Combustion Enhancement of Gel Propellant Containing High Concentration Aluminum Particles Based on Carbon Synergistic Effect
Abstract
:1. Introduction
2. Results and Discussion
2.1. Combustion Heat Detection
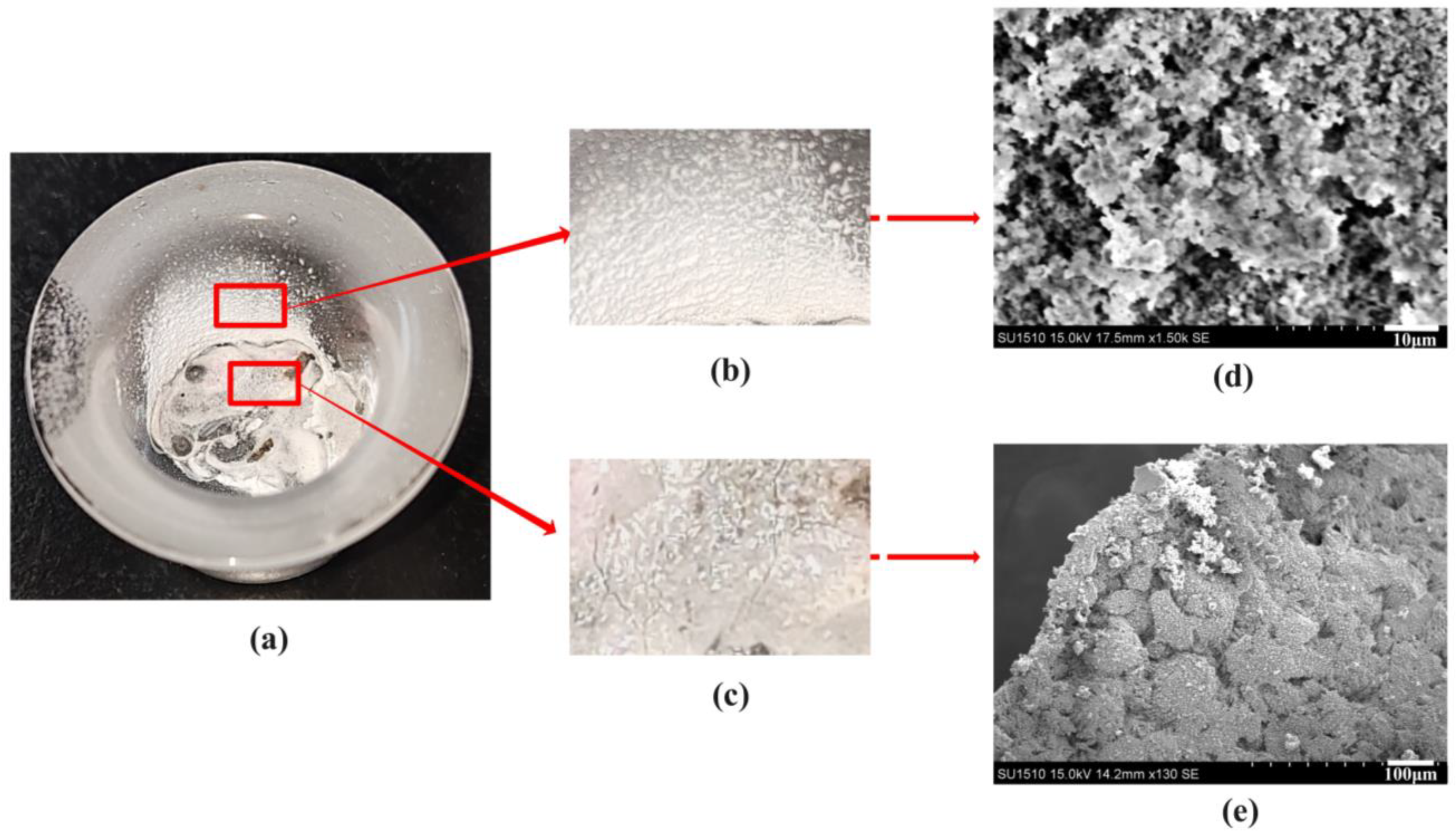
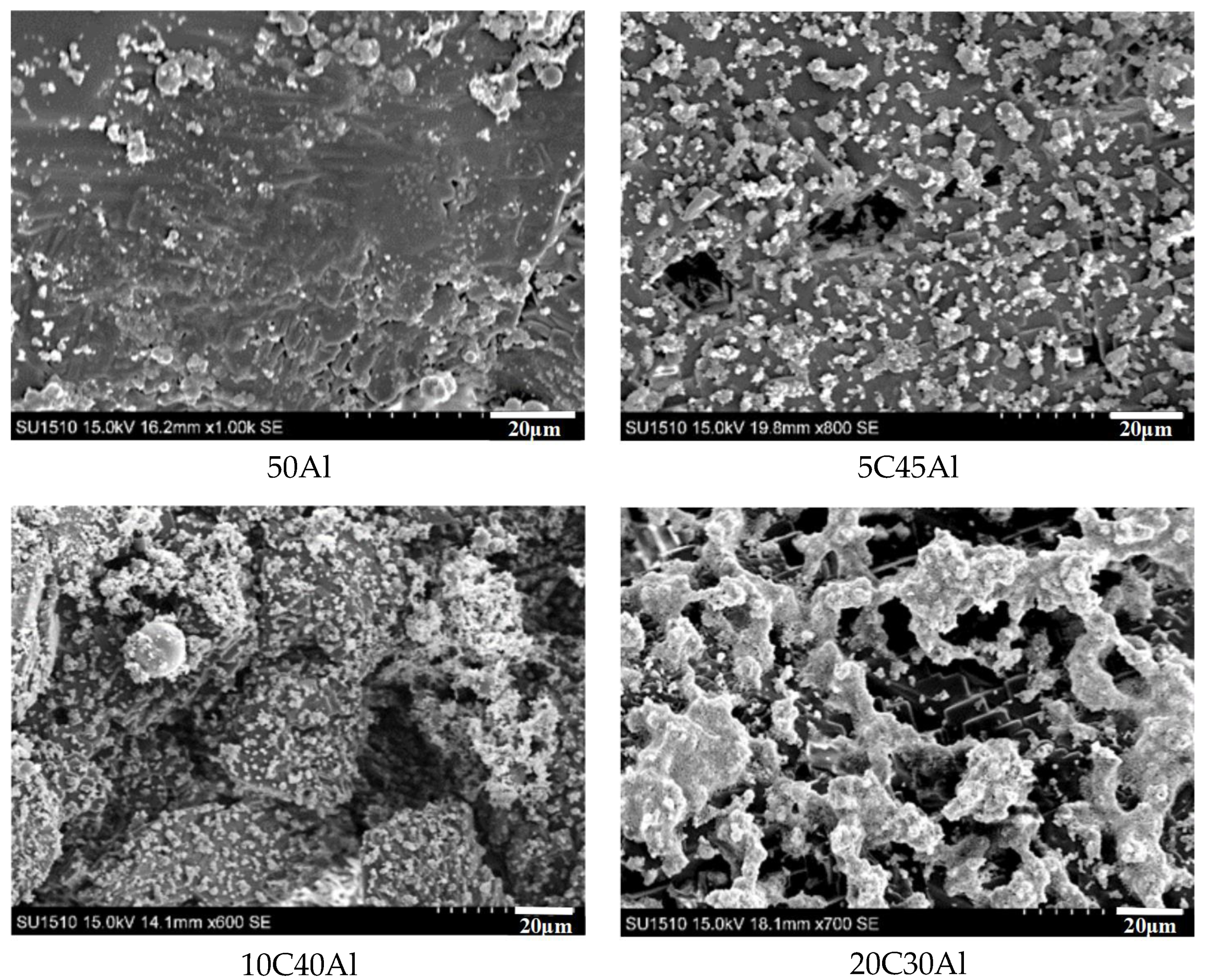
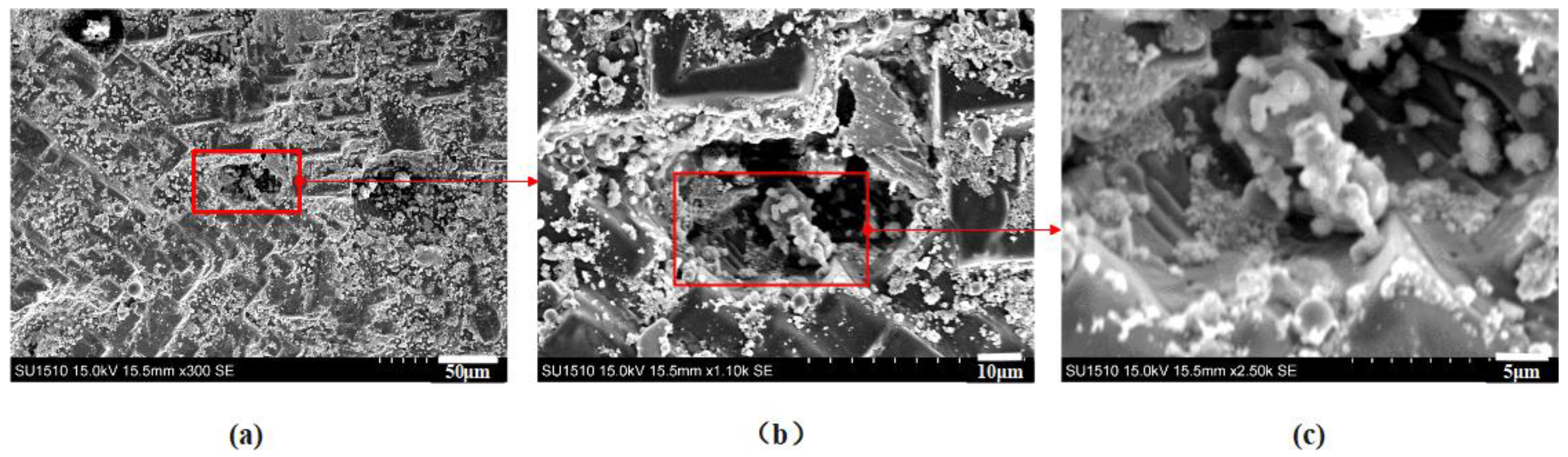
2.2. Scanning Electron Microscopy Analysis of Combustion Products
2.3. Mechanism of Carbon Particles Improving Aluminum Agglomeration
2.4. Rheological Study
2.4.1. Apparent Viscosity Test
2.4.2. Thixotropy
2.4.3. Amplitude Sweep
2.4.4. Frequency Sweep
3. Conclusions
4. Material and Methods
4.1. Materials
4.2. Equipment and Instruments
4.3. Preparation of Gel
4.4. Measurement and Characterization
4.4.1. Combustion Heat Detection
4.4.2. Scanning Electron Microscope (SEM)
4.4.3. Rheological Measurements
- (1)
- Shear viscosity test: The rheometer operates in rotation mode. Control the shear rate of the rotor in the range of 0.1–1000 s−1. By gradually increasing the shear rate of the paddle rotor according to a logarithmic law starting from 0.1 s−1, we can accurately record and analyze the relationship between the shear rate and viscosity of gels;
- (2)
- Thixotropic test: 3ITT (three Interval Thixotropic Test) [35] was used to detect the thixotropy of ethanolamine gel. A constant shear rate (300 s−1) was applied to the gel during 0–100 s. Remove the shear rate within 100–300 s and let the gel recover itself for 200 s. A constant shear rate (300 s−1) is still applied for 300–400 s. Finally, the curve of gel time and viscosity change is obtained;
- (3)
- Amplitude sweeping test: Set the oscillation frequency of the rotor to 1 Hz, the strain range to 0.1–1000%, and record the storage modulus (G’) and loss modulus (G”).
- (4)
- Frequency sweep test: constant strain is 1%, frequency is 1–100 Hz. Record the change of storage modulus (G’) and loss modulus (G”) at different frequencies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Na, H.; Kang, Y.W.; Park, C.S.; Jung, S.; Kim, H.Y.; Sun, J.Y. Hydrogel-based strong and fast actuators by electroosmotic turgor pressure. Science 2022, 376, 301–307. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, W.; Wang, X. Locking volatile organic molecules by subnanometer inorganic nanowire-based organogels. Science 2022, 377, 100–104. [Google Scholar] [CrossRef]
- Padwal, M.B.; Natan, B.; Mishra, D.P. Gel propellants. Prog. Energy Combust. Sci. 2021, 83, 100885. [Google Scholar] [CrossRef]
- Glushkov, D.O.; Paushkina, K.K.; Pleshko, A.O.; Vysokomorny, V.S. Characteristics of micro-explosive dispersion of gel fuel particles ignited in a high-temperature air medium. Fuel 2022, 313, 123024. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Design principles of food gels. Nat. Food 2020, 1, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [PubMed]
- Dennis, J.D.; Willits, J.D.; Pourpoint, T.L. Performance of neat and gelled monomethylhydrazine and red fuming nitric acid in an unlike-doublet combustor. J. Combust. Sci. Technol. 2018, 190, 1141–1157. [Google Scholar] [CrossRef]
- Arnold, R.; Santos, P.H.; Campanella, O.H.; Anderson, W.E. Rheological and Thermal Behavior of Gelled Hydrocarbon Fuels. J. Propuls. Power 2011, 27, 151–161. [Google Scholar] [CrossRef]
- Dennis, J.D.; Kubal, T.D.; Campanella, O.; Son, S.F.; Pourpoint, T.L. Rheological Characterization of Monomethylhydrazine Gels. J. Propuls. Power 2013, 29, 313–320. [Google Scholar] [CrossRef]
- Song, W.; Hwang, J.; Koo, J. Atomization of gelled kerosene by multi-hole pintle injector for rocket engines. Fuel 2021, 285, 119212. [Google Scholar] [CrossRef]
- Xue, K.; Cao, J.; Pan, L.; Zhang, X.; Zou, J.-J. Review on design, preparation and performance characterization of gelled fuels for advanced propulsion. J. Front. Chem. Sci. Eng. 2022, 16, 819–837. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, X.; Yang, Q.; Dou, S.; Wang, X.; Fu, Q.; Pan, L. Combustion Behavior of Hydrocarbon/Boron Gel-Fueled Scramjet. J. AIAA J. 2022, 60, 3834–3843. [Google Scholar] [CrossRef]
- Madlener, K.; Ciezki, H.K. Estimation of Flow Properties of Gelled Fuels with Regard to Propulsion Systems. J. Propuls. Power 2012, 28, 113–121. [Google Scholar] [CrossRef]
- Rahimi, S.; Peretz, A.; Natan, B. Rheological matching of gel propellants. J. Propuls. Power 2010, 26, 376–379. [Google Scholar] [CrossRef]
- Glushkov, D.; Paushkina, K.; Pleshko, A. Gel Fuels: Preparing, Rheology, Atomization, Combustion. Energies 2022, 16, 298. [Google Scholar] [CrossRef]
- Yoon, C.; Heister, S.; Xia, G.; Merkle, C.L. Numerical Modeling of Injection of Shear-thinning Gel Propellants Through Plain-orifice Atomizer. J. Propuls. Power 2011, 27, 944–954. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Pan, L.; Xue, K.; Zhang, X.; Zou, J.-J. High-energy-density gelled fuels with high stability and shear thinning performance. J. Chin. J. Chem. Eng. 2022, 43, 99–109. [Google Scholar] [CrossRef]
- Jyoti, B.V.S.; Baek, S.W. Rheological characterization of hydrogen peroxide gel propellant. Int. J. Aerosp. Eng. 2014, 15, 199–204. [Google Scholar] [CrossRef]
- Padwal, M.B.; Mishra, D.P. Interactions among synthesis, rheology, and atomization of a gelled propellant. Rheol. Acta 2016, 55, 177–186. [Google Scholar] [CrossRef]
- Negri, M.; Ciezki, H.K. Combustion of gelled propellants containing microsized and nanosized aluminum particles. J. Propuls. Power 2015, 31, 400–407. [Google Scholar] [CrossRef]
- Jyoti, B.V.S.; Naseem, M.S.; Baek, S.W. Hypergolicity and ignition delay study of pure and energized ethanol gel fuel with hydrogen peroxide. Combust. Flame 2017, 176, 318–325. [Google Scholar] [CrossRef]
- Gafni, G.; Kuznetsov, A.; Natan, B. Experimental Investigation of an Aluminized Gel Fuel Ramjet Combustor. J. Chem. Rocket Propuls. 2017, 31, 297–315. [Google Scholar]
- Ao, W.; Liu, X.; Rezaiguia, H.; Liu, H.; Wang, Z.; Liu, P. Aluminum agglomeration involving the second mergence of agglomerates on the solid propellants burning surface: Experiments and modeling. J. Acta Astronaut. 2017, 136, 219–229. [Google Scholar] [CrossRef]
- Jiang, Y.; Deng, S.; Hong, S.; Zhao, J.; Huang, S.; Wu, C.-C.; Gottfried, J.L.; Nomura, K.-I.; Li, Y.; Tiwari, S.; et al. Energetic Performance of Optically Activated Aluminum/Graphene Oxide Composites. J. ACS Nano 2018, 12, 11366–11375. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ao, W.; Wen, Z.; Zhang, Y.; Lv, X.; Qin, Z.; Liu, P. Combustion promotion and agglomeration reduction of the composite propellant using graphene. J. Aerosp. Sci. Technol. 2021, 118, 106988. [Google Scholar] [CrossRef]
- Ao, W.; Liu, P.; Liu, H.; Wu, S.; Tao, B.; Huang, X.; Li, L.K. Tuning the agglomeration and combustion characteristics of aluminized propellants via a new functionalized fluoropolymer. J. Chem. Eng. J. 2019, 382, 122987. [Google Scholar] [CrossRef]
- Belal, H.; Han, C.W.; Gunduz, I.E.; Ortalan, V.; Son, S.F. Ignition and Combustion Behavior of Mechanically Activated Al-Mg Particles in Composite Solid Propellants. J. Combust. Flame 2018, 194, 410–418. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, C.; Fang, Y.; Wang, X.; Wang, Z. Mechanism of Pyrolysis Reaction of Al-Rich Al/PTFE/TiH2Active Material. J. Polym. 2021, 13, 28–57. [Google Scholar]
- Elbasuney, S.; Fahd, A. Combustion wave of metalized extruded double-base propellants. Fuel 2019, 237, 1274–1280. [Google Scholar] [CrossRef]
- Botchu, J.; Baek, S.W. Flow and Dynamic Rheological Characterization of Ethanolamine Gel Propellant with Hybrid Gelling Agent. Sci. Technol. Energetic Mater. 2015, 76, 62–67. [Google Scholar]
- Botchu, J.; Baek, S.W. Rheological Characterization of Metalized and Non-Metalized Ethanol Gel Propellants. J. Propell. Explos. Pyrot. 2015, 39, 866–873. [Google Scholar]
- Li, M.-G.; Wu, Y.; Cao, Q.-L.; Yuan, X.-Y.; Chen, X.; Han, J.-L.; Wu, W.-T. Rheological Properties of Organic Kerosene Gel Fuel. Gels 2022, 8, 507. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-X.; Liu, Q.; Feng, B.; Yin, Q.; Li, Y.-C.; Wu, S.-Z.; Yu, Z.-S.; Huang, J.-Y.; Ren, X.-X. Improving the energy release characteristics of PTFE/Al by doping magnesium hydride. J. Def. Technol. 2022, 18, 219–228. [Google Scholar] [CrossRef]
- Terry, B.C.; Gunduz, I.E.; Pfeil, M.A.; Sippel, T.; Son, S. A Mechanism for Shattering Microexplosions and Dispersive Boiling Phenomena in Aluminum-Lithium Alloy Based Solid Propellant. J. Proc. Combust. Inst. 2016, 36, 2309–2316. [Google Scholar] [CrossRef]
- Ali, I.; Ali Shah, L.; Rehman, T.U.; Faizan, S. Investigation of the viscoelastic behavior of PVA-P (AAm/AMPS) IPN hydrogel with enhanced mechanical strength and excellent recoverability. J. Polym. Res. 2021, 29, 1–12. [Google Scholar] [CrossRef]
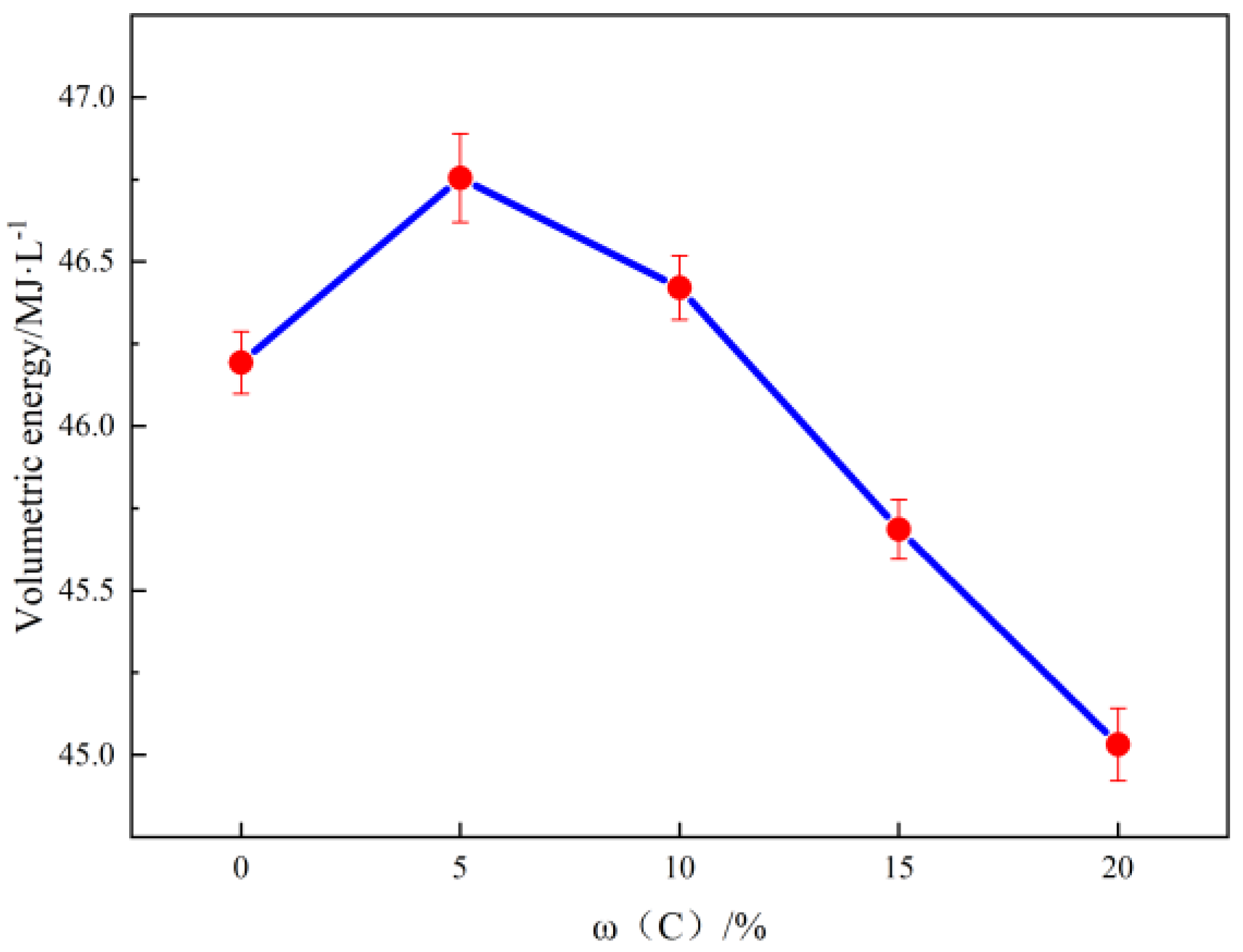
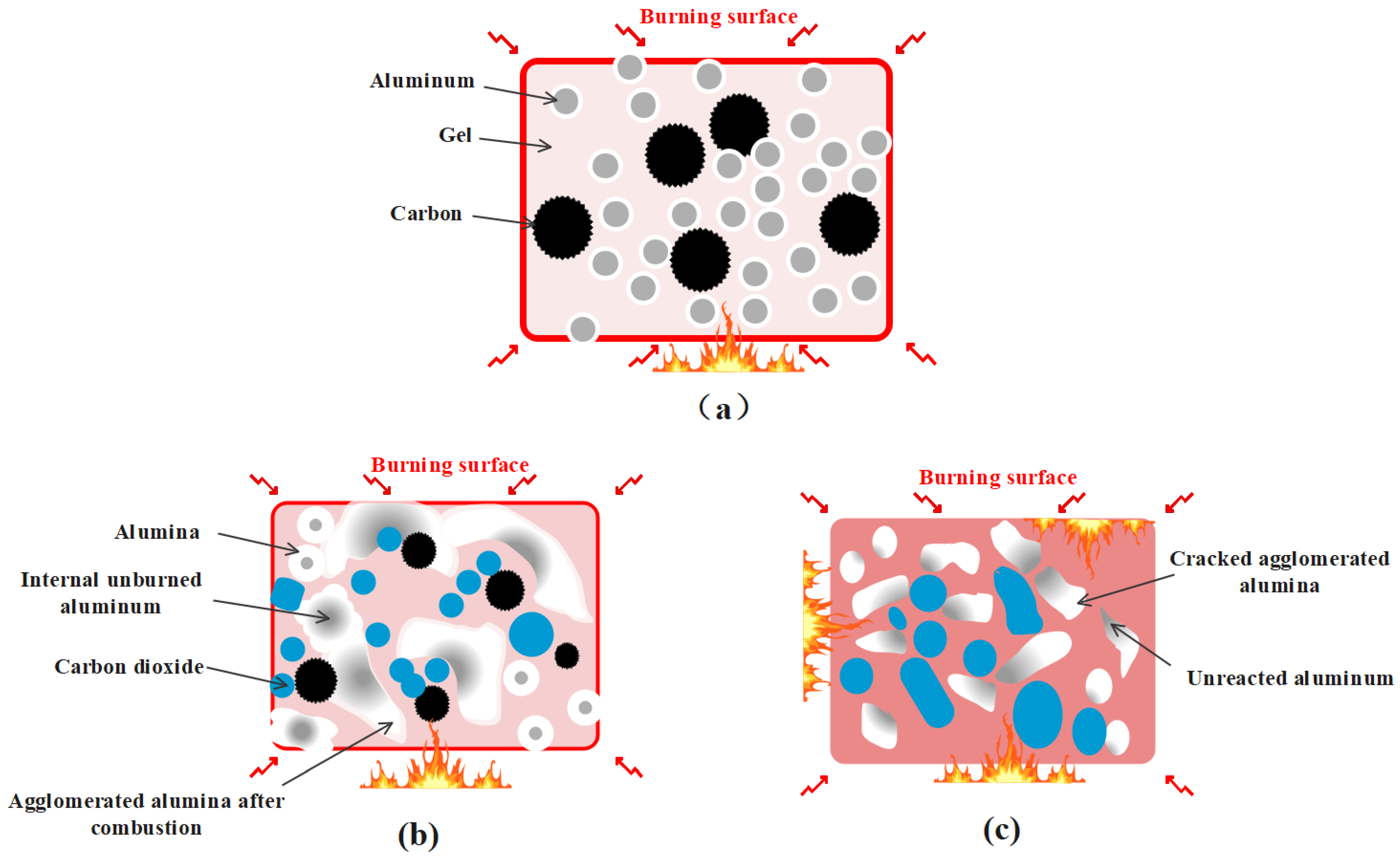

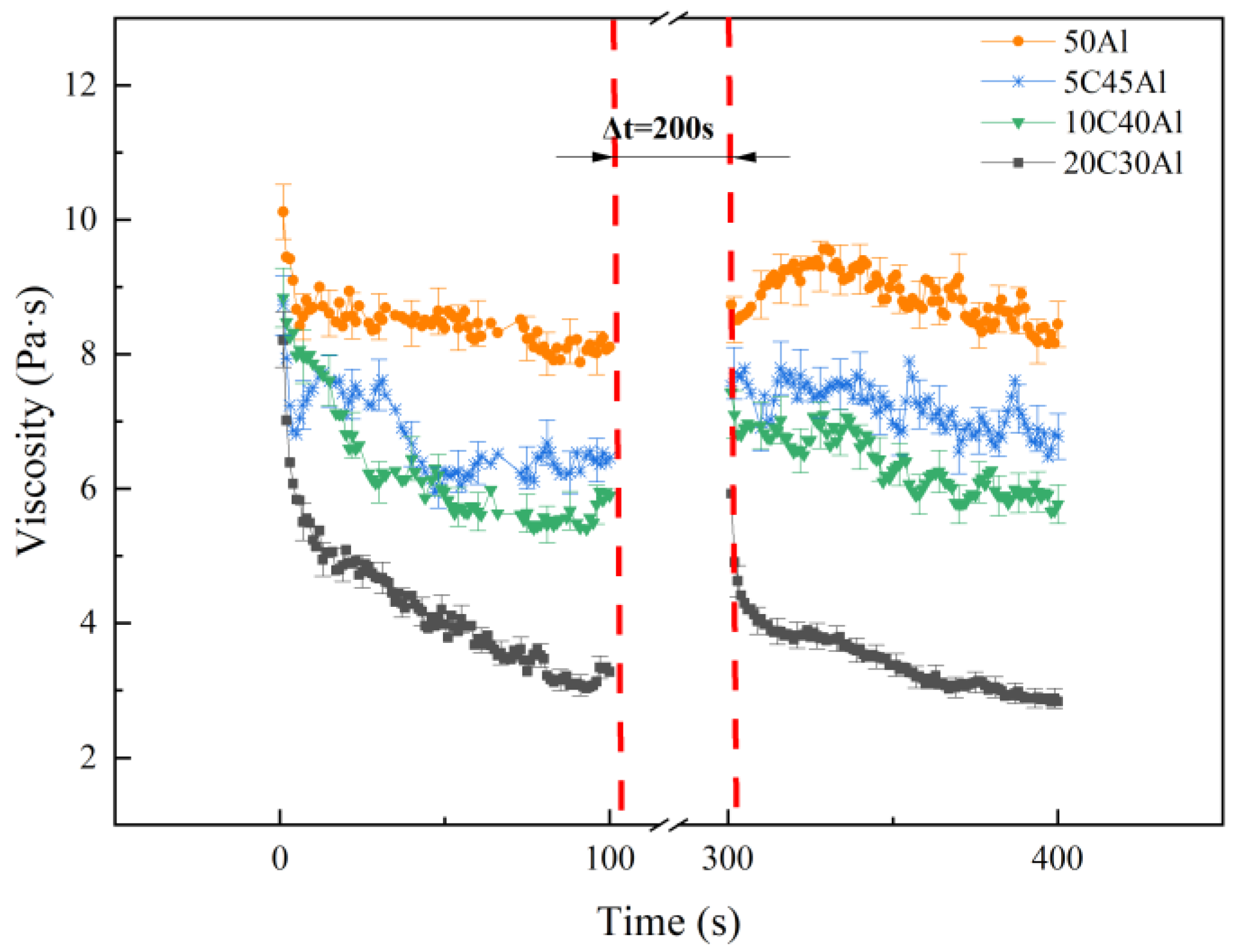


| Sample | Mass Heat of Combustion/MJ·kg−1 | Volume Calorific Value/MJ·L−1 | Density g/cm3 |
|---|---|---|---|
| 50Al | 25.578 | 46.193 | 1.805 |
| 5C45Al | 26.423 | 46.754 | 1.769 |
| 10C40Al | 26.787 | 46.421 | 1.732 |
| 15C35Al | 26.930 | 45.686 | 1.696 |
| 20C30Al | 27.128 | 45.031 | 1.659 |
| Sample | K | n | R2 |
|---|---|---|---|
| 50Al | 847.682 | 0.3602 | 0.99 |
| 5C45Al | 738.579 | 0.3551 | 0.99 |
| 10C40Al | 655.582 | 0.2729 | 0.99 |
| 20C30Al | 516.438 | 0.1953 | 0.99 |
| Sample | Viscosity before Recovery/Pa·s | Viscosity after Recovery/Pa·s | Recovery Ratio/% |
|---|---|---|---|
| 50Al | 8.248 | 8.517 | 3.26 |
| 5C45Al | 6.461 | 7.532 | 16.57 |
| 10C40Al | 5.897 | 7.435 | 26.07 |
| 20C30Al | 3.276 | 5.928 | 80.95 |
| Sample | Critical Strain/% | Yield Point Strain/% |
|---|---|---|
| 50Al | 22.80 | 163.00 |
| 5C45Al | 16.60 | 96.00 |
| 10C40Al | 4.67 | 51.25 |
| 20C30Al | 0.50 | 27.05 |
| Sample | w/% | |||
|---|---|---|---|---|
| C2H7NO | Agarose | Al | C | |
| 50Al | 46 | 4 | 50 | 0 |
| 5C-45Al | 46 | 4 | 45 | 5 |
| 10C-40Al | 46 | 4 | 40 | 10 |
| 15C-35Al | 46 | 4 | 35 | 15 |
| 20C-30Al | 46 | 4 | 30 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhao, H.; Li, W.; Liu, H. Combustion Enhancement of Gel Propellant Containing High Concentration Aluminum Particles Based on Carbon Synergistic Effect. Gels 2024, 10, 89. https://doi.org/10.3390/gels10020089
Chen J, Zhao H, Li W, Liu H. Combustion Enhancement of Gel Propellant Containing High Concentration Aluminum Particles Based on Carbon Synergistic Effect. Gels. 2024; 10(2):89. https://doi.org/10.3390/gels10020089
Chicago/Turabian StyleChen, Jiyuan, Hui Zhao, Weifeng Li, and Haifeng Liu. 2024. "Combustion Enhancement of Gel Propellant Containing High Concentration Aluminum Particles Based on Carbon Synergistic Effect" Gels 10, no. 2: 89. https://doi.org/10.3390/gels10020089
APA StyleChen, J., Zhao, H., Li, W., & Liu, H. (2024). Combustion Enhancement of Gel Propellant Containing High Concentration Aluminum Particles Based on Carbon Synergistic Effect. Gels, 10(2), 89. https://doi.org/10.3390/gels10020089






