Alginate-Based Emulsions and Hydrogels for Extending the Shelf Life of Banana Fruit
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Coating Formulations
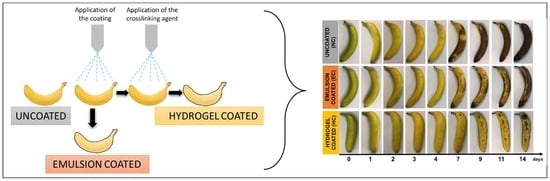
2.2. Effect of Coating Application on Fruit Ripening
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Edible Coating Formulation
4.3. Edible Coating Characterization
4.4. Shelf Life of Coated Fruit
4.5. Sensory Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baez-Sañudo, M.; Siller-Cepeda, J.; Muy-Rangel, D.; Heredia, J.B. Extending the shelf-life of bananas with 1-methylcyclopropene and a chitosan-based edible coating. J. Sci. Food Agric. 2009, 89, 2343–2349. [Google Scholar] [CrossRef]
- Al-Dairi, M.; Pathare, P.B.; Al-Yahyai, R.; Jayasuriya, H.; Al-Attabi, Z. Postharvest quality, technologies, and strategies to reduce losses along the supply chain of banana: A review. Trends Food Sci. Technol. 2023, 134, 177–191. [Google Scholar] [CrossRef]
- Kotiyal, A.; Singh, P. Applications of Edible Coatings to Extend Shelf-life of Fresh Fruits. In Food Process Engineering and Technology: Safety, Packaging, Nanotechnologies and Human Health; Malik, J.A., Goyal, M.R., Kumari, A., Eds.; Springer Nature Singapore: Singapore, 2023; pp. 99–118. [Google Scholar]
- Zaritzky, N. Edible coatings to improve food quality and safety. In Food Engineering Interfaces; Springer: New York, NY, USA, 2011; pp. 631–659. [Google Scholar]
- Dwivany, F.M.; Aprilyandi, A.N.; Suendo, V.; Sukriandi, N. Carrageenan edible coating application prolongs Cavendish banana shelf life. Int. J. Food Sci. 2020, 2020, 8861610. [Google Scholar] [CrossRef]
- Andrade, R.D.; Skurtys, O.; Osorio, F.A. Atomizing spray systems for application of edible coatings. Compr. Rev. Food Sci. Food Saf. 2012, 11, 323–337. [Google Scholar] [CrossRef]
- Cofelice, M.; Lopez, F.; Cuomo, F. Quality control of fresh-cut apples after coating application. Foods 2019, 8, 189. [Google Scholar] [CrossRef]
- Song, H.; Jang, A.R.; Lee, S.; Lee, S.-Y. Application of sodium alginate-based edible coating with citric acid to improve the safety and quality of fresh-cut melon (Cucumis melo L.) during cold storage. Food Sci. Biotechnol. 2024. [Google Scholar] [CrossRef]
- Turner, D.W.; Fortescue, J.A. Bananas (Musa spp.). In Crop Post-Harvest: Science and Technology; Blackwell Publishing Ltd.: Oxford, UK, 2012; pp. 24–42. [Google Scholar]
- Cofelice, M.; Iftikhar, A.; Lopez, F.; De Leonardis, A. Effect of edible coatings on quality parameters and phenol composition of ready-to-eat Salanova lettuce. Eur. Food Res. Technol. 2023, 250, 691–700. [Google Scholar] [CrossRef]
- Cofelice, M.; Cinelli, G.; Lopez, F.; Di Renzo, T.; Coppola, R.; Reale, A. Alginate-assisted lemongrass (Cymbopogon nardus) essential oil dispersions for antifungal activity. Foods 2021, 10, 1528. [Google Scholar] [CrossRef]
- Ahmed, Z.F.R.; Taha, E.M.A.; Abdelkareem, N.A.A.; Mohamed, W.M. Postharvest Properties of Unripe Bananas and the Potential of Producing Economic Nutritious Products. Int. J. Fruit Sci. 2020, 20, S995–S1014. [Google Scholar] [CrossRef]
- Kittur, F.; Saroja, N.; Habibunnisa; Tharanathan, R. Polysaccharide-based composite coating formulations for shelf-life extension of fresh banana and mango. Eur. Food Res. Technol. 2001, 213, 306–311. [Google Scholar] [CrossRef]
- Gol, N.B.; Ramana Rao, T. Banana fruit ripening as influenced by edible coatings. Int. J. Fruit Sci. 2011, 11, 119–135. [Google Scholar] [CrossRef]
- Maqbool, M.; Ali, A.; Alderson, P.G.; Zahid, N.; Siddiqui, Y. Effect of a novel edible composite coating based on gum arabic and chitosan on biochemical and physiological responses of banana fruits during cold storage. J. Agric. Food Chem. 2011, 59, 5474–5482. [Google Scholar] [CrossRef] [PubMed]
- La, D.D.; Nguyen-Tri, P.; Le, K.H.; Nguyen, P.T.; Nguyen, M.D.-B.; Vo, A.T.; Nguyen, M.T.; Chang, S.W.; Tran, L.D.; Chung, W.J. Effects of antibacterial ZnO nanoparticles on the performance of a chitosan/gum arabic edible coating for post-harvest banana preservation. Prog. Org. Coat. 2021, 151, 106057. [Google Scholar] [CrossRef]
- Lustriane, C.; Dwivany, F.M.; Suendo, V.; Reza, M. Effect of chitosan and chitosan-nanoparticles on post harvest quality of banana fruits. J. Plant Biotechnol. 2018, 45, 36–44. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Bowyer, M.; Singh, S.P.; Scarlett, C.J.; Stathopoulos, C.E.; Vuong, Q.V. A starch edible surface coating delays banana fruit ripening. LWT-Food Sci. Technol. 2019, 100, 341–347. [Google Scholar] [CrossRef]
- Deng, P.; Zhang, Y.; Deng, Q.; Sun, Y.; Li, Y.; Wang, Z.; Jiang, F. Multifunctional sodium alginate-based self-healing edible cross-linked coating for banana preservation. Food Hydrocoll. 2024, 151, 109753. [Google Scholar] [CrossRef]
- Yang, Z.; Li, M.; Zhai, X.; Zhao, L.; Tahir, H.E.; Shi, J.; Zou, X.; Huang, X.; Li, Z.; Xiao, J. Development and characterization of sodium alginate/tea tree essential oil nanoemulsion active film containing TiO2 nanoparticles for banana packaging. Int. J. Biol. Macromol. 2022, 213, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yu, M.; Wang, L. Physical and antimicrobial properties of sodium alginate/carboxymethyl cellulose films incorporated with cinnamon essential oil. Food Packag. Shelf Life 2018, 15, 35–42. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Castle, L.; Engel, K.-H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; et al. Safety assessment of titanium dioxide (E171) as a food additive. Efsa J. 2021, 19, e06585. [Google Scholar] [CrossRef] [PubMed]
- Senturk Parreidt, T.; Schott, M.; Schmid, M.; Müller, K. Effect of Presence and Concentration of Plasticizers, Vegetable Oils, and Surfactants on the Properties of Sodium-Alginate-Based Edible Coatings. Int. J. Mol. Sci. 2018, 19, 742. [Google Scholar] [CrossRef]
- Watharkar, R.B.; Pu, Y.; Ismail, B.B.; Srivastava, B.; Srivastav, P.P.; Liu, D. Change in physicochemical characteristics and volatile compounds during different stage of banana (Musa nana Lour vs. Dwarf Cavendish) Ripening. J. Food Meas. Charact. 2020, 14, 2040–2050. [Google Scholar] [CrossRef]
- Chen, C.; Ramaswamy, H. Color and texture change kinetics in ripening bananas. LWT-Food Sci. Technol. 2002, 35, 415–419. [Google Scholar] [CrossRef]
- Jaiswal, P.; Jha, S.N.; Kaur, P.P.; Bhardwaj, R.; Singh, A.K.; Wadhawan, V. Prediction of textural attributes using color values of banana (Musa sapientum) during ripening. J. Food Sci. Technol. 2014, 51, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Hakim, K.A.; Khairul, I.; Ibrahim, M.; Jamal, H.; Nure, A.; Kazi, M.; Faisal, H. Status of the behavrioral patteren of biochemoical properties of banana in storage condition. Int. J. Biosci. 2012, 2, 83–94. [Google Scholar]
- Cofelice, M.; Cuomo, F.; Chiralt, A. Alginate films encapsulating lemongrass essential oil as affected by spray calcium application. Colloids Interfaces 2019, 3, 58. [Google Scholar] [CrossRef]
- Sadler, G.D.; Murphy, P.A. pH and titratable acidity. Food Anal. 2010, 4, 219–238. [Google Scholar]
- Gafuma, S.; Byarugaba-Bazirake, G.; Mugampoza, E. Textural hardness of selected Ugandan banana cultivars under different processing treatments. J. Food Res. 2018, 7, 98–111. [Google Scholar] [CrossRef]







| L* | a* | b* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Days | NC | EC | HC | NC | EC | HC | NC | EC | HC |
| 0 | 72.35 ± 1.20 Ab | 71.14 ± 1.33 Aa | 73.42 ± 1.43 Aa | −5.42 ± 0.97 Ad | −5.02 ± 0.84 Ae | −5.93 ± 0.73 Ad | 35.76 ± 1.67 Ac | 36.46 ± 1.41 Ade | 33.14 ± 1.32 Bf |
| 1 | 71.51 ± 0.72 Ab | 70.16 ± 1.36 Aa | 71.37 ± 1.05 Aa | −5.90 ± 0.48 Ad | −6.17 ± 0.48 Af | −6.60 ± 0.44 Ae | 35.65 ± 1.45 Ac | 35.12 ± 1.18 Ae | 34.89 ± 1.71 Af |
| 2 | 72.33 ± 1.53 Ab | 72.01 ± 1.63 Aa | 71.33 ± 0.58 Aa | −1.65 ± 0.14 Ac | −3.89 ± 0.41 Bd | −6.48 ± 0.42 Ce | 36.81 ± 1.42 ABc | 34.67 ± 1.04 Be | 37.95 ± 1.76 Ae |
| 3 | 72.65 ± 1.11 Ab | 69.84 ± 1.01 Ba | 71.43 ± 0.80 Aa | 0.49 ± 0.34 Aa | −1.28 ± 0.34 Bc | −6.41 ± 0.24 Ce | 41.64 ± 1.19 Ab | 37.26 ± 1.24 Bd | 39.39 ± 1.10 ABde |
| 4 | 75.30 ± 0.93 Aa | 71.47 ± 1.06 Ba | 72.47 ± 1.10 Ba | 1.22 ± 0.12 Aa | −1.04 ± 0.03 Bc | −5.48 ± 0.25 Cd | 42.12 ± 1.03 Ab | 42.58 ± 1.06 Ac | 41.16 ± 1.19 Acd |
| 7 | 65.98 ± 2.27 Ac | 61.96 ± 1.44 Bb | 64.89 ± 0.13 Ab | 1.05 ± 0.65 Aa | 0.21 ± 0.34 Aab | −2.72 ± 0.31 Bc | 50.40 ± 2.46 Aa | 44.07 ± 2.24 Bbc | 42.27 ± 1.23 Bc |
| 9 | 57.61 ± 0.86 Cd | 63.67 ± 1.21 Bb | 67.29 ± 1.85 Ab | 1.19 ± 0.30 Aa | −0.27 ± 0.28 Bb | −1.74 ± 0.24 Cb | 50.44 ± 1.35 Aa | 47.64 ± 1.16 Bb | 47.00 ± 1.70 Bb |
| 11 | 55.61 ± 0.14 Ce | 60.33 ± 0.62 Bb | 64.96 ± 1.71 Ab | 1.45 ± 0.73 Aa | 0.91 ± 0.40 Aa | −0.77 ± 0.24 Ba | 50.28 ± 1.37 Aa | 50.43 ± 1.21 Aa | 48.43 ± 1.46 Aab |
| 14 | 53.28 ± 0.66 Cf | 55.67 ± 0.92 Bc | 62.62 ± 1.51 Ab | 1.30 ± 0.77 Aa | 0.89 ± 0.39 Aa | −0.61 ± 0.49 Ba | 50.89 ± 1.42 Aa | 50.60 ± 1.89 Aa | 50.43 ± 1.24 Aa |
| WL | TA | TSS | Firmness | |
|---|---|---|---|---|
| ΔE | 0.931 ** | −0.924 ** | 0.755 * | −0.923 * |
| WL | −0.976 ** | 0.796 * | −0.934 * | |
| TA | −0.821 * | 0.928 * | ||
| TSS | −0.870 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacovino, S.; Cofelice, M.; Sorrentino, E.; Cuomo, F.; Messia, M.C.; Lopez, F. Alginate-Based Emulsions and Hydrogels for Extending the Shelf Life of Banana Fruit. Gels 2024, 10, 245. https://doi.org/10.3390/gels10040245
Iacovino S, Cofelice M, Sorrentino E, Cuomo F, Messia MC, Lopez F. Alginate-Based Emulsions and Hydrogels for Extending the Shelf Life of Banana Fruit. Gels. 2024; 10(4):245. https://doi.org/10.3390/gels10040245
Chicago/Turabian StyleIacovino, Silvio, Martina Cofelice, Elena Sorrentino, Francesca Cuomo, Maria Cristina Messia, and Francesco Lopez. 2024. "Alginate-Based Emulsions and Hydrogels for Extending the Shelf Life of Banana Fruit" Gels 10, no. 4: 245. https://doi.org/10.3390/gels10040245