Cytotoxicity and Degradation Resistance of Cryo- and Hydrogels Based on Carboxyethylchitosan at Different pH Values
Abstract
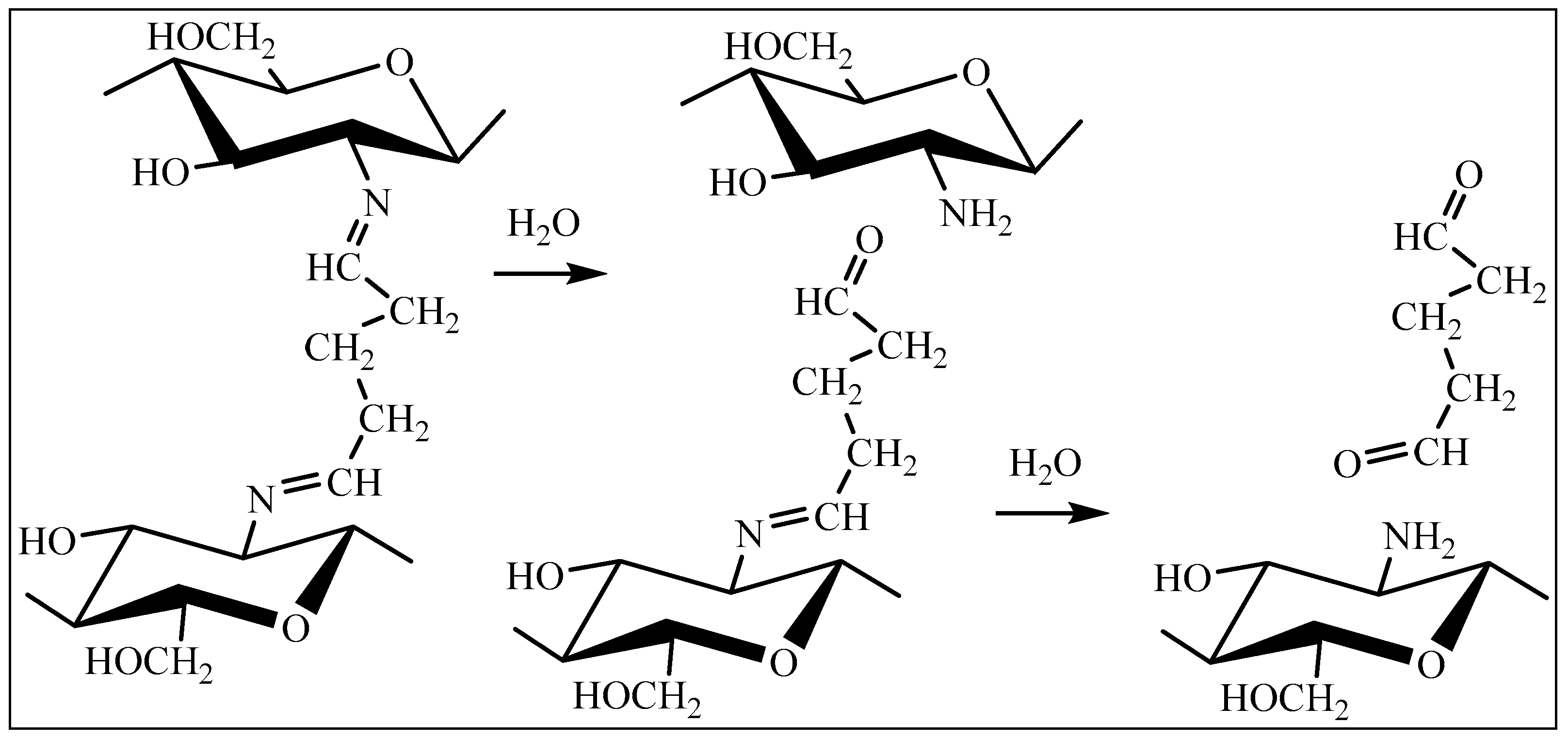
1. Introduction
2. Results and Discussion
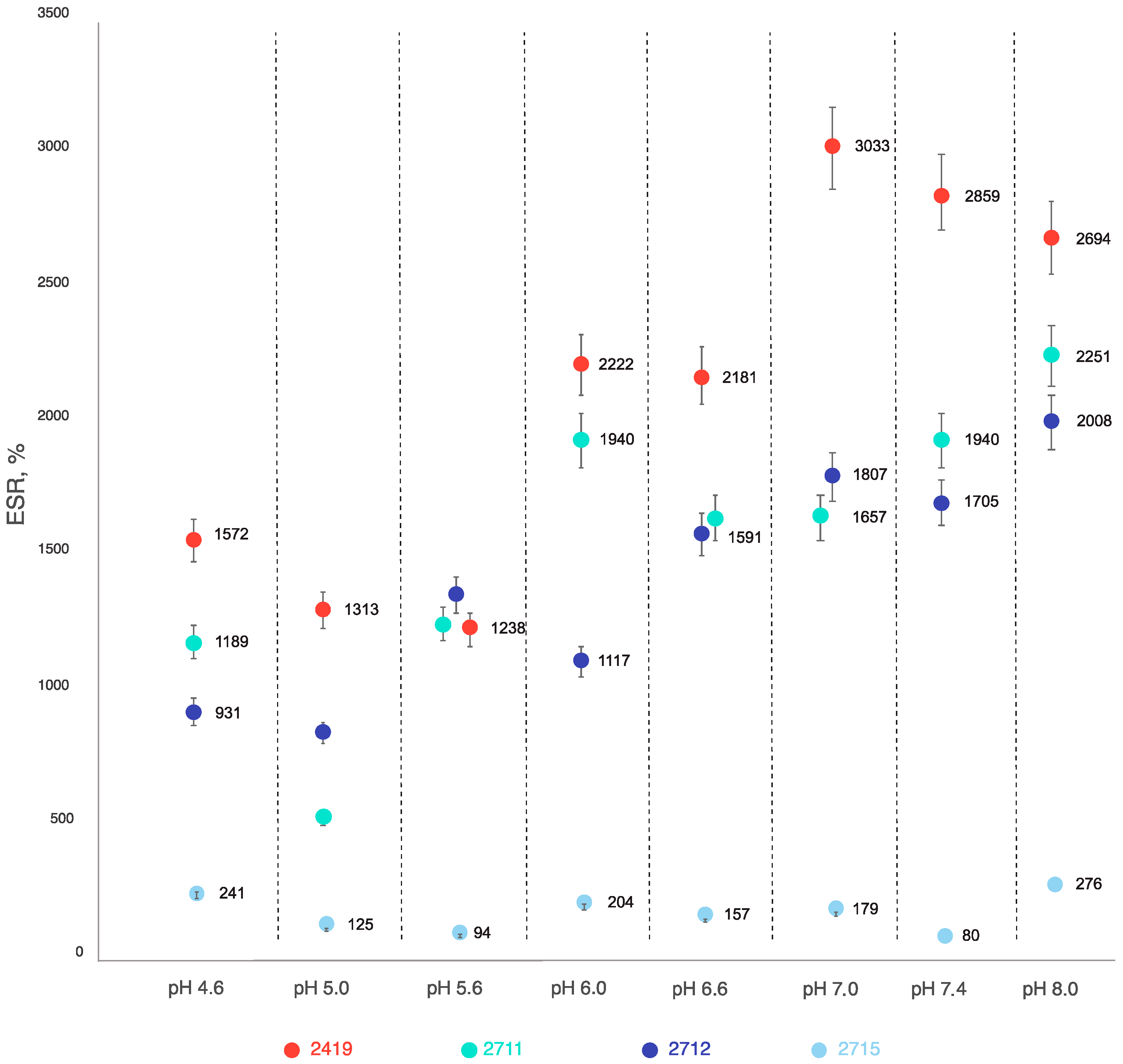
2.1. Stability and Swelling Properties of Sample 2419 Gel and Its Modifications—Samples 2711, 2712, and 2715 at Different pH Values at Room Temperature
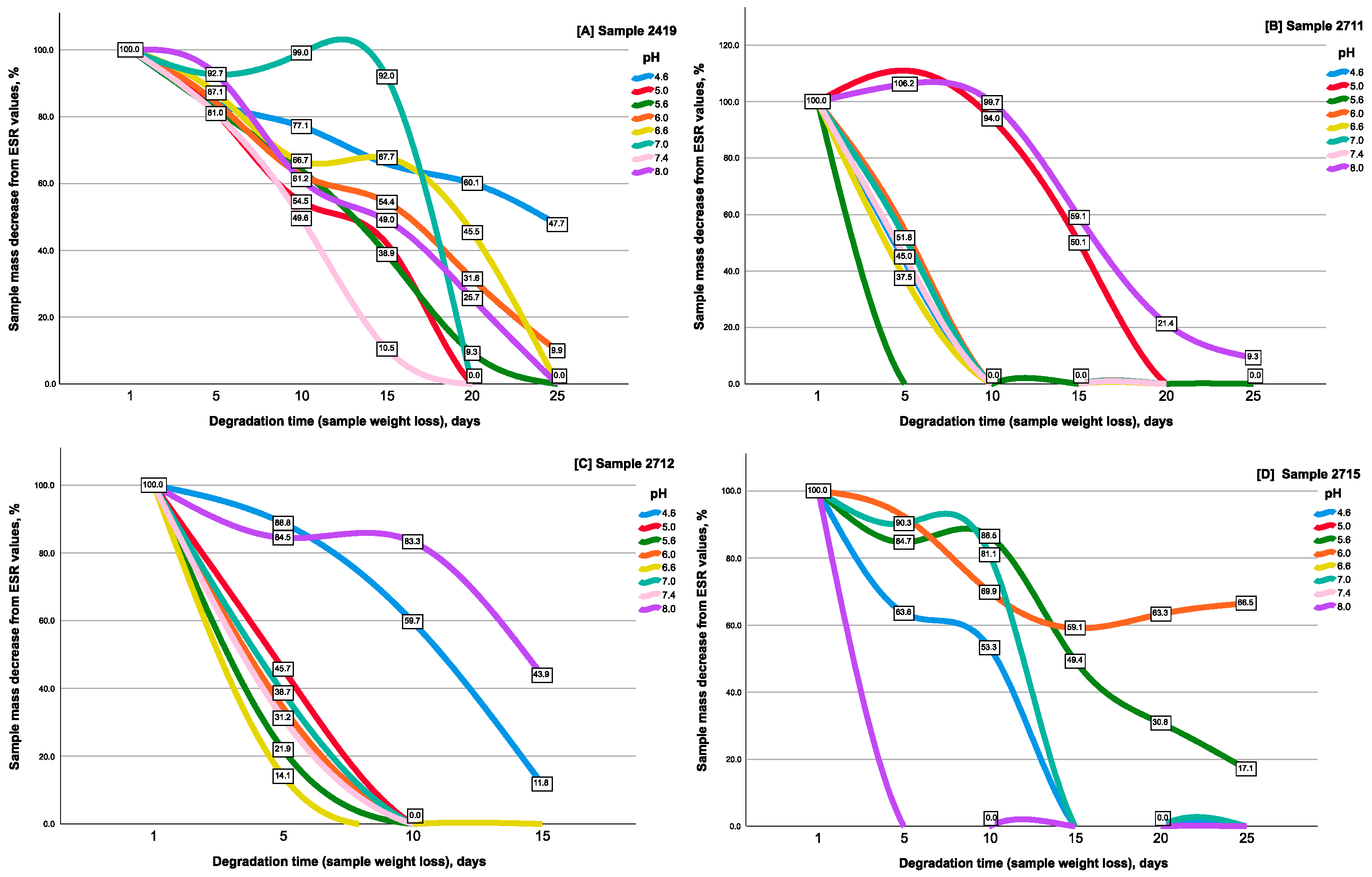
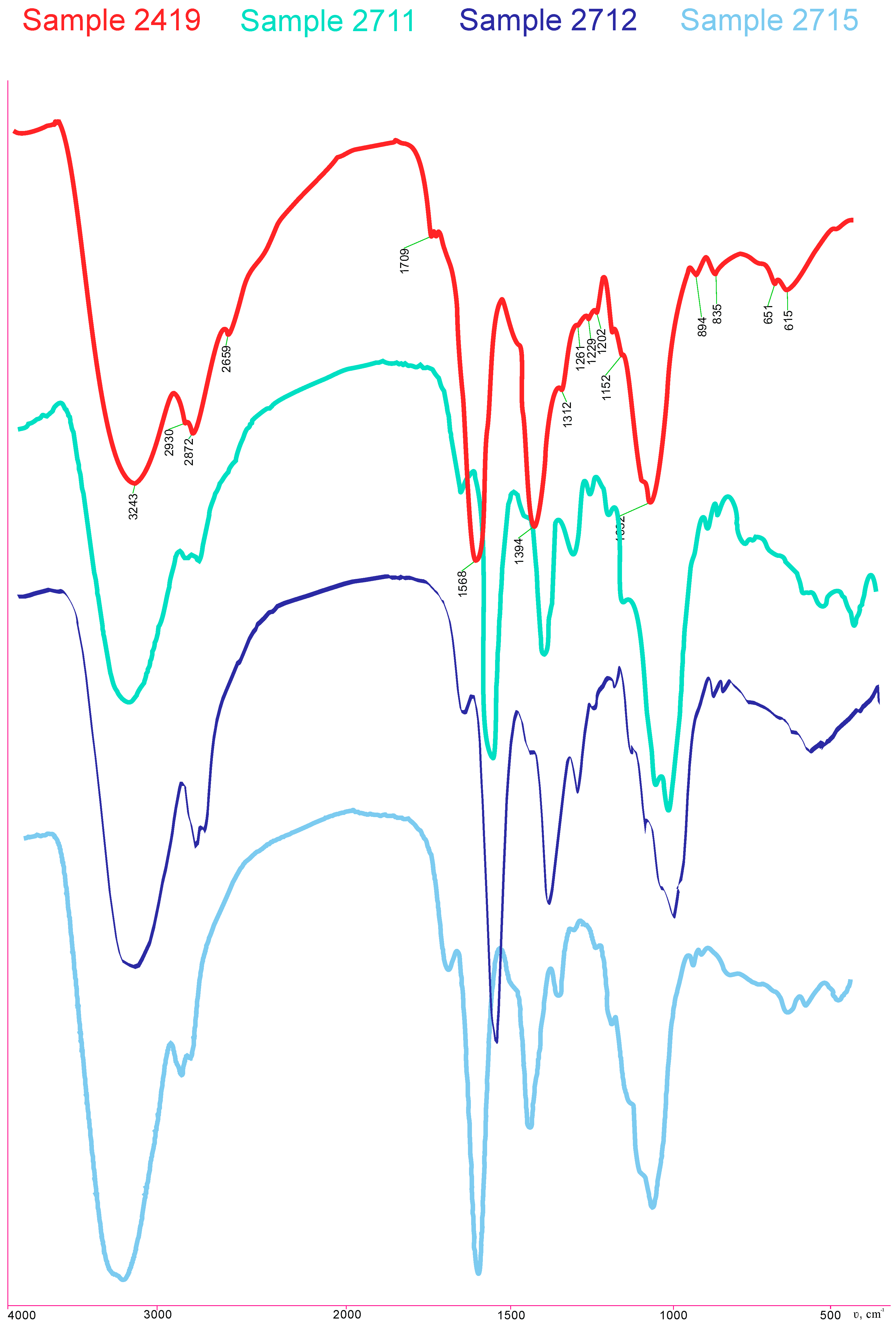
2.2. Evaluation of the Gels’ Resistance to Degradation Properties
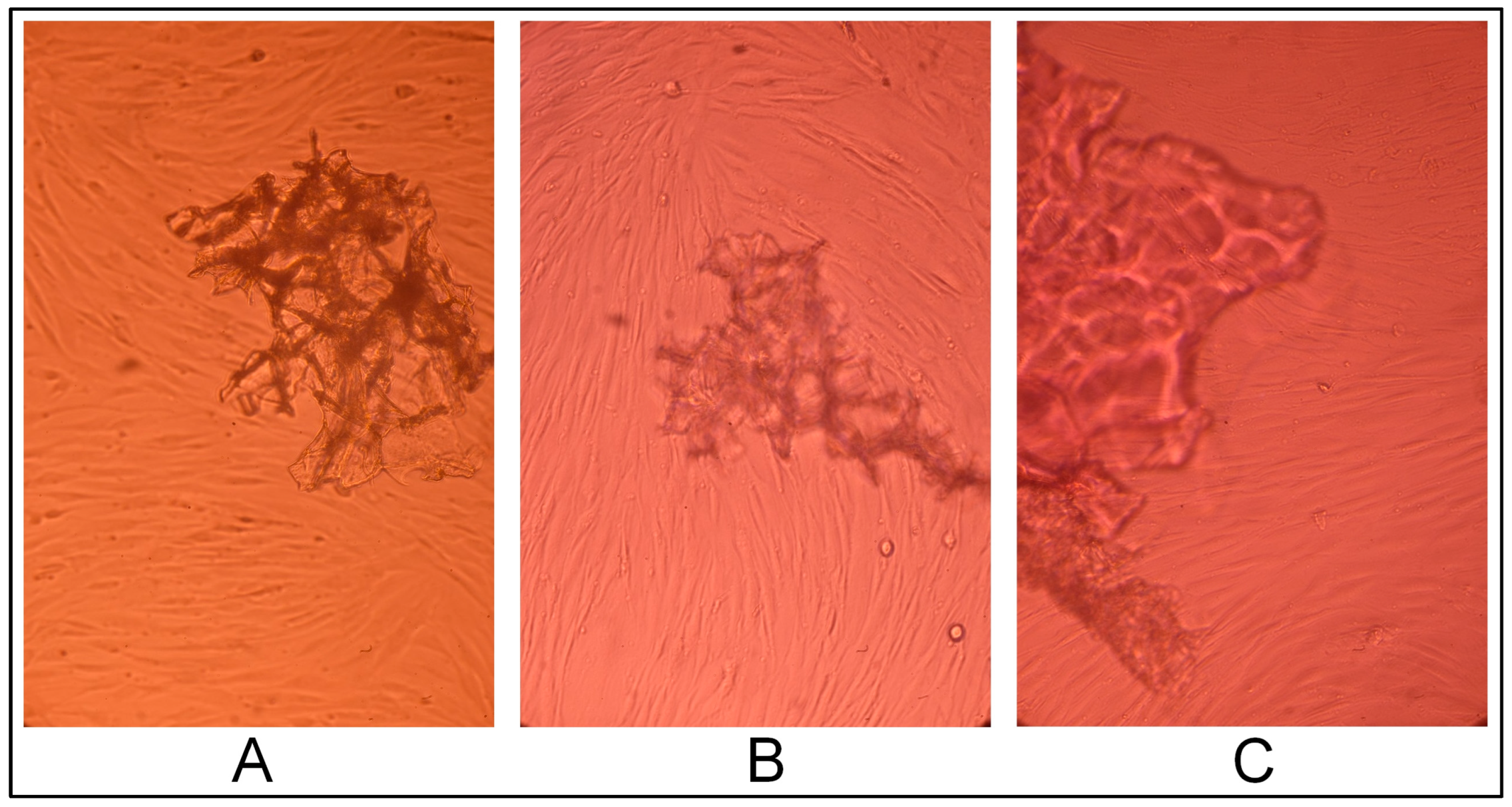
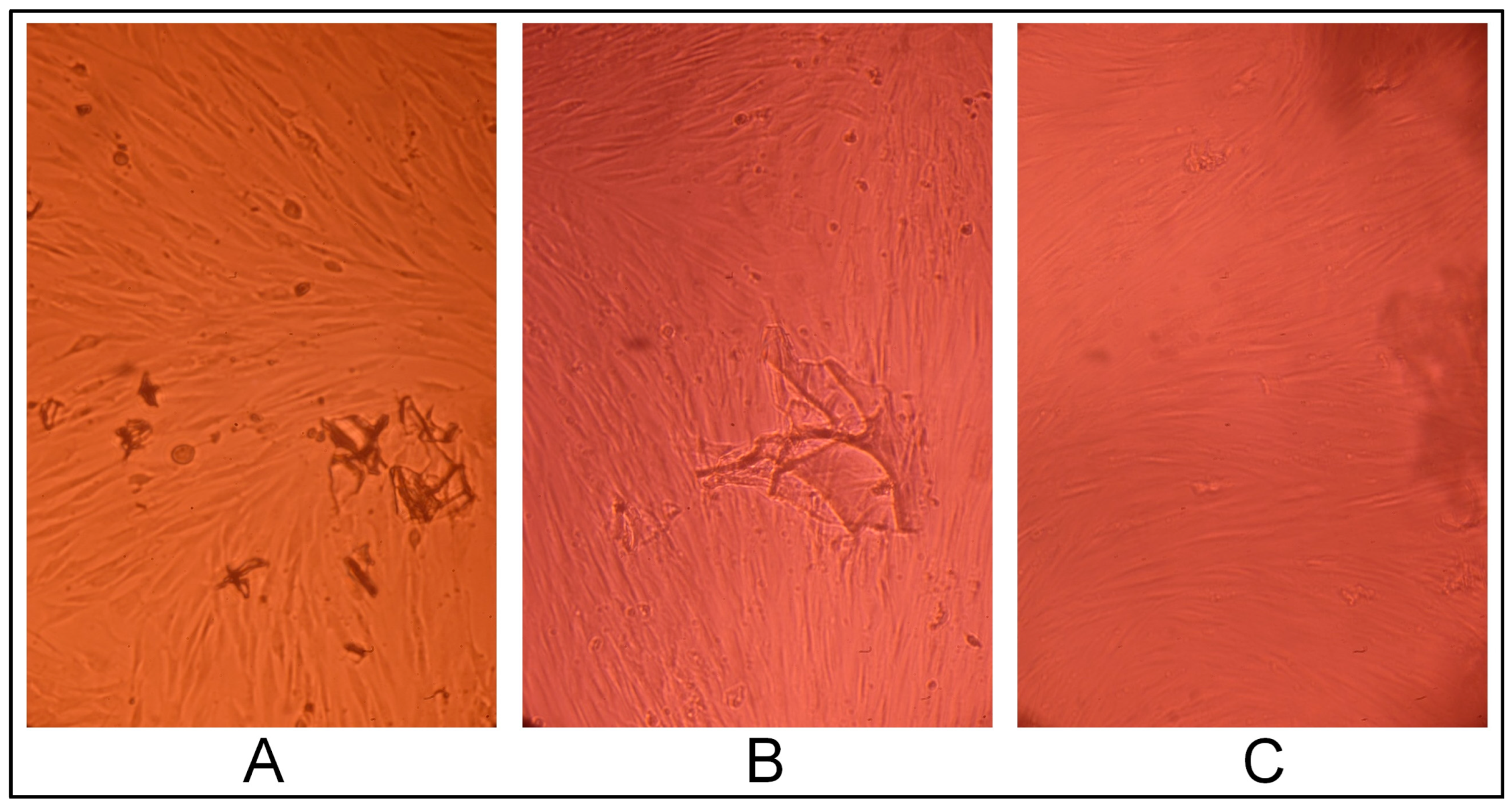
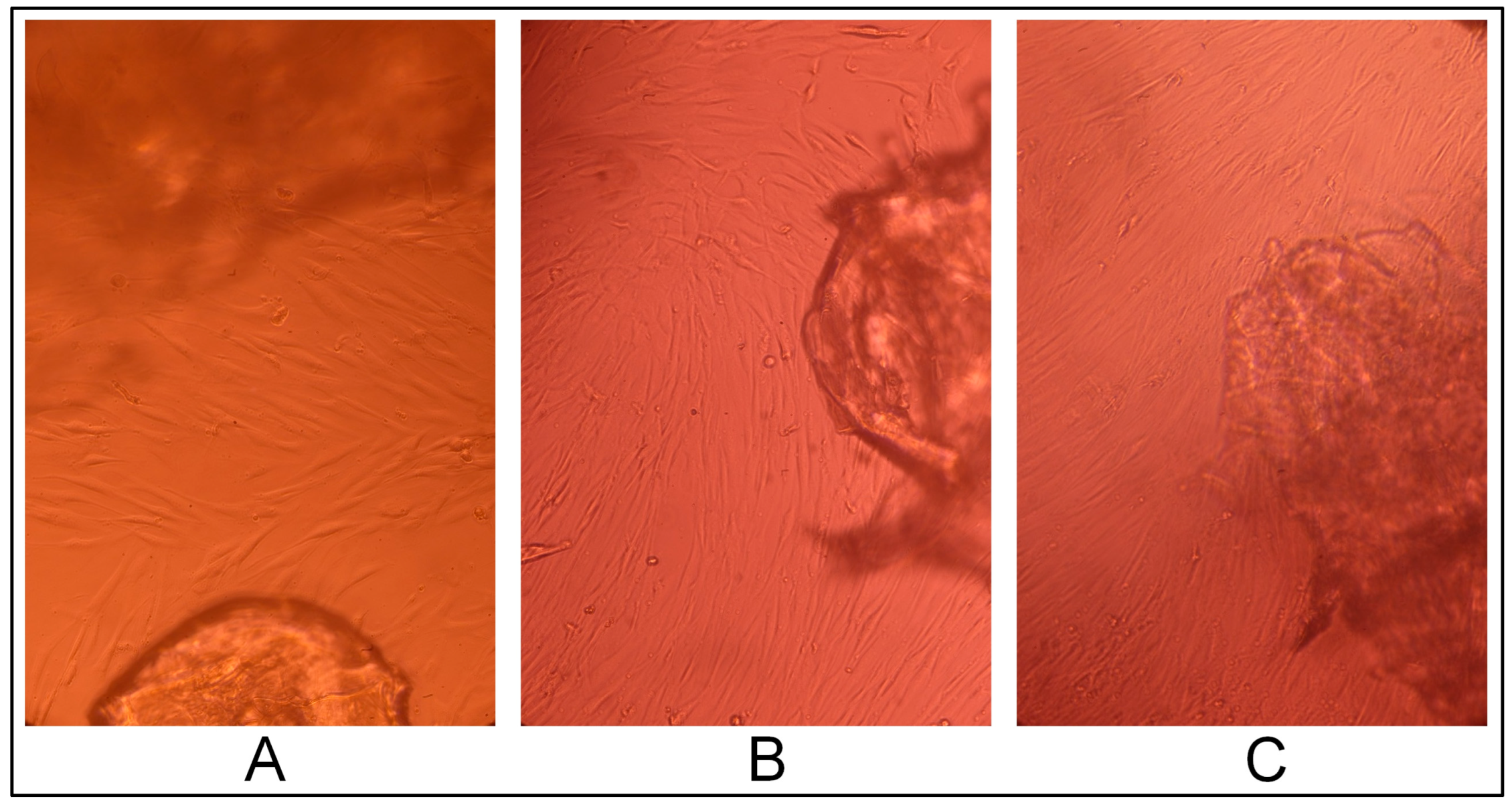
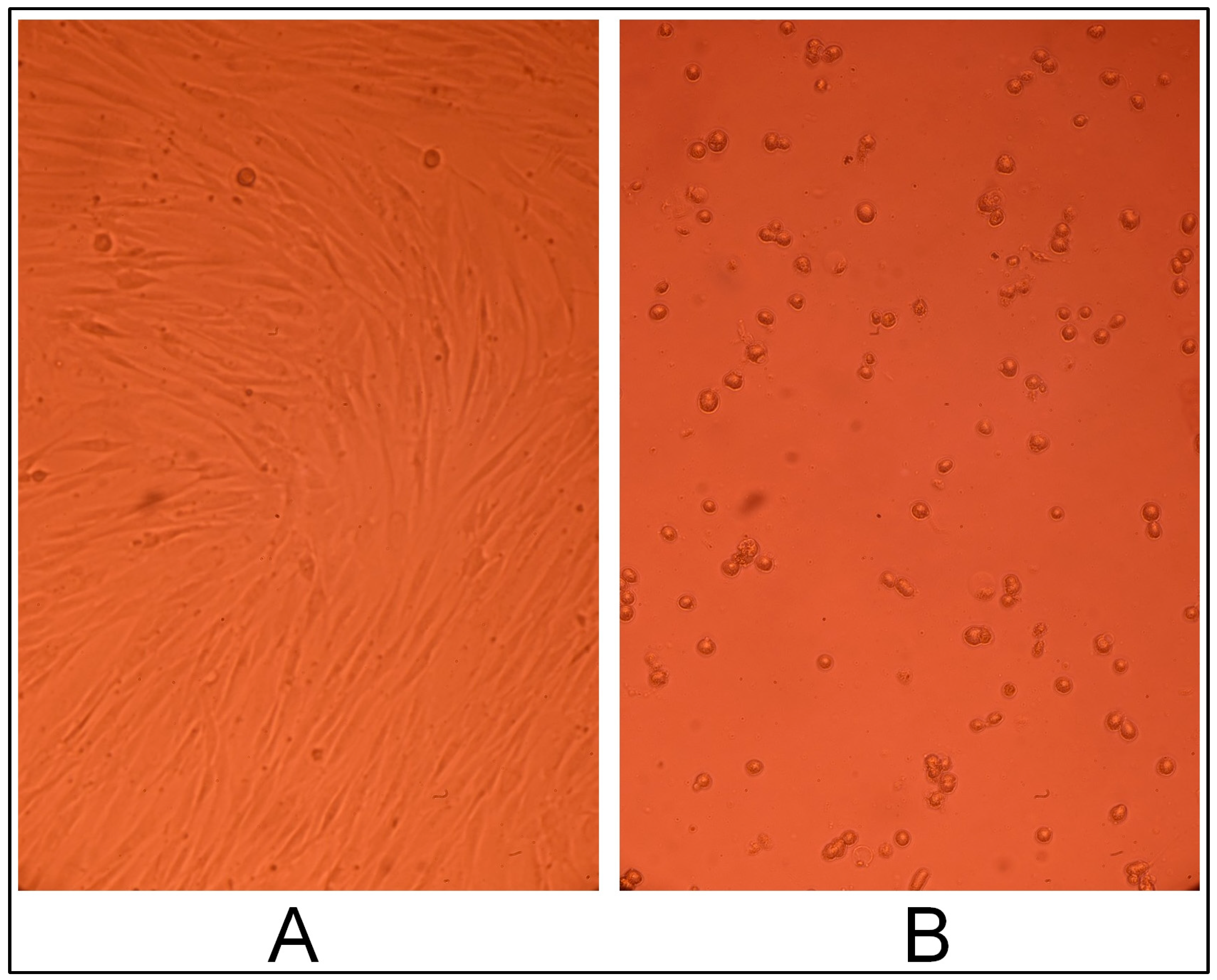
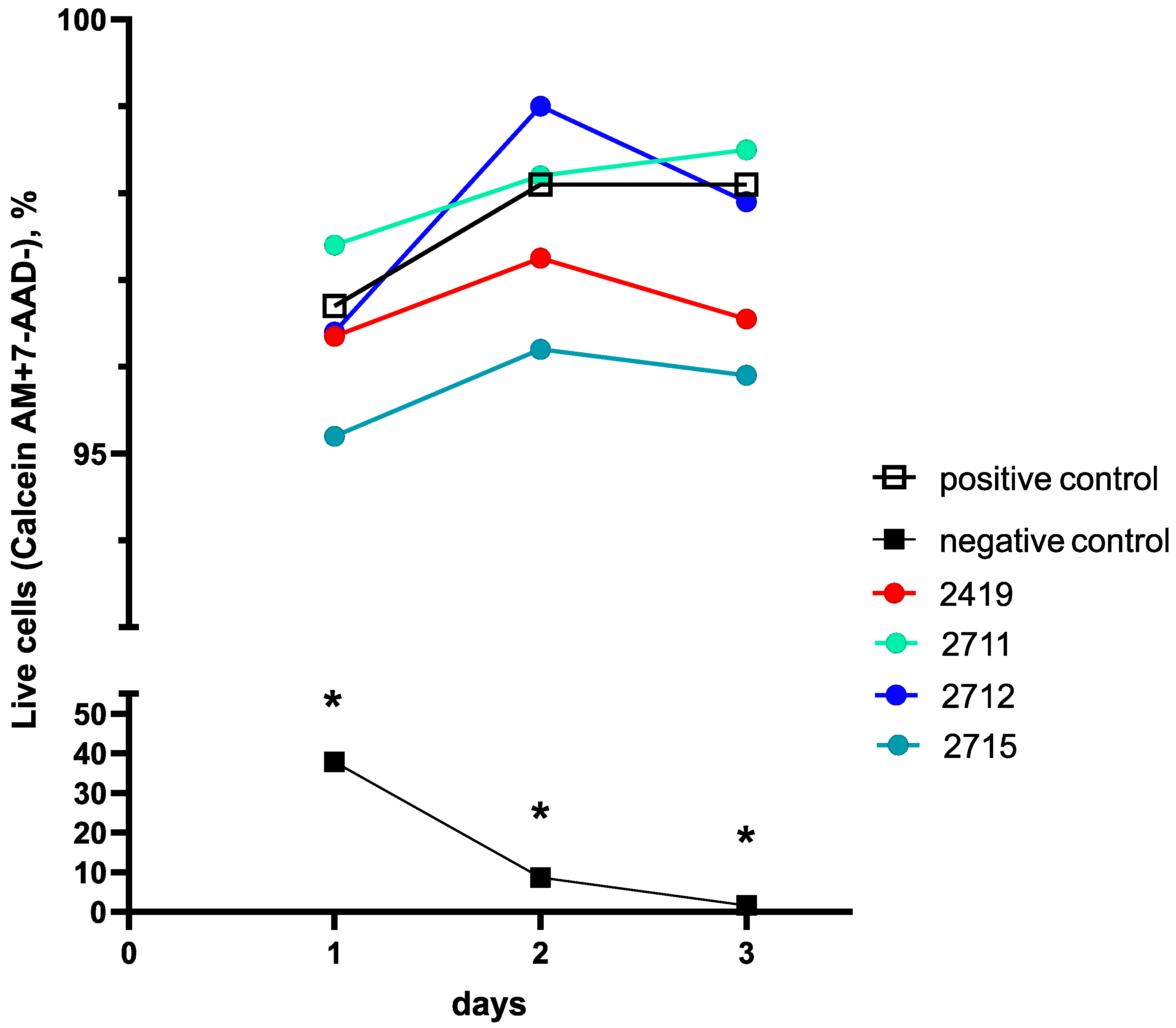
2.3. Gel Sample Cytotoxicity Evaluation on Human Fibroblast Culture In Vitro
2.3.1. Cell Line Morphology Results
2.3.2. Cell Line Flow Cytometry-Based Cytotoxicity Results
3. Conclusions
4. Materials and Methods
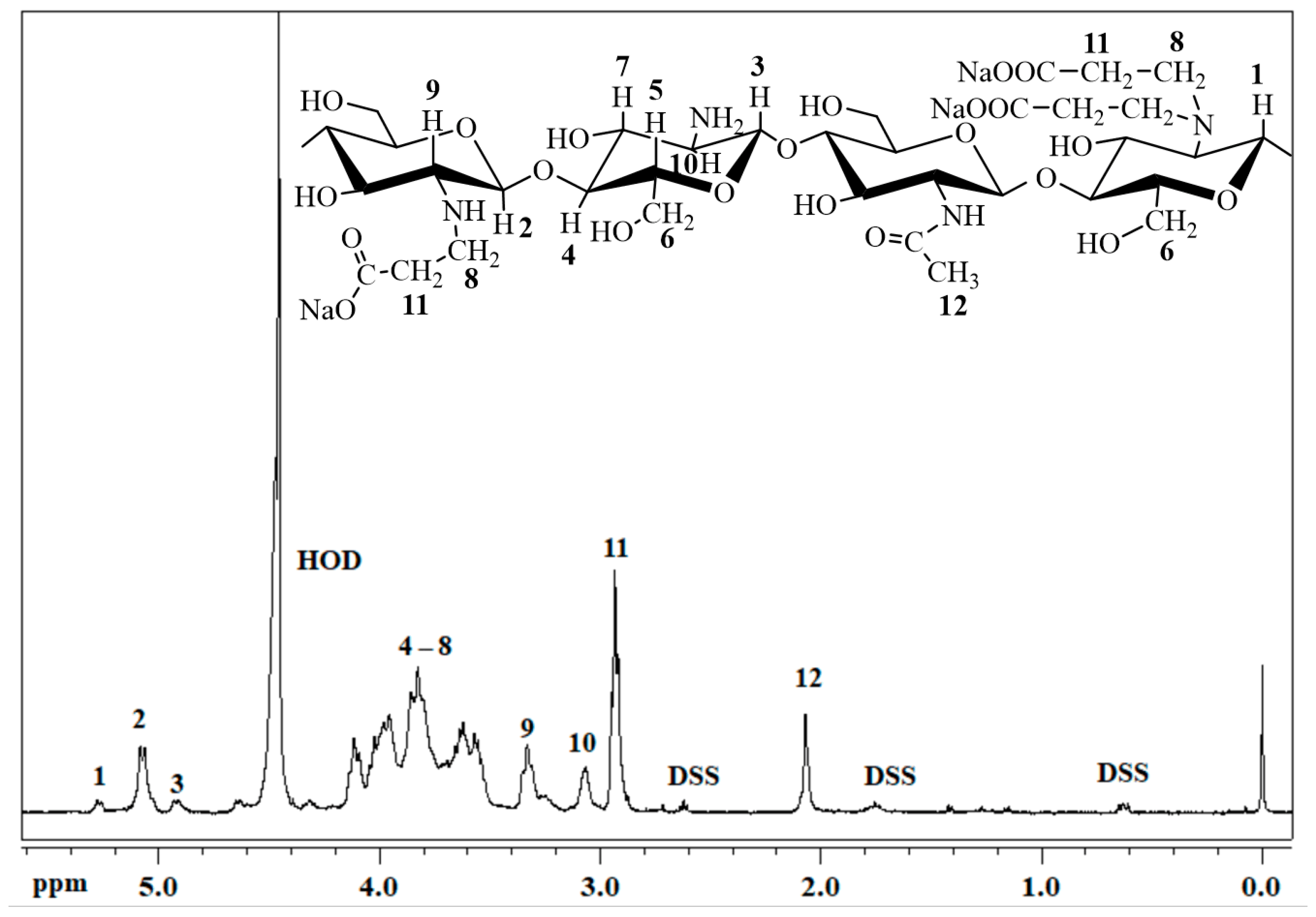
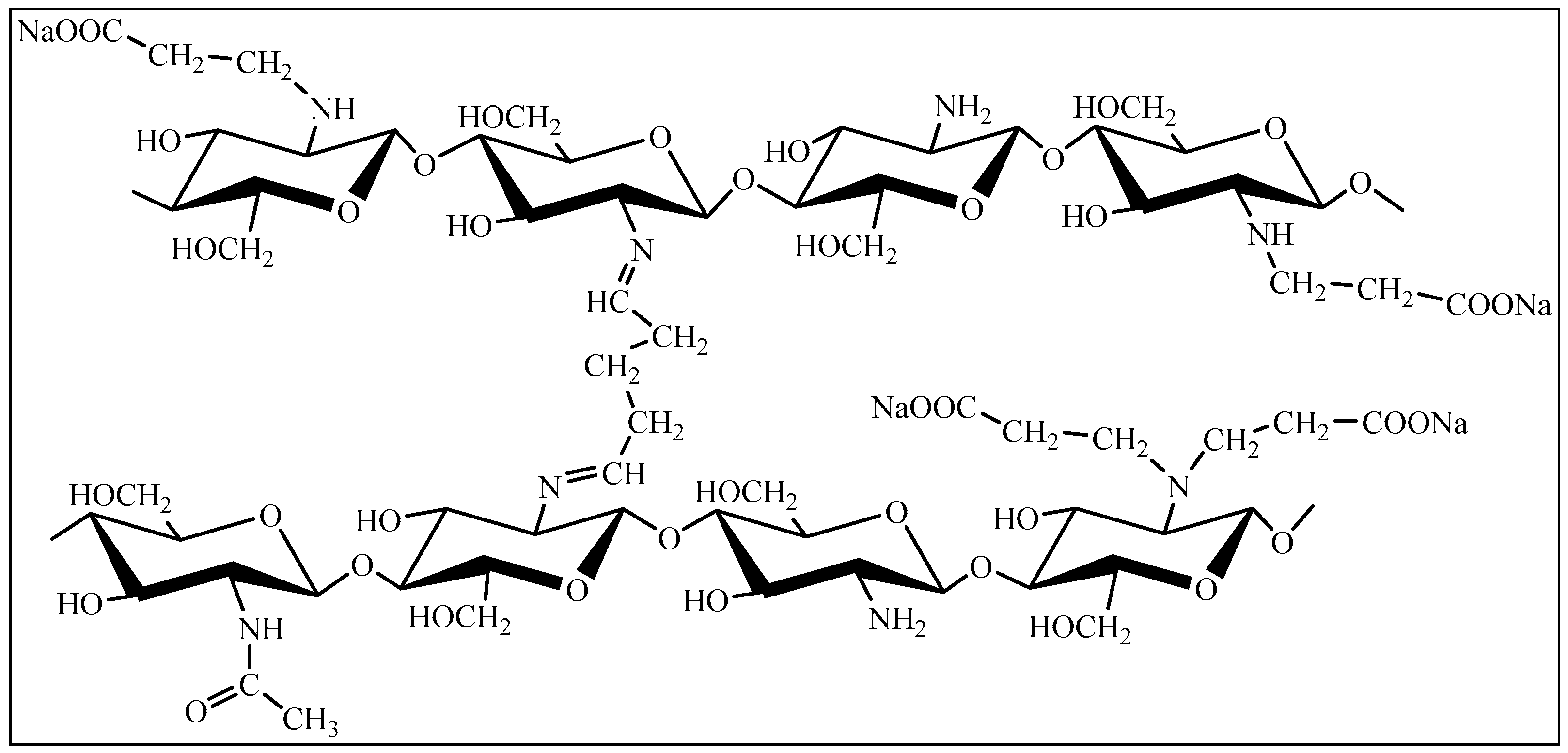
4.1. Bioresorbable Gel Carrier
- Sample 2419—CEC cryogel crosslinked with glutaraldehyde (CEC: aldehyde molar ratio of 10:1).
- Sample 2715—CEC hydrogel crosslinked with glutaraldehyde (CEC: aldehyde molar ratio of 10:1).
- Sample 2712—CEC cryogel crosslinked with glutaraldehyde (CEC: aldehyde molar ratio of 5:1).
- Sample 2711—CEC cryogel crosslinked with glutaraldehyde (CEC: aldehyde molar ratio of 20:1).
4.2. Swelling Analysis and Degradation Evaluation of CEC Gels under Various pHs
4.3. Cytotoxicity Evaluation
4.4. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, X.; Sun, H.; Qin, Z.; Che, P.; Yi, X.; Yu, Q.; Zhang, H.; Sun, X.; Yao, F.; Li, J. Fully Physically Crosslinked Pectin-Based Hydrogel with High Stretchability and Toughness for Biomedical Application. Int. J. Biol. Macromol. 2020, 149, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Mahdi, S.; Kaur, J.; Iqbal, Z.; Talegaonkar, S.; Ahmad, F.J. Advances and Potential Applications of Chitosan Derivatives as Mucoadhesive Biomaterials in Modern Drug Delivery. J. Pharm. Pharmacol. 2010, 58, 1021–1032. [Google Scholar] [CrossRef]
- Maksimova, Y.G.; Maksimov, A.Y.; Demakov, V.A.; Budnikov, V.I. The Influence of Polyacrylamide Gels on Soil Microflora. Bull. Perm Univ. Biol. 2010, 1, 45–49. Available online: https://www.elibrary.ru/item.asp?id=17024548&ysclid=lhittpnxv129807961 (accessed on 2 August 2023).
- Voskoboynikova, T.G.; Okolelova, A.A.; Manov, R.O. Increase of Radish Germination Using Hydrogel in Various Types of Soils. Belgorod State Univ. Sci. Bull. Nat. Sci. 2015, 206, 37–42. Available online: https://bsuedu.ru/upload/iblock/86e/N9(206)31_%D0%95%D0%9D.pdf (accessed on 2 August 2023).
- Ravi Kumar, M.N.V. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Vasilyev, A.V.; Kuznetsova, V.S.; Bukharova, T.B.; Zagoskin, Y.D.; Leonov, G.E.; Grigoriev, T.E.; Chvalun, S.N.; Goldshtein, D.V.; Kulakov, A.A. Chitosan Hydrogels Biocompatibility Improvement with the Perspective of Use as a Base for Osteoplastic Materials in Dentistry. Stomatologiia 2019, 98, 12. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of Natural Hydrogels for Regenerative Medicine Applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [PubMed]
- Apryatina, K.V. Polymer Compositions Based on Chitosan for Medical and Biological Purposes. Candidate of Science Thesis, Novgorod. 2018. Available online: https://diss.unn.ru/files/2018/839/autoref-839.pdf (accessed on 2 August 2023).
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. IJMS 2020, 21, 487. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on Modification of Chitosan Biopolymer and Its Potential Applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef]
- Henderson, T.M.A.; Ladewig, K.; Haylock, D.N.; McLean, K.M.; O’Connor, A.J. Cryogels for Biomedical Applications. J. Mater. Chem. B 2013, 1, 2682. [Google Scholar] [CrossRef] [PubMed]
- Savina, I.N.; Zoughaib, M.; Yergeshov, A.A. Design and Assessment of Biodegradable Macroporous Cryogels as Advanced Tissue Engineering and Drug Carrying Materials. Gels 2021, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Wu, J.; Dinh, N.-D.; Chen, C.-H. Gradient Porous Elastic Hydrogels with Shape-Memory Property and Anisotropic Responses for Programmable Locomotion. Adv. Funct. Mater. 2015, 25, 7272–7279. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, K.; Chang, Q.; Darabi, M.A.; Lin, B.; Zhong, W.; Xing, M. Highly Flexible and Resilient Elastin Hybrid Cryogels with Shape Memory, Injectability, Conductivity, and Magnetic Responsive Properties. Adv. Mater. 2016, 28, 7758–7767. [Google Scholar] [CrossRef] [PubMed]
- Shiekh, P.A.; Andrabi, S.M.; Singh, A.; Majumder, S.; Kumar, A. Designing Cryogels through Cryostructuring of Polymeric Matrices for Biomedical Applications. Eur. Polym. J. 2021, 144, 110234. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.; Chen, L.; Dai, B. Control of Ice Crystal Growth and Its Effect on Porous Structure of Chitosan Cryogels. Chem. Eng. Sci. 2019, 201, 50–57. [Google Scholar] [CrossRef]
- Singh, A.; Shiekh, P.A.; Das, M.; Seppälä, J.; Kumar, A. Aligned Chitosan-Gelatin Cryogel-Filled Polyurethane Nerve Guidance Channel for Neural Tissue Engineering: Fabrication, Characterization, and In Vitro Evaluation. Biomacromolecules 2019, 20, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, Q.; Sun, J.; Zhou, Q. Preparation of Poly(Ethylene Glycol) Aligned Porous Cryogels Using a Unidirectional Freezing Technique. Soft Matter 2012, 8, 3620. [Google Scholar] [CrossRef]
- Park, J.; Kim, D. Effect of Polymer Solution Concentration on the Swelling and Mechanical Properties of Glycol Chitosan Superporous Hydrogels. J. Appl. Polym. Sci. 2010, 115, 3434–3441. [Google Scholar] [CrossRef]
- Dinu, M.V.; Přádný, M.; Drăgan, E.S.; Michálek, J. Morphogical and Swelling Properties of Porous Hydrogels Based on Poly(Hydroxyethyl Methacrylate) and Chitosan Modulated by Ice-Templating Process and Porogen Leaching. J. Polym. Res. 2013, 20, 285. [Google Scholar] [CrossRef]
- Autissier, A.; Visage, C.L.; Pouzet, C.; Chaubet, F.; Letourneur, D. Fabrication of Porous Polysaccharide-Based Scaffolds Using a Combined Freeze-Drying/Cross-Linking Process. Acta Biomater. 2010, 6, 3640–3648. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Srivastava, A. Cell Separation Using Cryogel-Based Affinity Chromatography. Nat. Protoc. 2010, 5, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Nikonorov, V.V.; Ivanov, R.V.; Kil’deeva, N.R.; Bulatnikova, L.N.; Lozinskii, V.I. Synthesis and Characteristics of Cryogels of Chitosan Crosslinked by Glutaric Aldehyde. Polym. Sci. Ser. A 2010, 52, 828–834. [Google Scholar] [CrossRef]
- Demir, D.; Bölgen, N. Synthesis and Characterization of Injectable Chitosan Cryogel Microsphere Scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 686–696. [Google Scholar] [CrossRef]
- Beppu, M.M.; Vieira, R.S.; Aimoli, C.G.; Santana, C.C. Crosslinking of Chitosan Membranes Using Glutaraldehyde: Effect on Ion Permeability and Water Absorption. J. Membr. Sci. 2007, 301, 126–130. [Google Scholar] [CrossRef]
- Bratskaya, S.; Privar, Y.; Nesterov, D.; Modin, E.; Kodess, M.; Slobodyuk, A.; Marinin, D.; Pestov, A. Chitosan Gels and Cryogels Cross-Linked with Diglycidyl Ethers of Ethylene Glycol and Polyethylene Glycol in Acidic Media. Biomacromolecules 2019, 20, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Mejía, G.; Vázquez-Torres, N.A.; Castell-Rodríguez, A.; Del Río, J.M.; Corea, M.; Jiménez-Juárez, R. Synthesis of New Chitosan-Glutaraldehyde Scaffolds for Tissue Engineering Using Schiff Reactions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123658. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking Biopolymers for Biomedical Applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- Bratskaya, S.; Skatova, A.; Privar, Y.; Boroda, A.; Kantemirova, E.; Maiorova, M.; Pestov, A. Stimuli-Responsive Dual Cross-Linked N-Carboxyethylchitosan Hydrogels with Tunable Dissolution Rate. Gels 2021, 7, 188. [Google Scholar] [CrossRef]
- Pestov, A.V.; Zhuravlev, N.A.; Yatluk, Y.G. Synthesis in a Gel as a New Procedure for Preparing Carboxyethyl Chitosan. Russ. J. Appl. Chem. 2007, 80, 1154–1159. [Google Scholar] [CrossRef]
- Samokhin, A.G.; Tkachenko, V.O.; Kuznetsov, V.A.; Zemlyakova, E.O.; Nesterov, D.V.; Larionov, P.M.; Pestov, A.V. Selection of an Optimal Method for Sterilization of the Medical Grade Biodegradable Polymers. In Modern Synthetic Methodologies for Creating Drugs and Functional Materials (MOSM2018): Proceedings of the II International Conference, Yekaterinburg, Russia, 15–17 November 2018; AIP Publishing: Melville, NY, USA, 2019; p. 30017. [Google Scholar] [CrossRef]
- McIlvaine, T.C. A Buffer Solution for Colorimetric Comparison. J. Biol. Chem. 1921, 49, 183–186. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blinova, E.; Korel, A.; Zemlyakova, E.; Pestov, A.; Samokhin, A.; Zelikman, M.; Tkachenko, V.; Bets, V.; Arzhanova, E.; Litvinova, E. Cytotoxicity and Degradation Resistance of Cryo- and Hydrogels Based on Carboxyethylchitosan at Different pH Values. Gels 2024, 10, 272. https://doi.org/10.3390/gels10040272
Blinova E, Korel A, Zemlyakova E, Pestov A, Samokhin A, Zelikman M, Tkachenko V, Bets V, Arzhanova E, Litvinova E. Cytotoxicity and Degradation Resistance of Cryo- and Hydrogels Based on Carboxyethylchitosan at Different pH Values. Gels. 2024; 10(4):272. https://doi.org/10.3390/gels10040272
Chicago/Turabian StyleBlinova, Elena, Anastasia Korel, Ekaterina Zemlyakova, Alexander Pestov, Alexander Samokhin, Maxim Zelikman, Vadim Tkachenko, Viktoria Bets, Elena Arzhanova, and Ekaterina Litvinova. 2024. "Cytotoxicity and Degradation Resistance of Cryo- and Hydrogels Based on Carboxyethylchitosan at Different pH Values" Gels 10, no. 4: 272. https://doi.org/10.3390/gels10040272
APA StyleBlinova, E., Korel, A., Zemlyakova, E., Pestov, A., Samokhin, A., Zelikman, M., Tkachenko, V., Bets, V., Arzhanova, E., & Litvinova, E. (2024). Cytotoxicity and Degradation Resistance of Cryo- and Hydrogels Based on Carboxyethylchitosan at Different pH Values. Gels, 10(4), 272. https://doi.org/10.3390/gels10040272








