Advancements and Challenges in Hydrogel Engineering for Regenerative Medicine
Abstract
1. Introduction
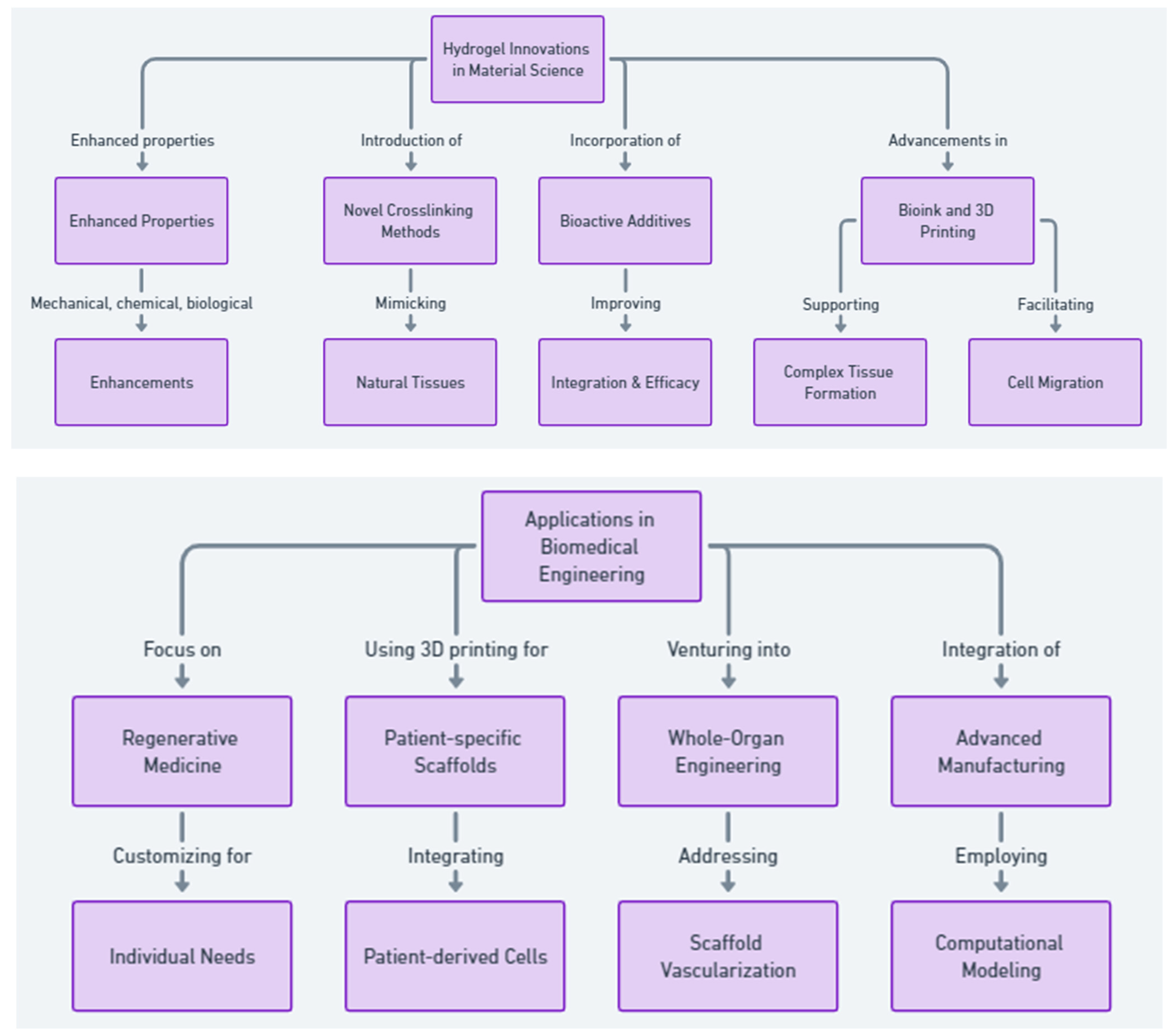
2. Engineering Advanced Hydrogels for Biomedical Applications
3. Common Hydrogel Materials in Regenerative Medicine
4. Trends in Hydrogel Research for Regenerative Medicine
5. Advancements in Hydrogel Applications for Tissue Engineering
6. Stem Cell Engineering and Differentiation
7. Cartilage Regeneration
8. Bone Regeneration
9. Neural and Spinal Cord Regeneration
10. Soft Tissue and Organ Regeneration
11. Other Tissue Engineering Applications
12. Collective Outcomes
13. Limitations
14. Future Directions
15. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Campos, F.; Bonhome-Espinosa, A.B.; Garcia-Martinez, L.; Duran, J.D.; Lopez-Lopez, M.T.; Alaminos, M.; Sanchez-Quevedo, M.C.; Carriel, V. Ex vivo characterization of a novel tissue-like cross-linked fibrin-agarose hydrogel for tissue engineering applications. Biomed. Mater. 2016, 11, 055004. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Teow, S.Y.; Pushpamalar, J. Application of Metal Nanoparticle(-)Hydrogel Composites in Tissue Regeneration. Bioengineering 2019, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Moller, L.; Krause, A.; Dahlmann, J.; Gruh, I.; Kirschning, A.; Drager, G. Preparation and evaluation of hydrogel-composites from methacrylated hyaluronic acid, alginate, and gelatin for tissue engineering. Int. J. Artif. Organs 2011, 34, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Rezaei Kolahchi, A.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Usprech, J.; Sun, Y.; Simmons, C.A. A microfabricated platform with hydrogel arrays for 3D mechanical stimulation of cells. Acta Biomater. 2016, 34, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Liu, C.; Lv, S.; Sun, D.; Qiao, Q.; Zhang, R.; Liu, Y.; Xiao, J.; Sun, G. Degradation prediction model and stem cell growth of gelatin-PEG composite hydrogel. J. Biomed. Mater. Res. A 2016, 104, 3149–3156. [Google Scholar] [CrossRef] [PubMed]
- Boere, K.W.; Visser, J.; Seyednejad, H.; Rahimian, S.; Gawlitta, D.; van Steenbergen, M.J.; Dhert, W.J.; Hennink, W.E.; Vermonden, T.; Malda, J. Covalent attachment of a three-dimensionally printed thermoplast to a gelatin hydrogel for mechanically enhanced cartilage constructs. Acta Biomater. 2014, 10, 2602–2611. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chang, Y.; Xu, Y.; Wu, P.; Mu, C.; Nie, A.; Qu, Y.; Duan, D.; Guo, X.; Liu, Z.; et al. Silicon-Phosphorus-Nanosheets-Integrated 3D-Printable Hydrogel as a Bioactive and Biodegradable Scaffold for Vascularized Bone Regeneration. Adv. Healthc. Mater. 2022, 11, e2101911. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Gastelum, G.; Ramos-Payan, R.; Lopez-Gutierrez, J.; Ayala-Ham, A.; Silva-Benitez, E.; Bermudez, M.; Romero-Quintana, J.G.; Sanchez-Schmitz, G.; Aguilar-Medina, M. An extracellular matrix hydrogel from porcine urinary bladder for tissue engineering: In vitro and in vivo analyses. Biomed. Mater. Eng. 2023, 34, 331–344. [Google Scholar] [CrossRef]
- Hong, H.; Seo, Y.B.; Kim, D.Y.; Lee, J.S.; Lee, Y.J.; Lee, H.; Ajiteru, O.; Sultan, M.T.; Lee, O.J.; Kim, S.H.; et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials 2020, 232, 119679. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, X.; Genin, G.M.; Lu, T.J.; Xu, F.; Lin, M. The relationship between thiol-acrylate photopolymerization kinetics and hydrogel mechanics: An improved model incorporating photobleaching and thiol-Michael addition. J. Mech. Behav. Biomed. Mater. 2018, 88, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Kadam, S.S.; Xiao, R.; Jivan, F.; Onn, T.M.; Fernandes, R.; Nguyen, T.D.; Gracias, D.H. Bio-origami hydrogel scaffolds composed of photocrosslinked PEG bilayers. Adv. Healthc. Mater. 2013, 2, 1142–1150. [Google Scholar] [CrossRef]
- Ahn, J.I.; Kim, G.A.; Kwon, H.S.; Ahn, J.Y.; Hubbell, J.A.; Song, Y.S.; Lee, S.T.; Lim, J.M. Culture of preantral follicles in poly(ethylene) glycol-based, three-dimensional hydrogel: A relationship between swelling ratio and follicular developments. J. Tissue Eng. Regen. Med. 2015, 9, 319–323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martinez-Garcia, F.D.; van Dongen, J.A.; Burgess, J.K.; Harmsen, M.C. Matrix Metalloproteases from Adipose Tissue-Derived Stromal Cells Are Spatiotemporally Regulated by Hydrogel Mechanics in a 3D Microenvironment. Bioengineering 2022, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Li, J.; Hartanto, Y.; Durham, M.; Tang, J.; Zhang, H.; Hooper, G.; Lim, K.; Woodfield, T. Advances in Extrusion 3D Bioprinting: A Focus on Multicomponent Hydrogel-Based Bioinks. Adv. Healthc. Mater. 2020, 9, e1901648. [Google Scholar] [CrossRef] [PubMed]
- Noh, I.; Kim, N.; Tran, H.N.; Lee, J.; Lee, C. 3D printable hyaluronic acid-based hydrogel for its potential application as a bioink in tissue engineering. Biomater. Res. 2019, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, H.; Ahlfeld, T.; Kilian, D.; Liu, Y.; Gelinsky, M.; Hu, Q. Evaluation of different crosslinking methods in altering the properties of extrusion-printed chitosan-based multi-material hydrogel composites. Bio-Des. Manuf. 2022, 6, 150–173. [Google Scholar] [CrossRef]
- Song, S.J.; Choi, J.; Park, Y.D.; Hong, S.; Lee, J.J.; Ahn, C.B.; Choi, H.; Sun, K. Sodium alginate hydrogel-based bioprinting using a novel multinozzle bioprinting system. Artif. Organs 2011, 35, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Choi, J.; Park, Y.D.; Lee, J.J.; Hong, S.Y.; Sun, K. A three-dimensional bioprinting system for use with a hydrogel-based biomaterial and printing parameter characterization. Artif. Organs 2010, 34, 1044–1048. [Google Scholar] [CrossRef]
- Moon, S.; Hasan, S.K.; Song, Y.S.; Xu, F.; Keles, H.O.; Manzur, F.; Mikkilineni, S.; Hong, J.W.; Nagatomi, J.; Haeggstrom, E.; et al. Layer by layer three-dimensional tissue epitaxy by cell-laden hydrogel droplets. Tissue Eng. Part C Methods 2010, 16, 157–166. [Google Scholar] [CrossRef]
- Calder, D.; Fathi, A.; Oveissi, F.; Maleknia, S.; Abrams, T.; Wang, Y.; Maitz, J.; Tsai, K.H.; Maitz, P.; Chrzanowski, W.; et al. Thermoresponsive and Injectable Hydrogel for Tissue Agnostic Regeneration. Adv. Healthc. Mater. 2022, 11, e2201714. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Gensini, L.; Serna, J.A.; Cifuentes, J.; Cruz, J.C.; Munoz-Camargo, C. Graphene Oxide-Embedded Extracellular Matrix-Derived Hydrogel as a Multiresponsive Platform for 3D Bioprinting Applications. Int. J. Bioprint. 2021, 7, 353. [Google Scholar] [CrossRef]
- Han, S.; Ham, T.R.; Haque, S.; Sparks, J.L.; Saul, J.M. Alkylation of human hair keratin for tunable hydrogel erosion and drug delivery in tissue engineering applications. Acta Biomater. 2015, 23, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Griveau, L.; Lafont, M.; le Goff, H.; Drouglazet, C.; Robbiani, B.; Berthier, A.; Sigaudo-Roussel, D.; Latif, N.; Visage, C.L.; Gache, V.; et al. Design and characterization of an in vivo injectable hydrogel with effervescently generated porosity for regenerative medicine applications. Acta Biomater. 2022, 140, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; Kuipers, E.; Gawlitta, D.; Dhert, W.J.; Alblas, J. Scaffold porosity and oxygenation of printed hydrogel constructs affect functionality of embedded osteogenic progenitors. Tissue Eng. Part A 2011, 17, 2473–2486. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, Y.; Fan, L.; Gao, C.; Liu, X.; Jing, X.; Zhang, H.; Huang, Y.; Guo, R.; Long, C.; et al. Cartilage Injury Repair by Human Umbilical Cord Wharton’s Jelly/Hydrogel Combined with Chondrocyte. Tissue Eng. Part C Methods 2023, 29, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, J.; Chen, C.; Li, X.; Zhang, H.; Xin, Y.; Tian, Q.; Wang, S. Emerging advances in hydrogel-based therapeutic strategies for tissue regeneration. Regen. Ther. 2023, 24, 459–471. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Qi, C.; Fang, Y.; Zhang, L.; Zhuo, R.; Jiang, X. Thermosensitive injectable in-situ forming carboxymethyl chitin hydrogel for three-dimensional cell culture. Acta Biomater. 2016, 35, 228–237. [Google Scholar] [CrossRef]
- Chung, I.M.; Enemchukwu, N.O.; Khaja, S.D.; Murthy, N.; Mantalaris, A.; Garcia, A.J. Bioadhesive hydrogel microenvironments to modulate epithelial morphogenesis. Biomaterials 2008, 29, 2637–2645. [Google Scholar] [CrossRef][Green Version]
- Jiang, Z.; Shaha, R.; McBride, R.; Jiang, K.; Tang, M.; Xu, B.; Goroncy, A.K.; Frick, C.; Oakey, J. Crosslinker length dictates step-growth hydrogel network formation dynamics and allows rapid on-chip photoencapsulation. Biofabrication 2020, 12, 035006. [Google Scholar] [CrossRef]
- Fan, C.; Wang, D.A. Macroporous Hydrogel Scaffolds for Three-Dimensional Cell Culture and Tissue Engineering. Tissue Eng. Part B Rev. 2017, 23, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Liu, X.; Zhu, K.; He, X. Hydrogel Encapsulation Facilitates Rapid-Cooling Cryopreservation of Stem Cell-Laden Core-Shell Microcapsules as Cell-Biomaterial Constructs. Adv. Healthc. Mater. 2017, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Placone, J.K.; Navarro, J.; Laslo, G.W.; Lerman, M.J.; Gabard, A.R.; Herendeen, G.J.; Falco, E.E.; Tomblyn, S.; Burnett, L.; Fisher, J.P. Development and Characterization of a 3D Printed, Keratin-Based Hydrogel. Ann. Biomed. Eng. 2017, 45, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Shamma, R.N.; Sayed, R.H.; Madry, H.; El Sayed, N.S.; Cucchiarini, M. Triblock Copolymer Bioinks in Hydrogel Three-Dimensional Printing for Regenerative Medicine: A Focus on Pluronic F127. Tissue Eng. Part B Rev. 2022, 28, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Nepal, S.; Si, J.; Ishikawa, S.; Nishikawa, M.; Sakai, Y.; Akimoto, A.M.; Okada, H.; Ohba, S.; Chung, U.I.; Sakai, T.; et al. Injectable phase-separated tetra-armed poly(ethylene glycol) hydrogel scaffold allows sustained release of growth factors to enhance the repair of critical bone defects. Regen. Ther. 2024, 25, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.M.; Sant, S.; Masaeli, M.; Kachouie, N.N.; Zamanian, B.; Lee, S.H.; Khademhosseini, A. Fabrication of three-dimensional porous cell-laden hydrogel for tissue engineering. Biofabrication 2010, 2, 035003. [Google Scholar] [CrossRef]
- Erickson, A.G.; Laughlin, T.D.; Romereim, S.M.; Sargus-Patino, C.N.; Pannier, A.K.; Dudley, A.T. A Tunable, Three-Dimensional In Vitro Culture Model of Growth Plate Cartilage Using Alginate Hydrogel Scaffolds. Tissue Eng. Part A 2018, 24, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Egorikhina, M.N.; Aleynik, D.Y.; Rubtsova, Y.P.; Levin, G.Y.; Charykova, I.N.; Semenycheva, L.L.; Bugrova, M.L.; Zakharychev, E.A. Hydrogel scaffolds based on blood plasma cryoprecipitate and collagen derived from various sources: Structural, mechanical and biological characteristics. Bioact. Mater. 2019, 4, 334–345. [Google Scholar] [CrossRef]
- Ji, Q.; Zhang, H.; Zhang, X.; Ma, Q.; Teng, L.; Qiu, L. Hydrosoluble collagen based biodegradable hybrid hydrogel for biomedical scaffold. J. Biomater. Sci. Polym. Ed. 2020, 31, 2199–2219. [Google Scholar] [CrossRef]
- Nadra, M.; Niu, W.; Kurisawa, M.; Rousson, D.; Spector, M. Platelet-Rich Plasma Lysate-Incorporating Gelatin Hydrogel as a Scaffold for Bone Reconstruction. Bioengineering 2022, 9, 15. [Google Scholar] [CrossRef]
- Pasini, C.; Pandini, S.; Ramorino, G.; Sartore, L. Tailoring the properties of composite scaffolds with a 3D-Printed lattice core and a bioactive hydrogel shell for tissue engineering. J. Mech. Behav. Biomed. Mater. 2024, 150, 106305. [Google Scholar] [CrossRef] [PubMed]
- Gnavi, S.; di Blasio, L.; Tonda-Turo, C.; Mancardi, A.; Primo, L.; Ciardelli, G.; Gambarotta, G.; Geuna, S.; Perroteau, I. Gelatin-based hydrogel for vascular endothelial growth factor release in peripheral nerve tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Ciftci, S.; Barthes, J.; Knopf-Marques, H.; Muller, C.B.; Debry, C.; Vrana, N.E.; Ghaemmaghami, A.M. A composite Gelatin/hyaluronic acid hydrogel as an ECM mimic for developing mesenchymal stem cell-derived epithelial tissue patches. J. Tissue Eng. Regen. Med. 2020, 14, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Tijore, A.; Irvine, S.A.; Sarig, U.; Mhaisalkar, P.; Baisane, V.; Venkatraman, S. Contact guidance for cardiac tissue engineering using 3D bioprinted gelatin patterned hydrogel. Biofabrication 2018, 10, 025003. [Google Scholar] [CrossRef] [PubMed]
- Naghizadeh, Z.; Karkhaneh, A.; Khojasteh, A. Simultaneous release of melatonin and methylprednisolone from an injectable in situ self-crosslinked hydrogel/microparticle system for cartilage tissue engineering. J. Biomed. Mater. Res. A 2018, 106, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Arha, M.; Choudhary, S.; Ashton, R.S.; Bhatia, S.R.; Schaffer, D.V.; Kane, R.S. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials 2009, 30, 4695–4699. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, H.; Asato, H.; Ogasawara, T.; Nishizawa, S.; Takahashi, T.; Nakatsuka, T.; Koshima, I.; Nakamura, K.; Kawaguchi, H.; Chung, U.I.; et al. Cartilage tissue engineering using human auricular chondrocytes embedded in different hydrogel materials. J. Biomed. Mater. Res. A 2006, 78, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Niu, C.; Zhang, L.; Li, G.; Yang, Y. Construction of polyacrylamide/graphene oxide/gelatin/sodium alginate composite hydrogel with bioactivity for promoting Schwann cells growth. J. Biomed. Mater. Res. A 2018, 106, 1951–1964. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gutierrez, J.; Ramos-Payan, R.; Romero-Quintana, J.G.; Ayala-Ham, A.; Castro-Salazar, Y.; Castillo-Ureta, H.; Jimenez-Gastelum, G.; Bermudez, M.; Aguilar-Medina, M. Evaluation of biocompatibility and angiogenic potential of extracellular matrix hydrogel biofunctionalized with the LL-37 peptide. Biomed. Mater. Eng. 2023, 34, 545–560. [Google Scholar] [CrossRef]
- Chen, C.; Huang, S.; Chen, Z.; Liu, Q.; Cai, Y.; Mei, Y.; Xu, Y.; Guo, R.; Yan, C. Kartogenin (KGN)/synthetic melanin nanoparticles (SMNP) loaded theranostic hydrogel scaffold system for multiparametric magnetic resonance imaging guided cartilage regeneration. Bioeng. Transl. Med. 2023, 8, e10364. [Google Scholar] [CrossRef]
- Lee, H.S.; Jeon, E.Y.; Nam, J.J.; Park, J.H.; Choi, I.C.; Kim, S.H.; Chung, J.J.; Lee, K.; Park, J.W.; Jung, Y. Development of a regenerative porous PLCL nerve guidance conduit with swellable hydrogel-based microgrooved surface pattern via 3D printing. Acta Biomater. 2022, 141, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; Radetzki, F.; Stirnweiss, A.; Mendel, T.; Ludtka, C.; Friedmann, A.; Baerthel, A.; Brehm, W.; Milosevic, J.; Meisel, H.J.; et al. Long-term pre-clinical evaluation of an injectable chitosan nanocellulose hydrogel with encapsulated adipose-derived stem cells in an ovine model for IVD regeneration. J. Tissue Eng. Regen. Med. 2021, 15, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, X.; Zhao, M.; Chen, Y.; Xu, Y.; Wang, K.; Bian, S.; Jiang, Q.; Fan, Y.; Zhang, X. Bioinspired supramolecular nanofiber hydrogel through self-assembly of biphenyl-tripeptide for tissue engineering. Bioact. Mater. 2022, 8, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, K.F.; Williams, R.J.; Nisbet, D.R. Dynamic and Responsive Growth Factor Delivery from Electrospun and Hydrogel Tissue Engineering Materials. Adv. Healthc. Mater. 2018, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Ma, L.; Wang, B.; Song, Y.; Luo, R.; Li, G.; Liao, Z.; Shi, Y.; Wang, K.; Feng, X.; et al. N-cadherin mimetic hydrogel enhances MSC chondrogenesis through cell metabolism. Acta Biomater. 2022, 150, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Barnett, H.; Shevchuk, M.; Peppas, N.A.; Caldorera-Moore, M. Influence of extracellular cues of hydrogel biomaterials on stem cell fate. J. Biomater. Sci. Polym. Ed. 2022, 33, 1324–1347. [Google Scholar] [CrossRef]
- Alaman-Diez, P.; Garcia-Gareta, E.; Arruebo, M.; Perez, M.A. A bone-on-a-chip collagen hydrogel-based model using pre-differentiated adipose-derived stem cells for personalized bone tissue engineering. J. Biomed. Mater. Res. A 2023, 111, 88–105. [Google Scholar] [CrossRef]
- Zhang, K.; Fu, Q.; Yoo, J.; Chen, X.; Chandra, P.; Mo, X.; Song, L.; Atala, A.; Zhao, W. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: An in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017, 50, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Munarin, F.; Kaiser, N.J.; Kim, T.Y.; Choi, B.R.; Coulombe, K.L.K. Laser-Etched Designs for Molding Hydrogel-Based Engineered Tissues. Tissue Eng. Part C Methods 2017, 23, 311–321. [Google Scholar] [CrossRef]
- Zhai, H.; Zhou, J.; Xu, J.; Sun, X.; Xu, Y.; Qiu, X.; Zhang, C.; Wu, Z.; Long, H.; Bai, Y.; et al. Mechanically strengthened hybrid peptide-polyester hydrogel and potential applications in spinal cord injury repair. Biomed. Mater. 2020, 15, 055031. [Google Scholar] [CrossRef]
- Makvandi, P.; Ashrafizadeh, M.; Ghomi, M.; Najafi, M.; Hossein, H.H.S.; Zarrabi, A.; Mattoli, V.; Varma, R.S. Injectable hyaluronic acid-based antibacterial hydrogel adorned with biogenically synthesized AgNPs-decorated multi-walled carbon nanotubes. Prog. Biomater. 2021, 10, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, M.; Kudlackova, R.; Zima, A.; Slosarczyk, A.; Ziabka, M.; Jelen, P.; Shkarina, S.; Cecilia, A.; Zuber, M.; Baumbach, T.; et al. Novel multicomponent organic-inorganic WPI/gelatin/CaP hydrogel composites for bone tissue engineering. J. Biomed. Mater. Res. A 2019, 107, 2479–2491. [Google Scholar] [CrossRef]
- Hong, Y.; Gong, Y.; Gao, C.; Shen, J. Collagen-coated polylactide microcarriers/chitosan hydrogel composite: Injectable scaffold for cartilage regeneration. J. Biomed. Mater. Res. A 2008, 85, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, C.; Zheng, G.; Zhang, L.; Zhong, Q.; Zhang, Y.; Zhao, W.; Qi, Y. Articular Cartilage Regeneration via Induced Chondrocyte Autophagy by Sustained Release of Leptin Inhibitor from Thermo-Sensitive Hydrogel through STAT3/REDD1/mTORC1 Cascade. Adv. Healthc. Mater. 2023, 12, e2302181. [Google Scholar] [CrossRef]
- Sa-Lima, H.; Tuzlakoglu, K.; Mano, J.F.; Reis, R.L. Thermoresponsive poly(N-isopropylacrylamide)-g-methylcellulose hydrogel as a three-dimensional extracellular matrix for cartilage-engineered applications. J. Biomed. Mater. Res. A 2011, 98, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Lucas de Lima, E.; Fittipaldi Vasconcelos, N.; da Silva Maciel, J.; Karine Andrade, F.; Silveira Vieira, R.; Andrade Feitosa, J.P. Injectable hydrogel based on dialdehyde galactomannan and N-succinyl chitosan: A suitable platform for cell culture. J. Mater. Sci. Mater. Med. 2019, 31, 5. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Nicholls, F.J.; Crum, W.R.; Ghuman, H.; Badylak, S.F.; Modo, M. Diamagnetic chemical exchange saturation transfer (diaCEST) affords magnetic resonance imaging of extracellular matrix hydrogel implantation in a rat model of stroke. Biomaterials 2017, 113, 176–190. [Google Scholar] [CrossRef]
- Drzeniek, N.M.; Mazzocchi, A.; Schlickeiser, S.; Forsythe, S.D.; Moll, G.; Geissler, S.; Reinke, P.; Gossen, M.; Gorantla, V.S.; Volk, H.D.; et al. Bio-instructive hydrogel expands the paracrine potency of mesenchymal stem cells. Biofabrication 2021, 13, 14. [Google Scholar] [CrossRef]
- Ferreira, L.S.; Gerecht, S.; Fuller, J.; Shieh, H.F.; Vunjak-Novakovic, G.; Langer, R. Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomaterials 2007, 28, 2706–2717. [Google Scholar] [CrossRef]
- Hodge, J.G.; Robinson, J.L.; Mellott, A.J. Novel hydrogel system eliminates subculturing and improves retention of nonsenescent mesenchymal stem cell populations. Regen. Med. 2023, 18, 23–36. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Xie, C.; Zhang, X.; Benoit, D.S. The effect of mesenchymal stem cells delivered via hydrogel-based tissue engineered periosteum on bone allograft healing. Biomaterials 2013, 34, 8887–8898. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, P.; Weir, M.D.; Reynolds, M.A.; Zhao, L.; Xu, H.H. Hydrogel fibers encapsulating human stem cells in an injectable calcium phosphate scaffold for bone tissue engineering. Biomed. Mater. 2016, 11, 065008. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xu, C.; Wo, K.; Wang, Y.; Zhang, J.; Lei, H.; Wang, X.; Shi, Y.; Fan, W.; Zhao, B.; et al. Wireless Electric Cues Mediate Autologous DPSC-Loaded Conductive Hydrogel Microspheres to Engineer the Immuno-Angiogenic Niche for Homologous Maxillofacial Bone Regeneration. Adv. Healthc. Mater. 2023, 13, e2303405. [Google Scholar] [CrossRef] [PubMed]
- Pahlevanzadeh, F.; Emadi, R.; Valiani, A.; Kharaziha, M.; Poursamar, A.; Bakhsheshi-Rad, H.R.; Fauzi Ismail, A.; Ramakrishna, S.; Berto, F. Three-Dimensional Printing Constructs Based on the Chitosan for Tissue Regeneration: State of the Art, Developing Directions and Prospect Trends. Materials 2020, 13, 2663. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Keller, K.A.; Sperduto, J.L.; Slater, J.H. Fundamentals of Laser-Based Hydrogel Degradation and Applications in Cell and Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, J.; Wang, L.; Zhang, D.; Zhang, J.; Guan, F.; Yao, M. Dual-enzymatically crosslinked hyaluronic acid hydrogel as a long-time 3D stem cell culture system. Biomed. Mater. 2020, 15, 045013. [Google Scholar] [CrossRef] [PubMed]
- King, S.N.; Hanson, S.E.; Chen, X.; Kim, J.; Hematti, P.; Thibeault, S.L. In vitro characterization of macrophage interaction with mesenchymal stromal cell-hyaluronan hydrogel constructs. J. Biomed. Mater. Res. A 2014, 102, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Truong, N.F.; Segura, T. Design of cell-matrix interactions in hyaluronic acid hydrogel scaffolds. Acta Biomater. 2014, 10, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Lee, J.Y.; Byun, H.; Ahmad, T.; Akashi, M.; Matsusaki, M.; Shin, H. One-step delivery of a functional multi-layered cell sheet using a thermally expandable hydrogel with controlled presentation of cell adhesive proteins. Biofabrication 2018, 10, 025001. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Z.; Gu, H.; Ge, Y.; Wu, X.; Zuo, F.; Du, Q.; Lei, Y.; Wang, Z.; Lin, H. Comparative study of differentiating human pluripotent stem cells into vascular smooth muscle cells in hydrogel-based culture methods. Regen. Ther. 2023, 22, 39–49. [Google Scholar] [CrossRef]
- Nelson, V.J.; Dinnunhan, M.F.K.; Turner, P.R.; Faed, J.M.; Cabral, J.D. A chitosan/dextran-based hydrogel as a delivery vehicle of human bone-marrow derived mesenchymal stem cells. Biomed. Mater. 2017, 12, 035012. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, S.; Chakravarti, A.R.; Modaresi, S.; Subham, S.; Burkey, K.; Kurlbaum, C.; Fang, M.; Neal, C.A.; Mellott, A.J.; Chakraborty, A.; et al. Investigation of human adipose-derived stem-cell behavior using a cell-instructive polydopamine-coated gelatin-alginate hydrogel. J. Biomed. Mater. Res. A 2021, 109, 2597–2610. [Google Scholar] [CrossRef] [PubMed]
- Antich, C.; Jimenez, G.; de Vicente, J.; Lopez-Ruiz, E.; Chocarro-Wrona, C.; Grinan-Lison, C.; Carrillo, E.; Montanez, E.; Marchal, J.A. Development of a Biomimetic Hydrogel Based on Predifferentiated Mesenchymal Stem-Cell-Derived ECM for Cartilage Tissue Engineering. Adv. Healthc. Mater. 2021, 10, e2001847. [Google Scholar] [CrossRef] [PubMed]
- Otto, I.A.; Levato, R.; Webb, W.R.; Khan, I.M.; Breugem, C.C.; Malda, J. Progenitor cells in auricular cartilage demonstrate cartilage-forming capacity in 3D hydrogel culture. Eur. Cell Mater. 2018, 35, 132–150. [Google Scholar] [CrossRef] [PubMed]
- Popa, E.G.; Caridade, S.G.; Mano, J.F.; Reis, R.L.; Gomes, M.E. Chondrogenic potential of injectable kappa-carrageenan hydrogel with encapsulated adipose stem cells for cartilage tissue-engineering applications. J. Tissue Eng. Regen. Med. 2015, 9, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Li, C.; Lee, M.; Marzvanyan, A.; Zhao, Z.; Ting, K.; Soo, C.; Zheng, Z. Photopolymerizable Hydrogel-Encapsulated Fibromodulin-Reprogrammed Cells for Muscle Regeneration. Tissue Eng. Part A 2020, 26, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Alcala-Orozco, C.R.; Baer, K.; Li, J.; Murphy, C.A.; Durham, M.; Lindberg, G.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B.F. 3D bioassembly of cell-instructive chondrogenic and osteogenic hydrogel microspheres containing allogeneic stem cells for hybrid biofabrication of osteochondral constructs. Biofabrication 2022, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Gawlitta, D.; Benders, K.E.; Toma, S.M.; Pouran, B.; van Weeren, P.R.; Dhert, W.J.; Malda, J. Endochondral bone formation in gelatin methacrylamide hydrogel with embedded cartilage-derived matrix particles. Biomaterials 2015, 37, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Diloksumpan, P.; de Ruijter, M.; Castilho, M.; Gbureck, U.; Vermonden, T.; van Weeren, P.R.; Malda, J.; Levato, R. Combining multi-scale 3D printing technologies to engineer reinforced hydrogel-ceramic interfaces. Biofabrication 2020, 12, 025014. [Google Scholar] [CrossRef]
- Gong, Y.; Su, K.; Lau, T.T.; Zhou, R.; Wang, D.A. Microcavitary hydrogel-mediating phase transfer cell culture for cartilage tissue engineering. Tissue Eng. Part A 2010, 16, 3611–3622. [Google Scholar] [CrossRef]
- Kwon, J.S.; Kim, S.W.; Kwon, D.Y.; Park, S.H.; Son, A.R.; Kim, J.H.; Kim, M.S. In vivo osteogenic differentiation of human turbinate mesenchymal stem cells in an injectable in situ-forming hydrogel. Biomaterials 2014, 35, 5337–5346. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, S.; Basu, S.; Berkland, C.; Wang, J.; Paul, A. Design of a cytocompatible hydrogel coating to modulate properties of ceramic-based scaffolds for bone repair. Cell Mol. Bioeng. 2018, 11, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, S.P.; Athirasala, A.; Tahayeri, A.; Abdelmoniem, R.; George, A.; Bertassoni, L.E. BoneMA-synthesis and characterization of a methacrylated bone-derived hydrogel for bioprinting ofin-vitrovascularized tissue constructs. Biofabrication 2021, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Gan, Y.; Bao, C.; Wu, W.; Wang, X.; Zhang, Z.; Zhou, Q.; Lin, Q.; Yang, Y.; Zhu, L. Photosensitive Hydrogel Creates Favorable Biologic Niches to Promote Spinal Cord Injury Repair. Adv. Healthc. Mater. 2019, 8, e1900013. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.Y.; Cheng, H.W.; Lo, Y.C.; Wang, W.C.; Chang, S.J.; Cheng, C.H.; Lin, Y.C.; Lu, H.E.; Sue, M.W.; Tsou, N.T.; et al. 4D spatiotemporal modulation of biomolecules distribution in anisotropic corrugated microwrinkles via electrically manipulated microcapsules within hierarchical hydrogel for spinal cord regeneration. Biomaterials 2021, 271, 120762. [Google Scholar] [CrossRef] [PubMed]
- Dumont, C.M.; Carlson, M.A.; Munsell, M.K.; Ciciriello, A.J.; Strnadova, K.; Park, J.; Cummings, B.J.; Anderson, A.J.; Shea, L.D. Aligned hydrogel tubes guide regeneration following spinal cord injury. Acta Biomater. 2019, 86, 312–322. [Google Scholar] [CrossRef]
- Kandalam, S.; Sindji, L.; Delcroix, G.J.; Violet, F.; Garric, X.; Andre, E.M.; Schiller, P.C.; Venier-Julienne, M.C.; des Rieux, A.; Guicheux, J.; et al. Pharmacologically active microcarriers delivering BDNF within a hydrogel: Novel strategy for human bone marrow-derived stem cells neural/neuronal differentiation guidance and therapeutic secretome enhancement. Acta Biomater. 2017, 49, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Vialli, N.; Fuh, J.Y.H.; Lu, W.F. Conductive collagen/polypyrrole-b-polycaprolactone hydrogel for bioprinting of neural tissue constructs. Int. J. Bioprint. 2019, 5, 229. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Borland, S.; Richardson, S.M.; Merry, C.L.R.; Saiani, A.; Gough, J.E. Self-assembling peptide hydrogel for intervertebral disc tissue engineering. Acta Biomater. 2016, 46, 29–40. [Google Scholar] [CrossRef]
- Augsornworawat, P.; Velazco-Cruz, L.; Song, J.; Millman, J.R. A hydrogel platform for in vitro three dimensional assembly of human stem cell-derived islet cells and endothelial cells. Acta Biomater. 2019, 97, 272–280. [Google Scholar] [CrossRef]
- Basurto, I.M.; Passipieri, J.A.; Gardner, G.M.; Smith, K.K.; Amacher, A.R.; Hansrisuk, A.I.; Christ, G.J.; Caliari, S.R. Photoreactive Hydrogel Stiffness Influences Volumetric Muscle Loss Repair. Tissue Eng. Part A 2022, 28, 312–329. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Zhu, Y.; Zhao, X.; Chen, J.; Tuan, R.S.; Chan, H.F. Efficient fabrication of monodisperse hepatocyte spheroids and encapsulation in hybrid hydrogel with controllable extracellular matrix effect. Biofabrication 2021, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Zhao, X.; Zhu, Y.; Tang, N.; Wang, R.; Zhang, X.; Qu, F.; Ho, Y.P.; Lee, W.Y.; Chen, J.; et al. Efficient hepatic differentiation of hydrogel microsphere-encapsulated human pluripotent stem cells for engineering prevascularized liver tissue. Biofabrication 2022, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Finklea, F.B.; Tian, Y.; Kerscher, P.; Seeto, W.J.; Ellis, M.E.; Lipke, E.A. Engineered cardiac tissue microsphere production through direct differentiation of hydrogel-encapsulated human pluripotent stem cells. Biomaterials 2021, 274, 120818. [Google Scholar] [CrossRef] [PubMed]
- Gwon, K.; Kim, E.; Tae, G. Heparin-hyaluronic acid hydrogel in support of cellular activities of 3D encapsulated adipose derived stem cells. Acta Biomater. 2017, 49, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, M.; Ramesh, P.; Toro, M.; Evans, E.; Moskwa, N.; Zhang, X.; Sharfstein, S.T.; Larsen, M.; Xie, Y. Alginate Hydrogel Microtubes for Salivary Gland Cell Organization and Cavitation. Bioengineering 2022, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, M.R.; Choi, C.; Kim, J.B.; Cha, C. Synergistic control of mechanics and microarchitecture of 3D bioactive hydrogel platform to promote the regenerative potential of engineered hepatic tissue. Biomaterials 2021, 270, 120688. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Zhang, X.; Xu, J.; Kang, J.; Li, B.; Zhao, B.; Wang, L. Cross-Linking Methods of the Silk Protein Hydrogel in Oral and Craniomaxillofacial Tissue Regeneration. Tissue Eng. Regen. Med. 2024, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Manoukian, O.S.; Matta, R.; Letendre, J.; Collins, P.; Mazzocca, A.D.; Kumbar, S.G. Electrospun Nanofiber Scaffolds and Their Hydrogel Composites for the Engineering and Regeneration of Soft Tissues. In Biomedical Nanotechnology: Methods and Protocols, 2nd ed.; Methods in Molecular Biology; Petrosko, S.H., Day, E.S., Eds.; Humana Press Inc.: Clifton, NJ, USA, 2017; Volume 1570, pp. 261–278. [Google Scholar]
- Rode, M.P.; Batti Angulski, A.B.; Gomes, F.A.; da Silva, M.M.; Jeremias, T.D.S.; de Carvalho, R.G.; Iucif Vieira, D.G.; Oliveira, L.F.C.; Fernandes Maia, L.; Trentin, A.G.; et al. Carrageenan hydrogel as a scaffold for skin-derived multipotent stromal cells delivery. J. Biomater. Appl. 2018, 33, 422–434. [Google Scholar] [CrossRef]
- Seo, Y.; Jung, Y.; Kim, S.H. Decellularized heart ECM hydrogel using supercritical carbon dioxide for improved angiogenesis. Acta Biomater. 2018, 67, 270–281. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, Z.; Sun, L.; Li, M.; Han, L.; Wang, J.; Wu, X.; Sang, S. Constructing epidermal rete ridges using a composite hydrogel to enhance multiple signaling pathways for the maintenance of epidermal stem cell niche. Acta Biomater. 2023, 169, 273–288. [Google Scholar] [CrossRef]
- Tomblyn, S.; Pettit Kneller, E.L.; Walker, S.J.; Ellenburg, M.D.; Kowalczewski, C.J.; Van Dyke, M.; Burnett, L.; Saul, J.M. Keratin hydrogel carrier system for simultaneous delivery of exogenous growth factors and muscle progenitor cells. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 864–879. [Google Scholar] [CrossRef]
- Wang, H.; Shi, J.; Wang, Y.; Yin, Y.; Wang, L.; Liu, J.; Liu, Z.; Duan, C.; Zhu, P.; Wang, C. Promotion of cardiac differentiation of brown adipose derived stem cells by chitosan hydrogel for repair after myocardial infarction. Biomaterials 2014, 35, 3986–3998. [Google Scholar] [CrossRef]
- Wang, Z.; Li, W.; Gou, L.; Zhou, Y.; Peng, G.; Zhang, J.; Liu, J.; Li, R.; Ni, H.; Zhang, W.; et al. Biodegradable and Antioxidant DNA Hydrogel as a Cytokine Delivery System for Diabetic Wound Healing. Adv. Healthc. Mater. 2022, 11, e2200782. [Google Scholar] [CrossRef]
- You, J.; Shin, D.S.; Patel, D.; Gao, Y.; Revzin, A. Multilayered heparin hydrogel microwells for cultivation of primary hepatocytes. Adv. Healthc. Mater. 2014, 3, 126–132. [Google Scholar] [CrossRef]
- Zaviskova, K.; Tukmachev, D.; Dubisova, J.; Vackova, I.; Hejcl, A.; Bystronova, J.; Pravda, M.; Scigalkova, I.; Sulakova, R.; Velebny, V.; et al. Injectable hydroxyphenyl derivative of hyaluronic acid hydrogel modified with RGD as scaffold for spinal cord injury repair. J. Biomed. Mater. Res. A 2018, 106, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Heher, P.; Hilborn, J.; Redl, H.; Ossipov, D.A. Hyaluronic acid-fibrin interpenetrating double network hydrogel prepared in situ by orthogonal disulfide cross-linking reaction for biomedical applications. Acta Biomater. 2016, 38, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, S.H.; Lee, J.E.; Park, S.J.; Kim, K.; Kim, I.S.; Lee, Y.S.; Hwang, N.S.; Kim, B.G. Tissue adhesive, rapid forming, and sprayable ECM hydrogel via recombinant tyrosinase crosslinking. Biomaterials 2018, 178, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, A.; Taguchi, T. Engineering thixotropic supramolecular gelatin-based hydrogel as an injectable scaffold for cell transplantation. Biomed. Mater. 2022, 18, 8. [Google Scholar] [CrossRef]
- Tian, A.; Yi, X.; Sun, N. Application of mesenchymal stem cells combined with nano-polypeptide hydrogel in tissue engineering blood vessel. Regen. Ther. 2022, 21, 277–281. [Google Scholar] [CrossRef]
- Vashist, A.; Kaushik, A.; Vashist, A.; Sagar, V.; Ghosal, A.; Gupta, Y.K.; Ahmad, S.; Nair, M. Advances in Carbon Nanotubes-Hydrogel Hybrids in Nanomedicine for Therapeutics. Adv. Healthc. Mater. 2018, 7, e1701213. [Google Scholar] [CrossRef] [PubMed]
- Vieira de Souza, T.; Malmonge, S.M.; Santos, A.R., Jr. Development of a chitosan and hyaluronic acid hydrogel with potential for bioprinting utilization: A preliminary study. J. Biomater. Appl. 2021, 36, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.T.; Diba, M.; Yang, S.; Freedman, B.R.; Elosegui-Artola, A.; Mooney, D.J. Hydrogel viscoelasticity modulates migration and fusion of mesenchymal stem cell spheroids. Bioeng. Transl. Med. 2023, 8, e10464. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Liu, Y.; Jiang, L.; Wang, X.; Chen, Y.; Li, L.; Song, M.; Zhang, H.; Zhang, Y.S.; Zhang, X. Injectable decellularized dental pulp matrix-functionalized hydrogel microspheres for endodontic regeneration. Acta Biomater. 2023, 156, 37–48. [Google Scholar] [CrossRef]
- Zuidema, J.M.; Pap, M.M.; Jaroch, D.B.; Morrison, F.A.; Gilbert, R.J. Fabrication and characterization of tunable polysaccharide hydrogel blends for neural repair. Acta Biomater. 2011, 7, 1634–1643. [Google Scholar] [CrossRef]

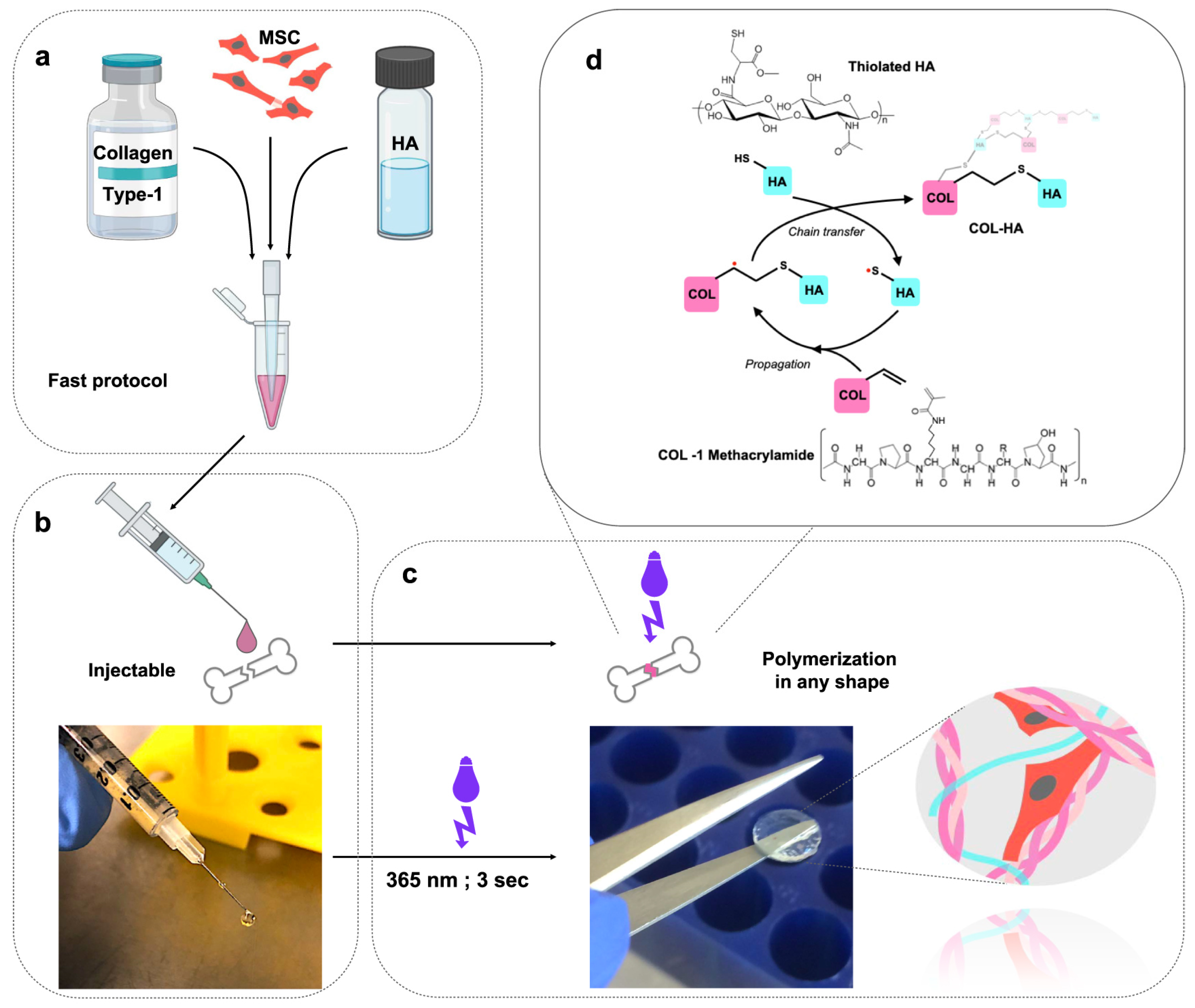
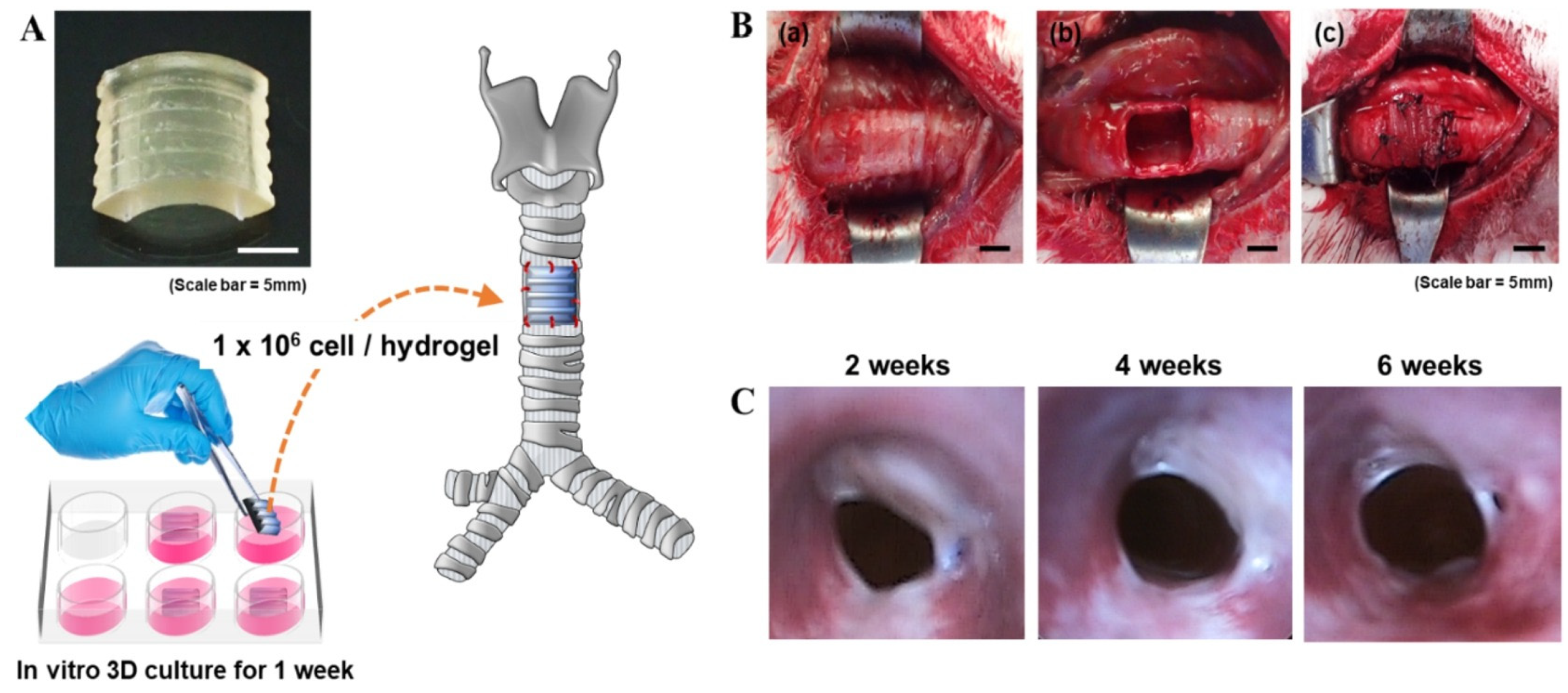
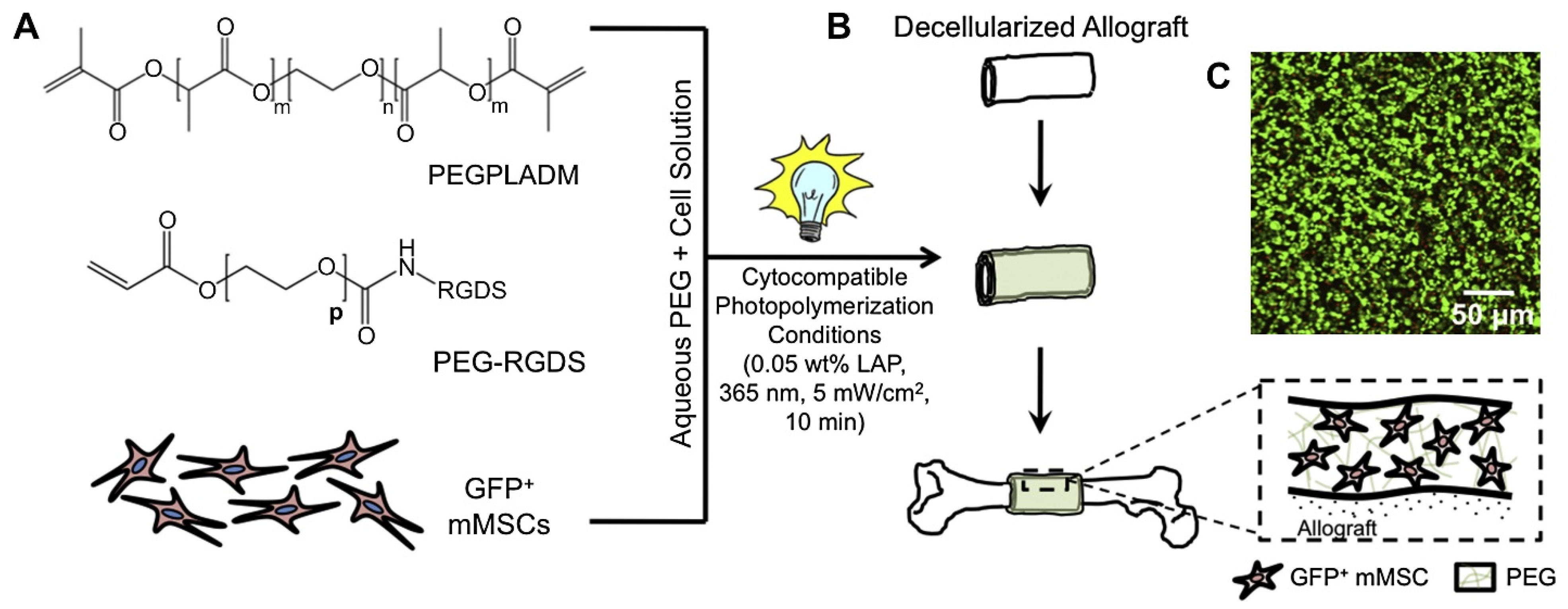
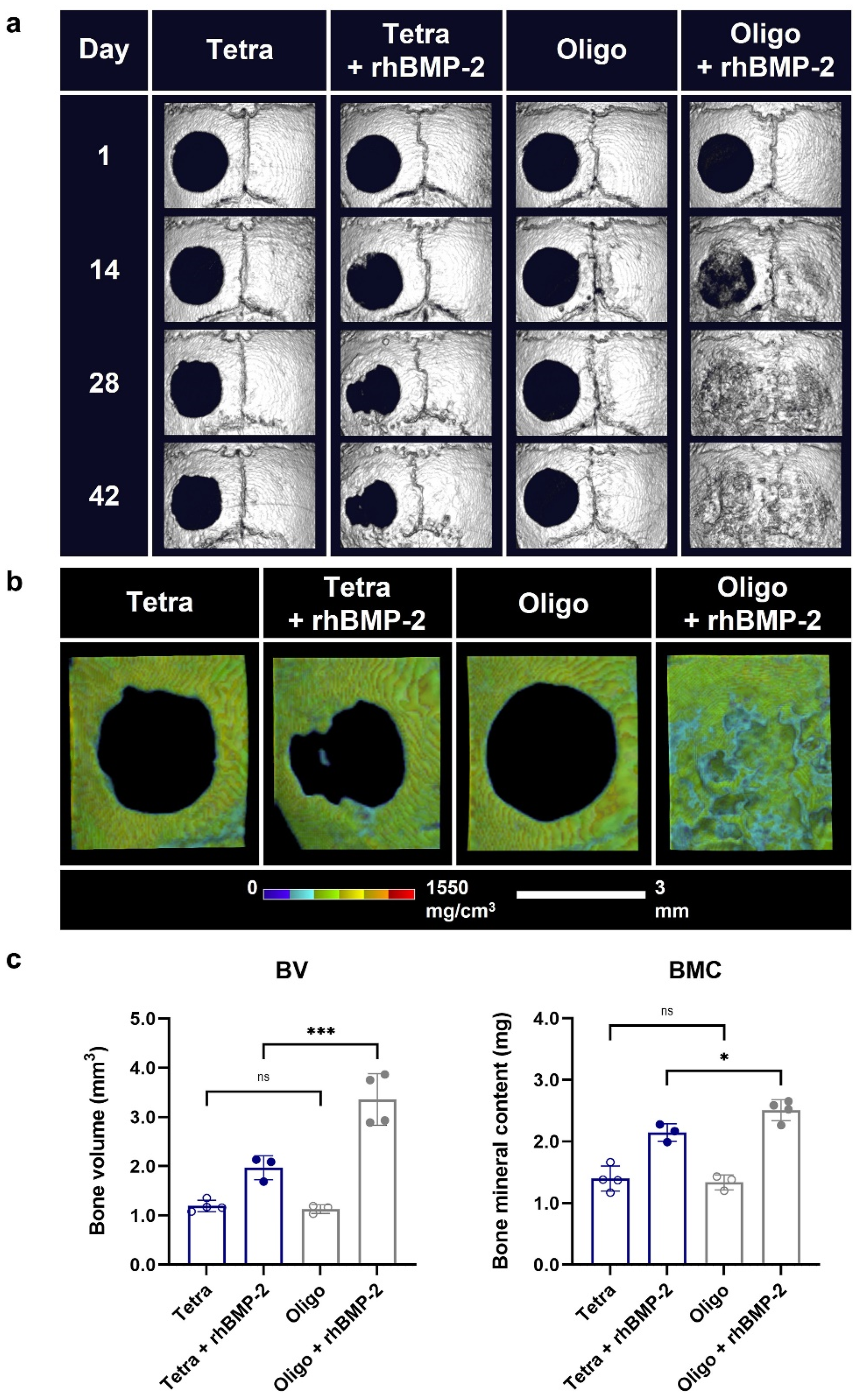
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omidian, H.; Chowdhury, S.D.; Wilson, R.L. Advancements and Challenges in Hydrogel Engineering for Regenerative Medicine. Gels 2024, 10, 238. https://doi.org/10.3390/gels10040238
Omidian H, Chowdhury SD, Wilson RL. Advancements and Challenges in Hydrogel Engineering for Regenerative Medicine. Gels. 2024; 10(4):238. https://doi.org/10.3390/gels10040238
Chicago/Turabian StyleOmidian, Hossein, Sumana Dey Chowdhury, and Renae L. Wilson. 2024. "Advancements and Challenges in Hydrogel Engineering for Regenerative Medicine" Gels 10, no. 4: 238. https://doi.org/10.3390/gels10040238
APA StyleOmidian, H., Chowdhury, S. D., & Wilson, R. L. (2024). Advancements and Challenges in Hydrogel Engineering for Regenerative Medicine. Gels, 10(4), 238. https://doi.org/10.3390/gels10040238







