Hypromellose-, Gelatin- and Gellan Gum-Based Gel Films with Chlorhexidine for Potential Application in Oral Inflammatory Diseases
Abstract
1. Introduction
2. Results and Discussion
2.1. Formulation Optimization
2.2. Water Loss after Drying
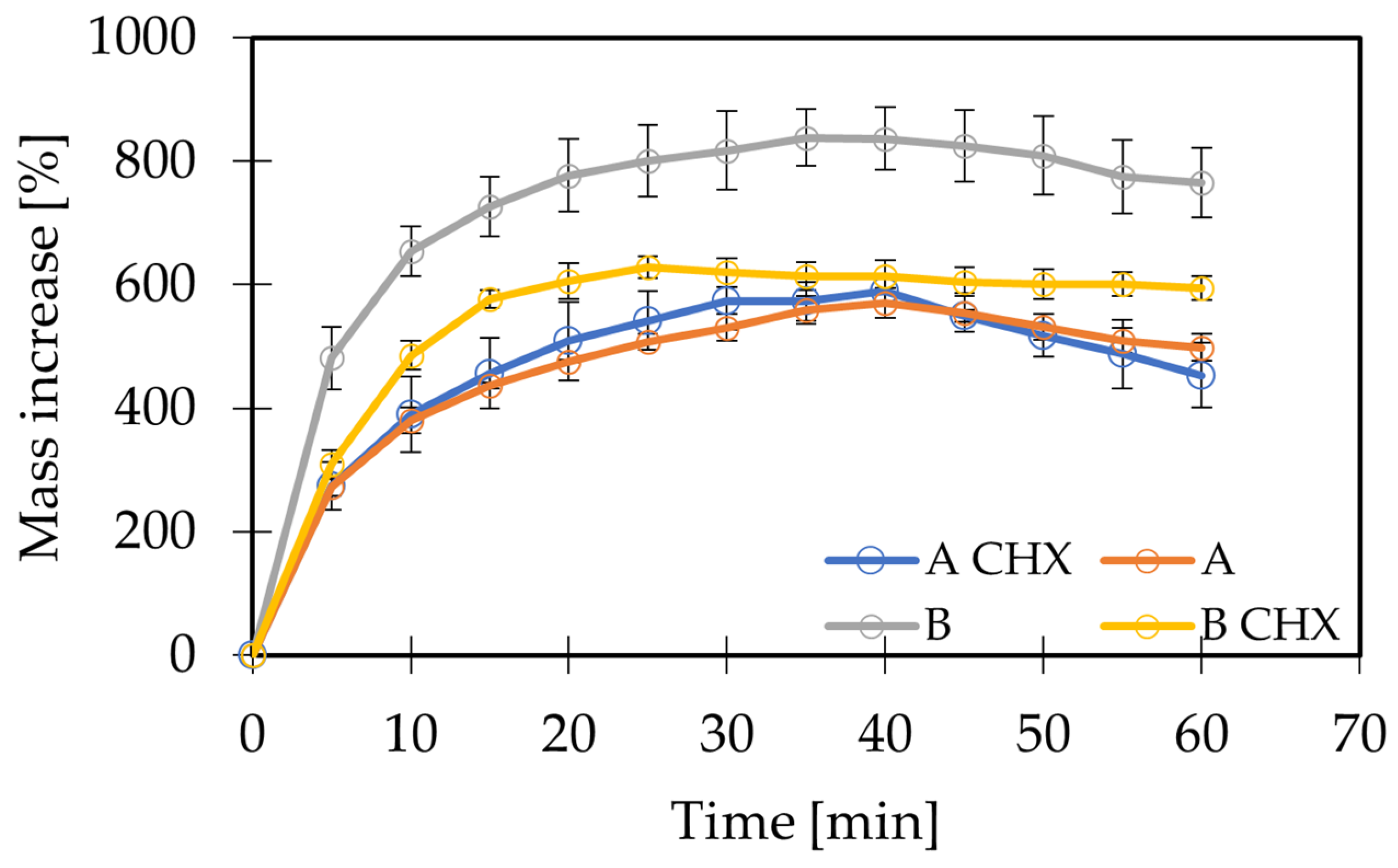
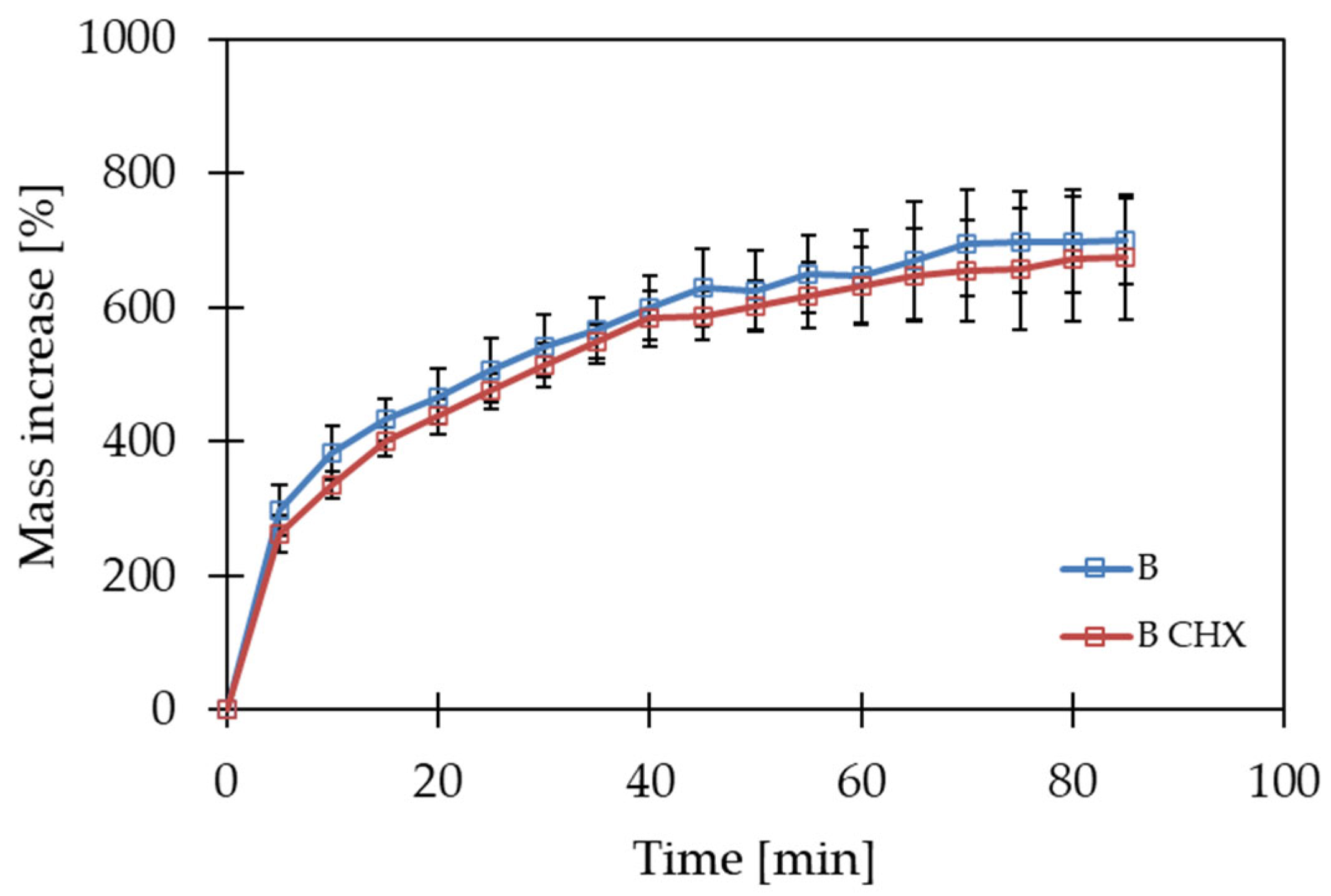
2.3. Swelling Analysis
2.4. Disintegration Time
2.5. Hygroscopicity Study
2.6. Tensile Strength
3. Conclusions
4. Materials and Methods
4.1. Polymer Film Preparation
4.2. Water Loss after Drying
4.3. Obtaining Film with Chlorhexidine
4.4. Characterization of Polymer Films
4.4.1. Swelling Study
4.4.2. Disintegration Time
4.4.3. Hygroscopicity of Films
4.4.4. Mechanical Strength
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shetty, S.S.; Maruthi, M.; Dhara, V.; de Arruda, J.A.A.; Abreu, L.G.; Mesquita, R.A.; Teixeira, A.L.; Silva, T.A.; Merchant, Y. Oral Mucositis: Current Knowledge and Future Directions. Dis. -A-Mon. 2022, 68, 101300. [Google Scholar] [CrossRef]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The Oral Microbiome: Role of Key Organisms and Complex Networks in Oral Health and Disease. Periodontology 2000 2021, 87, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Okada, K.; Kondo, M.; Matsushita, T.; Nakazawa, S.; Yamazaki, Y. Oral Health for Achieving Longevity. Geriatr. Gerontol. Int. 2020, 20, 526–538. [Google Scholar] [CrossRef]
- Tiensripojamarn, N.; Lertpimonchai, A.; Tavedhikul, K.; Udomsak, A.; Vathesatogkit, P.; Sritara, P.; Charatkulangkun, O. Periodontitis Is Associated with Cardiovascular Diseases: A 13-Year Study. J. Clin. Periodontol. 2021, 48, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, V.W.; Persson, G.R.; Berglund, J.S.; Renvert, S. Periodontitis Related to Cardiovascular Events and Mortality: A Long-Time Longitudinal Study. Clin. Oral Investig. 2021, 25, 4085–4095. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, G.M.; Horowitz, R.A.; Johnson, R.; Prestiano, R.A.; Klein, B.I. The Systemic Oral Health Connection: Biofilms. Medicine 2022, 101, e30517. [Google Scholar] [CrossRef]
- Sanz, M.; Marco del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and Cardiovascular Diseases: Consensus Report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, M.; Kozarov, E.; Song, H.; Whitlock, J.; Progulske-Fox, A. Both the Unique and Repeat Regions of the Porphyromonas Gingivalis Hemagglutin A Are Involved in Adhesion and Invasion of Host Cells. Anaerobe 2012, 18, 128–134. [Google Scholar] [CrossRef]
- Naderi, S.; Merchant, A.T. The Association Between Periodontitis and Cardiovascular Disease: An Update. Curr. Atheroscler. Rep. 2020, 22, 52. [Google Scholar] [CrossRef]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef]
- Kikutani, T.; Tamura, F.; Tashiro, H.; Yoshida, M.; Konishi, K.; Hamada, R. Relationship between Oral Bacteria Count and Pneumonia Onset in Elderly Nursing Home Residents. Geriatr. Gerontol. Int. 2015, 15, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Shay, K. Infectious Complications of Dental and Periodontal Diseases in the Elderly Population. Clin. Infect. Dis. 2002, 34, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Pulito, C.; Cristaudo, A.; Porta, C.L.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral Mucositis: The Hidden Side of Cancer Therapy. J. Exp. Clin. Cancer Res. 2020, 39, 210. [Google Scholar] [CrossRef] [PubMed]
- Trotti, A.; Bellm, L.A.; Epstein, J.B.; Frame, D.; Fuchs, H.J.; Gwede, C.K.; Komaroff, E.; Nalysnyk, L.; Zilberberg, M.D. Mucositis Incidence, Severity and Associated Outcomes in Patients with Head and Neck Cancer Receiving Radiotherapy with or without Chemotherapy: A Systematic Literature Review. Radiother. Oncol. 2003, 66, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Kusiak, A.; Jereczek-Fossa, B.A.; Cichońska, D.; Alterio, D. Oncological-Therapy Related Oral Mucositis as an Interdisciplinary Problem—Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 2464. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis Secondary to Cancer Therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.Á.; Warnakulasuriya, S.; González-Ruiz, I.; González-Ruiz, L.; Ayén, Á.; Lenouvel, D.; Ruiz-Ávila, I.; Ramos-García, P. Worldwide Prevalence of Oral Lichen Planus: A Systematic Review and Meta-Analysis. Oral Dis. 2021, 27, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.Z.; Bruce, A.J.; Rogers, R.S. Recurrent Aphthous Stomatitis. Clin. Dermatol. 2016, 34, 475–481. [Google Scholar] [CrossRef]
- Yogarajah, S.; Setterfield, J. Mouth Ulcers and Diseases of the Oral Cavity. Medicine 2021, 49, 407–413. [Google Scholar] [CrossRef]
- Jain, N.; Dutt, U.; Radenkov, I.; Jain, S. WHO’s Global Oral Health Status Report 2022: Actions, Discussion and Implementation. Oral Dis. 2024, 30, 73–79. [Google Scholar] [CrossRef]
- Poppolo Deus, F.; Ouanounou, A. Chlorhexidine in Dentistry: Pharmacology, Uses, and Adverse Effects. Int. Dent. J. 2022, 72, 269–277. [Google Scholar] [CrossRef]
- Bescos, R.; Ashworth, A.; Cutler, C.; Brookes, Z.L.; Belfield, L.; Rodiles, A.; Casas-Agustench, P.; Farnham, G.; Liddle, L.; Burleigh, M.; et al. Effects of Chlorhexidine Mouthwash on the Oral Microbiome. Sci. Rep. 2020, 10, 5254. [Google Scholar] [CrossRef]
- Puig-Asensio, M.; Marra, A.R.; Childs, C.A.; Perencevich, E.N.; Schweizer, M.L. Chlorhexidine Dressings to Prevent Catheter-Related Bloodstream Infections: A Systematic Literature Review and Meta-Analysis. Infect. Control Hosp. Epidemiol. 2020, 41, s165–s166. [Google Scholar] [CrossRef]
- Azzopardi, A.; Trapani, J. Chlorhexidine-Based versus Non-Chlorhexidine Dressings to Prevent Catheter-Related Bloodstream Infections: An Evidence-Based Review. Nurs. Crit. Care 2024, 29, 191–195. [Google Scholar] [CrossRef]
- Cheong, J.Z.A.; Liu, A.; Rust, C.J.; Tran, C.L.; Hassan, S.E.; Kalan, L.R.; Gibson, A.L.F. Robbing Peter to Pay Paul: Chlorhexidine Gluconate Demonstrates Short-Term Efficacy and Long-Term Cytotoxicity. Wound Repair Regen. 2022, 30, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Van den Poel, B.; Saegeman, V.; Schuermans, A. Increasing Usage of Chlorhexidine in Health Care Settings: Blessing or Curse? A Narrative Review of the Risk of Chlorhexidine Resistance and the Implications for Infection Prevention and Control. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 349–362. [Google Scholar] [CrossRef]
- Steinberg, D.; Friedman, M. Sustained-Release Delivery of Antimicrobial Drugs for the Treatment of Periodontal Diseases: Fantasy or Already Reality? Periodontology 2000 2020, 84, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.C.; Cunha, D.; Ferreira, D.C.; Reis, S.; Costa, P.J. Oromucosal Precursors of in Loco Hydrogels for Wound-Dressing and Drug Delivery in Oral Mucositis: Retain, Resist, and Release. Mater. Sci. Eng. C 2021, 118, 111413. [Google Scholar] [CrossRef] [PubMed]
- Nafee, N.A.; Ismail, F.A.; Boraie, N.A.; Mortada, L.M. Mucoadhesive Delivery Systems. I. Evaluation of Mucoadhesive Polymers for Buccal Tablet Formulation. Drug Dev. Ind. Pharm. 2004, 30, 985–993. [Google Scholar] [CrossRef]
- Ahmady, A.; Abu Samah, N.H. A Review: Gelatine as a Bioadhesive Material for Medical and Pharmaceutical Applications. Int. J. Pharm. 2021, 608, 121037. [Google Scholar] [CrossRef]
- Szekalska, M.; Citkowska, A.; Wróblewska, M.; Winnicka, K. The Impact of Gelatin on the Pharmaceutical Characteristics of Fucoidan Microspheres with Posaconazole. Materials 2021, 14, 4087. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Chetoni, P.; Giunchedi, P.; Rossi, S.; Ferrari, F.; Burgalassi, S.; Caramella, C. Carrageenan–Gelatin Mucoadhesive Systems for Ion-Exchange Based Ophthalmic Delivery: In Vitro and Preliminary In Vivo Studies. Eur. J. Pharm. Biopharm. 2004, 57, 465–472. [Google Scholar] [CrossRef]
- Li, A.; Khan, I.N.; Khan, I.U.; Yousaf, A.M.; Shahzad, Y. Gellan Gum-Based Bilayer Mucoadhesive Films Loaded with Moxifloxacin Hydrochloride and Clove Oil for Possible Treatment of Periodontitis. Drug Des. Dev. Ther. 2021, 15, 3937–3952. [Google Scholar] [CrossRef]
- de Oliveira Cardoso, V.M.; de Brito, N.A.P.; Ferreira, N.N.; Boni, F.I.; Ferreira, L.M.B.; Carvalho, S.G.; Gremião, M.P.D. Design of Mucoadhesive Gellan Gum and Chitosan Nanoparticles Intended for Colon-Specific Delivery of Peptide Drugs. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127321. [Google Scholar] [CrossRef]
- Gadziński, P.; Froelich, A.; Jadach, B.; Wojtyłko, M.; Tatarek, A.; Białek, A.; Krysztofiak, J.; Gackowski, M.; Otto, F.; Osmałek, T. Ionotropic Gelation and Chemical Crosslinking as Methods for Fabrication of Modified-Release Gellan Gum-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 108. [Google Scholar] [CrossRef]
- Borges, A.F.; Silva, C.; Coelho, J.F.J.; Simões, S. Oral Films: Current Status and Future Perspectives. J. Control. Release 2015, 206, 1–19. [Google Scholar] [CrossRef]
- Dixit, R.P.; Puthli, S.P. Oral Strip Technology: Overview and Future Potential. J. Control. Release 2009, 139, 94–107. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, M.; Li, G. Preparation and Characterization of Collagen/Hydroxypropyl Methylcellulose (HPMC) Blend Film. Carbohydr. Polym. 2015, 119, 194–201. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Leroux, J.-C. In Situ-Forming Hydrogels—Review of Temperature-Sensitive Systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef]
- Kowalski, Z.; Banach, M.; Makara, A. Preparation of Low Temperature Strongly Gelling Protein (Gelatine) by Chemical Methods. Chemik 2011, 65, 1085–1092. [Google Scholar]
- Foox, M.; Zilberman, M. Drug Delivery from Gelatin-Based Systems. Expert Opin. Drug Deliv. 2015, 12, 1547–1563. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and Bioactive Properties of Collagen and Gelatin from Alternative Sources: A Review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Morris, E.R.; Nishinari, K.; Rinaudo, M. Gelation of Gellan—A Review. Food Hydrocoll. 2012, 28, 373–411. [Google Scholar] [CrossRef]
- Kirchmajer, D.M.; Steinhoff, B.; Warren, H.; Clark, R.; in het Panhuis, M. Enhanced Gelation Properties of Purified Gellan Gum. Carbohydr. Res. 2014, 388, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Zia, K.M.; Tabasum, S.; Khan, M.F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent Trends on Gellan Gum Blends with Natural and Synthetic Polymers: A Review. Int. J. Biol. Macromol. 2018, 109, 1068–1087. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Amin, K.A.M.; Razali, M.H. Preparation of Gellan Gum (GG) Film: The Effect of GG, Calcium Chloride (CaCl2), Glycerol Concentration and Heat Treatment. IOP Conf. Ser. Mater. Sci. Eng. 2018, 440, 012006. [Google Scholar] [CrossRef]
- Swain, G.P.; Patel, S.; Gandhi, J.; Shah, P. Development of Moxifloxacin Hydrochloride Loaded In-Situ Gel for the Treatment of Periodontitis: In-Vitro Drug Release Study and Antibacterial Activity. J. Oral Biol. Craniofac. Res. 2019, 9, 190–200. [Google Scholar] [CrossRef]
- Tedesco, M.P.; Monaco-Lourenço, C.A.; Carvalho, R.A. Gelatin/Hydroxypropyl Methylcellulose Matrices—Polymer Interactions Approach for Oral Disintegrating Films. Mater. Sci. Eng. C 2016, 69, 668–674. [Google Scholar] [CrossRef]
- Colombo, P.; Bettini, R.; Santi, P.; Peppas, N.A. Swellable Matrices for Controlled Drug Delivery: Gel-Layer Behaviour, Mechanisms and Optimal Performance. Pharm. Sci. Technol. Today 2000, 3, 198–204. [Google Scholar] [CrossRef]
- Osmałek, T.Z.; Froelich, A.; Jadach, B.; Krakowski, M. Rheological Investigation of High-Acyl Gellan Gum Hydrogel and Its Mixtures with Simulated Body Fluids. J. Biomater. Appl. 2018, 32, 1435–1449. [Google Scholar] [CrossRef]
- Trastullo, R.; Abruzzo, A.; Saladini, B.; Gallucci, M.C.; Cerchiara, T.; Luppi, B.; Bigucci, F. Design and Evaluation of Buccal Films as Paediatric Dosage Form for Transmucosal Delivery of Ondansetron. Eur. J. Pharm. Biopharm. 2016, 105, 115–121. [Google Scholar] [CrossRef]
- Salunke, S.R.; Patil, S.B. Ion Activated in Situ Gel of Gellan Gum Containing Salbutamol Sulphate for Nasal Administration. Int. J. Biol. Macromol. 2016, 87, 41–47. [Google Scholar] [CrossRef]
- Gandhi, K. Formulation and Evaluation of Sol-Gel Drug Delivery System for Intracanal pH Sensitive Controlled Delivery of Chlorhexidine. JCDR 2017, 11, ZC68–ZC72. [Google Scholar] [CrossRef]
- Paolicelli, P.; Petralito, S.; Varani, G.; Nardoni, M.; Pacelli, S.; Di Muzio, L.; Tirillò, J.; Bartuli, C.; Cesa, S.; Casadei, M.A.; et al. Effect of Glycerol on the Physical and Mechanical Properties of Thin Gellan Gum Films for Oral Drug Delivery. Int. J. Pharm. 2018, 547, 226–234. [Google Scholar] [CrossRef]
- Allada, R.; Maruthapillai, A.; Palanisamy, K.; Chappa, P. Hygroscopicity Categorization of Pharmaceutical Solids by Gravimetric Sorption Analysis: A Systematic Approach. Asian J. Pharm. 2016, 10, 279–286. [Google Scholar] [CrossRef]
- Liew, K.B.; Tan, Y.T.F.; Peh, K.-K. Effect of Polymer, Plasticizer and Filler on Orally Disintegrating Film. Drug Dev. Ind. Pharm. 2014, 40, 110–119. [Google Scholar] [CrossRef]
- Adrover, A.; Varani, G.; Paolicelli, P.; Petralito, S.; Di Muzio, L.; Casadei, M.A.; Tho, I. Experimental and Modeling Study of Drug Release from HPMC-Based Erodible Oral Thin Films. Pharmaceutics 2018, 10, 222. [Google Scholar] [CrossRef]
- Peh, K.K.; Wong, C.F. Polymeric Films as Vehicle for Buccal Delivery: Swelling, Mechanical, and Bioadhesive Properties. J. Pharm. Pharm. Sci. 1999, 2, 53–61. [Google Scholar]
- Juliano, C.; Cossu, M.; Pigozzi, P.; Rassu, G.; Giunchedi, P. Preparation, In Vitro Characterization and Preliminary In Vivo Evaluation of Buccal Polymeric Films Containing Chlorhexidine. AAPS PharmSciTech 2008, 9, 1153–1158. [Google Scholar] [CrossRef]
- Characters Section in Monographs. In European Pharmacopeia; Council of Europe: Strasbourg, France, 2005; Volume 1, p. 565.


| Formulation | HPMC [%, w/w] | Gelatin [%, w/w] | Gellan Gum [%, w/w] | Glycerol [%, w/w] | Water [%, w/w] |
|---|---|---|---|---|---|
| F1 | 1.5 | - | - | - | 98.5 |
| F2 | 1.5 | 0.5 | - | 5.0 | 93.0 |
| F3 | 1.5 | 1.0 | - | 5.0 | 92.5 |
| F4 | 1.5 | 1.5 | - | 5.0 | 92.0 |
| F5 | 1.5 | - | 1 | 5.0 | 92.5 |
| F6 | 1.5 | - | 0.5 | 5.0 | 93.0 |
| F7 | 1.5 | - | 0.1 | 5.0 | 93.4 |
| F8 | 1.5 | - | 0.05 | 5.0 | 93.45 |
| Formulation | HPMC [%, w/w] | Gelatin [%, w/w] | Gellan Gum [%, w/w] | Glycerol [%, w/w] | 1% CHX Solution [%, w/w] | Water [%, w/w] |
|---|---|---|---|---|---|---|
| A(CHX) | 1.5 | 1.0 | - | 5.0 | 0.7 | 92.50 |
| B(CHX) | 1.5 | - | 0.05 | 5.0 | 0.63 | 92.82 |
| Formulation | Water Loss after Drying [%] * |
|---|---|
| A | 92.94 ± 0.04 |
| B | 93.71 ± 0.50 |
| Sample | Disintegration Time [min] |
|---|---|
| A | 106 ± 9 |
| A(CHX) | 77 ± 5 |
| B | 170 ± 17 |
| B(CHX) | >180 |
| B (AS) | 152 ± 16 |
| B(CHX) (AS) | >180 |
| Type of Film | Force [N] | Displacement [mm] | Elongation at Break [%] |
|---|---|---|---|
| A | 33.2 ± 2.4 | 24.0 ± 2.3 | 180.0 ± 7.7 |
| A(CHX) | 15.5 ± 1.9 | 14.8 ± 1.5 | 149.3 ± 5.0 |
| B | 22.9 ± 0.7 | 20.0± 2.4 | 166.7 ± 8.0 |
| B(CHX) | 8.7 ± 2.0 | 15.8 ± 2.8 | 152.7 ± 9.3 |
| Ingredient | Amount [g] |
|---|---|
| NaCl | 0.125 |
| KCl | 0.964 |
| KH2PO4 | 0.654 |
| Urea | 0.2 |
| NaHCO3 | 0.631 |
| Mucin | 10.0 |
| Water | ad 1000.0 |
| Hygroscopicity | Mass Increase |
|---|---|
| Soluble under the influence of air humidity | The sample absorbs moisture until it dissolves in the absorbed water |
| Large | ≥15% |
| Medium | ≥2% and <15% |
| Small | ≥0.2% and <2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtyłko, M.; Froelich, A.; Jadach, B. Hypromellose-, Gelatin- and Gellan Gum-Based Gel Films with Chlorhexidine for Potential Application in Oral Inflammatory Diseases. Gels 2024, 10, 265. https://doi.org/10.3390/gels10040265
Wojtyłko M, Froelich A, Jadach B. Hypromellose-, Gelatin- and Gellan Gum-Based Gel Films with Chlorhexidine for Potential Application in Oral Inflammatory Diseases. Gels. 2024; 10(4):265. https://doi.org/10.3390/gels10040265
Chicago/Turabian StyleWojtyłko, Monika, Anna Froelich, and Barbara Jadach. 2024. "Hypromellose-, Gelatin- and Gellan Gum-Based Gel Films with Chlorhexidine for Potential Application in Oral Inflammatory Diseases" Gels 10, no. 4: 265. https://doi.org/10.3390/gels10040265
APA StyleWojtyłko, M., Froelich, A., & Jadach, B. (2024). Hypromellose-, Gelatin- and Gellan Gum-Based Gel Films with Chlorhexidine for Potential Application in Oral Inflammatory Diseases. Gels, 10(4), 265. https://doi.org/10.3390/gels10040265