Hydrogel-Based Therapy for Age-Related Macular Degeneration: Current Innovations, Impediments, and Future Perspectives
Abstract
:1. Introduction
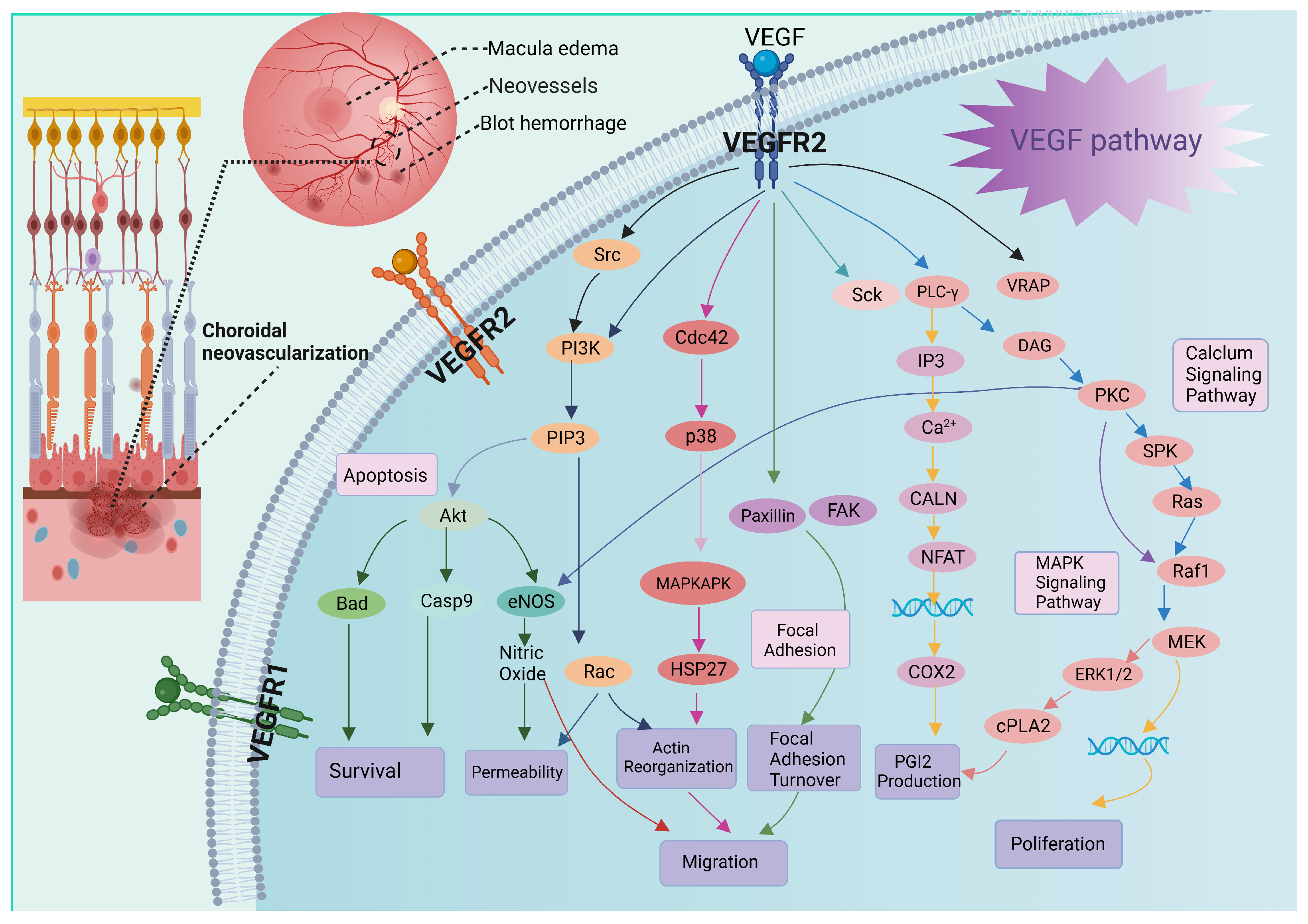
2. Pathology of AMD
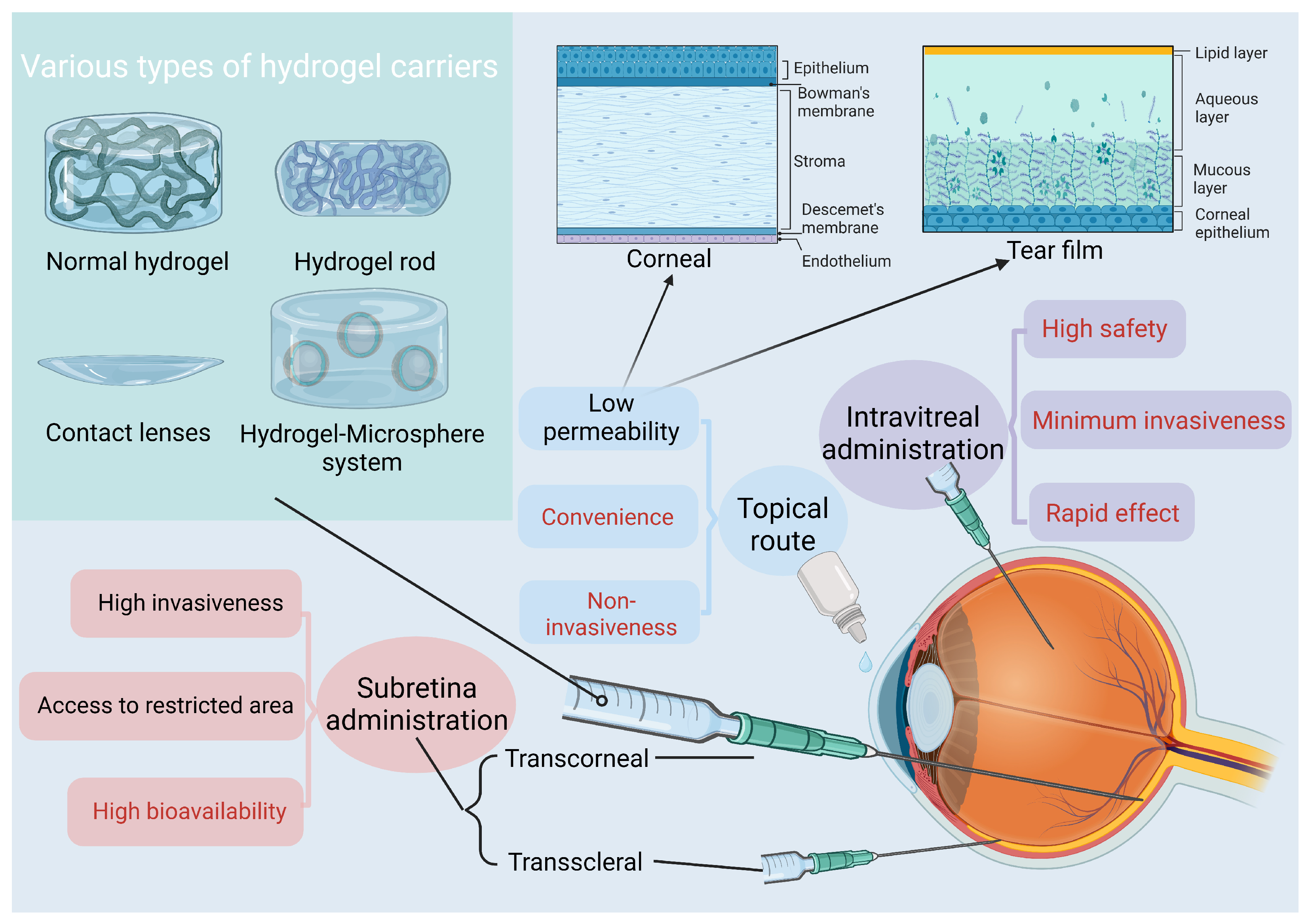
3. Hydrogels and Ocular Delivery: Properties and Potential
3.1. Classification of Hydrogels
3.2. Mechanisms of Drug Release in Hydrogels
3.3. Factors Influencing Drug Release in Hydrogels
3.4. Safety of Hydrogels for Ocular Delivery
3.5. Advantages and Limitations of Hydrogel Applications
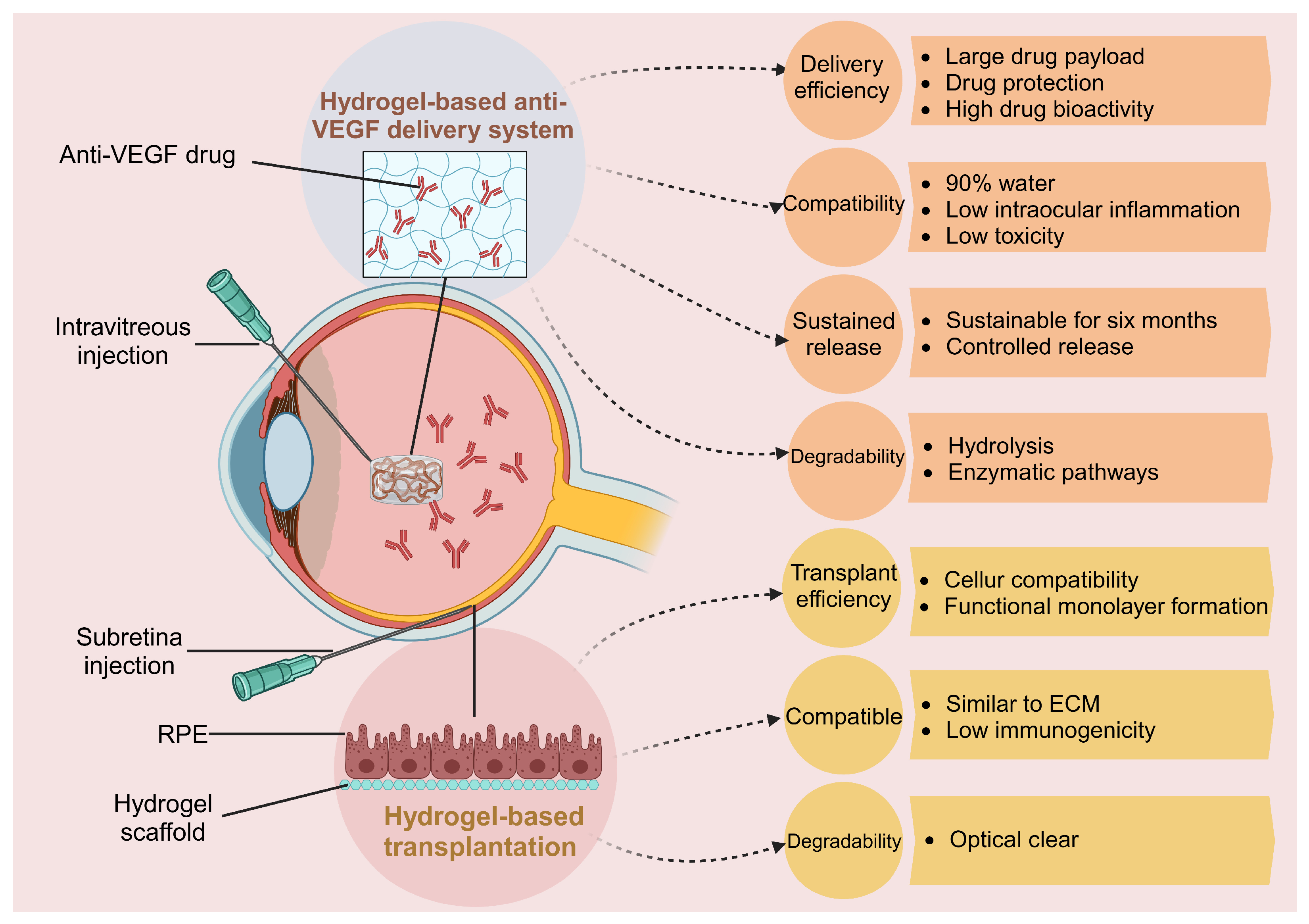
4. Treatments Based on Hydrogels for AMD
4.1. Hydrogel-Based Anti-VEGF Drug Delivery System
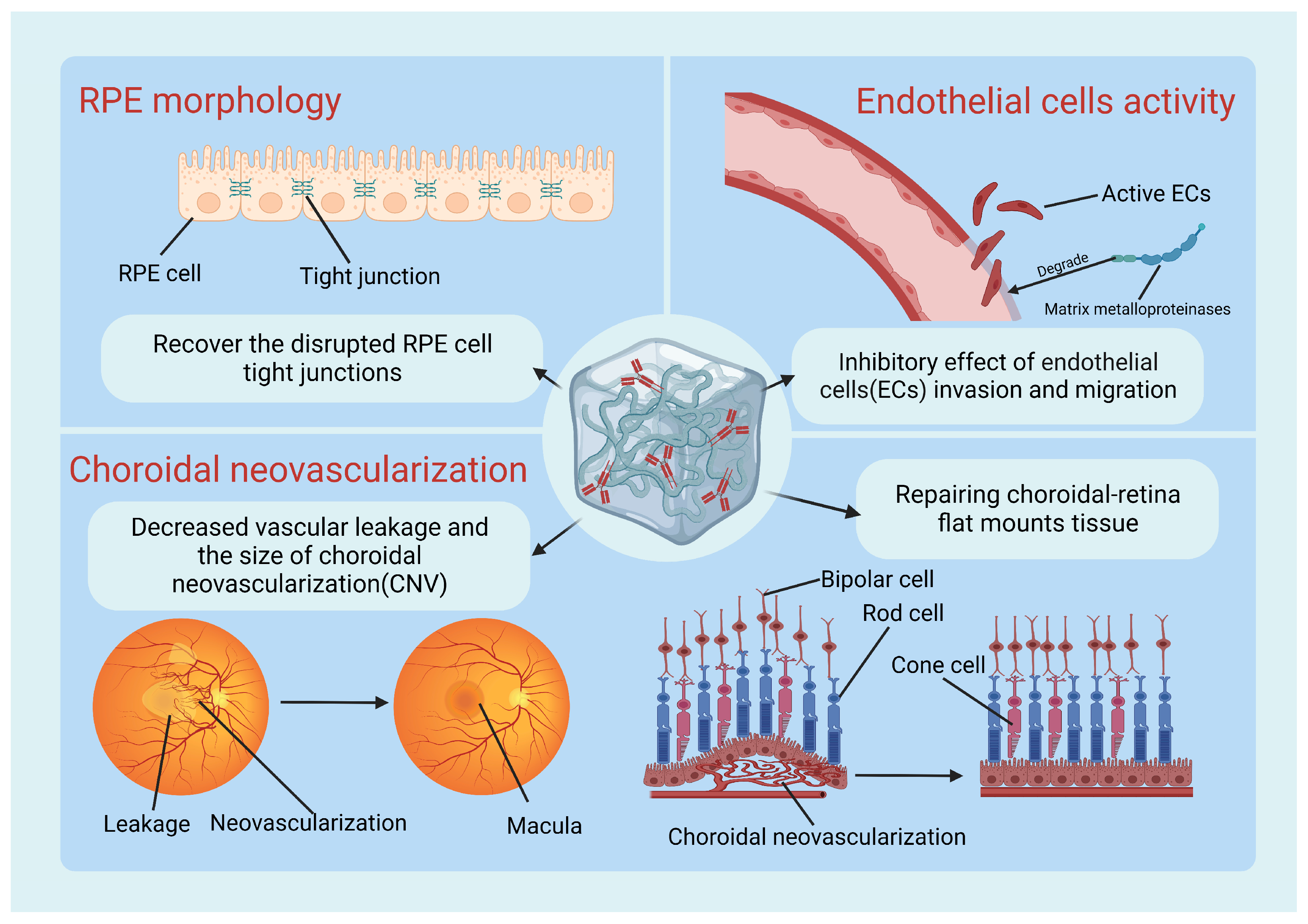
4.1.1. Pre-Clinical Trials
4.1.2. Clinical Trials
4.2. Hydrogel Applications in Tissue Engineering and RPE Transplantation
5. Future Perspectives of Hydrogel-Based Treatments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Mahroo, O.A.; Khan, R.S.; Mohamed, M.D.; McKibbin, M.; Bird, A.; Michaelides, M.; Tufail, A.; Moore, A.T. Differentiating drusen: Drusen and drusen-like appearances associated with ageing, age-related macular degeneration, inherited eye disease and other pathological processes. Prog. Retin. Eye Res. 2016, 53, 70–106. [Google Scholar] [CrossRef] [PubMed]
- Guymer, R.H.; Campbell, T.G. Age-related macular degeneration. Lancet 2023, 401, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Kahn, H.A.; Leibowitz, H.M.; Ganley, J.P.; Kini, M.M.; Colton, T.; Nickerson, R.S.; Dawber, T.R. The Framingham Eye Study. I. Outline and major prevalence findings. Am. J. Epidemiol. 1977, 106, 17–32. [Google Scholar] [CrossRef]
- Seah, I.; Zhao, X.; Lin, Q.; Liu, Z.; Su, S.Z.Z.; Yuen, Y.S.; Hunziker, W.; Lingam, G.; Loh, X.J.; Su, X. Use of biomaterials for sustained delivery of anti-VEGF to treat retinal diseases. Eye 2020, 34, 1341–1356. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Falavarjani, K.; Nguyen, Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye 2013, 27, 787–794. [Google Scholar] [CrossRef]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Weber, L.M.; Lopez, C.G.; Anseth, K.S. Effects of PEG hydrogel crosslinking density on protein diffusion and encapsulated islet survival and function. J. Biomed. Mater. Res. A 2009, 90, 720–729. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, S.G.; Boulton, M.E. Consequences of oxidative stress in age-related macular degeneration. Mol. Asp. Med. 2012, 33, 399–417. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Z.Z.; Cheng, Y.L.; Lin, W.; Qu, C. Resveratrol protects against oxidative damage of retinal pigment epithelium cells by modulating SOD/MDA activity and activating Bcl-2 expression. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 378–388. [Google Scholar] [PubMed]
- Melincovici, C.S.; Bosca, A.B.; Susman, S.; Marginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar] [PubMed]
- Hamilton, J.L.; Nagao, M.; Levine, B.R.; Chen, D.; Olsen, B.R.; Im, H.J. Targeting VEGF and Its Receptors for the Treatment of Osteoarthritis and Associated Pain. J. Bone Min. Res. 2016, 31, 911–924. [Google Scholar] [CrossRef]
- Dai, J.; Rabie, A.B. VEGF: An essential mediator of both angiogenesis and endochondral ossification. J. Dent. Res. 2007, 86, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Peach, C.; Mignone, V.; Arruda, M.; Alcobia, D.; Hill, S.; Kilpatrick, L.; Woolard, J. Molecular Pharmacology of VEGF-A Isoforms: Binding and Signalling at VEGFR2. Int. J. Mol. Sci. 2018, 19, 1264. [Google Scholar] [CrossRef] [PubMed]
- Porta, M.; Striglia, E. Intravitreal anti-VEGF agents and cardiovascular risk. Intern. Emerg. Med. 2020, 15, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Correa, S.; Grosskopf, A.K.; Lopez Hernandez, H.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef]
- Hesse, E.; Hefferan, T.E.; Tarara, J.E.; Haasper, C.; Meller, R.; Krettek, C.; Lu, L.; Yaszemski, M.J. Collagen type I hydrogel allows migration, proliferation, and osteogenic differentiation of rat bone marrow stromal cells. J. Biomed. Mater. Res. A 2010, 94, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Krajnak, T.; Cerna, E.; Suranova, M.; Samoril, T.; Zicha, D.; Vojtova, L.; Cechal, J. Replica-mold nanopatterned PHEMA hydrogel surfaces for ophthalmic applications. Sci. Rep. 2022, 12, 14497. [Google Scholar] [CrossRef]
- Tomar, N.; Tomar, M.; Gulati, N.; Nagaich, U. pHEMA hydrogels: Devices for ocular drug delivery. Int. J. Health Allied Sci. 2012, 1, 224. [Google Scholar] [CrossRef]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef]
- Sharifi, S.; Sharifi, H.; Akbari, A.; Chodosh, J. Systematic optimization of visible light-induced crosslinking conditions of gelatin methacryloyl (GelMA). Sci. Rep. 2021, 11, 23276. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.; Selvanathan, V.; Sonsudin, F.; Abouloula, C. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef]
- Ilochonwu, B.C.; van der Lugt, S.A.; Annala, A.; Di Marco, G.; Sampon, T.; Siepmann, J.; Siepmann, F.; Hennink, W.E.; Vermonden, T. Thermo-responsive Diels-Alder stabilized hydrogels for ocular drug delivery of a corticosteroid and an anti-VEGF fab fragment. J. Control. Release 2023, 361, 334–349. [Google Scholar] [CrossRef]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Xing, Y.; Zeng, B.; Yang, W. Light responsive hydrogels for controlled drug delivery. Front. Bioeng. Biotechnol. 2022, 10, 414. [Google Scholar] [CrossRef]
- Yang, R.; Jin, W.; Huang, C.; Liu, Y. Azobenzene Based Photo-Responsive Hydrogel: Synthesis, Self-Assembly, and Antimicrobial Activity. Gels 2022, 8, 414. [Google Scholar] [CrossRef]
- Liang, J.; Liu, B. ROS-responsive drug delivery systems. Bioeng. Transl. Med. 2016, 1, 239–251. [Google Scholar] [CrossRef]
- Rinaldi, A.; Caraffi, R.; Grazioli, M.V.; Oddone, N.; Giardino, L.; Tosi, G.; Vandelli, M.A.; Calza, L.; Ruozi, B.; Duskey, J.T. Applications of the ROS-Responsive Thioketal Linker for the Production of Smart Nanomedicines. Polymers 2022, 14, 687. [Google Scholar] [CrossRef]
- Knipe, J.M.; Peppas, N.A. Multi-responsive hydrogels for drug delivery and tissue engineering applications. Regen. Biomater. 2014, 1, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.J.; Gulfam, M.; Jo, S.H.; Gal, Y.S.; Oh, C.W.; Park, S.H.; Lim, K.T. Multi-stimuli responsive hydrogels derived from hyaluronic acid for cancer therapy application. Carbohydr. Polym. 2022, 286, 119303. [Google Scholar] [CrossRef] [PubMed]
- Dravid, A.; Raos, B.; Aqrawe, Z.; Parittotokkaporn, S.; O’Carroll, S.J.; Svirskis, D. A Macroscopic Diffusion-Based Gradient Generator to Establish Concentration Gradients of Soluble Molecules Within Hydrogel Scaffolds for Cell Culture. Front. Chem. 2019, 7, 638. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tawakol, A.P.; Rudeen, K.M.; Mieler, W.F.; Kang-Mieler, J.J. Treatment Efficacy and Biocompatibility of a Biodegradable Aflibercept-Loaded Microsphere-Hydrogel Drug Delivery System. Transl. Vis. Sci. Technol. 2020, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Bae, Y.H.; Okano, T. Hydrogels: Swelling, drug loading, and release. Pharm. Res. 1992, 9, 283–290. [Google Scholar] [CrossRef]
- Sheth, S.; Barnard, E.; Hyatt, B.; Rathinam, M.; Zustiak, S.P. Predicting Drug Release From Degradable Hydrogels Using Fluorescence Correlation Spectroscopy and Mathematical Modeling. Front. Bioeng. Biotechnol. 2019, 7, 410. [Google Scholar] [CrossRef]
- Rudeen, K.M.; Liu, W.; Mieler, W.F.; Kang-Mieler, J.J. Simultaneous Release of Aflibercept and Dexamethasone from an Ocular Drug Delivery System. Curr. Eye Res. 2022, 47, 1034–1042. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, Y.; Shen, J.; Cai, Z.; Zhao, C.; Chen, H.; Luo, X.; Hu, N.; Cui, W.; Huang, W. Injectable hydrogel microspheres with self-renewable hydration layers alleviate osteoarthritis. Sci. Adv. 2022, 8, eabl6449. [Google Scholar] [CrossRef]
- Werzer, O.; Tumphart, S.; Keimel, R.; Christian, P.; Coclite, A.M. Drug release from thin films encapsulated by a temperature-responsive hydrogel. Soft Matter 2019, 15, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-sensitive hydrogels for delivering biotherapeutic molecules: A review. Saudi Pharm. J. 2019, 27, 990–999. [Google Scholar] [CrossRef]
- Ilochonwu, B.C.; Urtti, A.; Hennink, W.E.; Vermonden, T. Intravitreal hydrogels for sustained release of therapeutic proteins. J. Control. Release 2020, 326, 419–441. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Rimpela, A.K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef]
- Dludla, S.B.K.; Mashabela, L.T.; Ng’andwe, B.; Makoni, P.A.; Witika, B.A. Current Advances in Nano-Based and Polymeric Stimuli-Responsive Drug Delivery Targeting the Ocular Microenvironment: A Review and Envisaged Future Perspectives. Polymers 2022, 14, 3580. [Google Scholar] [CrossRef]
- Srividya, B.; Cardoza, R.M.; Amin, P.D. Sustained ophthalmic delivery of ofloxacin from a pH triggered in situ gelling system. J. Control Release 2001, 73, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Abraham, B.L.; Toriki, E.S.; Tucker, N.J.; Nilsson, B.L. Electrostatic interactions regulate the release of small molecules from supramolecular hydrogels. J. Mater. Chem. B 2020, 8, 6366–6377. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hong, H.K.; Song, J.S.; Im Jeong, S.; Chung, J.Y.; Woo, S.J.; Park, K.D. Intravitreal injectable hydrogel rods with long-acting bevacizumab delivery to the retina. Acta Biomater. 2023, 171, 273–288. [Google Scholar] [CrossRef]
- Jung, J.H.; Kim, S.S.; Chung, H.; Hejri, A.; Prausnitz, M.R. Six-month sustained delivery of anti-VEGF from in-situ forming hydrogel in the suprachoroidal space. J. Control. Release 2022, 352, 472–484. [Google Scholar] [CrossRef]
- Gao, H.; Chen, M.; Liu, Y.; Zhang, D.; Shen, J.; Ni, N.; Tang, Z.; Ju, Y.; Dai, X.; Zhuang, A.; et al. Injectable Anti-Inflammatory Supramolecular Nanofiber Hydrogel to Promote Anti-VEGF Therapy in Age-Related Macular Degeneration Treatment. Adv. Mater. 2023, 35, e2204994. [Google Scholar] [CrossRef]
- Duan, N.; Mei, L.; Hu, L.; Yin, X.; Wei, X.; Li, Y.; Li, Q.; Zhao, G.; Zhou, Q.; Du, Z. Biomimetic, Injectable, and Self-Healing Hydrogels with Sustained Release of Ranibizumab to Treat Retinal Neovascularization. ACS Appl. Mater. Interfaces 2023, 15, 6371–6384. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.W.; Wei, R.L.; Cai, J.P.; Xi, G.L.; Zhu, H.; Li, Y.; Ma, X.Y. Efficacy of different intraocular lens materials and optic edge designs in preventing posterior capsular opacification: A meta-analysis. Am. J. Ophthalmol. 2007, 143, 428–436. [Google Scholar] [CrossRef]
- Kim, J.; Dunn, A.C. Soft hydrated sliding interfaces as complex fluids. Soft Matter 2016, 12, 6536–6546. [Google Scholar] [CrossRef] [PubMed]
- Kocen, R.; Gasik, M.; Gantar, A.; Novak, S. Viscoelastic behaviour of hydrogel-based composites for tissue engineering under mechanical load. Biomed. Mater. 2017, 12, 025004. [Google Scholar] [CrossRef]
- García-Quintanilla, L.; Luaces-Rodríguez, A.; Gil-Martínez, M.; Mondelo-García, C.; Maroñas, O.; Mangas-Sanjuan, V.; González-Barcia, M.; Zarra-Ferro, I.; Aguiar, P.; Otero-Espinar, F.J.; et al. Pharmacokinetics of Intravitreal Anti-VEGF Drugs in Age-Related Macular Degeneration. Pharmaceutics 2019, 11, 365. [Google Scholar] [CrossRef]
- Sharma, D.; Zachary, I.; Jia, H. Mechanisms of Acquired Resistance to Anti-VEGF Therapy for Neovascular Eye Diseases. Investig. Ophthalmol. Vis. Sci. 2023, 64, 28. [Google Scholar] [CrossRef]
- Tan, C.S.; Ngo, W.K.; Chay, I.W.; Ting, D.S.; Sadda, S.R. Neovascular Age-Related Macular Degeneration (nAMD): A Review of Emerging Treatment Options. Clin. Ophthalmol. 2022, 16, 917–933. [Google Scholar] [CrossRef] [PubMed]
- Nicolo, M.; Ferro Desideri, L.; Vagge, A.; Traverso, C.E. Faricimab: An investigational agent targeting the Tie-2/angiopoietin pathway and VEGF-A for the treatment of retinal diseases. Expert. Opin. Investig. Drugs 2021, 30, 193–200. [Google Scholar] [CrossRef]
- Liu, L.C.; Chen, Y.H.; Lu, D.W. Overview of Recent Advances in Nano-Based Ocular Drug Delivery. Int. J. Mol. Sci. 2023, 24, 15352. [Google Scholar] [CrossRef]
- Paliwal, H.; Prajapati, B.G.; Srichana, T.; Singh, S.; Patel, R.J. Novel Approaches in the Drug Development and Delivery Systems for Age-Related Macular Degeneration. Life 2023, 13, 568. [Google Scholar] [CrossRef]
- Arnold, J.J.; Campain, A.; Barthelmes, D.; Simpson, J.M.; Guymer, R.H.; Hunyor, A.P.; McAllister, I.L.; Essex, R.W.; Morlet, N.; Gillies, M.C.; et al. Two-Year Outcomes of “Treat and Extend” Intravitreal Therapy for Neovascular Age-Related Macular Degeneration. Ophthalmology 2015, 122, 1212–1219. [Google Scholar] [CrossRef]
- Yaylaci, S.; Dinc, E.; Aydin, B.; Tekinay, A.B.; Guler, M.O. Peptide Nanofiber System for Sustained Delivery of Anti-VEGF Proteins to the Eye Vitreous. Pharmaceutics 2023, 15, 1264. [Google Scholar] [CrossRef]
- Murakami, T.; Hoshi, S.; Okamoto, F.; Sakai, T.; Katashima, T.; Naito, M.; Oshika, T. Analysis of the sustained release ability of bevacizumab-loaded tetra-PEG gel. Exp. Eye Res. 2022, 223, 109206. [Google Scholar] [CrossRef] [PubMed]
- Osswald, C.R.; Guthrie, M.J.; Avila, A.; Valio, J.A., Jr.; Mieler, W.F.; Kang-Mieler, J.J. In Vivo Efficacy of an Injectable Microsphere-Hydrogel Ocular Drug Delivery System. Curr. Eye Res. 2017, 42, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Borrell, M.A.; Venerus, D.C.; Mieler, W.F.; Kang-Mieler, J.J. Characterization of Biodegradable Microsphere-Hydrogel Ocular Drug Delivery System for Controlled and Extended Release of Ranibizumab. Transl. Vis. Sci. Technol. 2019, 8, 12. [Google Scholar] [CrossRef]
- Chang, Y.H.; Hsing, C.H.; Chiu, C.J.; Wu, Y.R.; Hsu, S.M.; Hsu, Y.H. Protective role of IL-17-producing gammadelta T cells in a laser-induced choroidal neovascularization mouse model. J. Neuroinflamm. 2023, 20, 279. [Google Scholar] [CrossRef]
- Chen, Y.; Bounds, S.E.; Ma, X.; Karmoker, J.R.; Liu, Y.; Ma, J.X.; Cai, J. Interleukin-17-mediated protective cytokine signaling against degeneration of the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 2023, 120, e2311647120. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Zou, J.; Yoshida, S.; Jiang, B.; Zhou, Y. The Role of Inflammation in Age-Related Macular Degeneration. Int. J. Biol. Sci. 2020, 16, 2989–3001. [Google Scholar] [CrossRef]
- Won, J.Y.; Kim, J.; Gao, G.; Kim, J.; Jang, J.; Park, Y.H.; Cho, D.W. 3D printing of drug-loaded multi-shell rods for local delivery of bevacizumab and dexamethasone: A synergetic therapy for retinal vascular diseases. Acta Biomater. 2020, 116, 174–185. [Google Scholar] [CrossRef]
- Kass, L.E.; Nguyen, J. Nanocarrier-hydrogel composite delivery systems for precision drug release. WIREs Nanomed. Nanobiotechnol. 2021, 14, e1756. [Google Scholar] [CrossRef]
- Bento, C.S.; Gaspar, M.C.; Coimbra, P.; de Sousa, H.C.; Braga, M.E. A review of conventional and emerging technologies for hydrogels sterilization. Int. J. Pharm. 2023, 634, 122671. [Google Scholar] [CrossRef]
- Galante, R.; Pinto, T.J.A.; Colaço, R.; Serro, A.P. Sterilization of hydrogels for biomedical applications: A review. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2017, 106, 2472–2492. [Google Scholar] [CrossRef] [PubMed]
- Ocular Therapeutix, Inc. CLN-0046: Treatment of AMD Subjects with OTX-TKI. Available online: http://clinicaltrials.gov/ct2/show/NCT03630315 (accessed on 29 December 2023).
- Wong, J.G.; Chang, A.; Guymer, R.H.; Wickremasinghe, S.; Reilly, E.; Bell, N.; Vantipalli, S.; Moshfeghi, A.A.; Goldstein, M.H. Phase 1 Study of an Intravitreal Axitinib Hydrogel-Based Implant for the Treatment of Neovascular Age-Related Macular Degeneration (nAMD); 2021. Available online: https://ocutx.gcs-web.com/static-files/3c59d3b0-9995-4e63-a972-e233a7550b1b (accessed on 29 December 2023).
- Ocular Therapeutix, Inc. Study Evaluating the Treatment of OTX-TKI for Subjects with Neovascular Age-Related Macular Degeneration. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04989699 (accessed on 29 December 2023).
- Robert, L.; Avery, M.D. OTX-TKI, Sustained-Release Axitinib Hydrogel Implant, for Neovascular Age-Related Macular Degeneration. 2023. Available online: https://investors.ocutx.com/static-files/00a24bfa-e147-49fc-86cf-9135b9e4f37f (accessed on 29 December 2023).
- Nazari, H.; Zhang, L.; Zhu, D.H.; Chader, G.J.; Falabella, P.; Stefanini, F.; Rowland, T.; Clegg, D.O.; Kashani, A.H.; Hinton, D.R.; et al. Stem cell based therapies for age-related macular degeneration: The promises and the challenges. Prog. Retin. Eye Res. 2015, 48, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Rim, M.A.; Choi, J.H.; Park, A.; Youn, J.; Lee, S.; Kim, N.E.; Song, J.E.; Khang, G. Characterization of Gelatin/Gellan Gum/Glycol Chitosan Ternary Hydrogel for Retinal Pigment Epithelial Tissue Reconstruction Materials. ACS Appl. Bio Mater. 2020, 3, 6079–6087. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.; Choi, J.H.; Lee, S.; Lee, W.; Lee, S.W.; Kim, W.; Song, Y.; Tumursukh, N.E.; Song, J.E.; Khang, G. Fabrication and Evaluation of Gellan Gum/Hyaluronic Acid Hydrogel for Retinal Tissue Engineering Biomaterial and the Influence of Substrate Stress Relaxation on Retinal Pigment Epithelial Cells. Molecules 2022, 27, 5512. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.Y.; Park, J.H.; Shin, M.E.; Song, J.E.; Thangavelu, M.; Carlomagno, C.; Motta, A.; Migliaresi, C.; Khang, G. Injectable taurine-loaded alginate hydrogels for retinal pigment epithelium (RPE) regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109787. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Choi, J.H.; Lee, J.; Youn, J.; Kim, W.; Jeon, G.; Lee, S.W.; Song, J.E.; Khang, G. Dopamine-Functionalized Gellan Gum Hydrogel as a Candidate Biomaterial for a Retinal Pigment Epithelium Cell Delivery System. ACS Appl. Bio Mater. 2021, 4, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, J.K.; Manzar, Z.; Bachman, L.A.; Andrews-Pfannkoch, C.; Knudsen, T.; Hill, M.; Schmidt, H.; Iezzi, R.; Pulido, J.S.; Marmorstein, A.D. Fibrin hydrogels as a xenofree and rapidly degradable support for transplantation of retinal pigment epithelium monolayers. Acta Biomater. 2018, 67, 134–146. [Google Scholar] [CrossRef]
- Gandhi, J.K.; Mano, F.; Iezzi, R.; LoBue, S.A.; Holman, B.H.; Fautsch, M.P.; Olsen, T.W.; Pulido, J.S.; Marmorstein, A.D. Fibrin hydrogels are safe, degradable scaffolds for sub-retinal implantation. PLoS ONE 2020, 15, e0227641. [Google Scholar] [CrossRef]
- Wei, Y.; Alexandre, U.; Ma, X. Hydrogels to Support Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells. Brain Sci. 2022, 12, 1620. [Google Scholar] [CrossRef]
- Park, J.H.; Shin, E.Y.; Shin, M.E.; Choi, M.J.; Carlomagno, C.; Song, J.E.; Khang, G. Enhanced retinal pigment epithelium (RPE) regeneration using curcumin/alginate hydrogels: In vitro evaluation. Int. J. Biol. Macromol. 2018, 117, 546–552. [Google Scholar] [CrossRef]
- Mitrousis, N.; Hacibekiroglu, S.; Ho, M.T.; Sauve, Y.; Nagy, A.; van der Kooy, D.; Shoichet, M.S. Hydrogel-mediated co-transplantation of retinal pigmented epithelium and photoreceptors restores vision in an animal model of advanced retinal degeneration. Biomaterials 2020, 257, 120233. [Google Scholar] [CrossRef]
- Soroushzadeh, S.; Karamali, F.; Masaeli, E.; Atefi, A.; Nasr Esfahani, M.H. Scaffold free retinal pigment epithelium sheet engineering using modified alginate-RGD hydrogel. J. Biosci. Bioeng. 2022, 133, 579–586. [Google Scholar] [CrossRef]
- Hunt, N.C.; Hallam, D.; Karimi, A.; Mellough, C.B.; Chen, J.; Steel, D.H.W.; Lako, M. 3D culture of human pluripotent stem cells in RGD-alginate hydrogel improves retinal tissue development. Acta Biomater. 2017, 49, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, D.; Jeong, Y.W.; Choi, M.J.; Lee, G.W.; Thangavelu, M.; Song, J.E.; Khang, G. Engineering retinal pigment epithelial cells regeneration for transplantation in regenerative medicine using PEG/Gellan gum hydrogels. Int. J. Biol. Macromol. 2019, 130, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Baranov, P.; Aydin, A.; Abdelgawad, H.; Singh, D.; Niu, W.; Kurisawa, M.; Spector, M.; Young, M.J. In Situ Cross-linking Hydrogel as a Vehicle for Retinal Progenitor Cell Transplantation. Cell Transpl. 2019, 28, 596–606. [Google Scholar] [CrossRef]
- Jiang, F.; Tang, Z.; Zhang, Y.; Ju, Y.; Gao, H.; Sun, N.; Liu, F.; Gu, P.; Zhang, W. Enhanced proliferation and differentiation of retinal progenitor cells through a self-healing injectable hydrogel. Biomater. Sci. 2019, 7, 2335–2347. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Jiang, F.; Zhang, Y.; Zhang, Y.; Yang, Y.; Huang, X.; Wang, Y.; Zhang, D.; Ni, N.; Liu, F.; et al. Mussel-inspired injectable hydrogel and its counterpart for actuating proliferation and neuronal differentiation of retinal progenitor cells. Biomaterials 2019, 194, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.; Mitrousis, N.; Shoichet, M.S. Hydrogel for Simultaneous Tunable Growth Factor Delivery and Enhanced Viability of Encapsulated Cells in Vitro. Biomacromolecules 2016, 17, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zheng, M.; Lester, K.L.; Han, Z. Light-induced Nrf2−/− mice as atrophic age-related macular degeneration model and treatment with nanoceria laden injectable hydrogel. Sci. Rep. 2019, 9, 14573. [Google Scholar] [CrossRef]
- Brzozowski, P.; Penev, K.I.; Mequanint, K. Gellan gum gel tissue phantoms and gel dosimeters with tunable electrical, mechanical and dosimetric properties. Int. J. Biol. Macromol. 2021, 180, 332–338. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Diniz, B.; Thomas, P.; Thomas, B.; Ribeiro, R.; Hu, Y.T.; Brant, R.; Ahuja, A.; Zhu, D.H.; Liu, L.; Koss, M.; et al. Subretinal Implantation of Retinal Pigment Epithelial Cells Derived from Human Embryonic Stem Cells: Improved Survival When Implanted as a Monolayer. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5087–5096. [Google Scholar] [CrossRef] [PubMed]
- Stanzel, B.V.; Liu, Z.P.; Somboonthanakij, S.; Wongsawad, W.; Brinken, R.; Eter, N.; Corneo, B.; Holz, F.G.; Temple, S.; Stern, J.H.; et al. Human RPE Stem Cells Grown into Polarized RPE Monolayers on a Polyester Matrix Are Maintained after Grafting into Rabbit Subretinal Space. Stem Cell Rep. 2014, 2, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ayuso, D.; Di Pierdomenico, J.; Martinez-Vacas, A.; Vidal-Sanz, M.; Picaud, S.; Villegas-Perez, M.P. Taurine: A promising nutraceutic in the prevention of retinal degeneration. Neural Regen. Res. 2024, 19, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Cronin, T.; Chung, D.C.; Yang, Y.; Nandrot, E.F.; Bennett, J. The Signalling Role of the avbeta5-Integrin Can Impact the Efficacy of AAV in Retinal Gene Therapy. Pharmaceuticals 2012, 5, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.W.; Kondo, M.; Terasaki, H.; Lin, Y.; McCall, M.; Marc, R.E. Retinal remodeling. Jpn. J. Ophthalmol. 2012, 56, 289–306. [Google Scholar] [CrossRef]
- García-Ayuso, D.; Di Pierdomenico, J.; Vidal-Sanz, M.; Villegas-Pérez, M.P. Retinal Ganglion Cell Death as a Late Remodeling Effect of Photoreceptor Degeneration. Int. J. Mol. Sci. 2019, 20, 4649. [Google Scholar] [CrossRef]
| Drug | Date of FDA Approval | Type of Molecule | Molecular Weight (kDa) | Standard Dose |
|---|---|---|---|---|
| Ranibizumab (LucentisTM) | 2006 | Humanized monoclonal antibody fragment inhibitor of all isoforms of VEGF-A | 48 | 0.5 mg/50 μL |
| Aflibercept (EyleaTM) | 2011 | Recombinant fusion protein of VEGF receptors 1 and 2 | 115 | 2 mg/50 μL |
| Brolucizumab (BeovuTM) | 2019 | Humanized single-chain antibody fragment inhibitor of all isoforms of VEGF-A | 26 | 1.0 or 6.0 mg/eye |
| Bevacizumab (AvastinTM) | off-label | Humanized monoclonal antibody that targets all isoforms of VEGF-A | 149 | 1.25 mg/50 μL |
| Faricimab (VabysmoTM) | 2022 | Humanized bispecific immunoglobulin G monoclonal antibody inhibitor of VEGF-A and Ang-2 | 150 | 6 mg/50 μL |
| Type of Hydrogel | Bioactive Agents | Route | Experimental Models | Sustained Release | Bioactivity Assay | Biocompatibility | Ref. |
|---|---|---|---|---|---|---|---|
| Poly (ethylene glycol)-co-(L-lactic-acid diacrylate/N-isopropylacrylamide (PEG-PLLA-DA/NIPAAm) hydrogel | Aflibercept and dexamethasone | In vitro | In vitro | 224 days | ELISA and cell proliferation assay | - | [40] |
| Super-molecular nanofiber hydrogel | Betamethasone phosphate and an unspecified anti-VEGF drug | Intravitreal injection | Rats | At least 4 weeks | CCK-8, Transwell, EdU, and live/dead assays | Proven on histological examination | [51] |
| Hyaluronic acid (HA) /poly (ethylene glycol) diacrylate hydrogel | Bevacizumab | Suprachoroidal administration | Rabbits | At least 6 months | ELISA assay | Proven on histological examination | [50] |
| Tetra-armed polyethylene glycol (tetra-PEG) hydrogel | Bevacizumab | In vitro | In vitro | At least 2 weeks | ELISA, HPLC, and VEGF bioassay kit assay | - | [64] |
| Gelatin/PEG/tyramine (GPT) hydrogel | Bevacizumab | Intravitreal injection | Rabbits | At least 4 months | Cell proliferation assay | Proven on rabbit TNF-α DuoSet ELISA kit assay | [49] |
| Hyaluronic acid (HA)/pluronic 127 hydrogel | Ranibizumab | Intravitreal injection | Rabbits | At least 7 weeks | CCK-8 assay | Proven on histological examination | [52] |
| Peptide amphiphile hydrogel | Ranibizumab | Intravitreal injection | Rabbits | At least 7 days | MTT assay | - | [63] |
| Poly (lactic-co-glycolic acid) (PLGA) microsphere within PEG-PLLA-DA/NIPAAm hydrogel | Aflibercept | Intravitreal injection | Rats | At least 6 months | H&E staining assay | Proven on histological examination | [36] |
| Type of Hydrogel | Retinal Cell Type | Cellular Compatibility | Experimental Models | Biocompatibility | Ref. |
|---|---|---|---|---|---|
| Gellan gum/hyaluronic acid hydrogels | ARPE-19 | Live/dead assay | In vitro | In vitro | [80] |
| Taurine-loaded alginate hydrogels | Rabbit RPE | Live/dead assay and MTT | Nude mice | Immunohistochemistry analysis | [81] |
| Dopamine-functionalized gellan gum hydrogels | ARPE-19 | Live/dead staining and gene expression analysis | In vitro | In vitro | [82] |
| Fibrin hydrogels | iPSC-RPE cells | Live/dead assay and gene expression analysis | In vitro | In vitro | [83] |
| Fibrin hydrogels | Non-cells | - | Female pigs | H&E staining | [84] |
| Fibrin hydrogels | ARPE-19 and hESC-RPE cells | Live/dead assay | C57BL/6J mice | H&E staining | [85] |
| Curcumin/alginate hydrogels | Rabbit RPE | Live/dead assay, MTT analysis, and gene expression analysis | In vitro | In vitro | [86] |
| Hyaluronic acid/methylcellulose hydrogels | Rod photoreceptor and ARPE-19 | - | C57Bl/6J mice | Immunohistochemistry analysis | [87] |
| Arg-Gly-Asp/alginate hydrogels | hESC-RPE cells | - | RCS rats | H&E staining | [88] |
| Arg-Gly-Asp/alginate hydrogels | hESCs/hiPSCs embryoid bodies | Gene expression analysis | In vitro | In vitro | [89] |
| Polyethylene glycol/gellan gum hydrogels | ARPE-19 | MTT and gene expression analysis | In vitro | In vitro | [90] |
| Gelatin/gellan gum/glycol chitosan ternary hydrogels | ARPE-19 | Live/dead assay, MTT analysis, and gene expression analysis | In vitro | In vitro | [79] |
| Gelatin/hydroxyphenyl propionic acid hydrogels | Retinal progenitor cells (RPCs) | EthD-1 staining | Female Long Evans rats | Immunohistochemistry analysis | [91] |
| Chitosan hydrochloride/oxidized dextran hydrogels | Retinal progenitor cells (RPCs) | Live/dead assay and CCK-8 analysis | In vitro | In vitro | [92] |
| Gelatin/hyaluronic/polydopamine hydrogels | Retinal progenitor cells (RPCs) | Live/dead assay and inflammatory and apoptotic factor expression levels | Nude mice | H&E staining | [93] |
| IGF-1 loaded Src homology3-binding peptides hyaluronan/methylcellulose (HAMC) hydrogels | hESCs | Immunostaining | In vitro | In vitro | [94] |
| Glycol chitosan coated cerium oxide nanoparticles with alginate/gelatin hydrogels | ARPE-19 | Gene expression analysis | Light-induced Nrf2−/− mice | H&E staining | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wang, J.; Wu, H.; Fan, W.; Li, S.; Wei, D.; Song, Z.; Tao, Y. Hydrogel-Based Therapy for Age-Related Macular Degeneration: Current Innovations, Impediments, and Future Perspectives. Gels 2024, 10, 158. https://doi.org/10.3390/gels10030158
Zhang C, Wang J, Wu H, Fan W, Li S, Wei D, Song Z, Tao Y. Hydrogel-Based Therapy for Age-Related Macular Degeneration: Current Innovations, Impediments, and Future Perspectives. Gels. 2024; 10(3):158. https://doi.org/10.3390/gels10030158
Chicago/Turabian StyleZhang, Chengzhi, Jiale Wang, Hao Wu, Wenhui Fan, Siyu Li, Dong Wei, Zongming Song, and Ye Tao. 2024. "Hydrogel-Based Therapy for Age-Related Macular Degeneration: Current Innovations, Impediments, and Future Perspectives" Gels 10, no. 3: 158. https://doi.org/10.3390/gels10030158






