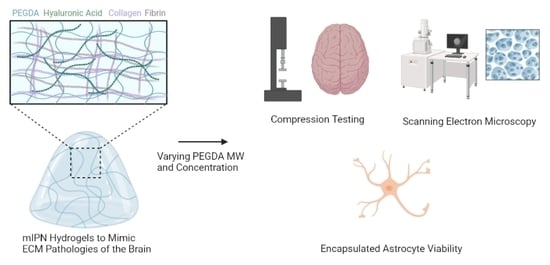
Fabrication and Characterization of Quad-Component Bioinspired Hydrogels to Model Elevated Fibrin Levels in Central Nervous Tissue Scaffolds
Abstract
1. Introduction
2. Results and Discussion
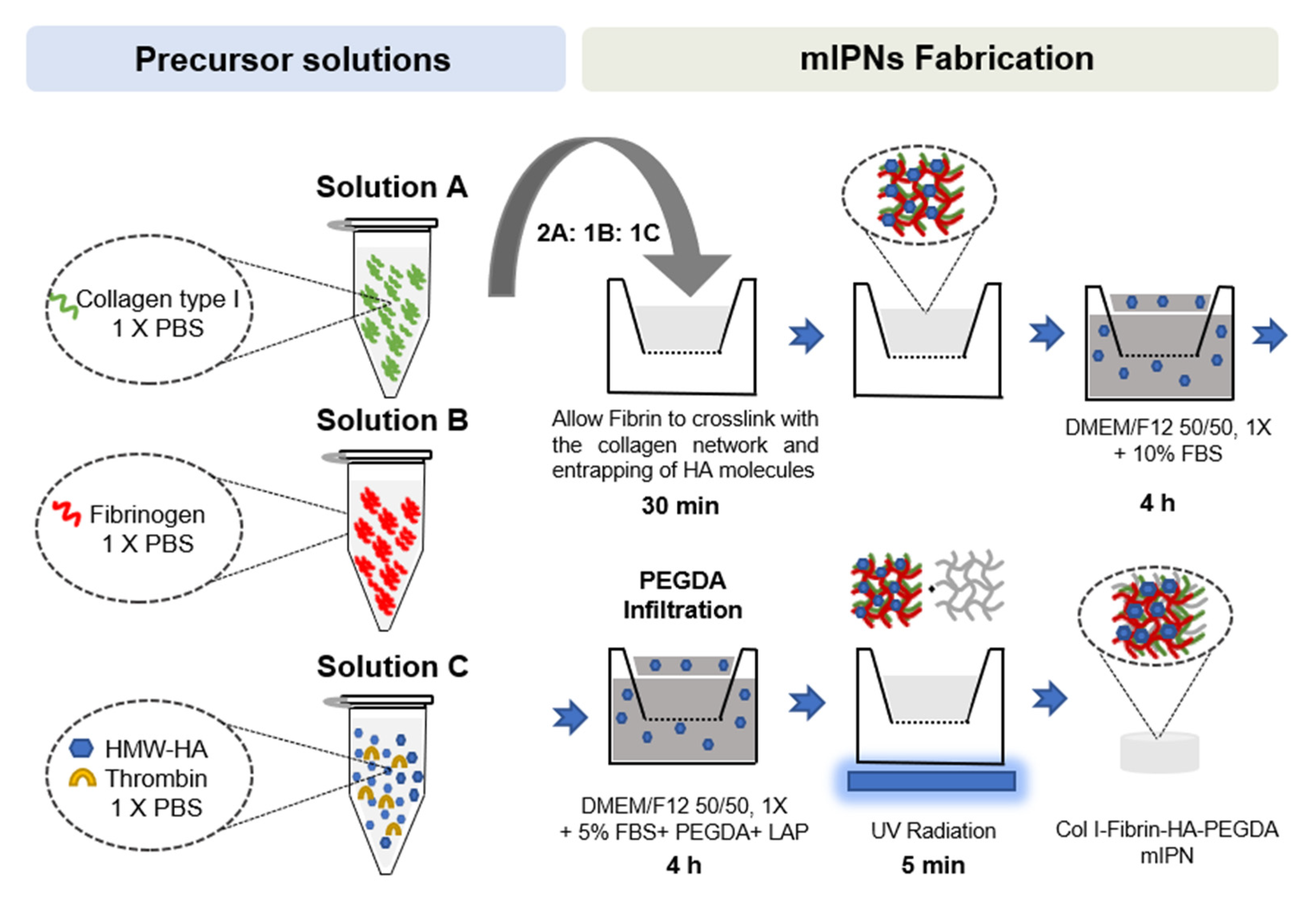
2.1. Fabrication of mIPNs
2.1.1. Tuning Thrombin-Fibrinogen Ratio
2.1.2. Incorporating Varying Fibrin Levels on Hydrogel Mechanical Performance
2.1.3. Tailoring the Mechanical Properties of mIPNs
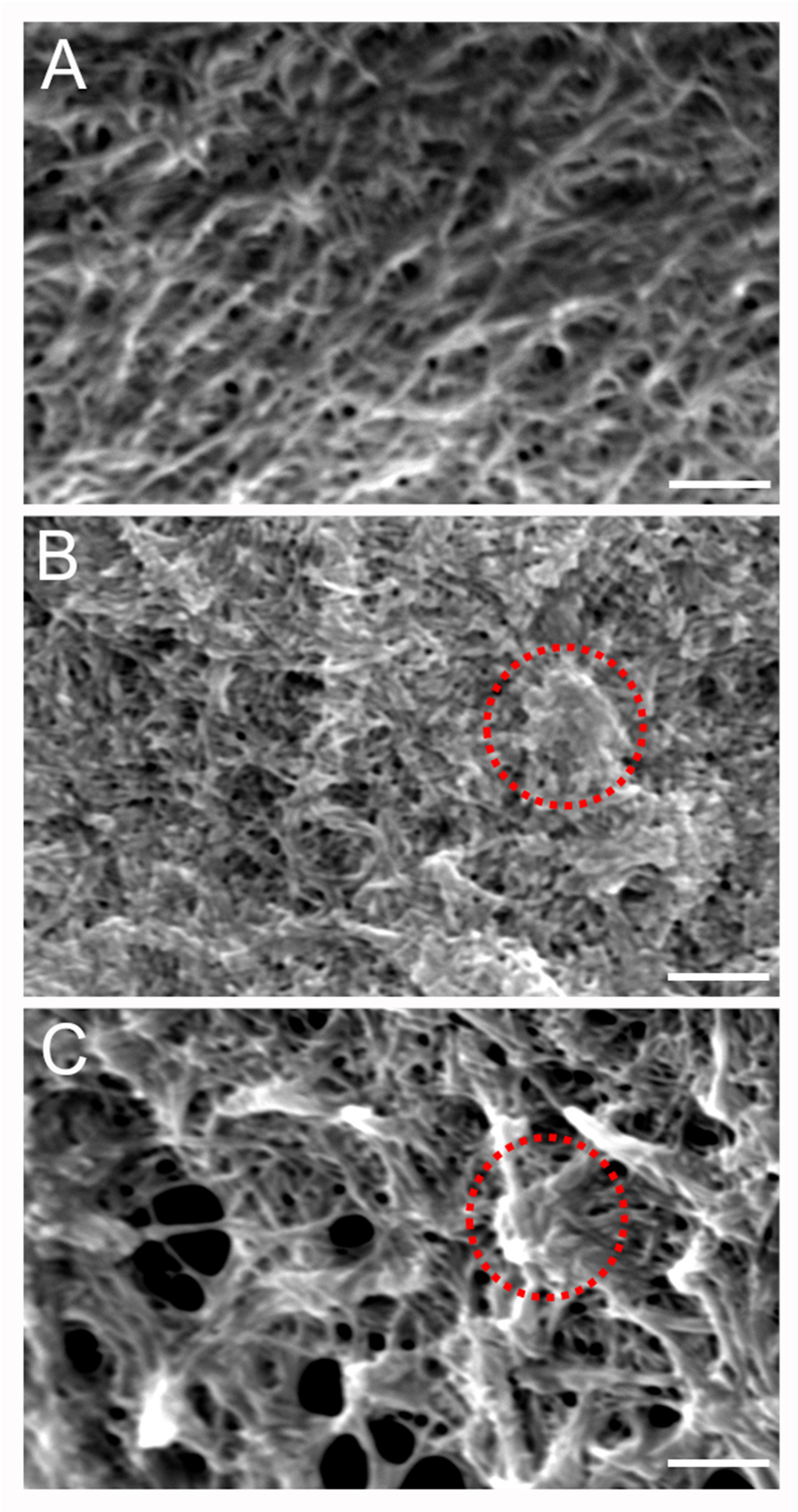
2.2. Microarchitecture of mIPNs
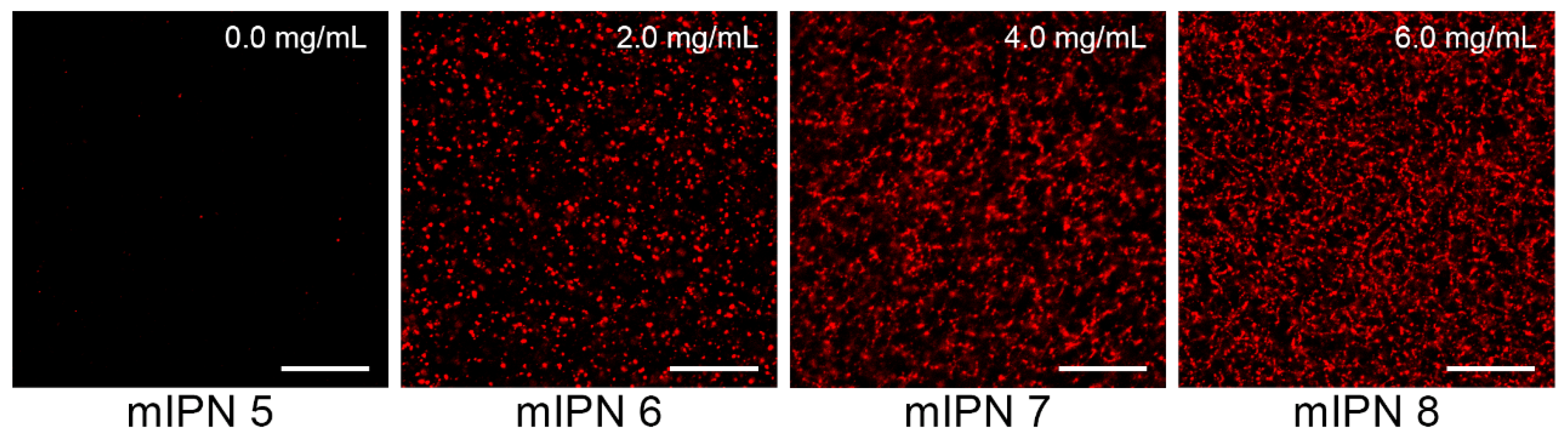
2.3. Cell Viability
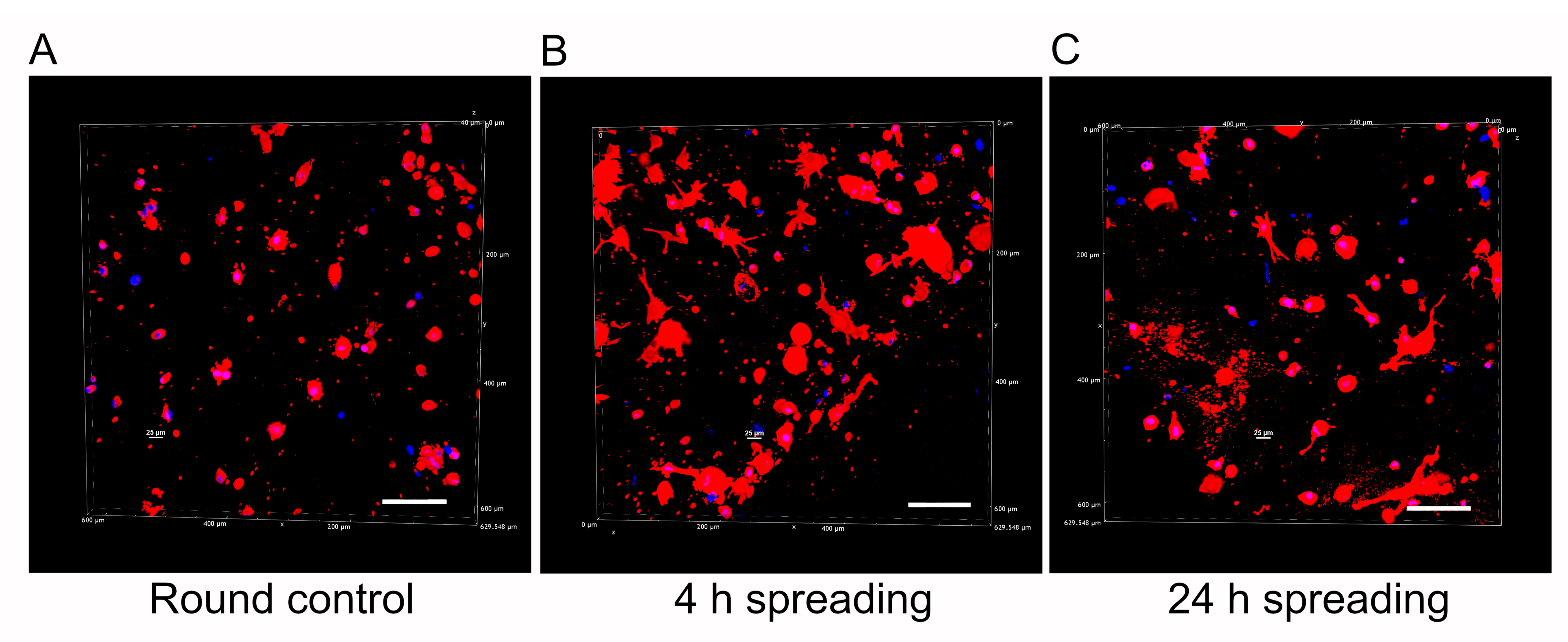
2.4. Qualitative Assessment of Cell Morphology
3. Conclusions
4. Materials and Methods
4.1. mIPNs Fabrication
- Solution A: collagen type I at a final concentration of 6.0 mg/mL in 1× phosphate-buffered saline (PBS) was obtained after the neutralization of collagen type I from rat tail at a concentration of 9.5 mg/mL (Corning, Bedford, MA, USA), using 1N NaOH (Amresco, Solon, OH, USA) and 10× PBS (Lonza, Walkersville, MD, USA). This solution was stored on ice until use.
- Solution B: fibrinogen (FG, Alfa Aesar, Ward Hill, MA, USA) at 4× the target fibrin levels in 1× PBS. This solution was prepared fresh at room temperature until the complete dissolution of FG, then sterile-filtered and stored on ice until use.
- Solution C: high molecular weight HA (HMW-HA; 1010 kDa–1800 kDa, Lifecore Biomedical, Chaska, MN, USA) at 4× the target concentration and appropriate thrombin levels to achieve 0.2 U of thrombin/mg of FG in 1× PBS. HMW-HA was dissolved at 4 °C for 12 h. The solution was sterile-filtered, and thrombin was added. Once thrombin was added, the solution was stored on ice until use.
4.2. Cell Culture and Encapsulation in mIPNs
4.3. Characterization of mIPN Mechanical Properties
4.4. Microarchitectural Characterization
4.5. Cell Viability Assessment
4.6. Cell Morphology Characterization
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González-Díaz, E.C.; Varghese, S. Hydrogels as extracellular matrix analogs. Gels 2016, 2, 20. [Google Scholar] [CrossRef]
- Vorwald, C.E.; Gonzalez-Fernandez, T.; Joshee, S.; Sikorski, P.; Leach, J.K. Tunable fibrin-alginate interpenetrating network hydrogels to support cell spreading and network formation. Acta Biomater. 2020, 108, 142–152. [Google Scholar] [CrossRef]
- Lin, W.; Kluzek, M.; Iuster, N.; Shimoni, E.; Kampf, N.; Goldberg, R.; Klein, J. Cartilage-inspired, lipid-based boundary-lubricated hydrogels. Science 2020, 370, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Soles, A.; Selimovic, A.; Sbrocco, K.; Ghannoum, F.; Hamel, K.; Moncada, E.L.; Gilliat, S.; Cvetanovic, M. Extracellular Matrix Regulation in Physiology and in Brain Disease. Int. J. Mol. Sci. 2023, 24, 7049. [Google Scholar] [CrossRef] [PubMed]
- Chelyshev, Y.A.; Kabdesh, I.M.; Mukhamedshina, Y.O. Extracellular matrix in neural plasticity and regeneration. Cell. Mol. Neurobiol. 2022, 42, 647–664. [Google Scholar] [CrossRef] [PubMed]
- George, N.; Geller, H.M. Extracellular matrix and traumatic brain injury. J. Neurosci. Res. 2018, 96, 573–588. [Google Scholar] [CrossRef]
- Hebisch, M.; Klostermeier, S.; Wolf, K.; Boccaccini, A.R.; Wolf, S.E.; Tanzi, R.E.; Kim, D.Y. The Impact of the Cellular Environment and Aging on Modeling Alzheimer’s Disease in 3D Cell Culture Models. Adv. Sci. 2023, 10, 2205037. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Popovich, P.G. Extracellular matrix regulation of inflammation in the healthy and injured spinal cord. Exp. Neurol. 2014, 258, 24–34. [Google Scholar] [CrossRef]
- Wu, F.; Liu, L.; Zhou, H. Endothelial cell activation in central nervous system inflammation. J. Leukoc. Biol. 2017, 101, 1119–1132. [Google Scholar] [CrossRef]
- Benarroch, E.E. Astrocyte signaling and synaptic homeostasis: I: Membrane channels, transporters, and receptors in astrocytes. Neurology 2016, 87, 324–330. [Google Scholar] [CrossRef]
- Placone, A.L.; McGuiggan, P.M.; Bergles, D.E.; Guerrero-Cazares, H.; Quiñones-Hinojosa, A.; Searson, P.C. Human astrocytes develop physiological morphology and remain quiescent in a novel 3D matrix. Biomaterials 2015, 42, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Michinaga, S.; Koyama, Y. Pathophysiological responses and roles of astrocytes in traumatic brain injury. Int. J. Mol. Sci. 2021, 22, 6418. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Balordi, F.; Su, N.; Chen, L.; Fishell, G.; Hébert, J.M. Astrocyte activation is suppressed in both normal and injured brain by FGF signaling. Proc. Natl. Acad. Sci. USA 2014, 111, E2987–E2995. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, D.V.; Astakhova, A.A.; Azbukina, N.V.; Goriainov, S.V.; Chistyakov, V.V.; Sergeeva, M.G. High and low molecular weight hyaluronic acid differentially influences oxylipins synthesis in course of neuroinflammation. Int. J. Mol. Sci. 2019, 20, 3894. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.; Strickland, S.; Melchor, J.P. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer’s disease. J. Exp. Med. 2007, 204, 1999–2008. [Google Scholar] [CrossRef] [PubMed]
- Lohmeier, J.; Silva, R.V.; Tietze, A.; Taupitz, M.; Kaneko, T.; Prüss, H.; Paul, F.; Infante-Duarte, C.; Hamm, B.; Caravan, P. Fibrin-targeting molecular MRI in inflammatory CNS disorders. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3692–3704. [Google Scholar] [CrossRef]
- Li, F.; Ducker, M.; Sun, B.; Szele, F.G.; Czernuszka, J.T. Interpenetrating polymer networks of collagen, hyaluronic acid, and chondroitin sulfate as scaffolds for brain tissue engineering. Acta Biomater. 2020, 112, 122–135. [Google Scholar] [CrossRef]
- Abbasi Aval, N.; Emadi, R.; Valiani, A.; Kharaziha, M.; Finne-Wistrand, A. An aligned fibrous and thermosensitive hyaluronic acid-puramatrix interpenetrating polymer network hydrogel with mechanical properties adjusted for neural tissue. J. Mater. Sci. 2022, 57, 2883–2896. [Google Scholar] [CrossRef]
- Hakim Khalili, M.; Zhang, R.; Wilson, S.; Goel, S.; Impey, S.A.; Aria, A.I. Additive Manufacturing and Physicomechanical Characteristics of PEGDA Hydrogels: Recent Advances and Perspective for Tissue Engineering. Polymers 2023, 15, 2341. [Google Scholar] [CrossRef]
- Munoz-Pinto, D.J.; Jimenez-Vergara, A.C.; Gharat, T.P.; Hahn, M.S. Characterization of sequential collagen-poly (ethylene glycol) diacrylate interpenetrating networks and initial assessment of their potential for vascular tissue engineering. Biomaterials 2015, 40, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Saleh, B.M.; Pourmostafa, A.; Patrawalla, N.Y.; Kishore, V. Xeno-Free Biomimetic ECM Model for Investigation of Matrix Composition and Stiffness on Astrocyte Cell Response. J. Funct. Biomater. 2023, 14, 256. [Google Scholar] [CrossRef]
- Van Drunen, R.; Jimenez-Vergara, A.C.; Tsai, E.H.; Tchen, R.; Cagle, T.; Agee, A.B.; Roberts, J.; Steele, J.M.; Munoz-Pinto, D.J. Collagen based multicomponent interpenetrating networks as promising scaffolds for 3D culture of human neural stem cells, human astrocytes, and human microglia. ACS Appl. Bio Mater. 2019, 2, 975–980. [Google Scholar] [CrossRef]
- Jimenez-Vergara, A.C.; Van Drunen, R.; Cagle, T.; Munoz-Pinto, D.J. Modeling the effects of hyaluronic acid degradation on the regulation of human astrocyte phenotype using multicomponent interpenetrating polymer networks (mIPNs). Sci. Rep. 2020, 10, 20734. [Google Scholar] [CrossRef]
- Petersen, M.A.; Ryu, J.K.; Akassoglou, K. Fibrinogen in neurological diseases: Mechanisms, imaging and therapeutics. Nat. Rev. Neurosci. 2018, 19, 283–301. [Google Scholar] [CrossRef]
- Crosby, C.O.; Stern, B.; Kalkunte, N.; Pedahzur, S.; Ramesh, S.; Zoldan, J. Interpenetrating polymer network hydrogels as bioactive scaffolds for tissue engineering. Rev. Chem. Eng. 2022, 38, 347–361. [Google Scholar] [CrossRef]
- Coradin, T.; Wang, K.; Law, T.; Trichet, L. Type I collagen-fibrin mixed hydrogels: Preparation, properties and biomedical applications. Gels 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Torres, J.E.; Hakim, M.; Babiak, P.M.; Pal, P.; Battistoni, C.M.; Nguyen, M.; Panitch, A.; Solorio, L.; Liu, J.C. Collagen-and hyaluronic acid-based hydrogels and their biomedical applications. Mater. Sci. Eng. R Rep. 2021, 146, 100641. [Google Scholar] [CrossRef]
- Montero, A.; Atienza, C.; Elvira, C.; Jorcano, J.L.; Velasco, D. Hyaluronic acid-fibrin hydrogels show improved mechanical stability in dermo-epidermal skin substitutes. Mater. Sci. Eng. C 2021, 128, 112352. [Google Scholar] [CrossRef]
- Bindi, B.; Perioli, A.; Melo, P.; Mattu, C.; Ferreira, A.M. Bioinspired Collagen/Hyaluronic Acid/Fibrin-Based Hydrogels for Soft Tissue Engineering: Design, Synthesis, and In Vitro Characterization. J. Funct. Biomater. 2023, 14, 495. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-H.; Lin, R.-Z.; Melero-Martin, J.M.; Chen, Y.-C. Comparison of covalently and physically cross-linked collagen hydrogels on mediating vascular network formation for engineering adipose tissue. Artif. Cells Nanomed. Biotechnol. 2018, 46, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Gansau, J.; Buckley, C.T. Incorporation of collagen and hyaluronic acid to enhance the bioactivity of fibrin-based hydrogels for nucleus pulposus regeneration. J. Funct. Biomater. 2018, 9, 43. [Google Scholar] [CrossRef]
- Vilar, R.; Fish, R.J.; Casini, A.; Neerman-Arbez, M. Fibrin (ogen) in human disease: Both friend and foe. Haematologica 2020, 105, 284. [Google Scholar] [CrossRef]
- Sulimai, N.; Lominadze, D. Fibrinogen and neuroinflammation during traumatic brain injury. Mol. Neurobiol. 2020, 57, 4692–4703. [Google Scholar] [CrossRef]
- Sjölin, K.; Kultima, K.; Larsson, A.; Freyhult, E.; Zjukovskaja, C.; Alkass, K.; Burman, J. Distribution of five clinically important neuroglial proteins in the human brain. Mol. Brain 2022, 15, 52. [Google Scholar] [CrossRef]
- Cortes-Canteli, M.; Mattei, L.; Richards, A.T.; Norris, E.H.; Strickland, S. Fibrin deposited in the Alzheimer’s disease brain promotes neuronal degeneration. Neurobiol. Aging 2015, 36, 608–617. [Google Scholar] [CrossRef]
- Rowe, S.L.; Lee, S.; Stegemann, J.P. Influence of thrombin concentration on the mechanical and morphological properties of cell-seeded fibrin hydrogels. Acta Biomater. 2007, 3, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Wachendörfer, M.; Buhl, E.M.; Messaoud, G.B.; Richtering, W.; Fischer, H. pH and Thrombin Concentration Are Decisive in Synthesizing Stiff, Stable, and Open-Porous Fibrin-Collagen Hydrogel Blends without Chemical Cross-Linker. Adv. Healthc. Mater. 2023, 12, 2203302. [Google Scholar] [CrossRef] [PubMed]
- Tomasch, J.; Maleiner, B.; Heher, P.; Rufin, M.; Andriotis, O.G.; Thurner, P.J.; Redl, H.; Fuchs, C.; Teuschl-Woller, A.H. Changes in elastic moduli of fibrin hydrogels within the myogenic range alter behavior of murine C2C12 and human C25 myoblasts differently. Front. Bioeng. Biotechnol. 2022, 10, 836520. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.; Wu, B.; Tawil, B. Modulation of 3D fibrin matrix stiffness by intrinsic fibrinogen–thrombin compositions and by extrinsic cellular activity. Tissue Eng. A 2009, 15, 1865–1876. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Zakusilo, F.T.; O’Banion, M.K.; Gelbard, H.A.; Seluanov, A.; Gorbunova, V. Matters of size: Roles of hyaluronan in CNS aging and disease. Ageing Res. Rev. 2021, 72, 101485. [Google Scholar] [CrossRef]
- Norris, E.H.; Strickland, S. Fibrinogen in the nervous system: Glia beware. Neuron 2017, 96, 951–953. [Google Scholar] [CrossRef]
- Lin, S.; Sangaj, N.; Razafiarison, T.; Zhang, C.; Varghese, S. Influence of physical properties of biomaterials on cellular behavior. Pharm. Res. 2011, 28, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Van Oss, C. Surface properties of fibrinogen and fibrin. J. Protein Chem. 1990, 9, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ma, L.; Zhou, J.; Mao, Z.; Gao, C.; Shen, J. Fabrication and physical and biological properties of fibrin gel derived from human plasma. Biomed. Mater. 2007, 3, 015001. [Google Scholar] [CrossRef] [PubMed]
- Seyedhassantehrani, N.; Li, Y.; Yao, L. Dynamic behaviors of astrocytes in chemically modified fibrin and collagen hydrogels. Integr. Biol. 2016, 8, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Gradišnik, L.; Bošnjak, R.; Maver, T.; Velnar, T. Advanced bio-based polymers for astrocyte cell models. Materials 2021, 14, 3664. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Vergara, A.C.; Lewis, J.; Hahn, M.S.; Munoz-Pinto, D.J. An improved correlation to predict molecular weight between crosslinks based on equilibrium degree of swelling of hydrogel networks. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1339–1348. [Google Scholar] [CrossRef]
- Clevenger, A.J.; Jimenez-Vergara, A.C.; Tsai, E.H.; de Barros Righes, G.; Díaz-Lasprilla, A.M.; Ramírez-Caballero, G.E.; Munoz-Pinto, D.J. Growth Factor Binding Peptides in Poly (Ethylene Glycol) Diacrylate (PEGDA)-Based Hydrogels for an Improved Healing Response of Human Dermal Fibroblasts. Gels 2022, 9, 28. [Google Scholar] [CrossRef]



| Fibrinogen [mg/mL] | Thrombin [U/mg fibrinogen] | T [°C] | Curing Time [min] | Hydrogel Formation |
|---|---|---|---|---|
| 6.0 | 1.0 | 25 | 0.5 1 | Successful |
| 6.0 | 0.4 | 25 | 1.0 1 | Successful |
| 6.0 | 0.2 | 37 | 30.0 | Successful |
| 6.0 | 0.1 | 37 | 30.0 | Unsuccessful |
| Formulation | HA | Fibrin | PEGDA | Complex Modulus | |
|---|---|---|---|---|---|
| [mg/mL] | [mg/mL] | [kDa] | [%w/w] | [kPa] | |
| AD Brain cortex | - | - | - | - | 7.35 ± 0.06 |
| mIPN 1 | 0.0 | 2.0 | 0.0 | 0.00 | ND |
| mIPN 2 | 0.0 | 4.0 | 0.0 | 0.00 | 4.78 ± 1.30 * |
| mIPN 3 | 0.0 | 6.0 | 0.0 | 0.00 | 6.96 ± 0.66 # |
| mIPN 4 | 0.0 | 8.0 | 0.0 | 0.00 | 7.34 ± 0.10 # |
| mIPN 5 | 0.0 | 0.0 | 10.0 | 6.25 | 7.26 ± 1.42 |
| mIPN 6 | 0.0 | 2.0 | 6.0 | 5.26 | 7.28 ± 1.44 |
| mIPN 7 | 0.0 | 4.0 | 6.0 | 5.26 | 7.73 ± 0.38 |
| mIPN 8 | 0.0 | 6.0 | 6.0 | 5.26 | 8.47 ± 1.59 |
| mIPN 9 | 2.0 | 0.0 | 3.4 | 5.75 | 7.23 ± 1.42 |
| mIPN 10 | 2.0 | 2.0 | 6.0 | 5.75 | 6.58 ± 0.53 |
| mIPN 11 | 2.0 | 4.0 | 6.0 | 5.75 | 6.34 ± 2.02 |
| mIPN 12 | 2.0 | 6.0 | 6.0 | 5.75 | 7.24 ± 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-Lasprilla, A.M.; McKee, M.; Jimenez-Vergara, A.C.; Ravi, S.; Bellamy, D.; Ortega, W.; Crosby, C.O.; Steele, J.; Plascencia-Villa, G.; Perry, G.; et al. Fabrication and Characterization of Quad-Component Bioinspired Hydrogels to Model Elevated Fibrin Levels in Central Nervous Tissue Scaffolds. Gels 2024, 10, 203. https://doi.org/10.3390/gels10030203
Diaz-Lasprilla AM, McKee M, Jimenez-Vergara AC, Ravi S, Bellamy D, Ortega W, Crosby CO, Steele J, Plascencia-Villa G, Perry G, et al. Fabrication and Characterization of Quad-Component Bioinspired Hydrogels to Model Elevated Fibrin Levels in Central Nervous Tissue Scaffolds. Gels. 2024; 10(3):203. https://doi.org/10.3390/gels10030203
Chicago/Turabian StyleDiaz-Lasprilla, Ana M., Meagan McKee, Andrea C. Jimenez-Vergara, Swathisri Ravi, Devon Bellamy, Wendy Ortega, Cody O. Crosby, Jennifer Steele, Germán Plascencia-Villa, George Perry, and et al. 2024. "Fabrication and Characterization of Quad-Component Bioinspired Hydrogels to Model Elevated Fibrin Levels in Central Nervous Tissue Scaffolds" Gels 10, no. 3: 203. https://doi.org/10.3390/gels10030203
APA StyleDiaz-Lasprilla, A. M., McKee, M., Jimenez-Vergara, A. C., Ravi, S., Bellamy, D., Ortega, W., Crosby, C. O., Steele, J., Plascencia-Villa, G., Perry, G., & Munoz-Pinto, D. J. (2024). Fabrication and Characterization of Quad-Component Bioinspired Hydrogels to Model Elevated Fibrin Levels in Central Nervous Tissue Scaffolds. Gels, 10(3), 203. https://doi.org/10.3390/gels10030203