Cross-Linked Hyaluronan Derivatives in the Delivery of Phycocyanin
Abstract
1. Introduction
2. Results and Discussion
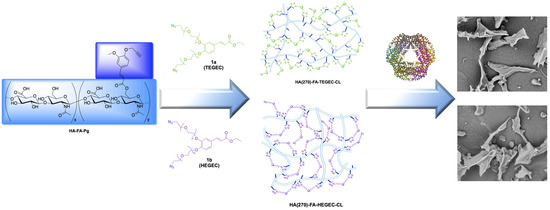
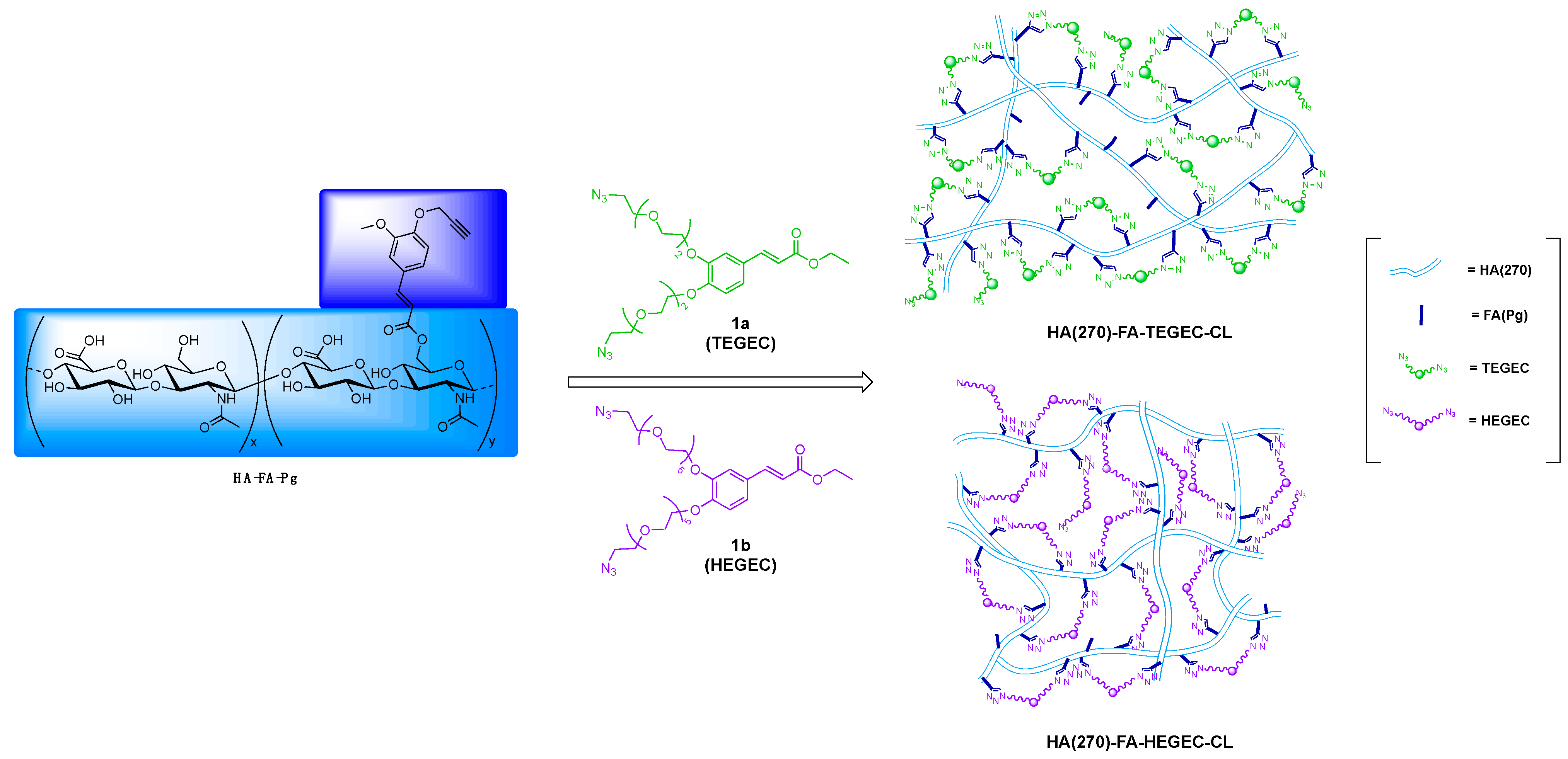
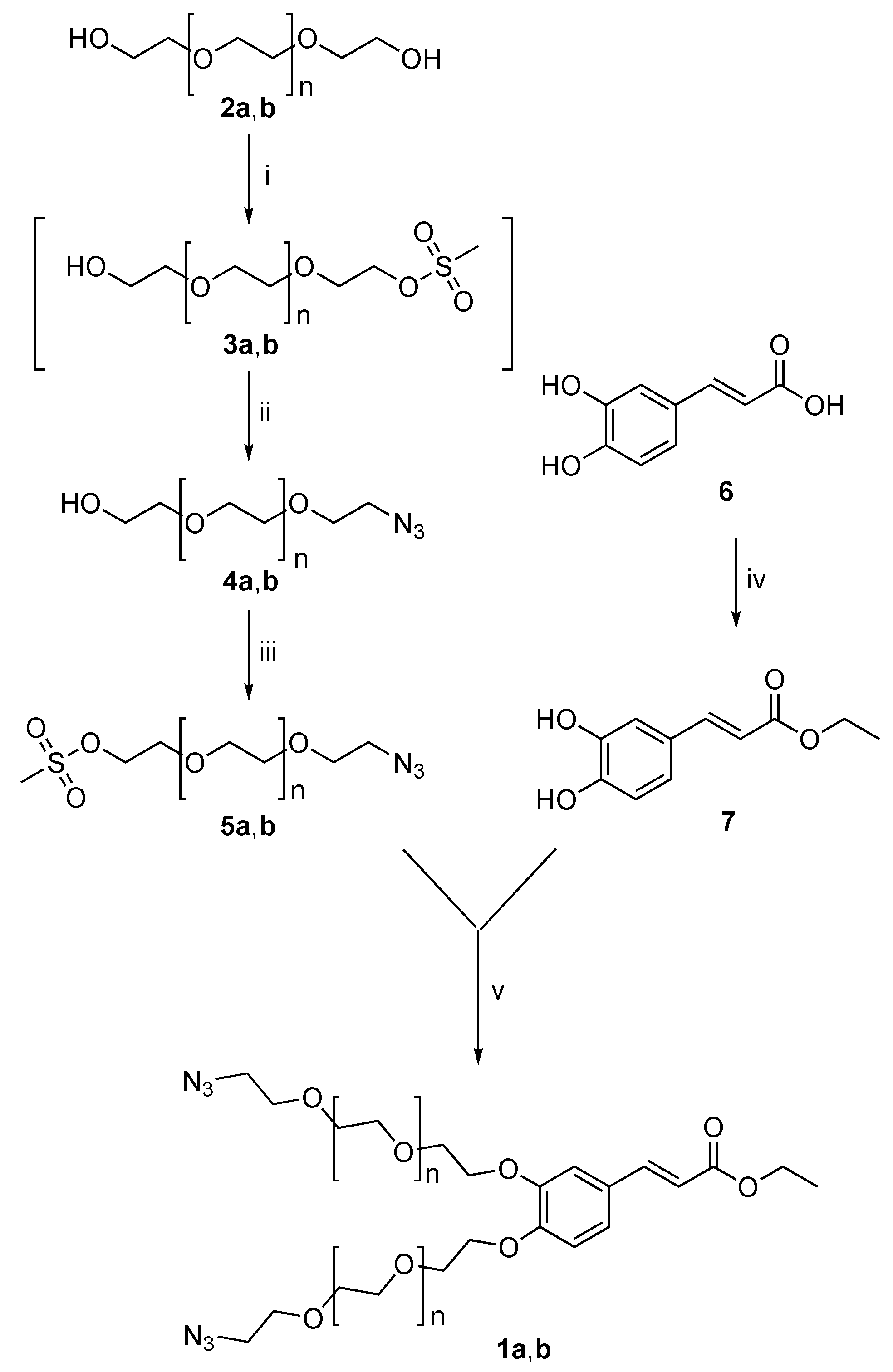
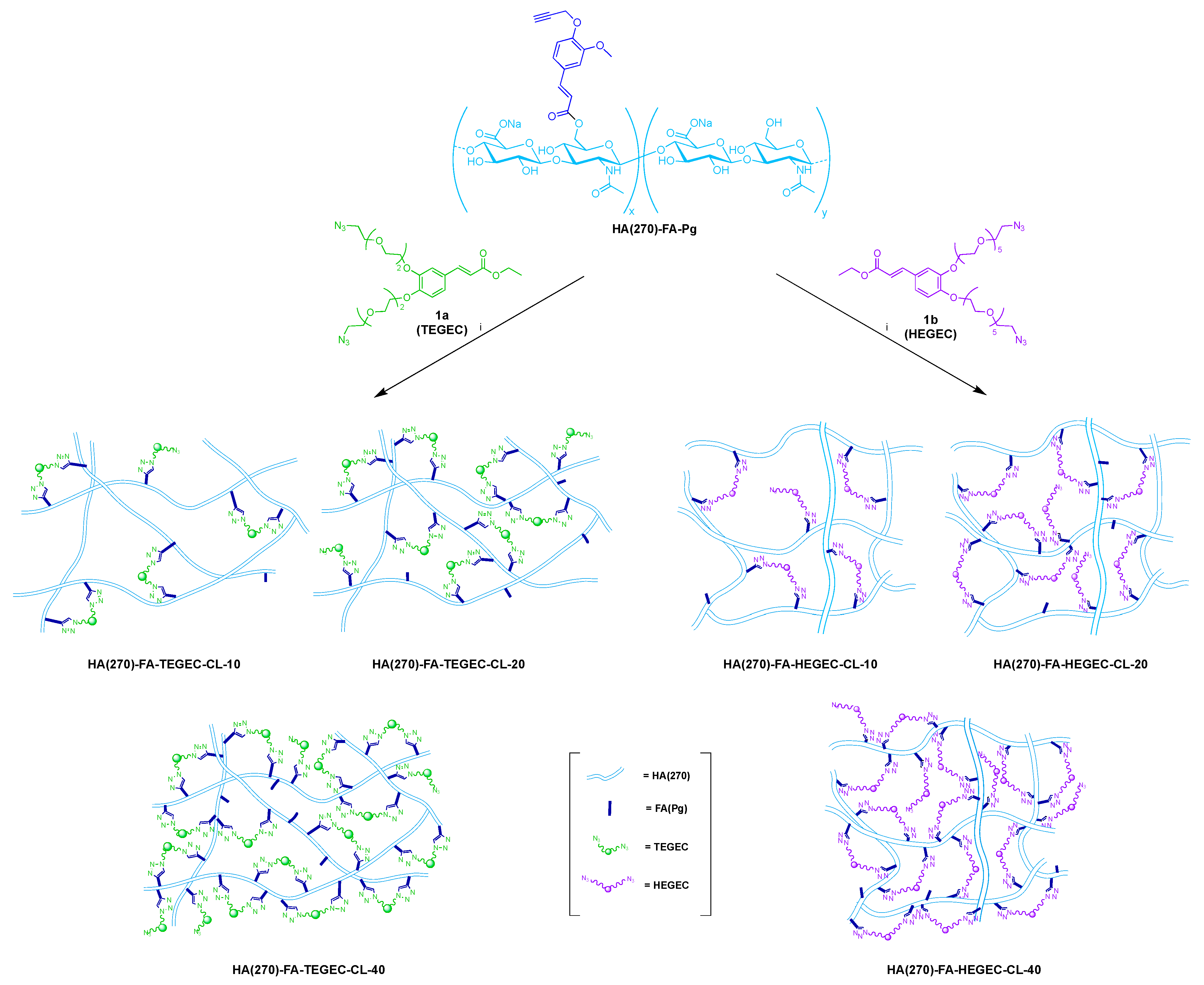
2.1. Synthesis of HA(270)-FA-TEGEC-CL and HA(270)-FA-HEGEC-CL Derivatives
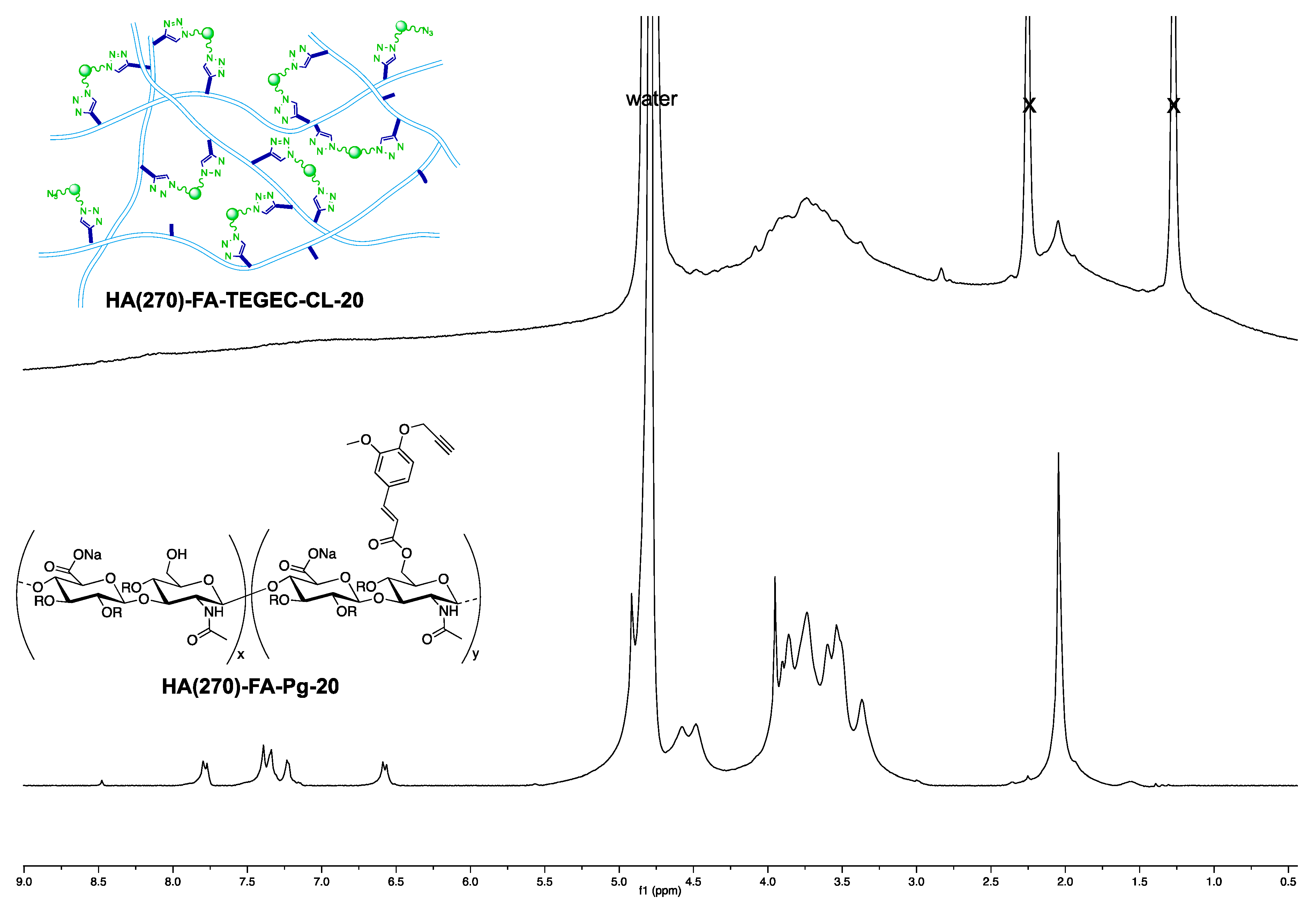
2.2. Structure of HA(270)-FA-TEGEC-CL and HA(270)-FA-HEGEC-CL Materials
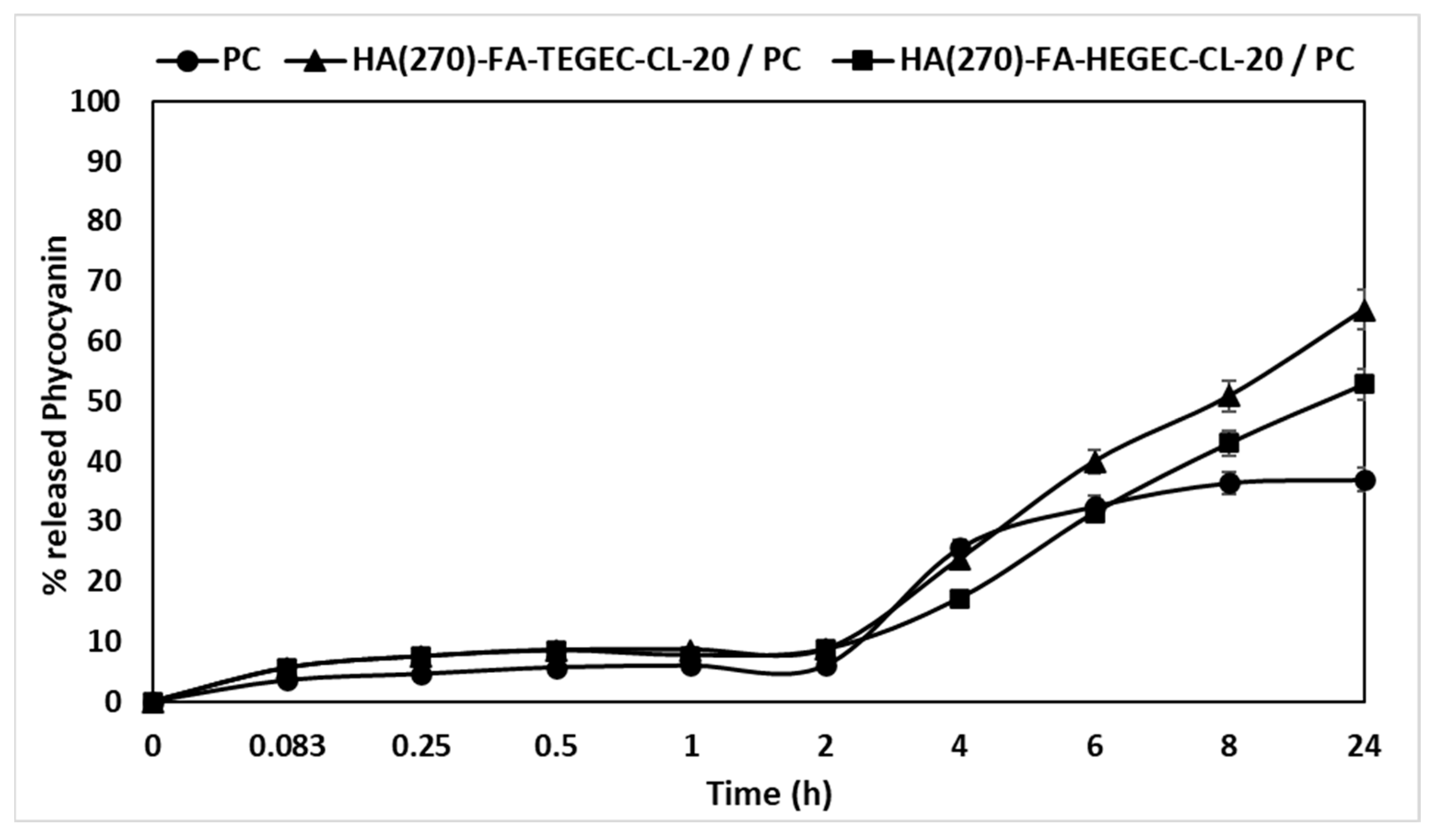
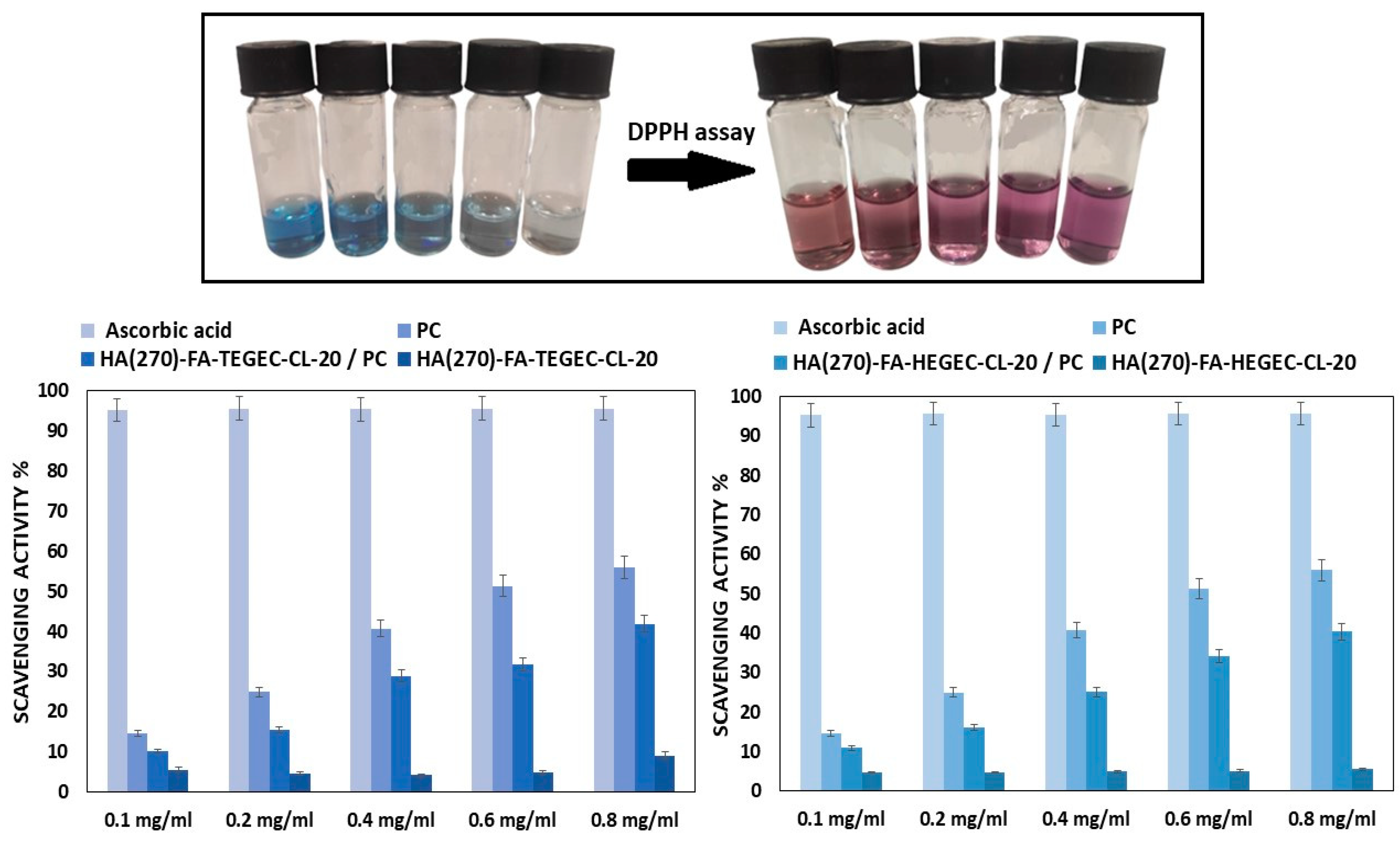
2.3. Application of HA(270)-FA-TEGEC-CL and HA(270)-FA-HEGEC-CL Crosslinked Materials in the Delivery of Phycocyanin
3. Conclusions
4. Materials and Methods
4.1. Synthesis and Characterization
- 2-(2-(2-Hydroxyethoxy)ethoxy)ethyl methanesulfonate (3a).
- 17-Hydroxy-3,6,9,12,15-pentaoxaheptadecyl methanesulfonate (3b).
- 2-(2-(2-Azidoethoxy)ethoxy)ethan-1-ol (4a).
- 17-Azido-3,6,9,12,15-pentaoxaheptadecan-1-ol (4b).
- 2-(2-(2-Azidoethoxy)ethoxy)ethyl methanesulfonate (5a).
- 17-Azido-3,6,9,12,15-pentaoxaheptadecyl methanesulfonate (5b).
- Ethyl (E)-3-(3,4-dihydroxyphenyl)acrylate (7) [82].
- Ethyl (E)-3-(3,4-bis(2-(2-(2-azidoethoxy)ethoxy)ethoxy)phenyl)acrylate (1a).
- Ethyl (E)-3-(3,4-bis((17-azido-3,6,9,12,15-pentaoxaheptadecyl)oxy)phenyl)acrylate (1b).
4.2. The General Procedure of Click-Chemistry Crosslinking of HA(270)-FA-Pg Graft Copolymers
- HA(270)-FA-TEGEC-CL-10 material
- HA(270)-FA-TEGEC-CL-20 material
- HA(270)-FA-TEGEC-CL-40 material
- HA(270)-FA-HEGEC-CL-10 material
- HA(270)-FA-HEGEC-CL-20 material
- HA(270)-FA-HEGEC-CL-40 material
4.3. Preparation of Phycocyanin-Loaded HA(270)-FA-TEGEC-CL-20 and HA(270)-FA-HEGEC-CL-20 Materials
4.4. Drug Loading Evaluation
4.5. Dynamic Light Scattering and Zeta Potential Measurements
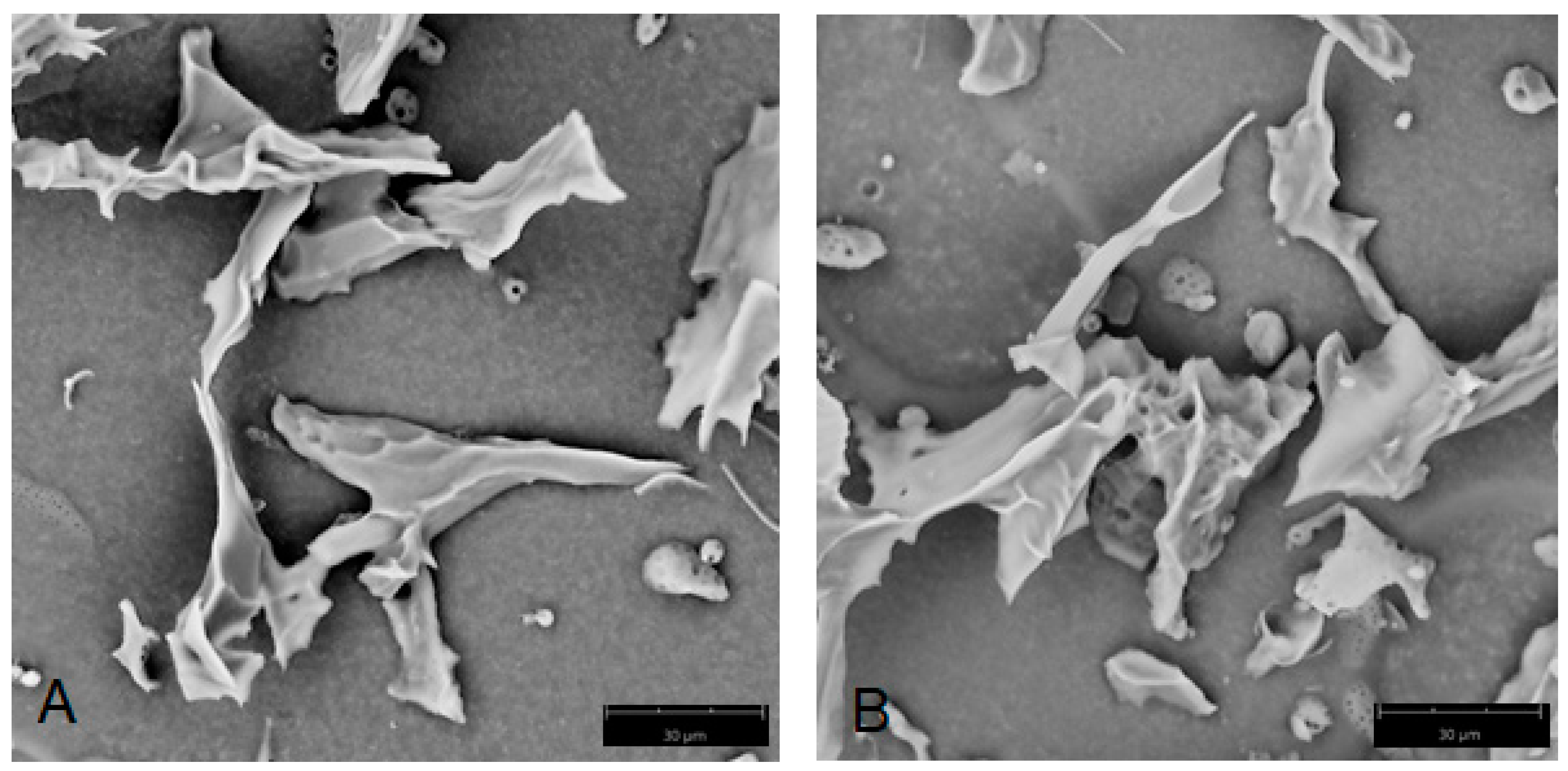
4.6. Scanning Electron Microscopy
4.7. In Vitro Dissolution Studies
4.8. DPPH Free Radical Scavenging Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, D.H.; Oh, J.-H.; Chung, J.H. Glycosaminoglycan and proteoglycan in skin aging. J. Dermatol. Sci. 2016, 83, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.S.; Svechkarev, D.; Souchek, J.J.; Hill, T.K.; Taylor, M.A.; Natarajan, A.; Mohs, A.M. Impact of structurally modifying hyaluronic acid on CD44 interaction. J. Mater. Chem. B 2017, 5, 8183–8192. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Cockerill, G.W.; Gamble, J.R.; Vadas, M.A. Angiogenesis: Models and Modulators. Int. Rev. Cytol. 1995, 159, 113–160. [Google Scholar]
- Mast, B.A.; Diegelmann, R.F.; Krummel, T.M.; Cohen, I.K. Hyaluronic Acid Modulates Proliferation, Collagen and Protein Synthesis of Cultured Fetal Fibroblasts. Matrix 1993, 13, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Bourguignon, L.Y.W. Interaction between CD44 and the repeat domain of ankyrin promotes hyaluronic acid-mediated ovarian tumor cell migration. J. Cell. Physiol. 2000, 183, 182–195. [Google Scholar] [CrossRef]
- Wasteson, Å.; Westermark, B.; Lindahl, U.; Pontén, J. Aggregation of feline lymphoma cells by hyaluronic acid. Int. J. Cancer 1973, 12, 169–178. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef]
- Salwowska, N.M.; Bebenek, K.A.; Żądło, D.A.; Wcisło-Dziadecka, D.L. Physiochemical properties and application of hyaluronic acid: A systematic review. J. Cosmet. Dermatol. 2016, 15, 520–526. [Google Scholar] [CrossRef]
- Solchaga, L.A.; Dennis, J.E.; Goldberg, V.M.; Caplan, A.I. Hyaluronic acid-based polymers as cell carriers for tissue-engineered repair of bone and cartilage. J. Orthop. Res. 1999, 17, 205–213. [Google Scholar] [CrossRef]
- Mabuchi, K.; Tsukamoto, Y.; Obara, T.; Yamaguchi, T. The effect of additive hyaluronic acid on animal joints with experimentally reduced lubricating ability. J. Biomed. Mater. Res. 1994, 28, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Joester, D.; Geiger, B.; Addadi, L. Spatial and Temporal Sequence of Events in Cell Adhesion: From Molecular Recognition to Focal Adhesion Assembly. ChemBioChem 2004, 5, 1393–1399. [Google Scholar] [CrossRef]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef]
- Tripodo, G.; Trapani, A.; Torre, M.L.; Giammona, G.; Trapani, G.; Mandracchia, D. Hyaluronic acid and its derivatives in drug delivery and imaging: Recent advances and challenges. Eur. J. Pharm. Biopharm. 2015, 97, 400–416. [Google Scholar] [CrossRef]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Trombino, S.; Servidio, C.; Curcio, F.; Cassano, R. Strategies for Hyaluronic Acid-Based Hydrogel Design in Drug Delivery. Pharmaceutics 2019, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Gilarska, A.; Lewandowska-Łańcucka, J.; Horak, W.; Nowakowska, M. Collagen/chitosan/hyaluronic acid—Based injectable hydrogels for tissue engineering applications—Design, physicochemical and biological characterization. Colloids Surf. B Biointerfaces 2018, 170, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8, 2041731417726464. [Google Scholar] [CrossRef]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Hyaluronic acid-based hydrogels: From a natural polysaccharide to complex networks. Soft Matter 2012, 8, 3280–3294. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, H.; Xu, C.; Yu, D.; Xu, H.; Hu, Y. Synthesis of hyaluronic acid hydrogels by crosslinking the mixture of high-molecular-weight hyaluronic acid and low-molecular-weight hyaluronic acid with 1,4-butanediol diglycidyl ether. RSC Adv. 2020, 10, 7206–7213. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant Properties of Ferulic Acid and Its Related Compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef]
- Adeyi, O.E.; Somade, O.T.; Ajayi, B.O.; James, A.S.; Adeboye, T.R.; Olufemi, D.A.; Oyinlola, E.V.; Sanyaolu, E.T.; Mufutau, I.O. The anti-inflammatory effect of ferulic acid is via the modulation of NFκB-TNF-α-IL-6 and STAT1-PIAS1 signaling pathways in 2-methoxyethanol-induced testicular inflammation in rats. Phytomedicine Plus 2023, 3, 100464. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.; Xu, F.; Chu, C.; Li, X.; Shi, X.; Zheng, W.; Wang, Z.; Jia, Y.; Xiao, W. Use of Ferulic Acid in the Management of Diabetes Mellitus and Its Complications. Molecules 2022, 27, 6010. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.; Kwok, K.-C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Iiyama, K.; Lam, T.B.T.; Stone, B.A. Covalent Cross-Links in the Cell Wall. Plant Physiol. 1994, 104, 315–320. [Google Scholar] [CrossRef]
- Buanafina, M.M.d.O. Feruloylation in Grasses: Current and Future Perspectives. Mol. Plant 2009, 2, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Grabber, J.H.; Ralph, J.; Hatfield, R.D. Cross-Linking of Maize Walls by Ferulate Dimerization and Incorporation into Lignin. J. Agric. Food Chem. 2000, 48, 6106–6113. [Google Scholar] [CrossRef] [PubMed]
- Wallace, G.; Fry, S.C. Phenolic Components of the Plant Cell Wall. Int. Rev. Cytol. 1994, 151, 229–267. [Google Scholar] [PubMed]
- Mathew, S.; Abraham, T.E. Ferulic Acid: An Antioxidant Found Naturally in Plant Cell Walls and Feruloyl Esterases Involved in its Release and Their Applications. Crit. Rev. Biotechnol. 2004, 24, 59–83. [Google Scholar] [CrossRef]
- Bramanti, E.; Fulgentini, L.; Bizzarri, R.; Lenci, F.; Sgarbossa, A. β-Amyloid Amorphous Aggregates Induced by the Small Natural Molecule Ferulic Acid. J. Phys. Chem. B 2013, 117, 13816–13821. [Google Scholar] [CrossRef]
- Noel, A.; Borguet, Y.P.; Raymond, J.E.; Wooley, K.L. Poly(carbonate–amide)s Derived from Bio-Based Resources: Poly(ferulic acid-co-tyrosine). Macromolecules 2014, 47, 2974–2983. [Google Scholar] [CrossRef] [PubMed]
- Pepi, S.; Paolino, M.; Saletti, M.; Venditti, J.; Talarico, L.; Andreassi, M.; Giuliani, G.; Caselli, G.; Artusi, R.; Cappelli, A.; et al. Ferulated Poly(vinyl alcohol) based hydrogels. Heliyon 2023, 9, e22330. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Grisci, G.; Paolino, M.; Giuliani, G.; Donati, A.; Mendichi, R.; Artusi, R.; Demiranda, M.; Zanardi, A.; Giorgi, G.; et al. Hyaluronan derivatives bearing variable densities of ferulic acid residues. J. Mater. Chem. B 2014, 2, 4489–4499. [Google Scholar] [CrossRef]
- Valacchi, G.; Grisci, G.; Sticozzi, C.; Lim, Y.; Paolino, M.; Giuliani, G.; Mendichi, R.; Belmonte, G.; Artusi, R.; Zanardi, A.; et al. Wound healing properties of hyaluronan derivatives bearing ferulate residues. J. Mater. Chem. B 2015, 3, 7037–7045. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Paolino, M.; Grisci, G.; Razzano, V.; Giuliani, G.; Donati, A.; Bonechi, C.; Mendichi, R.; Battiato, S.; Samperi, F.; et al. Hyaluronan-coated polybenzofulvene brushes as biomimetic materials. Polym. Chem. 2016, 7, 6529–6544. [Google Scholar] [CrossRef]
- Licciardi, M.; Scialabba, C.; Giammona, G.; Paolino, M.; Razzano, V.; Grisci, G.; Giuliani, G.; Makovec, F.; Cappelli, A. Design and development of hyaluronan-functionalized polybenzofulvene nanoparticles as CD44 receptor mediated drug delivery system. J. Nanoparticle Res. 2017, 19, 197. [Google Scholar] [CrossRef]
- Cappelli, A.; Paolino, M.; Reale, A.; Razzano, V.; Grisci, G.; Giuliani, G.; Donati, A.; Bonechi, C.; Lamponi, S.; Mendichi, R.; et al. Hyaluronan-based graft copolymers bearing aggregation-induced emission fluorogens. RSC Adv. 2018, 8, 5864–5881. [Google Scholar] [CrossRef]
- Paolino, M.; Licciardi, M.; Savoca, C.; Giammona, G.; Modica De Mohac, L.; Reale, A.; Giuliani, G.; Komber, H.; Donati, A.; Leone, G.; et al. Hyaluronan Graft Copolymers Bearing Fatty-Acid Residues as Self-Assembling Nanoparticles for Olanzapine Delivery. Pharmaceutics 2019, 11, 675. [Google Scholar] [CrossRef]
- Atrei, A.; Innocenti, C.; Lamponi, S.; Paesano, S.; Leone, G.; Reale, A.; Paolino, M.; Cappelli, A. Covalent hyaluronic-based coating of magnetite nanoparticles: Preparation, physicochemical and biological characterization. Mater. Sci. Eng. C 2020, 107, 110271. [Google Scholar] [CrossRef]
- Paolino, M.; Varvarà, P.; Saletti, M.; Reale, A.; Gentile, M.; Paccagnini, E.; Giuliani, G.; Komber, H.; Licciardi, M.; Cappelli, A. Hyaluronan-coated poly(propylene imine) dendrimers as biomimetic nanocarriers of doxorubicin. J. Appl. Polym. Sci. 2023, 140, e53300. [Google Scholar] [CrossRef]
- Saletti, M.; Paolino, M.; Ballerini, L.; Giuliani, G.; Leone, G.; Lamponi, S.; Andreassi, M.; Bonechi, C.; Donati, A.; Piovani, D.; et al. Click-Chemistry Cross-Linking of Hyaluronan Graft Copolymers. Pharmaceutics 2022, 14, 1041. [Google Scholar] [CrossRef]
- Li, X.; Xiong, Y. Application of “Click” Chemistry in Biomedical Hydrogels. ACS Omega 2022, 7, 36918–36928. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, X.; Zhang, X. Recent Developments in the Area of Click-Crosslinked Nanocarriers for Drug Delivery. Macromol. Rapid Commun. 2019, 40, 1800541. [Google Scholar] [CrossRef]
- Li, C.; Tang, J.; Xie, J. Synthesis of crosslinking amino acids by click chemistry. Tetrahedron 2009, 65, 7935–7941. [Google Scholar] [CrossRef]
- Schellinger, J.G.; Kudupudi, A.; Natarajan, A.; Du, W.; DeNardo, S.J.; Gervay-Haguea, J. A general chemical synthesis platform for crosslinking multivalent single chain variable fragments. Org. Biomol. Chem. 2012, 10, 1521–1526. [Google Scholar] [CrossRef]
- Pierre-Antoine, A.; François, B.; Rachida, Z. Crosslinked cellulose developed by CuAAC, a route to new materials. Carbohydr. Res. 2012, 356, 247–251. [Google Scholar] [CrossRef]
- Fernandes, R.; Campos, J.; Serra, M.; Fidalgo, J.; Almeida, H.; Casas, A.; Toubarro, D.; Barros, A.I.R.N.A. Exploring the Benefits of Phycocyanin: From Spirulina Cultivation to Its Widespread Applications. Pharmaceuticals 2023, 16, 592. [Google Scholar] [CrossRef] [PubMed]
- Lupatini, A.L.; Colla, L.M.; Canan, C.; Colla, E. Potential application of microalga Spirulina platensis as a protein source. J. Sci. Food Agric. 2017, 97, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Mosulishvili, L.M.; Kirkesali, E.I.; Belokobylsky, A.I.; Khizanishvili, A.I.; Frontasyeva, M.V.; Pavlov, S.S.; Gundorina, S.F. Experimental substantiation of the possibility of developing selenium- and iodine-containing pharmaceuticals based on blue–green algae Spirulina platensis. J. Pharm. Biomed. Anal. 2002, 30, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Grover, P.; Bhatnagar, A.; Kumari, N.; Narayan Bhatt, A.; Kumar Nishad, D.; Purkayastha, J. C-Phycocyanin-a novel protein from Spirulina platensis- In vivo toxicity, antioxidant and immunomodulatory studies. Saudi J. Biol. Sci. 2021, 28, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Sun, J.; Bi, Y.; Xu, X.; Zang, X. Fluorescence and antioxidant activity of heterologous expression of phycocyanin and allophycocyanin from Arthrospira platensis. Front. Nutr. 2023, 10, 1127422. [Google Scholar] [CrossRef]
- Li, Y. The Bioactivities of Phycocyanobilin from Spirulina. J. Immunol. Res. 2022, 2022, 4008991. [Google Scholar] [CrossRef]
- Liu, R.-Z.; Li, W.-J.; Zhang, J.-J.; Liu, Z.-Y.; Li, Y.; Liu, C.; Qin, S. The Inhibitory Effect of Phycocyanin Peptide on Pulmonary Fibrosis In Vitro. Mar. Drugs 2022, 20, 696. [Google Scholar] [CrossRef]
- Lemos, P.V.F.; Opretzka, L.C.F.; Almeida, L.S.; Cardoso, L.G.; da Silva, J.B.A.; de Souza, C.O.; Villarreal, C.F.; Druzian, J.I. Preparation and characterization of C-phycocyanin coated with STMP/STPP cross-linked starches from different botanical sources. Int. J. Biol. Macromol. 2020, 159, 739–750. [Google Scholar] [CrossRef]
- Fernández-Rojas, B.; Hernández-Juárez, J.; Pedraza-Chaverri, J. Nutraceutical properties of phycocyanin. J. Funct. Foods 2014, 11, 375–392. [Google Scholar] [CrossRef]
- Bhayani, K.; Mitra, M.; Ghosh, T.; Mishra, S. C-Phycocyanin as a potential biosensor for heavy metals like Hg2+ in aquatic systems. RSC Adv. 2016, 6, 111599–111605. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Y.; Zhang, R.; Cai, T.; Cai, Y. Medical Application of Spirulina platensis Derived C-Phycocyanin. Evid.-Based Complement. Altern. Med. 2016, 2016, 7803846. [Google Scholar]
- Ashaolu, T.J.; Samborska, K.; Lee, C.C.; Tomas, M.; Capanoglu, E.; Tarhan, Ö.; Taze, B.; Jafari, S.M. Phycocyanin, a super functional ingredient from algae; properties, purification characterization, and applications. Int. J. Biol. Macromol. 2021, 193, 2320–2331. [Google Scholar] [CrossRef] [PubMed]
- Morya, S.; Kumar Chattu, V.; Khalid, W.; Zubair Khalid, M.; Siddeeg, A. Potential protein phycocyanin: An overview on its properties, extraction, and utilization. Int. J. Food Prop. 2023, 26, 3160–3176. [Google Scholar] [CrossRef]
- González, R.; Rodríguez, S.; Romay, C.; González, A.; Armesto, J.; Remirez, D.; Merino, N. Anti-Inflammatory Activity Of Phycocyanin Extract In Acetic Acid-Induced Colitis In Rats. Pharmacol. Res. 1999, 39, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, G.; Sampathkumar, P.; Kavisri, M.; Moovendhan, M. Extraction and characterization of phycocyanin from Spirulina platensis and evaluation of its anticancer, antidiabetic and antiinflammatory effect. Int. J. Biol. Macromol. 2020, 153, 256–263. [Google Scholar] [CrossRef]
- Pentón-Rol, G.; Marín-Prida, J.; Pardo-Andreu, G.; Martínez-Sánchez, G.; Acosta-Medina, E.F.; Valdivia-Acosta, A.; Lagumersindez-Denis, N.; Rodríguez-Jiménez, E.; Llópiz-Arzuaga, A.; López-Saura, P.A.; et al. C-Phycocyanin is neuroprotective against global cerebral ischemia/reperfusion injury in gerbils. Brain Res. Bull. 2011, 86, 42–52. [Google Scholar] [CrossRef]
- Vadiraja, B.B.; Gaikwad, N.W.; Madyastha, K.M. Hepatoprotective Effect of C-Phycocyanin: Protection for Carbon Tetrachloride and R-(+)-Pulegone-Mediated Hepatotoxicty in Rats. Biochem. Biophys. Res. Commun. 1998, 249, 428–431. [Google Scholar] [CrossRef]
- Chen, H.-W.; Yang, T.-S.; Chen, M.-J.; Chang, Y.-C.; Wang, E.I.-C.; Ho, C.-L.; Lai, Y.-J.; Yu, C.-C.; Chou, J.-C.; Chao, L.K.-P.; et al. Purification and immunomodulating activity of C-phycocyanin from Spirulina platensis cultured using power plant flue gas. Process Biochem. 2014, 49, 1337–1344. [Google Scholar] [CrossRef]
- Fernández-Rojas, B.; Medina-Campos, O.N.; Hernández-Pando, R.; Negrette-Guzmán, M.; Huerta-Yepez, S.; Pedraza-Chaverri, J. C-Phycocyanin prevents cisplatin-induced nephrotoxicity through inhibition of oxidative stress. Food Funct. 2014, 5, 480–490. [Google Scholar] [CrossRef]
- Liu, R.; Qin, S.; Li, W. Phycocyanin: Anti-inflammatory effect and mechanism. Biomed. Pharmacother. 2022, 153, 113362. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Li, Z.; Shan, H.; Dashnyam, B.; Xu, X.; McClements, D.J.; Zhang, B.; Tan, M.; Wang, Z.; Cao, C. A review of recent strategies to improve the physical stability of phycocyanin. Curr. Res. Food Sci. 2022, 5, 2329–2337. [Google Scholar] [CrossRef]
- Braune, S.; Krüger-Genge, A.; Kammerer, S.; Jung, F.; Küpper, J.-H. Phycocyanin from Arthrospira platensis as Potential Anti-Cancer Drug: Review of In Vitro and In Vivo Studies. Life 2021, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wong, Y.-S. In Vitro Antioxidant and Antiproliferative Activities of Selenium-Containing Phycocyanin from Selenium-Enriched Spirulina platensis. J. Agric. Food Chem. 2008, 56, 4352–4358. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, W.; Qin, S. Therapeutic effect of phycocyanin on acute liver oxidative damage caused by X-ray. Biomed. Pharmacother. 2020, 130, 110553. [Google Scholar] [CrossRef]
- Fratelli, C.; Burck, M.; Amarante, M.C.A.; Braga, A.R.C. Antioxidant potential of nature’s “something blue”: Something new in the marriage of biological activity and extraction methods applied to C-phycocyanin. Trends Food Sci. Technol. 2021, 107, 309–323. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, B.J.; Wong, R.N.S.; Jiang, Y. Characterization and antioxidant activity of selenium-containing phycocyanin isolated from Spirulina platensis. Food Chem. 2007, 100, 1137–1143. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- Pez Jaeschke, D.; Rocha Teixeira, I.; Damasceno Ferreira Marczak, L.; Domeneghini Mercali, G. Phycocyanin from Spirulina: A review of extraction methods and stability. Food Res. Int. 2021, 143, 110314. [Google Scholar] [CrossRef] [PubMed]
- Selig, M.J.; Malchione, N.M.; Gamaleldin, S.; Padilla-Zakour, O.I.; Abbaspourrad, A. Protection of blue color in a spirulina derived phycocyanin extract from proteolytic and thermal degradation via complexation with beet-pectin. Food Hydrocoll. 2018, 74, 46–52. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Abbaspourrad, A. Improvement of the colloidal stability of phycocyanin in acidified conditions using whey protein-phycocyanin interactions. Food Hydrocoll. 2020, 105, 105747. [Google Scholar] [CrossRef]
- Singh, G.; Majeed, A.; Singh, R.; George, N.; Singh, G.; Gupta, S.; Singh, H.; Kaur, G.; Singh, J. CuAAC ensembled 1,2,3-triazole linked nanogels for targeted drug delivery: A review. RSC Adv. 2023, 13, 2912–2936. [Google Scholar] [CrossRef]
- Meldal, M. Polymer “Clicking” by CuAAC Reactions. Macromol. Rapid Commun. 2008, 29, 1016–1051. [Google Scholar] [CrossRef]
- Rojas-Franco, P.; Garcia-Pliego, E.; Vite-Aquino, A.G.; Franco-Colin, M.; Serrano-Contreras, J.I.; Paniagua-Castro, N.; Gallardo-Casas, C.A.; Blas-Valdivia, V.; Cano-Europa, E. The Nutraceutical Antihypertensive Action of C-Phycocyanin in Chronic Kidney Disease Is Related to the Prevention of Endothelial Dysfunction. Nutrients 2022, 14, 1464. [Google Scholar] [CrossRef]
- Puglisi, R.; Biazzi, E.; Gesmundo, D.; Vanni, R.; Tava, A.; Cenadelli, S. The Antioxidant Activity of a Commercial and a Fractionated Phycocyanin on Human Skin Cells In Vitro. Molecules 2022, 27, 5276. [Google Scholar] [CrossRef]
- Suárez-Escobedo, L.; Gotor-Fernández, V. Solvent role in the lipase-catalysed esterification of cinnamic acid and derivatives. Optimisation of the biotransformation conditions. Tetrahedron 2021, 81, 131873. [Google Scholar] [CrossRef]
- Terracina, F.; Caruana, R.; Bonomo, F.P.; Montalbano, F.; Licciardi, M. Gastro-Resistant Microparticles Produced by Spray-Drying as Controlled Release Systems for Liposoluble Vitamins. Pharmaceutics 2022, 14, 1480. [Google Scholar] [CrossRef] [PubMed]
- Puleo, G.; Terracina, F.; Catania, V.; Sciré, S.; Schillaci, D.; Licciardi, M. Electrosprayed Poly-butyl-succinate microparticles for sustained release of Ciprofloxacin as an antimicrobial delivery system. Powder Technol. 2024, 432, 119152. [Google Scholar] [CrossRef]
- Agrawal, M.; Bansal, S.; Chopra, K. Evaluation of the in vitro and in vivo antioxidant potentials of food grade Phycocyanin. J. Food Sci. Technol. 2021, 58, 4382–4390. [Google Scholar] [CrossRef] [PubMed]


| Sample | Z-Average (nm) | PDI | Zeta Potential (mV) | DL% (p/p) |
|---|---|---|---|---|
| HA(270)-FA-TEGEC-CL-20 | 332.5 ± 22.6 | 0.338 ± 0.012 | −39.1 ± 0.7 | / |
| HA(270)-FA-HEGEC-CL-20 | 348.6 ± 18.4 | 0.279 ± 0.015 | −41.5 ± 0.5 | / |
| HA(270)-FA-TEGEC-CL-20/PC | / | / | −1.23 ± 0.07 | 41% ± 2 |
| HA(270)-FA-HEGEC-CL-20/PC | / | / | −1.73 ± 0.03 | 42% ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terracina, F.; Saletti, M.; Paolino, M.; Venditti, J.; Giuliani, G.; Bonechi, C.; Licciardi, M.; Cappelli, A. Cross-Linked Hyaluronan Derivatives in the Delivery of Phycocyanin. Gels 2024, 10, 91. https://doi.org/10.3390/gels10020091
Terracina F, Saletti M, Paolino M, Venditti J, Giuliani G, Bonechi C, Licciardi M, Cappelli A. Cross-Linked Hyaluronan Derivatives in the Delivery of Phycocyanin. Gels. 2024; 10(2):91. https://doi.org/10.3390/gels10020091
Chicago/Turabian StyleTerracina, Francesca, Mario Saletti, Marco Paolino, Jacopo Venditti, Germano Giuliani, Claudia Bonechi, Mariano Licciardi, and Andrea Cappelli. 2024. "Cross-Linked Hyaluronan Derivatives in the Delivery of Phycocyanin" Gels 10, no. 2: 91. https://doi.org/10.3390/gels10020091
APA StyleTerracina, F., Saletti, M., Paolino, M., Venditti, J., Giuliani, G., Bonechi, C., Licciardi, M., & Cappelli, A. (2024). Cross-Linked Hyaluronan Derivatives in the Delivery of Phycocyanin. Gels, 10(2), 91. https://doi.org/10.3390/gels10020091