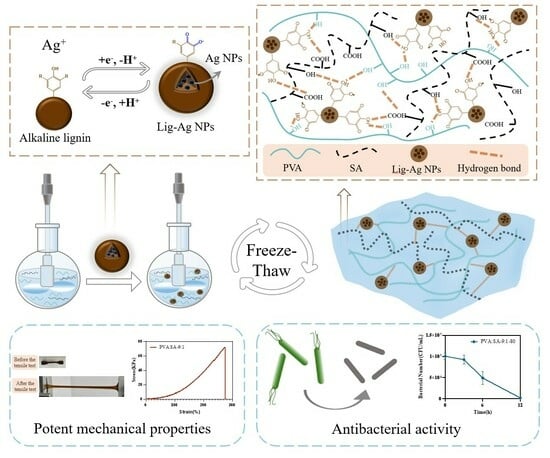
A Lignin Silver Nanoparticles/Polyvinyl Alcohol/Sodium Alginate Hybrid Hydrogel with Potent Mechanical Properties and Antibacterial Activity
Abstract
1. Introduction
2. Result and Discussion
2.1. Hydrogel Formation
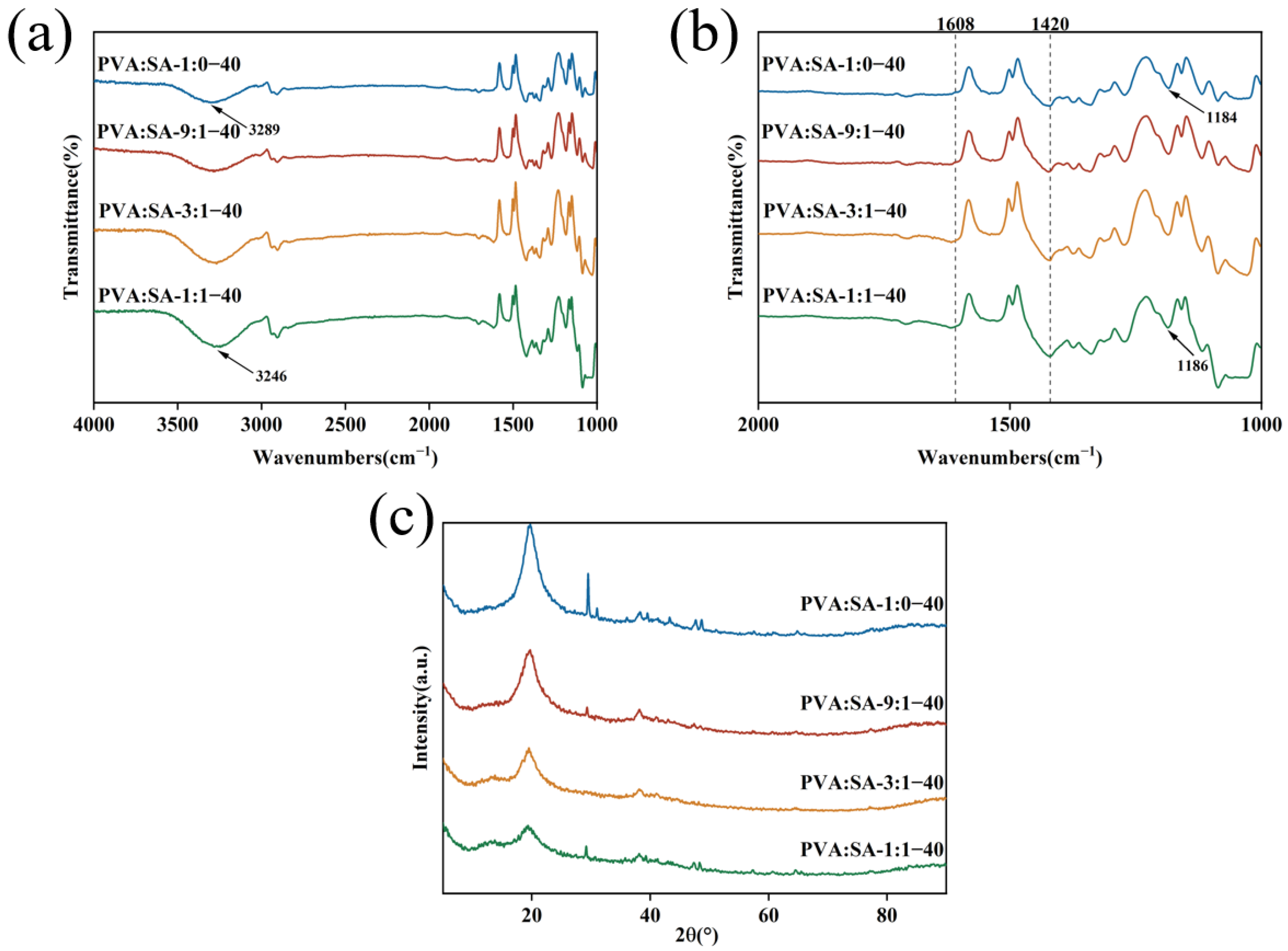
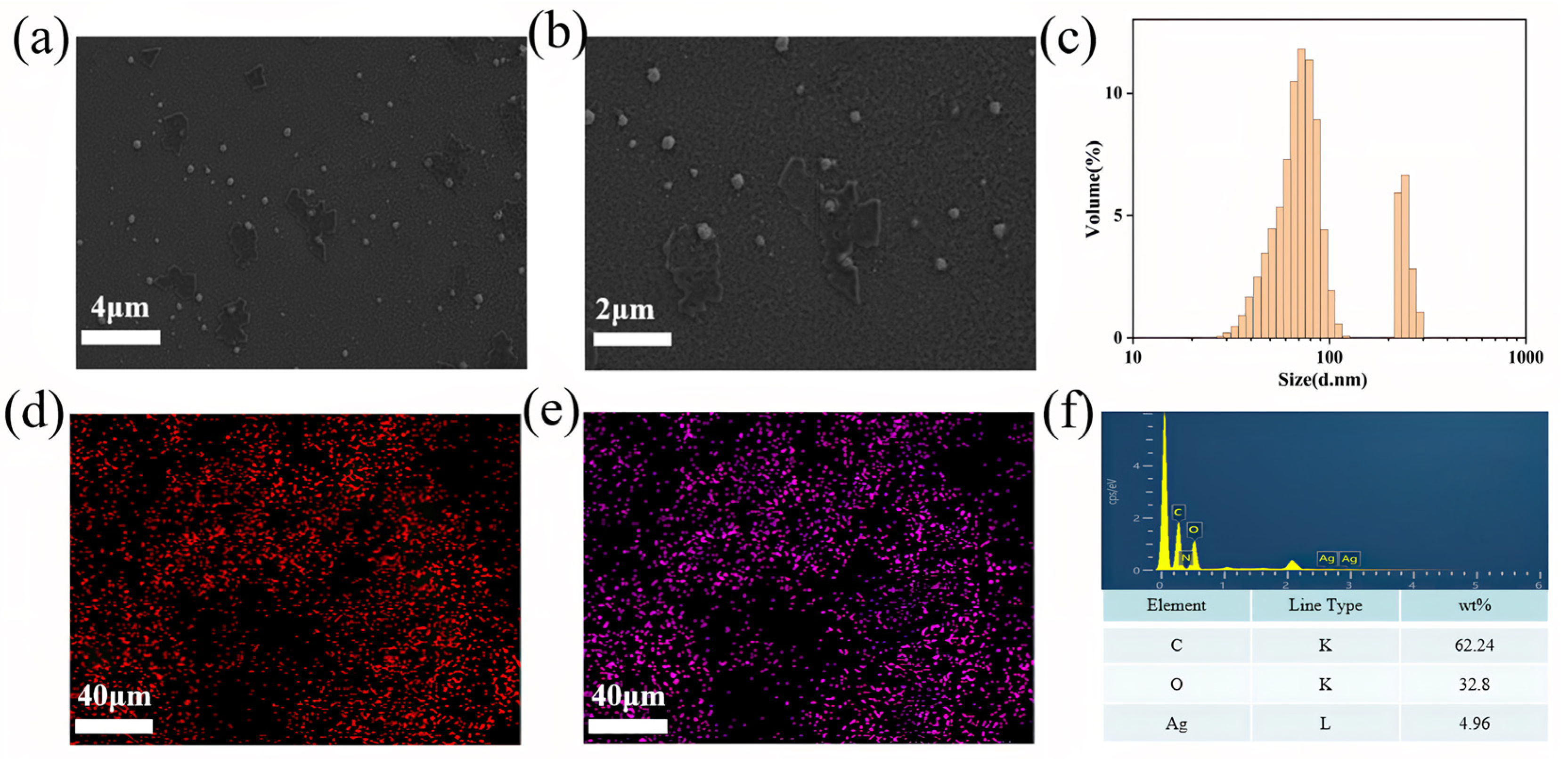
2.2. Characterization of Hydrogels
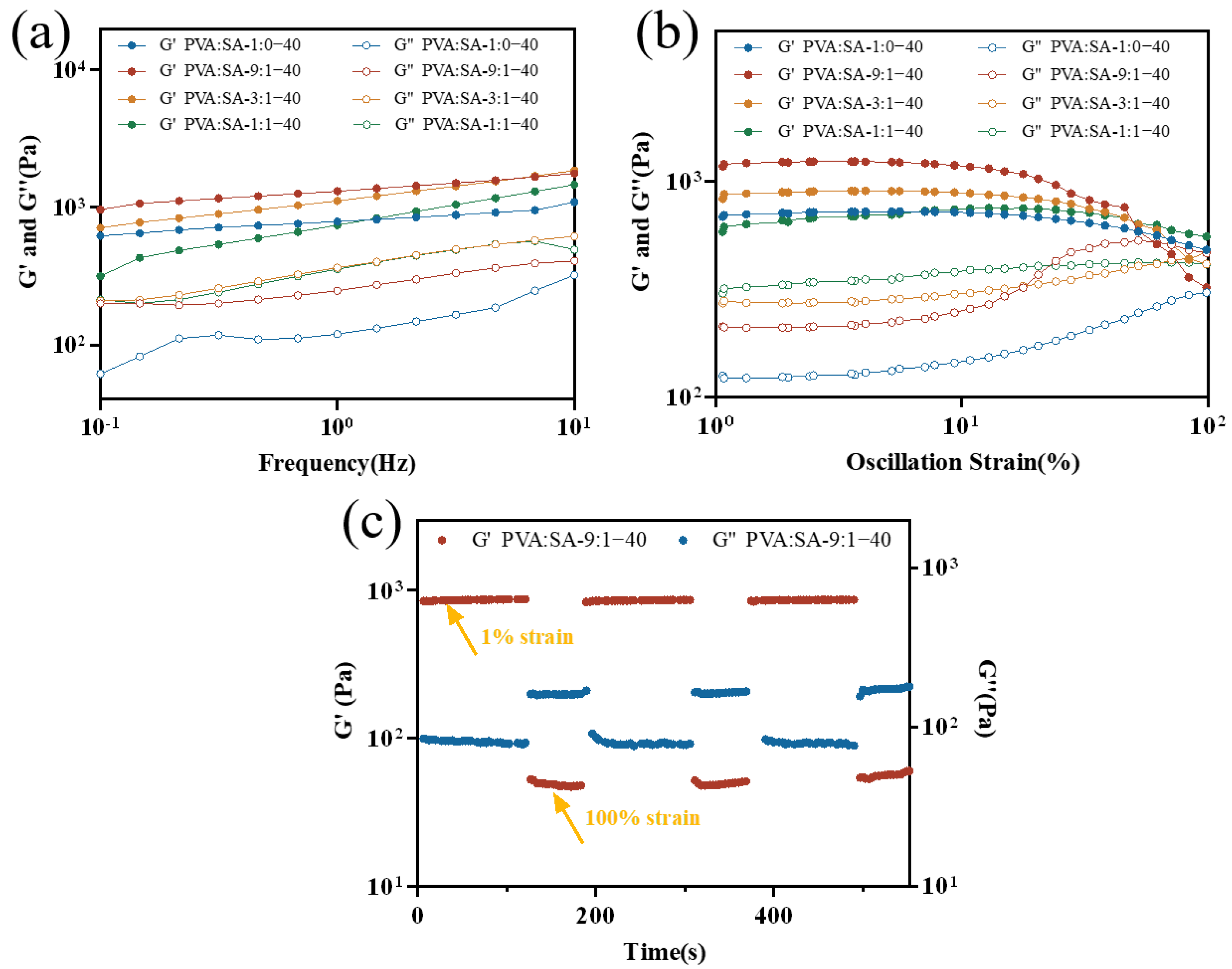
2.3. Rheological Properties
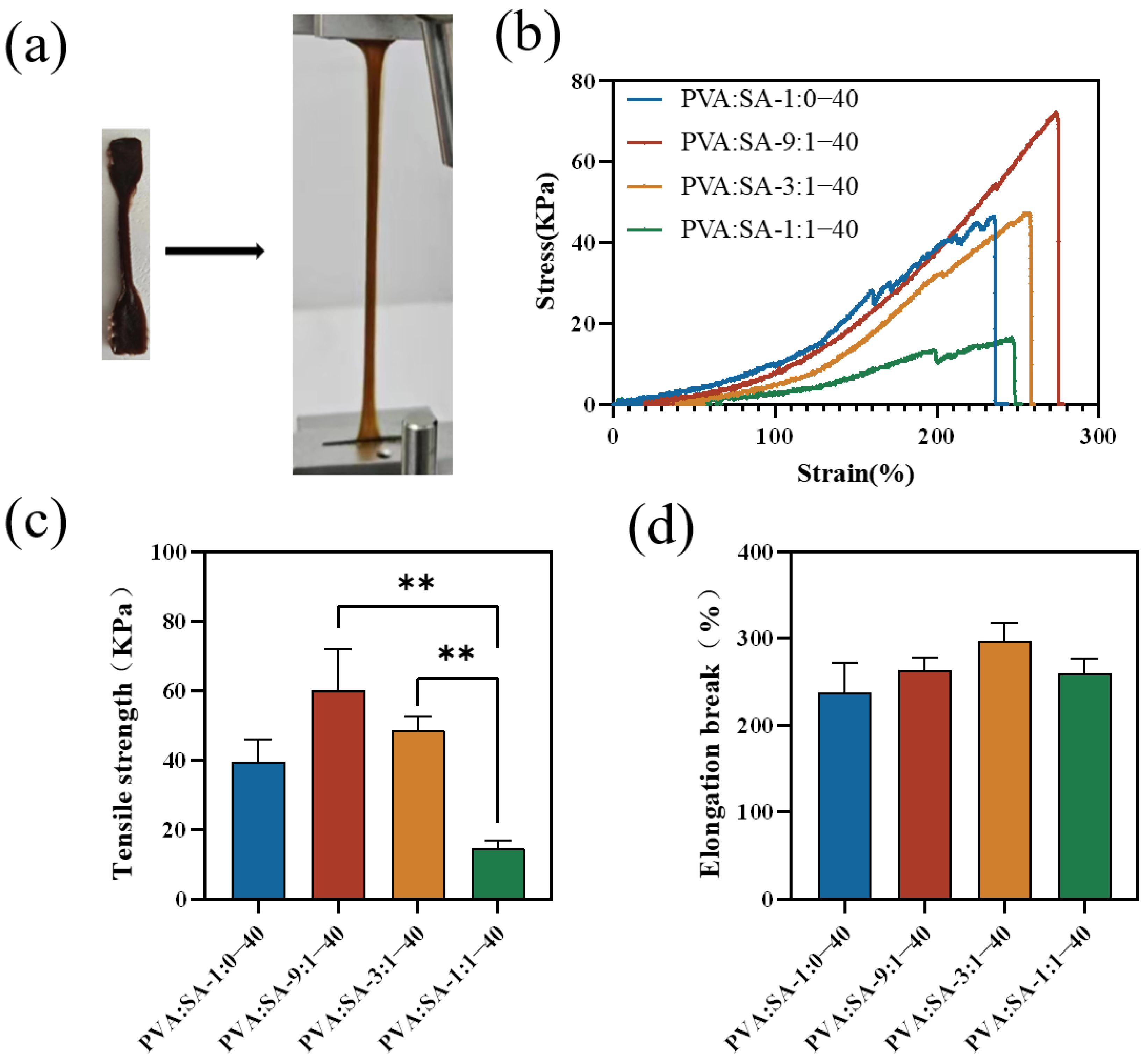
2.4. Mechanical Properties
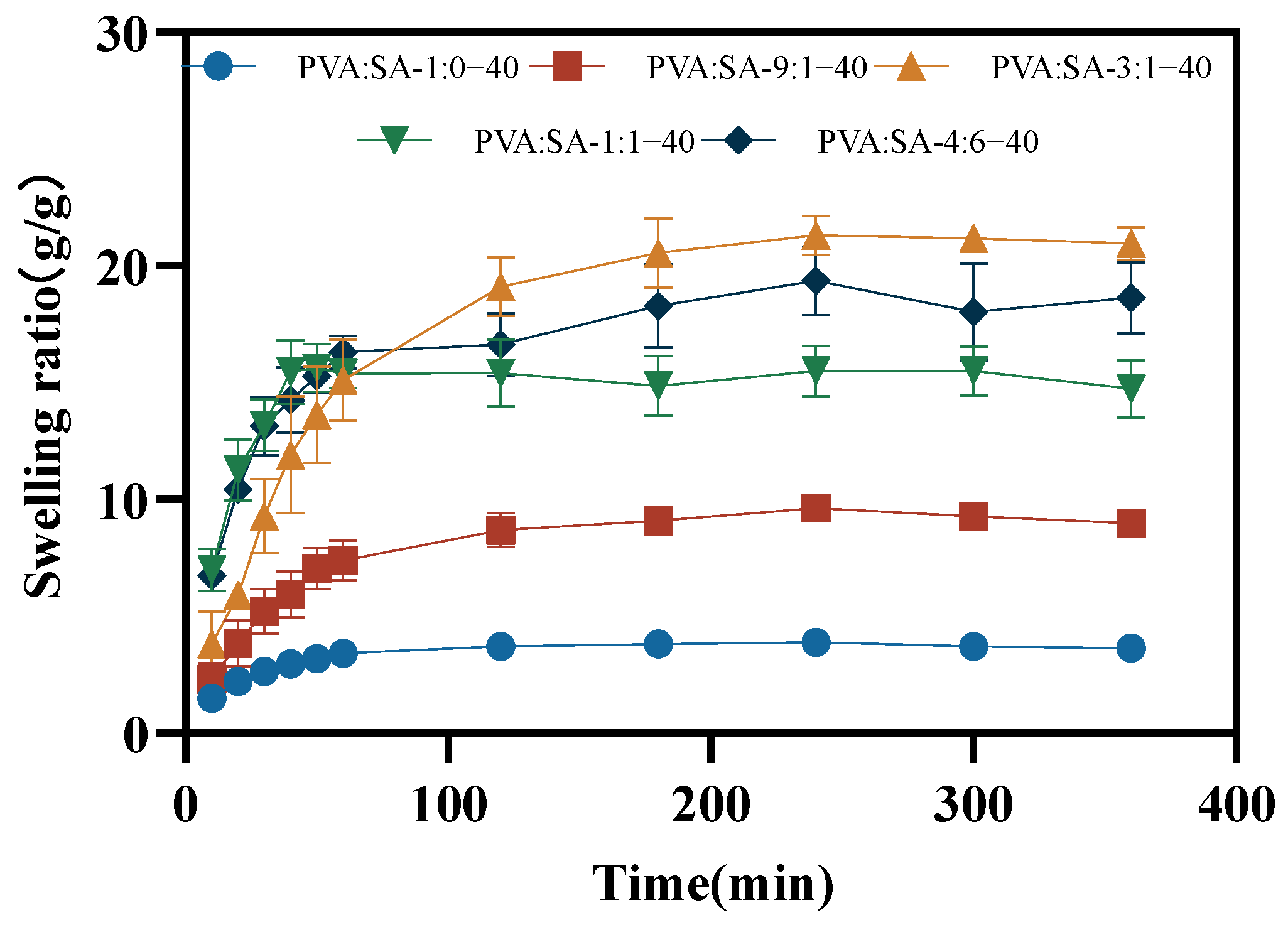
2.5. Swelling Properties
2.6. Antibacterial Properties
3. Conclusions
4. Materials and Methods
4.1. Materials and Chemicals
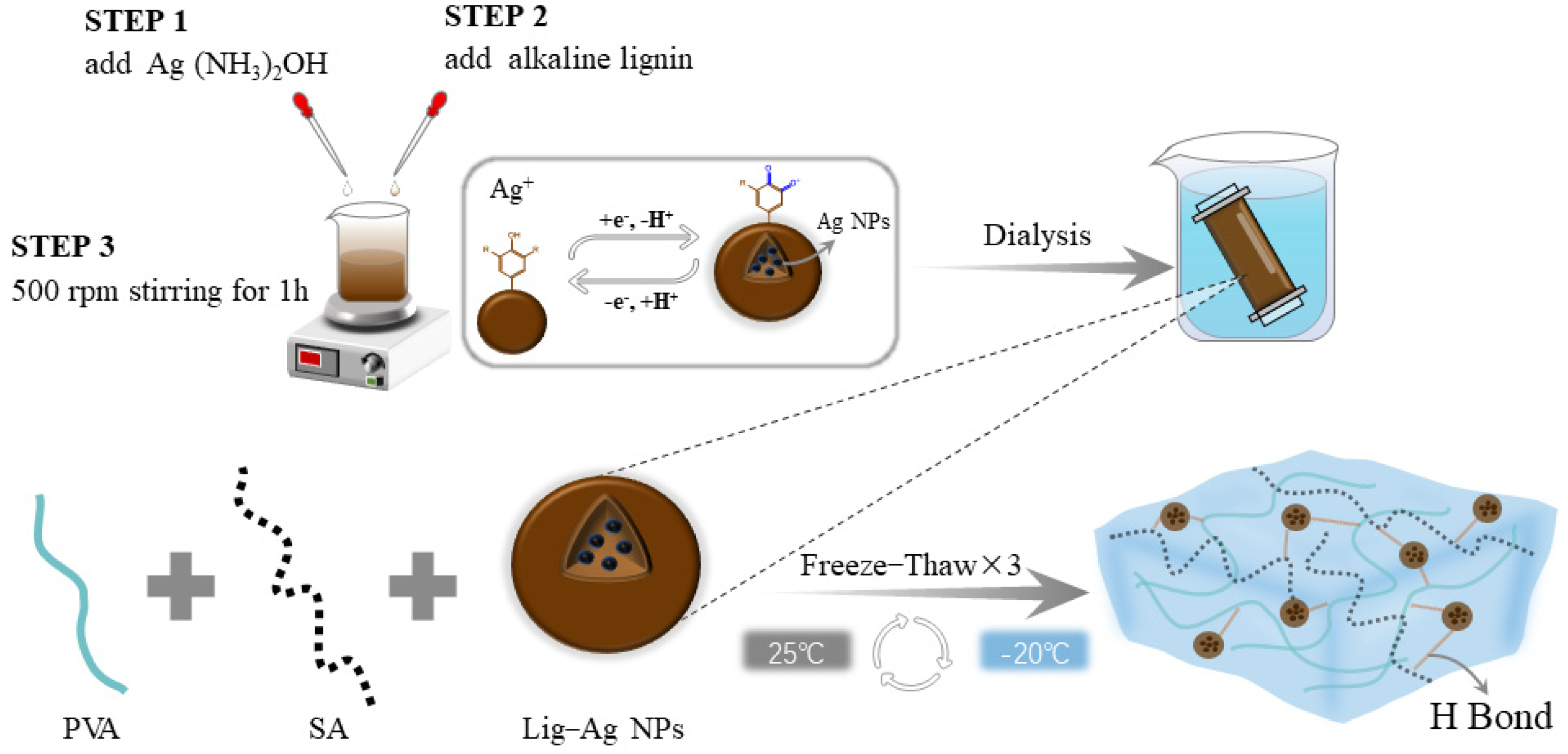
4.2. Synthesis of Lig-Ag NPs
4.3. Preparation of PVA/SA/Lig-Ag NPs Hydrogels
4.4. Characterization
4.5. Mechanical and Rheological Measurements
4.6. Swelling Test
4.7. Antibacterial Evaluation
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Allegranzi, B.; Bagheri Nejad, S.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet 2011, 377, 228–241. [Google Scholar] [PubMed]
- Potter, C.W. A history of influenza. J. Appl. Microbiol. 2001, 91, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Hooper, D.C. Hospital-Acquired Infections Due to Gram-Negative Bacteria. N. Engl. J. Med. 2010, 362, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Luo, Y.; Li, Z.; Tan, L.; Liu, X.; Li, C.; Zheng, Y.; Cui, Z.; Yeung, K.W.K.; Liang, Y.; et al. Antibacterial Hybrid Hydrogels. Macromol. Biosci. 2020, 21, 2000252. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, W.; Xu, Q.; Zheng, Y. Progress in Antibacterial Hydrogel Dressing. Gels 2022, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Timofejeva, A.; D’Este, M.; Loca, D. Calcium phosphate/polyvinyl alcohol composite hydrogels: A review on the freeze-thawing synthesis approach and applications in regenerative medicine. Eur. Polym. J. 2017, 95, 547–565. [Google Scholar]
- Kumar, A.; Sood, A.; Han, S.S. Poly (vinyl alcohol)-alginate as potential matrix for various applications: A focused review. Carbohydr. Polym. 2022, 277, 118881. [Google Scholar] [PubMed]
- Kim, J.O.; Park, J.K.; Kim, J.H.; Jin, S.G.; Yong, C.S.; Li, D.X.; Choi, J.Y.; Woo, J.S.; Yoo, B.K.; Lyoo, W.S.; et al. Development of polyvinyl alcohol-sodium alginate gel-matrix-based wound dressing system containing nitrofurazone. Int. J. Pharm. 2008, 359, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Muangsri, R.; Chuysinuan, P.; Thanyacharoen, T.; Techasakul, S.; Sukhavattanakul, P.; Ummartyotin, S. Utilization of freeze thaw process for polyvinyl alcohol/sodium alginate (PVA/SA) hydrogel composite. J. Met. Mater. Miner. 2022, 32, 34–41. [Google Scholar] [CrossRef]
- Ionita, M.; Pandele, M.A.; Iovu, H. Sodium alginate/graphene oxide composite films with enhanced thermal and mechanical properties. Carbohydr. Polym. 2013, 94, 339–344. [Google Scholar] [CrossRef]
- Seok, J.M.; Oh, S.H.; Lee, S.J.; Lee, J.H.; Kim, W.D.; Park, S.-H.; Nam, S.Y.; Shin, H.; Park, S.A. Fabrication and characterization of 3D scaffolds made from blends of sodium alginate and poly(vinyl alcohol). Mater. Today Commun. 2019, 19, 56–61. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Tamer, T.M.; El-Meligy, M.A.; Mohy Eldin, M.S. Poly (vinyl alcohol)-alginate physically crosslinked hydrogel membranes for wound dressing applications: Characterization and bio-evaluation. Arab. J. Chem. 2015, 8, 38–47. [Google Scholar] [CrossRef]
- Zhang, S.; Han, D.; Ding, Z.; Wang, X.; Zhao, D.; Hu, Y.; Xiao, X. Fabrication and Characterization of One Interpenetrating Network Hydrogel Based on Sodium Alginate and Polyvinyl Alcohol. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019, 34, 744–751. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial Hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef]
- Moritz, M.; Geszke-Moritz, M. The newest achievements in synthesis, immobilization and practical applications of antibacterial nanoparticles. Chem. Eng. J. 2013, 228, 596–613. [Google Scholar] [CrossRef]
- Tan, H.L.; Teow, S.Y.; Pushpamalar, J. Application of Metal Nanoparticle(-)Hydrogel Composites in Tissue Regeneration. Bioengineering 2019, 6, 17. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Guo, Z.; Xiao, Y.; Zhang, Y.; Sun, X.; Zhe, T.; Cao, Y.; Wang, L.; Lu, Q.; et al. Silver nanoparticle-embedded hydrogel as a photothermal platform for combating bacterial infections. Chem. Eng. J. 2020, 382, 122990. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, B.; Wang, J.; Zhang, S.; Lin, Q.; Gong, P.; Ma, L.; Yang, S. A Novel Wound Dressing Based on Ag/Graphene Polymer Hydrogel: Effectively Kill Bacteria and Accelerate Wound Healing. Adv. Funct. Mater. 2014, 24, 3933–3943. [Google Scholar] [CrossRef]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle-Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef]
- Juby, K.A.; Dwivedi, C.; Kumar, M.; Kota, S.; Misra, H.S.; Bajaj, P.N. Silver nanoparticle-loaded PVA/gum acacia hydrogel: Synthesis, characterization and antibacterial study. Carbohydr. Polym. 2012, 89, 906–913. [Google Scholar] [CrossRef]
- Stojkovska, J.; Kostić, D.; Jovanović, Ž.; Vukašinović-Sekulić, M.; Mišković-Stanković, V.; Obradović, B. A comprehensive approach to in vitro functional evaluation of Ag/alginate nanocomposite hydrogels. Carbohydr. Polym. 2014, 111, 305–314. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Han, S.S. Dual-crosslinked poly(vinyl alcohol)/sodium alginate/silver nanocomposite beads—A promising antimicrobial material. Food Chem. 2017, 234, 103–110. [Google Scholar] [CrossRef]
- Xu, L.; Li, X.; Takemura, T.; Hanagata, N.; Wu, G.; Chou, L.L. Genotoxicity and molecular response of silver nanoparticle (NP)-based hydrogel. J. Nanobiotechnol. 2012, 10, 16. [Google Scholar] [CrossRef]
- Kawata, K.; Osawa, M.; Okabe, S. In vitro toxicity of silver nanoparticles at noncytotoxic doses to HepG2 human hepatoma cells. Environ. Sci. Technol. 2009, 43, 6046–6051. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Y.; Jin, M.; Yuan, Z.; Bond, P.; Guo, J. Both silver ions and silver nanoparticles facilitate the horizontal transfer of plasmid-mediated antibiotic resistance genes. Water Res. 2020, 169, 115229. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal oxide nanoparticles as antimicrobial agents: A promise for the future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef]
- Chen, J.; An, L.; Bae, J.H.; Heo, J.W.; Han, S.Y.; Kim, Y.S. Green and facile synthesis of aminated lignin-silver complex and its antibacterial activity. Ind. Crops Prod. 2021, 173, 114102. [Google Scholar] [CrossRef]
- Gan, D.; Xing, W.; Jiang, L.; Fang, J.; Zhao, C.; Ren, F.; Fang, L.; Wang, K.; Lu, X. Plant-inspired adhesive and tough hydrogel based on Ag-Lignin nanoparticles-triggered dynamic redox catechol chemistry. Nat. Commun. 2019, 10, 1487. [Google Scholar] [CrossRef]
- Ran, F.; Li, C.; Hao, Z.; Zhang, X.; Dai, L.; Si, C.; Shen, Z.; Qiu, Z.; Wang, J. Combined bactericidal process of lignin and silver in a hybrid nanoparticle on E. coli. Adv. Compos. Hybrid Mater. 2022, 5, 1841–1851. [Google Scholar] [CrossRef]
- Han, X.; Lv, Z.; Ran, F.; Dai, L.; Li, C.; Si, C. Green and stable piezoresistive pressure sensor based on lignin-silver hybrid nanoparticles/polyvinyl alcohol hydrogel. Int. J. Biol. Macromol. 2021, 176, 78–86. [Google Scholar] [CrossRef]
- Yang, J.M.; Panda, P.K.; Jie, C.J.; Dash, P.; Chang, Y.H. Poly (vinyl alcohol)/chitosan/sodium alginate composite blended membrane: Preparation, characterization, and water-induced shape memory phenomenon. Polym. Eng. Sci. 2022, 62, 1526–1537. [Google Scholar] [CrossRef]
- Hsiao, Y.-C.; Lee, L.-C.; Lin, Y.-T.; Hong, S.-H.; Wang, K.-C.; Tung, S.-H.; Liu, C.-L. Stretchable polyvinyl alcohol and sodium alginate double network ionic hydrogels for low-grade heat harvesting with ultrahigh thermopower. Mater. Today Energy 2023, 37, 101383. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zhen, M.; Wu, Y.; Ma, M.; Cheng, Y.; Jin, Y. Effect of catechin and tannins on the structural and functional properties of sodium alginate/gelatin/ poly(vinylalcohol) blend films. Food Hydrocoll. 2023, 135, 108141. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Cai, J.; Huang, J.; Qiu, X. Equip the hydrogel with armor: Strong and super tough biomass reinforced hydrogels with excellent conductivity and anti-bacterial performance. J. Mater. Chem. A 2019, 7, 26917–26926. [Google Scholar] [CrossRef]
- Yesilyurt, V.; Webber, M.J.; Appel, E.A.; Godwin, C.; Langer, R.; Anderson, D.G. Injectable Self-Healing Glucose-Responsive Hydrogels with pH-Regulated Mechanical Properties. Adv. Mater. 2016, 28, 86–91. [Google Scholar] [CrossRef]
- Chen, Y.; Diaz-Dussan, D.; Wu, D.; Wang, W.; Peng, Y.Y.; Asha, A.B.; Hall, D.G.; Ishihara, K.; Narain, R. Bioinspired Self-Healing Hydrogel Based on Benzoxaborole-Catechol Dynamic Covalent Chemistry for 3D Cell Encapsulation. ACS Macro Lett. 2018, 7, 904–908. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Y.; Wang, Z.; Chen, S.; Ma, Y.; Wei, H.; Lü, S. An Anti-Swellable Hydrogel Strain Sensor for Underwater Motion Detection. Adv. Funct. Mater. 2021, 32, 2107404. [Google Scholar] [CrossRef]
- Lin, F.; Wang, Z.; Shen, Y.; Tang, L.; Zhang, P.; Wang, Y.; Chen, Y.; Huang, B.; Lu, B. Natural skin-inspired versatile cellulose biomimetic hydrogels. J. Mater. Chem. A 2019, 7, 26442–26455. [Google Scholar] [CrossRef]
- Jiang, X.; Xiang, N.; Zhang, H.; Sun, Y.; Lin, Z.; Hou, L. Preparation and characterization of poly(vinyl alcohol)/sodium alginate hydrogel with high toughness and electric conductivity. Carbohydr. Polym. 2018, 186, 377–383. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, T.; Chen, S.; Wang, X.; He, J.; Luo, Y. Construction and characterization of chitosan/poly(acrylamide-[2-(methacryloyloxy)ethyl]trimethylammonium chloride) double-network hydrogel with enhanced antibacterial activity. Adv. Compos. Hybrid Mater. 2023, 6, 192. [Google Scholar] [CrossRef]
- Huang, B.; Liu, W.; Lan, Y.; Huang, Y.; Fu, L.; Lin, B.; Xu, C. Highly ion-conducting, robust and environmentally stable poly(vinyl alcohol) eutectic gels designed by natural polyelectrolytes for flexible wearable sensors and supercapacitors. Chem. Eng. J. 2024, 480, 147888. [Google Scholar] [CrossRef]
- Liu, K.; Dai, L.; Li, C. A lignocellulose-based nanocomposite hydrogel with pH-sensitive and potent antibacterial activity for wound healing. Int. J. Biol. Macromol. 2021, 191, 1249–1254. [Google Scholar] [CrossRef]
- Liu, J.; He, H.; Yu, Z.; Suryawanshi, A.; Li, Y.; Lin, X.; Sun, Z. Investigation of temperature-responsive and thermo-physiological comfort of modified polyester fabric with Sericin/PNIPAAm/Ag NPs interpenetrating polymer network hydrogel. Text. Res. J. 2020, 90, 2622–2638. [Google Scholar] [CrossRef]
- Cao, C.; Yang, N.; Zhao, Y.; Yang, D.; Hu, Y.; Yang, D.; Song, X.; Wang, W.; Dong, X. Biodegradable hydrogel with thermo-response and hemostatic effect for photothermal enhanced anti-infective therapy. Nano Today 2021, 39, 101165. [Google Scholar] [CrossRef]
- Duan, C.; Liu, C.; Meng, X.; Gao, K.; Lu, W.; Zhang, Y.; Dai, L.; Zhao, W.; Xiong, C.; Wang, W.; et al. Facile synthesis of Ag NPs@ MIL-100(Fe)/guar gum hybrid hydrogel as a versatile photocatalyst for wastewater remediation: Photocatalytic degradation, water/oil separation and bacterial inactivation. Carbohydr Polym 2020, 230, 115642. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Xu, L.; Yan, J.; Wang, X. Preparation and Properties of Temperature-Sensitive Silver-Loaded Antibacterial Sericin/poly (N-isopropylacrylamide) Hydrogel. J. Macromol. Sci. Part B 2023, 1–20. [Google Scholar] [CrossRef]
- Liu, C.; Ling, J.; Yang, L.Y.; Ouyang, X.K.; Wang, N. Chitosan-based carbon nitride-polydopamine-silver composite dressing with antibacterial properties for wound healing. Carbohydr. Polym. 2023, 303, 120436. [Google Scholar] [CrossRef]
- Kandile, N.G.; Elzamly, R.A.; Mohamed, M.I.; Zaky, H.T.; Harding, D.R.K.; Mohamed, H.M. New sustainable antimicrobial chitosan hydrogels based on sulfonamides and its nanocomposites: Fabrication and characterization. Int. J. Biol. Macromol. 2023, 239, 124280. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Ran, F.; Li, C.; Hao, Z.; He, H.; Dai, L.; Wang, J.; Yang, W. A Lignin Silver Nanoparticles/Polyvinyl Alcohol/Sodium Alginate Hybrid Hydrogel with Potent Mechanical Properties and Antibacterial Activity. Gels 2024, 10, 240. https://doi.org/10.3390/gels10040240
Yu J, Ran F, Li C, Hao Z, He H, Dai L, Wang J, Yang W. A Lignin Silver Nanoparticles/Polyvinyl Alcohol/Sodium Alginate Hybrid Hydrogel with Potent Mechanical Properties and Antibacterial Activity. Gels. 2024; 10(4):240. https://doi.org/10.3390/gels10040240
Chicago/Turabian StyleYu, Jie, Fangli Ran, Chenyu Li, Zhenxin Hao, Haodong He, Lin Dai, Jingfeng Wang, and Wenjuan Yang. 2024. "A Lignin Silver Nanoparticles/Polyvinyl Alcohol/Sodium Alginate Hybrid Hydrogel with Potent Mechanical Properties and Antibacterial Activity" Gels 10, no. 4: 240. https://doi.org/10.3390/gels10040240
APA StyleYu, J., Ran, F., Li, C., Hao, Z., He, H., Dai, L., Wang, J., & Yang, W. (2024). A Lignin Silver Nanoparticles/Polyvinyl Alcohol/Sodium Alginate Hybrid Hydrogel with Potent Mechanical Properties and Antibacterial Activity. Gels, 10(4), 240. https://doi.org/10.3390/gels10040240