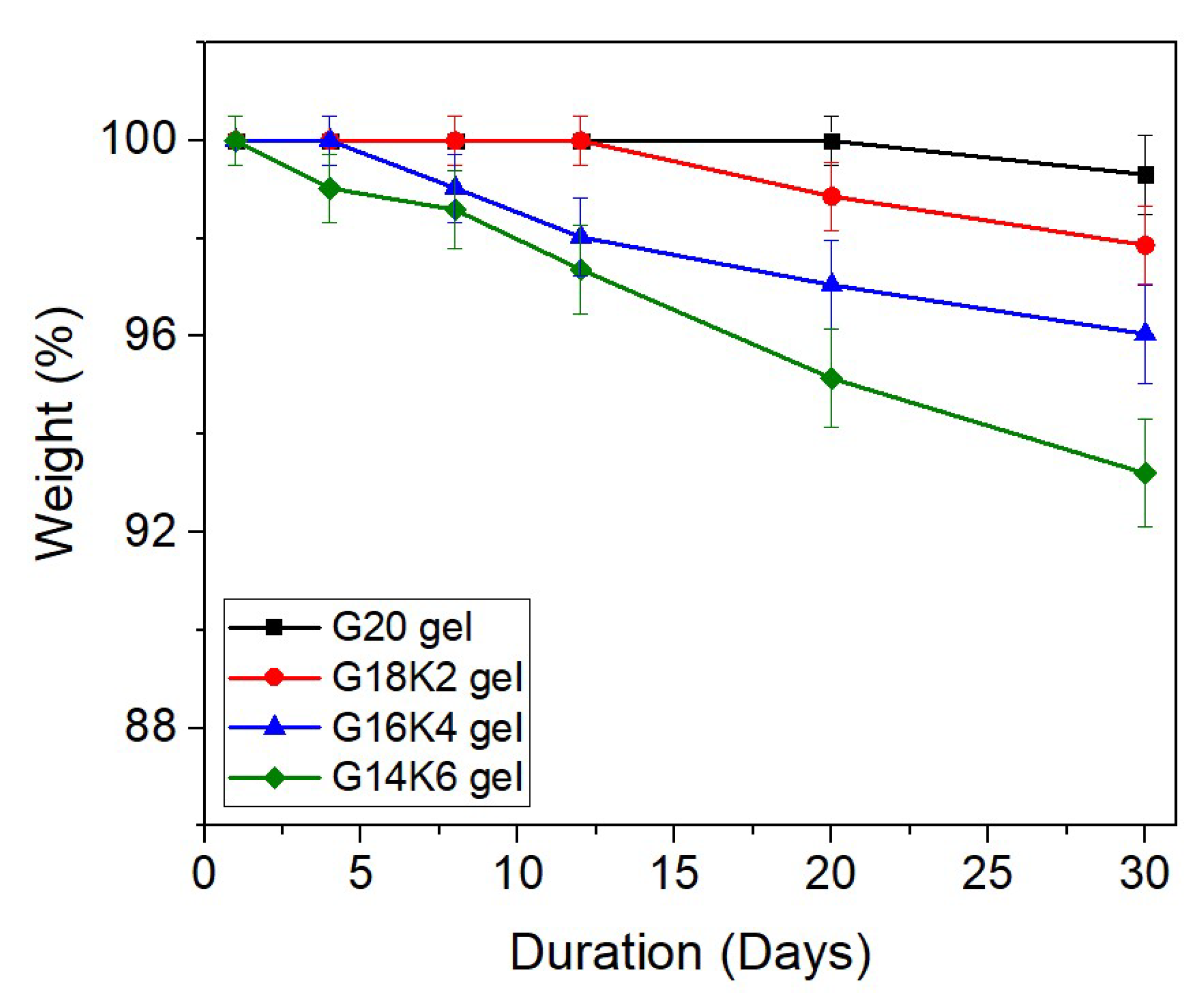
2.5. Hydrolytic Degradation of Gluten/Keratin Hybrid Film
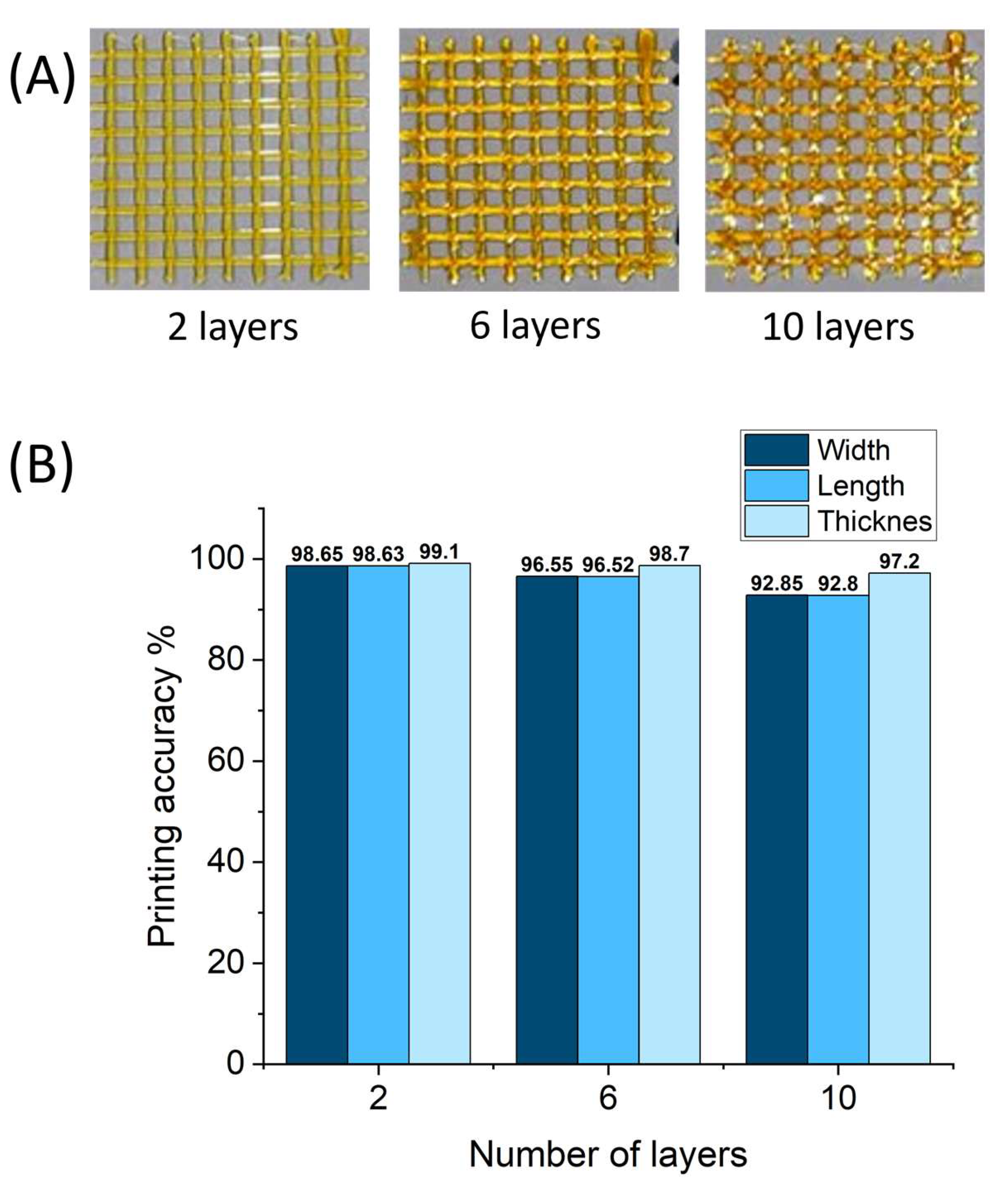
2.6. 3D Printing of Gluten/Keratin Hybrids
| Properties and Parameters | This Study (Gluten & Keratin) | Studies on Gluten [58,59,60] | Studies on Keratin [61,62,63] | |
|---|---|---|---|---|
| Secondary structure from FTIR | β-sheets | 40–47 (%) | 47 (%) | 28 (%) |
| random coils and α- helix | 47–57 (%) | 45 (%) | 65 (%) | |
| β-turns | 0.4–2.7 (%) | 8 (%) | 7 (%) | |
| Tg from DSC | 173–180 (°C) | 130–180 (°C) | 150 (°C) | |
| Water uptake capacity * | 80–186 (%) | 72 (%) | 597 (%) | |
| Micropore size from SEM | 1–6 µm | - | - | |
| Weight loss after 30 days from hydrolytic degradation | 0.7–6.8 wt.% | - | - | |
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Fractionated Gluten Protein Solution
4.3. Preparation of Keratin Protein Solution
4.4. Fabrication of Gluten/Keratin Hybrid Films
4.5. Fourier-Transform Infrared (FTIR) Spectroscopy
4.6. Equilibrium Water Swelling Study
4.7. Scanning Electron Microscopy (SEM)
4.8. Small-Angle and Ultrasmall-Angle Neutron Scattering
4.9. Rheology
4.10. Differential Scanning Calorimetry (DSC)
4.11. Hydrolytic Degradation Study
4.12. 3D Printing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- John, M.; Thomas, S. Biofibres and Biocomposites. Carbohydr. Polym. 2008, 71, 343–364. [Google Scholar] [CrossRef]
- Feroz, S.; Muhammad, N.; Ranayake, J.; Dias, G. Keratin-Based Materials for Biomedical Applications. Bioact. Mater. 2019, 5, 496–509. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and New Opportunities on Barrier Performance of Biodegradable Polymers for Sustainable Packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Joseph, A.M.; George, B. Handbook of Biopolymers; Springer: Singapore, 2023; pp. 1135–1172. [Google Scholar] [CrossRef]
- Talan, A.; Tiwari, B.; Yadav, B.; Tyagi, R.D.; Wong, J.W.C.; Drogui, P. Food Waste Valorization: Energy Production Using Novel Integrated Systems. Bioresour. Technol. 2021, 322, 124538. [Google Scholar] [CrossRef]
- Pagga, U. Biodegradability and Compostability of Polymeric Materials in the Context of the European Packaging Regulation. Polym. Degrad. Stabil. 1998, 59, 371–376. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent Advances in Biodegradable Polymers for Sustainable Applications. npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Biesiekierski, J.R. What Is Gluten? J. Gastroenterol. Hepatol. 2017, 32, 78–81. [Google Scholar] [CrossRef]
- Wieser, H.; Koehler, P.; Scherf, K.A. Chemistry of Wheat Gluten Proteins: Qualitative Composition. Ceéreéal. Chem. 2023, 100, 23–35. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Mahmood, S.; Saffe, S.N.B.M.; Arifin, M.A.B.; Gupta, A.; Sikkandar, M.Y.; Begum, S.S.; Narasaiah, B. Extraction and Application of Keratin from Natural Resources: A Review. 3 Biotech 2021, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Martelli, S.M.; Laurindo, J.B. Chicken Feather Keratin Films Plasticized with Polyethylene Glycol. Int. J. Polym. Mater. 2012, 61, 17–29. [Google Scholar] [CrossRef]
- Wu, S.; Chen, X.; Li, T.; Cui, Y.; Yi, M.; Ge, J.; Yin, G.; Li, X.; He, M. Improving the Performance of Feather Keratin/Polyvinyl Alcohol/Tris(Hydroxymethyl)Aminomethane Nanocomposite Films by Incorporating Graphene Oxide or Graphene. Nanomaterials 2020, 10, 327. [Google Scholar] [CrossRef]
- Tilley, K.A.; Benjamin, R.E.; Bagorogoza, K.E.; Okot-Kotber, B.M.; Prakash, O.; Kwen, H. Tyrosine Cross-Links: Molecular Basis of Gluten Structure and Function. J. Agric. Food Chem. 2001, 49, 2627–2632. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, W.; McKittrick, J.; Meyers, M.A. Keratin: Structure, Mechanical Properties, Occurrence in Biological Organisms, and Efforts at Bioinspiration. Prog. Mater. Sci. 2016, 76, 229–318. [Google Scholar] [CrossRef]
- Placone, J.K.; Navarro, J.; Laslo, G.W.; Lerman, M.J.; Gabard, A.R.; Herendeen, G.J.; Falco, E.E.; Tomblyn, S.; Burnett, L.; Fisher, J.P. Development and Characterization of a 3D Printed, Keratin-Based Hydrogel. Ann. Biomed. Eng. 2017, 45, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, S. 3D Printing of Soy Protein- and Gluten-Based Gels Facilitated by Thermosensitive Cocoa Butter in a Model Study. ACS Food Sci. Technol. 2021, 1, 1990–1996. [Google Scholar] [CrossRef]
- Peloquin, J.; Han, Y.; Gall, K. Printability and Mechanical Behavior as a Function of Base Material, Structure, and a Wide Range of Porosities for Polymer Lattice Structures Fabricated by Vat-Based 3d Printing. Addit. Manuf. 2023, 78, 103892. [Google Scholar] [CrossRef]
- Zhang, X.N.; Zheng, Q.; Wu, Z.L. Recent Advances in 3D Printing of Tough Hydrogels: A Review. Compos. Part B Eng. 2022, 238, 109895. [Google Scholar] [CrossRef]
- Banjo, A.D.; Agrawal, V.; Auad, M.L.; Celestine, A.-D.N. Moisture-Induced Changes in the Mechanical Behavior of 3D Printed Polymers. Compos. Part C Open Access 2022, 7, 100243. [Google Scholar] [CrossRef]
- Kong, U.; Rawi, N.F.M.; Tay, G.S. The Potential Applications of Reinforced Bioplastics in Various Industries: A Review. Polymers 2023, 15, 2399. [Google Scholar] [CrossRef]
- Lamp, A.; Kaltschmitt, M.; Dethloff, J. Options to Improve the Mechanical Properties of Protein-Based Materials. Molecules 2022, 27, 446. [Google Scholar] [CrossRef]
- Li, Y.; Ren, X.; Zhu, L.; Li, C. Biomass 3D Printing: Principles, Materials, Post-Processing and Applications. Polymers 2023, 15, 2692. [Google Scholar] [CrossRef]
- Schuller, T.; Jalaal, M.; Fanzio, P.; Galindo-Rosales, F.J. Optimal Shape Design of Printing Nozzles for Extrusion-Based Additive Manufacturing. arXiv 2024, arXiv:2401.02298. [Google Scholar]
- Dizon, J.R.C.; Gache, C.C.L.; Cascolan, H.M.S.; Cancino, L.T.; Advincula, R.C. Post-Processing of 3D-Printed Polymers. Technologies 2021, 9, 61. [Google Scholar] [CrossRef]
- Tiwari, K.; Kumar, S. Analysis of the Factors Affecting the Dimensional Accuracy of 3D Printed Products. Mater. Today Proc. 2018, 5, 18674–18680. [Google Scholar] [CrossRef]
- Olariu, L.; Dumitriu, B.G.; Gaidau, C.; Stanca, M.; Tanase, L.M.; Ene, M.D.; Stanculescu, I.-R.; Tablet, C. Bioactive Low Molecular Weight Keratin Hydrolysates for Improving Skin Wound Healing. Polymers 2022, 14, 1125. [Google Scholar] [CrossRef]
- Kłosok, K.; Welc, R.; Fornal, E.; Nawrocka, A. Effects of Physical and Chemical Factors on the Structure of Gluten, Gliadins and Glutenins as Studied with Spectroscopic Methods. Molecules 2021, 26, 508. [Google Scholar] [CrossRef]
- Georget, D.M.R.; Belton, P.S. Effects of Temperature and Water Content on the Secondary Structure of Wheat Gluten Studied by FTIR Spectroscopy. Biomacromolecules 2006, 7, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Matveev, Y.I.; Grinberg, V.Y.; Sochava, I.V.; Tolstoguzov, V.B. Glass Transition Temperature of Proteins. Calculation Based on the Additive Contribution Method and Experimental Data. Food Hydrocoll. 1997, 11, 125–133. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, E.; Hamlet, S.; George, R.; Sharma, A.; Love, R. Biofunctional Approaches of Wool-Based Keratin for Tissue Engineering. J. Sci. Adv. Mater. Devices 2021, 7, 100398. [Google Scholar] [CrossRef]
- Ooms, N.; Delcour, J.A. How to Impact Gluten Protein Network Formation during Wheat Flour Dough Making. Curr. Opin. Food Sci. 2019, 25, 88–97. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Balu, R.; Knott, R.; de Campo, L.; Mata, J.P.; Rehm, C.; Hill, A.J.; Dutta, N.K.; Choudhury, N.R. Structural Evolution of Photocrosslinked Silk Fibroin and Silk Fibroin-Based Hybrid Hydrogels: A Small Angle and Ultra-Small Angle Scattering Investigation. Int. J. Biol. Macromol. 2018, 114, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Balu, R.; Mata, J.P.; Knott, R.; Elvin, C.M.; Hill, A.J.; Choudhury, N.R.; Dutta, N.K. Effects of Crowding and Environment on the Evolution of Conformational Ensembles of the Multi-Stimuli-Responsive Intrinsically Disordered Protein, Rec1-Resilin: A Small-Angle Scattering Investigation. J. Phys. Chem. B 2016, 120, 6490–6503. [Google Scholar] [CrossRef] [PubMed]
- Balu, R.; Wanasingha, N.; Mata, J.P.; Rekas, A.; Barrett, S.; Dumsday, G.; Thornton, A.W.; Hill, A.J.; Choudhury, N.R.; Dutta, N.K. Crowder-Directed Interactions and Conformational Dynamics in Multistimuli-Responsive Intrinsically Disordered Protein. Sci. Adv. 2022, 8, eabq2202. [Google Scholar] [CrossRef] [PubMed]
- Balu, R.; Reeder, S.; Knott, R.; Mata, J.; de Campo, L.; Dutta, N.K.; Choudhury, N.R. Tough Photocrosslinked Silk Fibroin/Graphene Oxide Nanocomposite Hydrogels. Langmuir 2018, 34, 9238–9251. [Google Scholar] [CrossRef] [PubMed]
- Dorishetty, P.; Balu, R.; Sreekumar, A.; de Campo, L.; Mata, J.P.; Choudhury, N.R.; Dutta, N.K. Robust and Tunable Hybrid Hydrogels from Photo-Cross-Linked Soy Protein Isolate and Regenerated Silk Fibroin. ACS Sustain. Chem. Eng. 2019, 7, 9257–9271. [Google Scholar] [CrossRef]
- Urade, R.; Sato, N.; Sugiyama, M. Gliadins from Wheat Grain: An Overview, from Primary Structure to Nanostructures of Aggregates. Biophys. Rev. 2018, 10, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, B. A New Guinier–Porod Model. J. Appl. Crystallogr. 2010, 43, 716–719. [Google Scholar] [CrossRef]
- Lee, J.; MacOsko, C.W.; Urry, D.W. Phase Transition and Elasticity of Protein-Based Hydrogels. J. Biomater. Sci. Polym. Ed. 2001, 12, 229–242. [Google Scholar] [CrossRef]
- Simões, A.; Miranda, M.; Cardoso, C.; Veiga, F.; Vitorino, C. Rheology by Design: A Regulatory Tutorial for Analytical Method Validation. Pharmaceutics 2020, 12, 820. [Google Scholar] [CrossRef]
- Huang, J.; Fu, P.; Li, W.; Xiao, L.; Chen, J.; Nie, X. Influence of Crosslinking Density on the Mechanical and Thermal Properties of Plant Oil-Based Epoxy Resin. RSC Adv. 2022, 12, 23048–23056. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, C.-C.; Ma, Y.; Chao, Y.-Y.; Tseng, C.-L.; Wei, Y. Study on Keratin/PEGDA Composite Hydrogel with the Addition of Varied Hair Protein Fractions. ACS Appl. Polym. Mater. 2022, 4, 3426–3437. [Google Scholar] [CrossRef]
- Cui, H.; Nowicki, M.; Fisher, J.P.; Zhang, L.G. 3D Bioprinting for Organ Regeneration. Adv. Healthc. Mater. 2017, 6, 1601118. [Google Scholar] [CrossRef]
- Smith, P.T.; Basu, A.; Saha, A.; Nelson, A. Chemical Modification and Printability of Shear-Thinning Hydrogel Inks for Direct-Write 3D Printing. Polymer 2018, 152, 42–50. [Google Scholar] [CrossRef]
- Park, S.; Shou, W.; Makatura, L.; Matusik, W.; Fu, K.K. 3D Printing of Polymer Composites: Materials, Processes, and Applications. Matter 2022, 5, 43–76. [Google Scholar] [CrossRef]
- Agrawal, A.; Hussain, C.M. 3D-Printed Hydrogel for Diverse Applications: A Review. Gels 2023, 9, 960. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Rani, M.; Sogi, D.S.; Gill, B.S. Characterization of Gliadin, Secalin and Hordein Fractions Using Analytical Techniques. Sci. Rep. 2021, 11, 23135. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.; Pérez-Andrés, J.; Martínez-Blanco, H.; Ferrero, M.A.; Vaquero, L.; Vivas, S.; Casqueiro, J.; Rodríguez-Aparicio, L.B. The Human Digestive Tract Has Proteases Capable of Gluten Hydrolysis. Mol. Metab. 2017, 6, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, T.K.; Sharma, A.; Sharma, V.; Chandra, S. Keratin; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 10850–10877. [Google Scholar] [CrossRef] [PubMed]
- Dorishetty, P.; Balu, R.; Gelmi, A.; Mata, J.P.; Dutta, N.K.; Choudhury, N.R. 3D Printable Soy/Silk Hybrid Hydrogels for Tissue Engineering Applications. Biomacromolecules 2021, 22, 3668–3678. [Google Scholar] [CrossRef] [PubMed]
- Czyżewski, P.; Marciniak, D.; Nowinka, B.; Borowiak, M.; Bieliński, M. Influence of Extruder’s Nozzle Diameter on the Improvement of Functional Properties of 3D-Printed PLA Products. Polymers 2022, 14, 356. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhao, Z.; Wang, T.; Pan, Y.-T. Three-Dimensional Printing of Large Ceramic Products and Process Simulation. Materials 2023, 16, 3815. [Google Scholar] [CrossRef]
- Croom, B.P.; Abbott, A.; Kemp, J.W.; Rueschhoff, L.; Smieska, L.; Woll, A.; Stoupin, S.; Koerner, H. Mechanics of Nozzle Clogging during Direct Ink Writing of Fiber-Reinforced Composites. Addit. Manuf. 2021, 37, 101701. [Google Scholar] [CrossRef]
- Jang, S.; Boddorff, A.; Jang, D.J.; Lloyd, J.; Wagner, K.; Thadhani, N.; Brettmann, B. Effect of Material Extrusion Process Parameters on Filament Geometry and Inter-Filament Voids in as-Fabricated High Solids Loaded Polymer Composites. Addit. Manuf. 2021, 47, 102313. [Google Scholar] [CrossRef]
- Nawrocka, A.; Miś, A.; Niewiadomski, Z. Dehydration of Gluten Matrix as a Result of Dietary Fibre Addition—A Study on Model Flour with Application of FT-IR Spectroscopy. J. Cereal Sci. 2017, 74, 86–94. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y. Wheat Gluten-Based Coatings and Films: Preparation, Properties, and Applications. J. Food Sci. 2022, 88, 582–594. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Zarate-Ramírez, L.S.; Romero, A.; Bengoechea, C.; Partal, P.; Guerrero, A. Bioplastics Based on Wheat Gluten Processed by Extrusion. J. Clean. Prod. 2019, 239, 117994. [Google Scholar] [CrossRef]
- Milczarek, P.; Zielinski, M.; Garcia, M.L. The Mechanism and Stability of Thermal Transitions in Hair Keratin. Colloid. Polym. Sci. 1992, 270, 1106–1115. [Google Scholar] [CrossRef]
- Tinoco, A.; Rodrigues, R.M.; Machado, R.; Pereira, R.N.; Cavaco-Paulo, A.; Ribeiro, A. Ohmic Heating as an Innovative Approach for the Production of Keratin Films. Int. J. Biol. Macromol. 2020, 150, 671–680. [Google Scholar] [CrossRef]
- Fernández-d’Arlas, B. Tough and Functional Cross-Linked Bioplastics from Sheep Wool Keratin. Sci. Rep. 2019, 9, 14810. [Google Scholar] [CrossRef] [PubMed]
- Wieser, H. Chemistry of Gluten Proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Cha, J.-K.; Lee, S.-M.; Kabange, N.R.; Lee, J.-H. Rapid and Easy High-Molecular-Weight Glutenin Subunit Identification System by Lab-on-a-Chip in Wheat (Triticum aestivum L.). Plants 2020, 9, 1517. [Google Scholar] [CrossRef]
- Marsh, M.N.; Tatham, A.S.; Gilbert, S.M.; Fido, R.J.; Shewry, P.R. Extraction, Separation, and Purification of Wheat Gluten Proteins and Related Proteins of Barley, Rye, and Oats. Methods Mol. Med. 2000, 41, 55–73. [Google Scholar] [CrossRef]
- Truong, M.Y.; Dutta, N.K.; Choudhury, N.R.; Kim, M.; Elvin, C.M.; Nairn, K.M.; Hill, A.J. The Effect of Hydration on Molecular Chain Mobility and the Viscoelastic Behavior of Resilin-Mimetic Protein-Based Hydrogels. Biomaterials 2011, 32, 8462–8473. [Google Scholar] [CrossRef]
- Wood, K.; Mata, J.P.; Garvey, C.J.; Wu, C.-M.; Hamilton, W.A.; Abbeywick, P.; Bartlett, D.; Bartsch, F.; Baxter, P.; Booth, N.; et al. QUOKKA, the Pinhole Small-angle Neutron Scattering Instrument at the OPAL Research Reactor, Australia: Design, Performance, Operation and Scientific Highlights. J. Appl. Crystallogr. 2018, 51, 294–314. [Google Scholar] [CrossRef]
- Rehm, C.; de Campo, L.; Brûlé, A.; Darmann, F.; Bartsch, F.; Berry, A. Design and Performance of the Variable-wavelength Bonse–Hart Ultra-small-angle Neutron Scattering Diffractometer KOOKABURRA at ANSTO. J. Appl. Crystallogr. 2018, 51, 1–8. [Google Scholar] [CrossRef]
- Kaulich, B.; Gianoncelli, A.; Beran, A.; Eichert, D.; Kreft, I.; Pongrac, P.; Regvar, M.; Vogel-Miku, K.; Kiskinova, M. Low-Energy X-ray Fluorescence Microscopy Opening New Opportunities for Bio-Related Research. J. R. Soc. Interface 2009, 6 (Suppl. 5), S641–S647. [Google Scholar] [CrossRef]
- Balu, R.; Choudhury, N.R.; Mata, J.P.; de Campo, L.; Rehm, C.; Hill, A.J.; Dutta, N.K. Evolution of the Interfacial Structure of a Catalyst Ink with the Quality of the Dispersing Solvent: A Contrast Variation Small-Angle and Ultrasmall-Angle Neutron Scattering Investigation. ACS Appl. Mater. Interfaces 2019, 11, 9934–9946. [Google Scholar] [CrossRef]
- Balu, R.; Dorishetty, P.; Mata, J.P.; Hill, A.J.; Dutta, N.K.; Choudhury, N.R. Tuning the Hierarchical Structure and Resilience of Resilin-like Polypeptide Hydrogels Using Graphene Oxide. ACS Appl. Bio Mater. 2020, 3, 8688–8697. [Google Scholar] [CrossRef]
- Zhang, J.; Allardyce, B.J.; Rajkhowa, R.; Zhao, Y.; Dilley, R.J.; Redmond, S.L.; Wang, X.; Liu, X. 3D Printing of Silk Particle-Reinforced Chitosan Hydrogel Structures and Their Properties. Acs Biomater. Sci. Eng. 2018, 4, 3036–3046. [Google Scholar] [CrossRef] [PubMed]
- Dorishetty, P.; Balu, R.; Athukoralalage, S.S.; Greaves, T.L.; Mata, J.; de Campo, L.; Saha, N.; Zannettino, A.C.W.; Dutta, N.K.; Choudhury, N.R. Tunable Biomimetic Hydrogels from Silk Fibroin and Nanocellulose. Acs Sustain. Chem. Eng. 2020, 8, 2375–2389. [Google Scholar] [CrossRef]
- Cardamone, J.M. Investigating the microstructure of keratin extracted from wool: Peptide sequence (MALDI-TOF/TOF) and protein conformation (FTIR). J. Mol. Struct. 2010, 969, 97–105. [Google Scholar] [CrossRef]
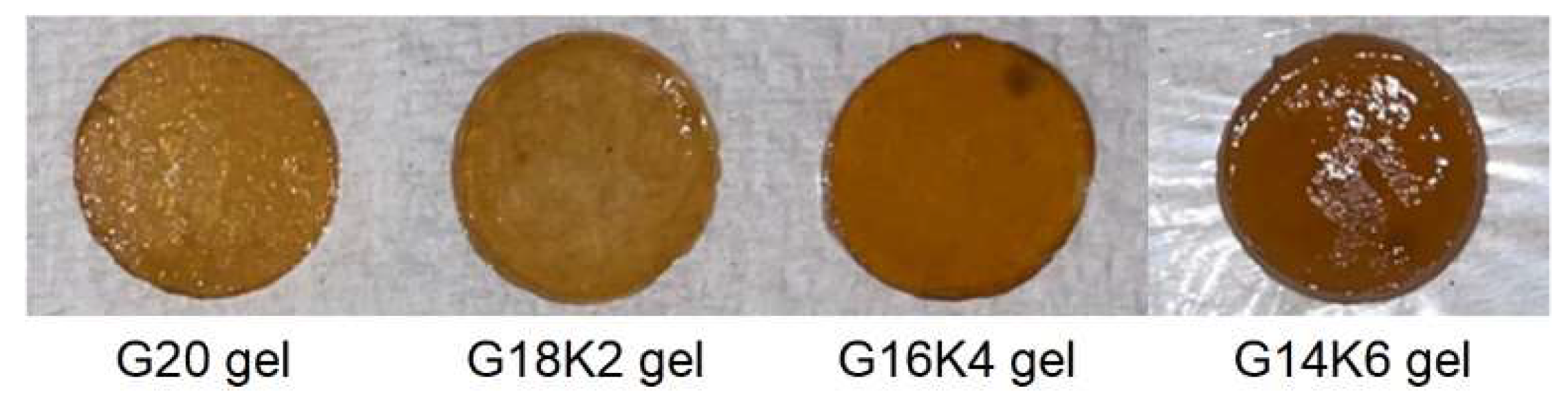


| Sample Name | Gluten:Keratin (Weight Ratio) | Equilibrium Swelling Study | SEM | |
|---|---|---|---|---|
| Water Uptake Capacity (%) | Crosslink Density (×10−3 mol/cm3) | Pore Size (µm) | ||
| G20 gel | 100:0 | 83.61 ± 3.76 | 0.365 ± 0.001 | 1.65 ± 0.41 |
| G18K2 gel | 90:10 | 105.59 ± 5.89 | 0.359 ± 0.001 | 3.42 ± 1.12 |
| G16K4 gel | 80:20 | 122.30 ± 5.49 | 0.355 ± 0.001 | 3.81 ± 1.13 |
| G14K6 gel | 70:30 | 181.64 ± 4.89 | 0.347 ± 0.000 | 4.63 ± 2.02 |
| Sample | SANS High-Q (Guinier-Porod 1 Fit) | SANS Mid-Q (Guinier-Porod 2 Fit) | SANS Mid-Q (Guinier-Porod 3 Fit) | SANS Low-Q (Power Law Fit) | USANS Very Low-Q (2π/Q) | |||
|---|---|---|---|---|---|---|---|---|
| Porod Slope | Rg (Å) | Porod Slope | Rg (Å) | Porod Slope | Rg (Å) | Porod Slope | Pore Size (µm) | |
| G20 gel | 4.00 ± 0.01 | 10.00 ± 0.01 | 2.76 ± 0.07 | 20.99 ± 0.08 | 2.25 ± 0.04 | 59.03 ± 0.23 | 2.93 ± 0.05 | ~1.24 |
| G18K2 gel | 4.00 ± 0.01 | 10.00 ± 0.01 | 2.56 ± 0.05 | 20.40 ± 0.07 | 2.24 ± 0.04 | 58.80 ± 0.22 | 2.82 ± 0.03 | ~1.64 |
| G16K4 gel | 4.00 ± 0.01 | 10.00 ± 0.01 | 2.46 ± 0.04 | 20.37 ± 0.05 | 2.23 ± 0.05 | 58.69 ± 0.27 | 2.79 ± 0.02 | ~2.06 |
| G14K6 gel | 4.00 ± 0.01 | 10.00 ± 0.01 | 2.40 ± 0.02 | 20.35 ± 0.05 | 2.09 ± 0.04 | 58.59 ± 0.25 | 2.65 ± 0.03 | ~2.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshehhi, J.R.M.H.; Wanasingha, N.; Balu, R.; Mata, J.; Shah, K.; Dutta, N.K.; Choudhury, N.R. 3D-Printable Sustainable Bioplastics from Gluten and Keratin. Gels 2024, 10, 136. https://doi.org/10.3390/gels10020136
Alshehhi JRMH, Wanasingha N, Balu R, Mata J, Shah K, Dutta NK, Choudhury NR. 3D-Printable Sustainable Bioplastics from Gluten and Keratin. Gels. 2024; 10(2):136. https://doi.org/10.3390/gels10020136
Chicago/Turabian StyleAlshehhi, Jumana Rashid Mohammed Haroub, Nisal Wanasingha, Rajkamal Balu, Jitendra Mata, Kalpit Shah, Naba K. Dutta, and Namita Roy Choudhury. 2024. "3D-Printable Sustainable Bioplastics from Gluten and Keratin" Gels 10, no. 2: 136. https://doi.org/10.3390/gels10020136
APA StyleAlshehhi, J. R. M. H., Wanasingha, N., Balu, R., Mata, J., Shah, K., Dutta, N. K., & Choudhury, N. R. (2024). 3D-Printable Sustainable Bioplastics from Gluten and Keratin. Gels, 10(2), 136. https://doi.org/10.3390/gels10020136








