High Modulus, Strut-like poly(ether ether ketone) Aerogels Produced from a Benign Solvent
Abstract
1. Introduction
2. Results and Discussion
2.1. Gelation of PEEK in DPA
2.2. Morphology of PEEK Aerogels
2.3. Porosity and Surface Area of PEEK Aerogels
2.4. Mechanical Properties of PEEK Aerogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. PEEK Gel Preparation
4.3. PEEK Aerogel Preparation
4.4. Characterization
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nijenhuis, K.t. Thermoreversible networks: Viscoelastic properties and structure of gels. In Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1997; Volume 130. [Google Scholar]
- Song, G.; Zhang, L.; He, C.; Fang, D.-C.; Whitten, P.G.; Wang, H. Facile fabrication of tough hydrogels physically cross-linked by strong cooperative hydrogen bonding. Macromolecules 2013, 46, 7423–7435. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, S.; Wang, J.; Li, X.; Bi, Z.; Jin, Y.; Fan, L.; Wang, L.; Wu, Y.; Gan, F. Polyimide solution with reversible sol-gel transition by construction of dynamic π-π stacking. Polymer 2023, 278, 126006. [Google Scholar] [CrossRef]
- Itagaki, H.; Tokami, T.; Mochizuki, J. A trial to clarify a cause of forming physical gels: Morphology of syndiotactic polystyrene in n-alkylbenzene. Polymer 2012, 53, 5304–5312. [Google Scholar] [CrossRef]
- Cirkel, P.A.; Okada, T. A comparison of mechanical and electrical percolation during the gelling of Nafion solutions. Macromolecules 2000, 33, 4921–4925. [Google Scholar] [CrossRef]
- Hikmet, R.; Callister, S.; Keller, A. Thermoreversible gelation of atactic polystyrene: Phase transformation and morphology. Polymer 1988, 29, 1378–1388. [Google Scholar] [CrossRef]
- Guenet, J.-M. Thermoreversible Gelation of Polymers and Biopolymers; Academic Press: London, UK, 1992. [Google Scholar]
- Hong, P.-D.; Chen, J.-H. Structure and properties of polyvinyl chloride physical gels. Polymer 1998, 39, 711–717. [Google Scholar] [CrossRef]
- Matsuo, M.; Miyoshi, S.; Azuma, M.; Bin, Y.; Agari, Y.; Sato, Y.; Kondo, A. Phase separation of several kinds of polyethylene solution under the gelation/crystallization process. Macromolecules 2005, 38, 6688–6699. [Google Scholar] [CrossRef]
- Daniel, C.; Longo, S.; Guerra, G. High porosity polyethylene aerogels. Polyolefins J. 2015, 2, 49–55. [Google Scholar]
- Domszy, R.; Alamo, R.; Edwards, C.; Mandelkern, L. Thermoreversible gelation and crystallization of homopolymers and copolymers. Macromolecules 1986, 19, 310–325. [Google Scholar] [CrossRef]
- Matsuda, H.; Inoue, T.; Okabe, M.; Ukaji, T. Study of polyolefin gel in organic solvents I. Structure of isotactic polypropylene gel in organic solvents. Polym. J. 1987, 19, 323–329. [Google Scholar] [CrossRef][Green Version]
- Xiao, Z.; Sun, N. Crystallization behavior for metallocene-catalyzed isotactic polypropylene in alkane solvents of various molecular sizes. J. Therm. Anal. Calorim. 2016, 124, 295–303. [Google Scholar] [CrossRef]
- Daniel, C.; Dammer, C.; Guenet, J.-M. On the definition of thermoreversible gels: The case of syndiotactic polystyrene. Polymer 1994, 35, 4243–4246. [Google Scholar] [CrossRef]
- Daniel, C.; Deluca, M.; Guenet, J.-M.; Brulet, A.; Menelle, A. Thermoreversible gelation of syndiotactic polystyrene in benzene. Polymer 1996, 37, 1273–1280. [Google Scholar] [CrossRef]
- Daniel, C.; Menelle, A.; Brulet, A.; Guenet, J.-M. Thermoreversible gelation of syndiotactic polystyrene in toluene and chloroform. Polymer 1997, 38, 4193–4199. [Google Scholar] [CrossRef]
- D’Aniello, C.; Daniel, C.; Guerra, G. ε form gels and aerogels of syndiotactic polystyrene. Macromolecules 2015, 48, 1187–1193. [Google Scholar] [CrossRef]
- Ma, P.X.; Zhang, R. Synthetic nano-scale fibrous extracellular matrix. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. 1999, 46, 60–72. [Google Scholar] [CrossRef]
- Fang, X.; Wyatt, T.; Shi, J.; Yao, D. Fabrication of high-strength polyoxymethylene fibers by gel spinning. J. Mater. Sci. 2018, 53, 11901–11916. [Google Scholar] [CrossRef]
- Stamhuis, J.E.; Pennings, A.J. Gelation and Crystallisation of Nylon-6 from Dilute Solution. Br. Polym. J. 1978, 10, 221–225. [Google Scholar] [CrossRef]
- Tazaki, M.; Wada, R.; Abe, M.O.; Homma, T. Crystallization and gelation of poly(vinylidene fluoride) in organic solvents. J. Appl. Polym. Sci. 1997, 65, 1517–1524. [Google Scholar] [CrossRef]
- Yadav, P.J.P.; Patra, A.K.; Sastry, P.U.; Ghorai, B.K.; Maiti, P. Solvent Retention, Thermodynamics, Rheology and Small Angle X-ray Scattering Studies on Thermoreversible Poly(vinylidene fluoride) Gels. J. Phys. Chem. B 2010, 114, 11420–11429. [Google Scholar] [CrossRef]
- Xue, G.; Ji, G.; Li, Y. Rapid crystallization and thermoreversible gelation of poly(ethylene terephthalate) in polymer/oligomer binary system. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 1219–1225. [Google Scholar] [CrossRef]
- Daniel, C.; Longo, S.; Fasano, G.; Vitillo, J.G.; Guerra, G. Nanoporous Crystalline Phases of Poly(2,6-Dimethyl-1,4-phenylene)oxide. Chem. Mater. 2011, 23, 3195–3200. [Google Scholar] [CrossRef]
- Daniel, C.; Longo, S.; Cardea, S.; Vitillo, J.G.; Guerra, G. Monolithic nanoporous–crystalline aerogels based on PPO. RSC Adv. 2012, 2, 12011–12018. [Google Scholar] [CrossRef]
- Buyse, K.; Berghmans, H.; Bosco, M.; Paoletti, S. Mechanistic aspects of the thermoreversible gelation of syndiotactic poly(methyl methacrylate) in toluene. Macromolecules 1998, 31, 9224–9230. [Google Scholar] [CrossRef]
- Saiani, A.; Spěváček, J.; Guenet, J.-M. Phase behavior and polymer/solvent interactions in thermoreversible gels of syndiotactic poly(methyl methacrylate). Macromolecules 1998, 31, 703–710. [Google Scholar] [CrossRef]
- Godshall, G.F.; Spiering, G.A.; Crater, E.R.; Moore, R.B. Low-Density, Semicrystalline Poly(phenylene sulfide) Aerogels Fabricated Using a Benign Solvent. ACS Appl. Polym. Mater. 2023, 5, 7994–8004. [Google Scholar] [CrossRef]
- Mochizuki, J.; Sano, T.; Tokami, T.; Itagaki, H. Decisive properties of solvent able to form gels with syndiotactic polystyrene. Polymer 2015, 67, 118–127. [Google Scholar] [CrossRef]
- Kobayashi, M. Structure of gels, characterization techniques. In Gels Handbook; Elsevier: Amsterdam, The Netherlands, 2001; pp. 172–412. [Google Scholar]
- Okabe, M.; Wada, R.; Tazaki, M.; Homma, T. The Flory-Huggins interaction parameter and thermoreversible gelation of poly(vinylidene fluoride) in organic solvents. Polym. J. 2003, 35, 798–803. [Google Scholar] [CrossRef]
- Talley, S.J.; AndersonSchoepe, C.L.; Berger, C.J.; Leary, K.A.; Snyder, S.A.; Moore, R.B. Mechanically robust and superhydrophobic aerogels of poly(ether ether ketone). Polymer 2017, 126, 437–445. [Google Scholar] [CrossRef]
- Talley, S.J.; Yuan, X.; Moore, R.B. Thermoreversible Gelation of Poly(ether ether ketone). ACS Macro Lett. 2017, 6, 262–266. [Google Scholar] [CrossRef]
- Talley, S.J.; Vivod, S.L.; Nguyen, B.A.; Meador, M.A.B.; Radulescu, A.; Moore, R.B. Hierarchical Morphology of Poly(ether ether ketone) Aerogels. ACS Appl. Mater. Interfaces 2019, 11, 31508–31519. [Google Scholar] [CrossRef]
- Substances Added to Food (Formerly EAFUS): 1,3-Diphenyl-2-propanone. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=FoodSubstances&id=DIPHENYLPROPANONE (accessed on 27 March 2024).
- Pomatto, M.E.; Crater, E.R.; Godshall, G.F.; Moore, R.B. Blocky bromination of poly(ether ketone ketone) as a means to preserve crystallizability and rapid crystallization kinetics. Polym. Chem. 2024, 15, 609–621. [Google Scholar] [CrossRef]
- Kim, S.S.; Lloyd, D.R. Microporous membrane formation via thermally-induced phase separation. III. Effect of thermodynamic interactions on the structure of isotactic polypropylene membranes. J. Membr. Sci. 1991, 64, 13–29. [Google Scholar] [CrossRef]
- Matsuyama, H.; Maki, T.; Teramoto, M.; Asano, K. Effect of polypropylene molecular weight on porous membrane formation by thermally induced phase separation. J. Membr. Sci. 2002, 204, 323–328. [Google Scholar] [CrossRef]
- Gu, M.; Zhang, J.; Wang, X.; Tao, H.; Ge, L. Formation of poly(vinylidene fluoride) (PVDF) membranes via thermally induced phase separation. Desalination 2006, 192, 160–167. [Google Scholar] [CrossRef]
- Tanaka, T.; Lloyd, D.R. Formation of poly(l-lactic acid) microfiltration membranes via thermally induced phase separation. J. Membr. Sci. 2004, 238, 65–73. [Google Scholar] [CrossRef]
- Matsuyama, H.; Okafuji, H.; Maki, T.; Teramoto, M.; Kubota, N. Preparation of polyethylene hollow fiber membrane via thermally induced phase separation. J. Membr. Sci. 2003, 223, 119–126. [Google Scholar] [CrossRef]
- Jeon, S.; Karkhanechi, H.; Fang, L.-F.; Cheng, L.; Ono, T.; Nakamura, R.; Matsuyama, H. Novel preparation and fundamental characterization of polyamide 6 self-supporting hollow fiber membranes via thermally induced phase separation (TIPS). J. Membr. Sci. 2018, 546, 1–14. [Google Scholar] [CrossRef]
- Yang, Z.; Li, P.; Xie, L.; Wang, Z.; Wang, S.-C. Preparation of iPP hollow-fiber microporous membranes via thermally induced phase separation with co-solvents of DBP and DOP. Desalination 2006, 192, 168–181. [Google Scholar] [CrossRef]
- Rusakov, D.; Menner, A.; Bismarck, A. High-Performance Polymer Foams by Thermally Induced Phase Separation. Macromol. Rapid Commun. 2020, 41, 2000110. [Google Scholar] [CrossRef]
- Onder, O.C.; Yilgor, E.; Yilgor, I. Critical parameters controlling the properties of monolithic poly(lactic acid) foams prepared by thermally induced phase separation. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 98–108. [Google Scholar] [CrossRef]
- Su, M.; Pan, Y.; Zheng, G.; Liu, C.; Shen, C.; Liu, X. An ultra-light, superhydrophobic and thermal insulation ultra-high molecular weight polyethylene foam. Polymer 2021, 218, 123528. [Google Scholar] [CrossRef]
- Su, D.; Yang, J.; Liu, S.; Ren, L.; Qin, S. Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation. e-Polymers 2022, 22, 553–565. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, G.; Yang, J.; Wang, X. Formation of isotactic polypropylene membranes with bicontinuous structure and good strength via thermally induced phase separation method. Desalination 2009, 236, 8–15. [Google Scholar] [CrossRef]
- Lin, Y.; Tang, Y.; Ma, H.; Yang, J.; Tian, Y.; Ma, W.; Wang, X. Formation of a bicontinuous structure membrane of polyvinylidene fluoride in diphenyl carbonate diluent via thermally induced phase separation. J. Appl. Polym. Sci. 2009, 114, 1523–1528. [Google Scholar] [CrossRef]
- Lloyd, D.R.; Kinzer, K.E.; Tseng, H. Microporous membrane formation via thermally induced phase separation. I. Solid-liquid phase separation. J. Membr. Sci. 1990, 52, 239–261. [Google Scholar] [CrossRef]
- Caplan, M.R.; Chiang, C.-Y.; Lloyd, D.R.; Yen, L.Y. Formation of microporous Teflon® PFA membranes via thermally induced phase separation. J. Membr. Sci. 1997, 130, 219–237. [Google Scholar] [CrossRef]
- Chiang, C.-y.; Lloyd, D.R. Effects of process conditions on the formation of microporous membranes via solid-liquid thermally induced phase separation. J. Porous Mater. 1996, 2, 273–285. [Google Scholar] [CrossRef]
- Rezabeigi, E.; Drew, R.A.L.; Wood-Adams, P.M. Highly Porous Polymer Structures Fabricated via Rapid Precipitation from Ternary Systems. Ind. Eng. Chem. Res. 2017, 56, 11451–11459. [Google Scholar] [CrossRef]
- Rusakov, D.; Menner, A.; Spieckermann, F.; Wilhelm, H.; Bismarck, A. Morphology and properties of foamed high crystallinity PEEK prepared by high temperature thermally induced phase separation. J. Appl. Polym. Sci. 2022, 139, 51423. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, J.; Yan, M.; Tian, X. Poly ether ether ketone and its composite powder prepared by thermally induced phase separation for high temperature selective laser sintering. Mater. Des. 2021, 201, 109510. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, J.; Zhao, S.; Qi, D.; Xu, P.; Wu, S.; Wang, L.; Yue, X.; Jiang, Z. A Novel PEEK Foam with Ultra-High Temperature-Resistant by Temperature Induced Phase Separation. Macromol. Mater. Eng. 2023, 308, 2200559. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, Q.; Tian, Y.; Shi, Y.; Liu, B. Preparation of porous structure in the system of PEEK/PPS/diphenyl ketone via thermally induced phase separation. J. Appl. Polym. Sci. 2007, 104, 1523–1530. [Google Scholar] [CrossRef]
- Bisson, A.; Rigacci, A.; Lecomte, D.; Rodier, E.; Achard, P. Drying of silica gels to obtain aerogels: Phenomenology and basic techniques. Dry. Technol. 2003, 21, 593–628. [Google Scholar] [CrossRef]
- Job, N.; Pirard, R.; Marien, J.; Pirard, J.-P. Porous carbon xerogels with texture tailored by pH control during sol–gel process. Carbon 2004, 42, 619–628. [Google Scholar] [CrossRef]
- Scherer, G.W. Freezing gels. J. Non-Cryst. Solids 1993, 155, 1–25. [Google Scholar] [CrossRef]
- Hüsing, N.; Schubert, U. Aerogels—Airy materials: Chemistry, structure, and properties. Angew. Chem. Int. Ed. 1998, 37, 22–45. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef]
- Fesmire, J.E. Aerogel insulation systems for space launch applications. Cryogenics 2006, 46, 111–117. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Aerogel insulation for building applications: A state-of-the-art review. Energy Build. 2011, 43, 761–769. [Google Scholar] [CrossRef]
- Cuce, E.; Cuce, P.M.; Wood, C.J.; Riffat, S.B. Toward aerogel based thermal superinsulation in buildings: A comprehensive review. Renew. Sustain. Energy Rev. 2014, 34, 273–299. [Google Scholar] [CrossRef]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring Mechanical Properties of Aerogels for Aerospace Applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, M.; Chen, Z.; Ramakrishna, S.; Liu, Y.; Wang, X.; Hu, X.; Wei, Y. Recent advances in the development of nanofiber-based aerogel for oil-water separation: A review. Fuel 2023, 354, 129338. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, L.; Yang, Y.; Liu, X. Porous carbon nanospheres aerogel based molecularly imprinted polymer for efficient phenol adsorption and removal from wastewater. Sep. Purif. Technol. 2021, 274, 119029. [Google Scholar] [CrossRef]
- Tian, C.; She, J.; Wu, Y.; Luo, S.; Wu, Q.; Qing, Y. Reusable and cross-linked cellulose nanofibrils aerogel for the removal of heavy metal ions. Polym. Compos. 2018, 39, 4442–4451. [Google Scholar] [CrossRef]
- Maatar, W.; Boufi, S. Microporous cationic nanofibrillar cellulose aerogel as promising adsorbent of acid dyes. Cellulose 2017, 24, 1001–1015. [Google Scholar] [CrossRef]
- Hees, T.; Zhong, F.; Rudolph, T.; Walther, A.; Mülhaupt, R. Nanocellulose aerogels for supporting iron catalysts and in situ formation of polyethylene nanocomposites. Adv. Funct. Mater. 2017, 27, 1605586. [Google Scholar] [CrossRef]
- Yu, H.; Oh, S.; Han, Y.; Lee, S.; Jeong, H.S.; Hong, H.-J. Modified cellulose nanofibril aerogel: Tunable catalyst support for treatment of 4-Nitrophenol from wastewater. Chemosphere 2021, 285, 131448. [Google Scholar] [CrossRef]
- Kim, S.J.; Chase, G.; Jana, S.C. Polymer aerogels for efficient removal of airborne nanoparticles. Sep. Purif. Technol. 2015, 156, 803–808. [Google Scholar] [CrossRef]
- Zhai, C.; Jana, S.C. Tuning porous networks in polyimide aerogels for airborne nanoparticle filtration. ACS Appl. Mater. Interfaces 2017, 9, 30074–30082. [Google Scholar] [CrossRef]
- Noble, K.F.; Troya, D.; Talley, S.J.; Ilavsky, J.; Moore, R.B. High-Resolution Comonomer Sequencing of Blocky Brominated Syndiotactic Polystyrene Copolymers Using 13C NMR Spectroscopy and Computer Simulations. Macromolecules 2020, 53, 9539–9552. [Google Scholar] [CrossRef]
- Kasprzak, C.R.; Anderson, L.J.; Moore, R.B. Tailored sequencing of highly brominated Poly(ether ether ketone) as a means to preserve crystallizability and enhance Tg. Polymer 2022, 251, 124918. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, Y.; Ma, W.; Wang, X. A review on microporous polyvinylidene fluoride membranes fabricated via thermally induced phase separation for MF/UF application. J. Membr. Sci. 2021, 639, 119759. [Google Scholar] [CrossRef]
- Lloyd, D.R.; Kim, S.S.; Kinzer, K.E. Microporous membrane formation via thermally-induced phase separation. II. Liquid—Liquid phase separation. J. Membr. Sci. 1991, 64, 1–11. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Van Krevelen, D.W.; Te Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Stefanis, E.; Panayiotou, C. Prediction of Hansen solubility parameters with a new group-contribution method. Int. J. Thermophys. 2008, 29, 568–585. [Google Scholar] [CrossRef]
- Flory, P.J. Thermodynamics of crystallization in high polymers. IV. A theory of crystalline states and fusion in polymers, copolymers, and their mixtures with diluents. J. Chem. Phys. 1949, 17, 223–240. [Google Scholar] [CrossRef]
- Blundell, D.J.; Osborn, B.N. The morphology of poly(aryl-ether-ether-ketone). Polymer 1983, 24, 953–958. [Google Scholar] [CrossRef]
- Verma, R.; Marand, H.; Hsiao, B. Morphological changes during secondary crystallization and subsequent melting in poly(ether ether ketone) as studied by real time small angle X-ray scattering. Macromolecules 1996, 29, 7767–7775. [Google Scholar] [CrossRef]
- Wang, W.; Schultz, J.M.; Hsiao, B.S. Time-resolved simultaneous SAXS/WAXS studies of PEEK during isothermal crystallization, melting, and subsequent cooling. J. Macromol. Sci. Part B Phys. 1998, 37, 667–682. [Google Scholar] [CrossRef]
- Fougnies, C.; Damman, P.; Dosiere, M.; Koch, M. Time-resolved SAXS, WAXS, and DSC study of melting of poly(aryl ether ether ketone)(PEEK) annealed from the amorphous state. Macromolecules 1997, 30, 1392–1399. [Google Scholar] [CrossRef]
- Hsiao, B.S.; Sauer, B.B.; Verma, R.K.; Zachmann, H.G.; Seifert, S.; Chu, B.; Harney, P. New Insight of Isothermal Melt Crystallization in Poly(aryl ether ether ketone) via Time-Resolved Simultaneous Small-Angle X-ray Scattering/Wide-Angle X-ray Diffraction Measurements. Macromolecules 1995, 28, 6931–6936. [Google Scholar] [CrossRef]
- Hay, J.; Langford, J.; Lloyd, J. Variation in unit cell parameters of aromatic polymers with crystallization temperature. Polymer 1989, 30, 489–493. [Google Scholar] [CrossRef]
- Beaucage, G. Approximations leading to a unified exponential/power-law approach to small-angle scattering. J. Appl. Crystallogr. 1995, 28, 717–728. [Google Scholar] [CrossRef]
- Ogawa, T. Small-angle X-ray scattering from the surfaces of polymer crystals. J. Phys. Soc. Jpn. 1990, 59, 3642–3649. [Google Scholar] [CrossRef][Green Version]
- Vonk, C.G. Computerization of Ruland’s X-ray method for determination of the crystallinity in polymers. J. Appl. Crystallogr. 1973, 6, 148–152. [Google Scholar] [CrossRef]
- James, M.; Anderson, D. Determination of crystallinity in graphite fiber-reinforced thermoplastic composites. Adv. X-ray Anal. 1985, 29, 291–303. [Google Scholar] [CrossRef]
- Kumar, S.; Anderson, D.P.; Adams, W.W. Crystallization and morphology of poly(aryl-ether-ether-ketone). Polymer 1986, 27, 329–336. [Google Scholar] [CrossRef]
- Spahr, D.; Schultz, J.M. Determination of matrix crystallinity of composites by X-ray diffraction. Polym. Compos. 1990, 11, 201–210. [Google Scholar] [CrossRef]
- Sing, K.S.; Everett, D.; Haul, R.; Moscou, L.; Pierotti, R.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Nikonovich, M.; Costa, J.F.S.; Fonseca, A.C.; Ramalho, A.; Emami, N. Structural, thermal, and mechanical characterisation of PEEK-based composites in cryogenic temperature. Polym. Test. 2023, 125, 108139. [Google Scholar] [CrossRef]
- Gibson, I.; Ashby, M.F. The mechanics of three-dimensional cellular materials. Proc. R. Soc. Lond. A Math. Phys. Sci. 1982, 382, 43–59. [Google Scholar]
- Woignier, T.; Reynes, J.; Alaoui, A.H.; Beurroies, I.; Phalippou, J. Different kinds of structure in aerogels: Relationships with the mechanical properties. J. Non-Cryst. Solids 1998, 241, 45–52. [Google Scholar] [CrossRef]
- Fan, H.; Hartshorn, C.; Buchheit, T.; Tallant, D.; Assink, R.; Simpson, R.; Kissel, D.J.; Lacks, D.J.; Torquato, S.; Brinker, C.J. Modulus–density scaling behaviour and framework architecture of nanoporous self-assembled silicas. Nat. Mater. 2007, 6, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Fricke, J. Aerogels—highly tenuous solids with fascinating properties. J. Non-Cryst. Solids 1988, 100, 169–173. [Google Scholar] [CrossRef]
- Williams, J.C.; Meador, M.A.B.; McCorkle, L.; Mueller, C.; Wilmoth, N. Synthesis and Properties of Step-Growth Polyamide Aerogels Cross-linked with Triacid Chlorides. Chem. Mater. 2014, 26, 4163–4171. [Google Scholar] [CrossRef]
- Guo, H.; Meador, M.A.B.; McCorkle, L.; Quade, D.J.; Guo, J.; Hamilton, B.; Cakmak, M.; Sprowl, G. Polyimide Aerogels Cross-Linked through Amine Functionalized Polyoligomeric Silsesquioxane. ACS Appl. Mater. Interfaces 2011, 3, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Leventis, N.; Sotiriou-Leventis, C.; Chandrasekaran, N.; Mulik, S.; Larimore, Z.J.; Lu, H.; Churu, G.; Mang, J.T. Multifunctional polyurea aerogels from isocyanates and water. A structure− property case study. Chem. Mater. 2010, 22, 6692–6710. [Google Scholar] [CrossRef]
- Ilavsky, J.; Jemian, P.R. Irena: Tool suite for modeling and analysis of small-angle scattering. J. Appl. Crystallogr. 2009, 42, 347–353. [Google Scholar] [CrossRef]
- ASTM D695-23; Standard Test Method for Compressive Properties of Rigid Plastics. American Society for Testing and Materials: West Conshohocken, PA, USA, 2023.
- Ruland, W. X-ray determination of crystallinity and diffuse disorder scattering. Acta Crystallogr. 1961, 14, 1180–1185. [Google Scholar] [CrossRef]
- Roe, R.-J. Methods of X-ray and Neutron Scattering in Polymer Science; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Tence-Girault, S.; Quibel, J.; Cherri, A.; Roland, S.; Fayolle, B.; Bizet, S.; Iliopoulos, I. Quantitative structural study of cold-crystallized PEKK. ACS Appl. Poly. Mater. 2021, 3, 1795–1808. [Google Scholar] [CrossRef]
- Prince, E. International Tables for Crystallography, Volume C: Mathematical, Physical and Chemical Tables; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Hubbell, J.H.; Veigele, W.J.; Briggs, E.; Brown, R.; Cromer, D.; Howerton, d.R. Atomic form factors, incoherent scattering functions, and photon scattering cross sections. J. Phys. Chem. Ref. Data 1975, 4, 471–538. [Google Scholar] [CrossRef]

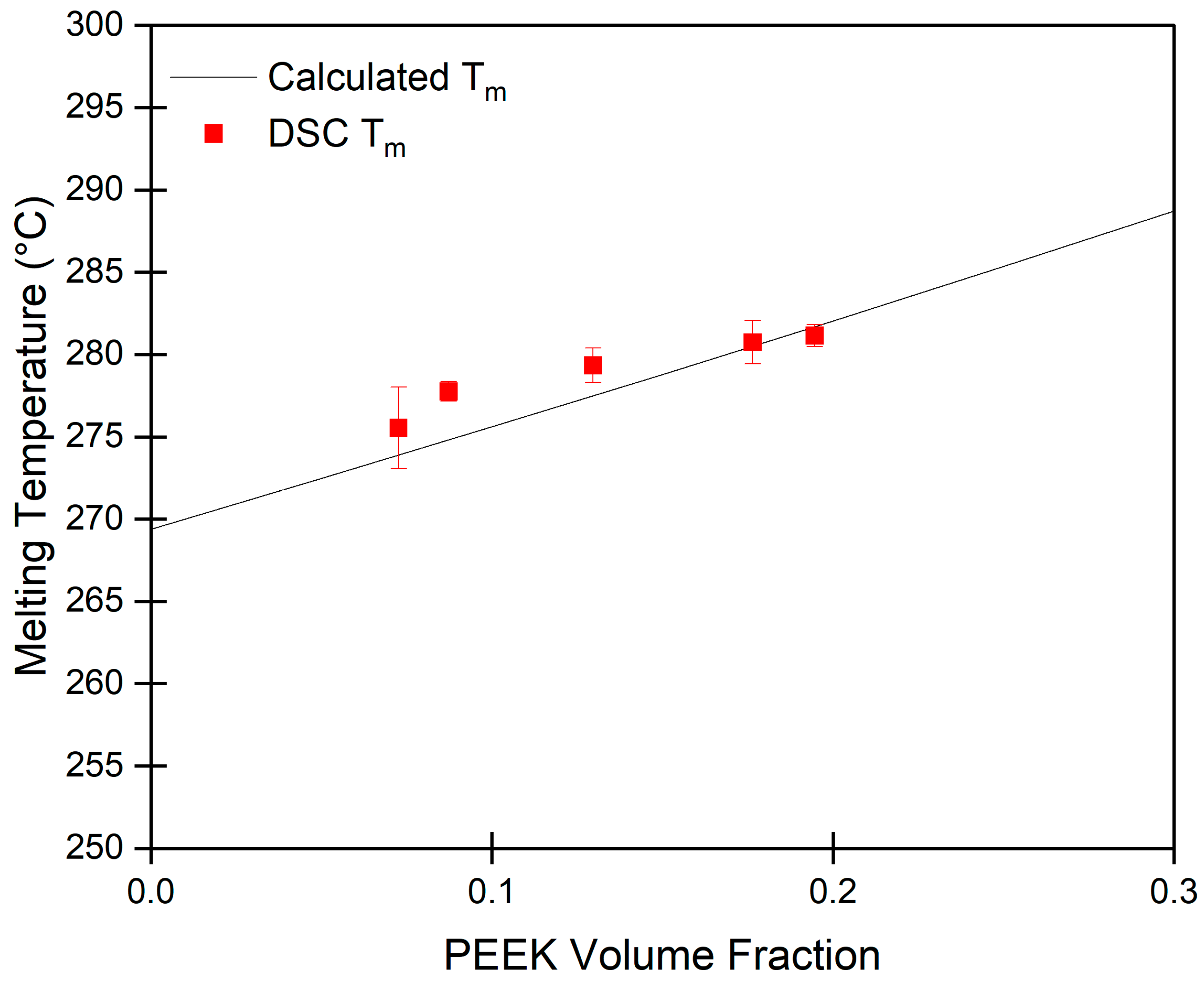
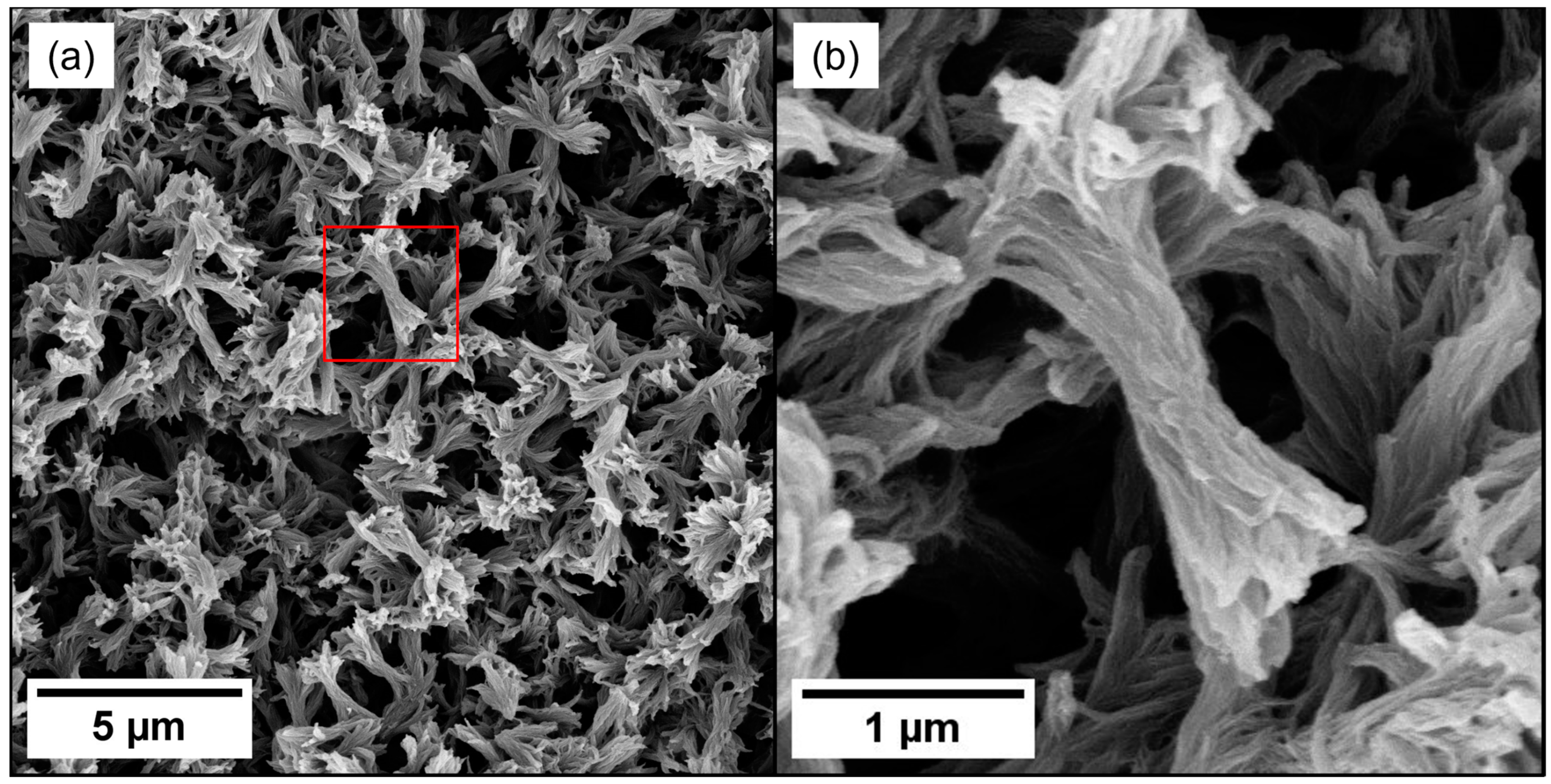

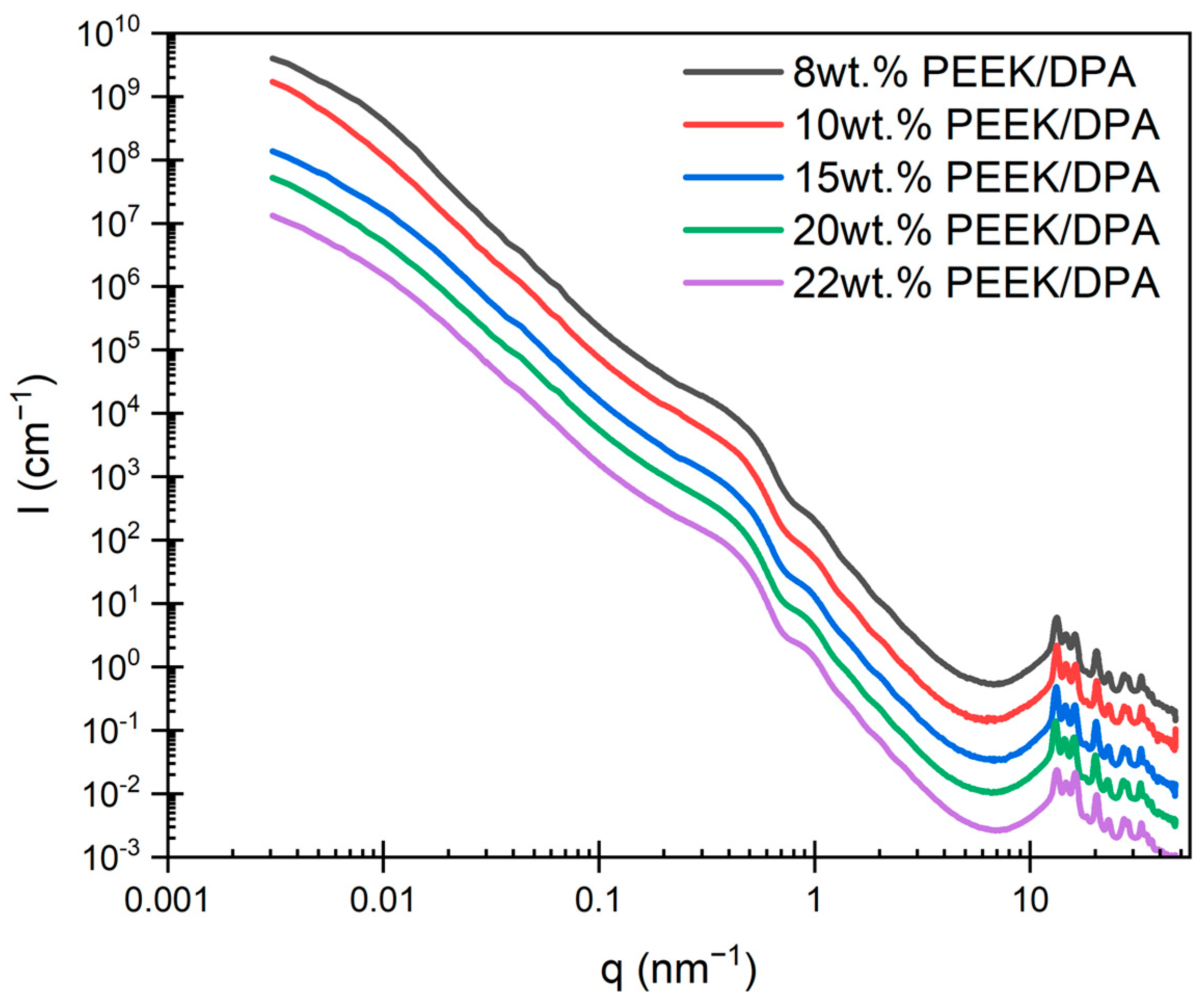
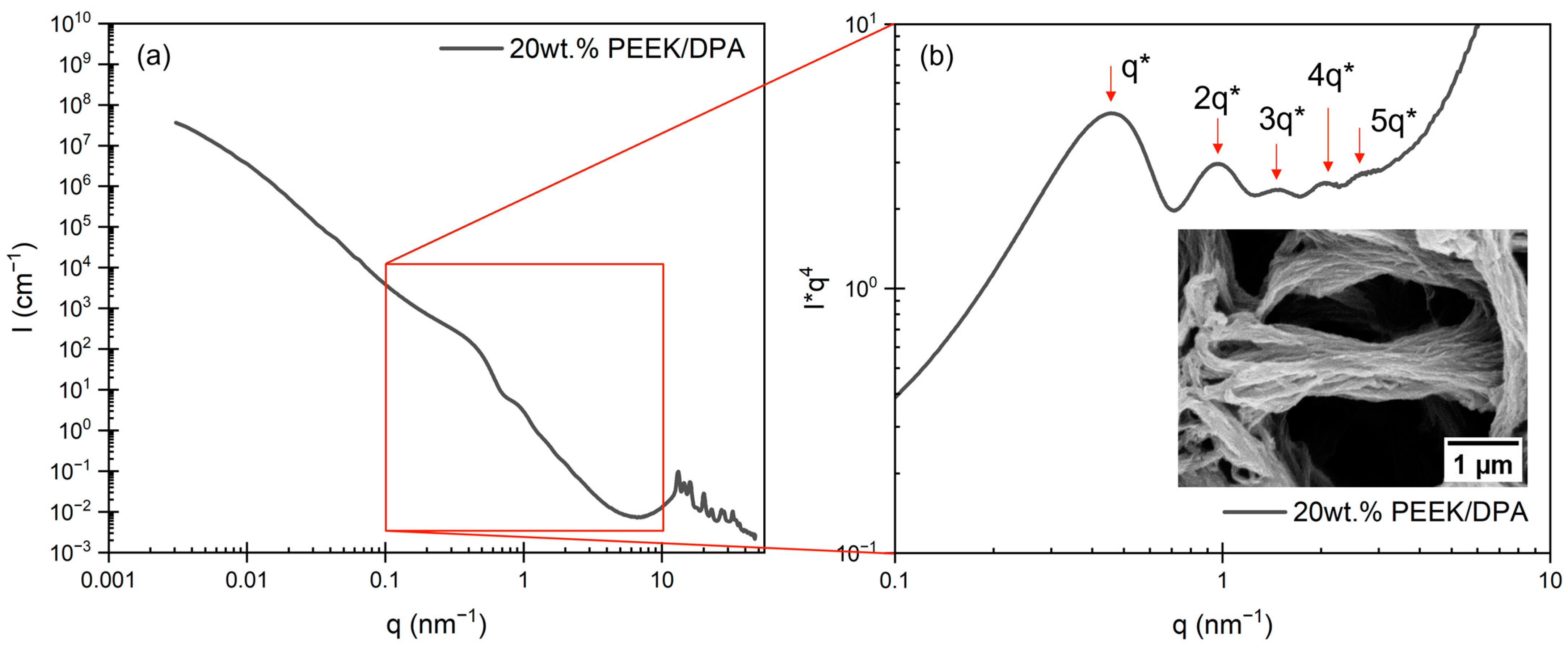
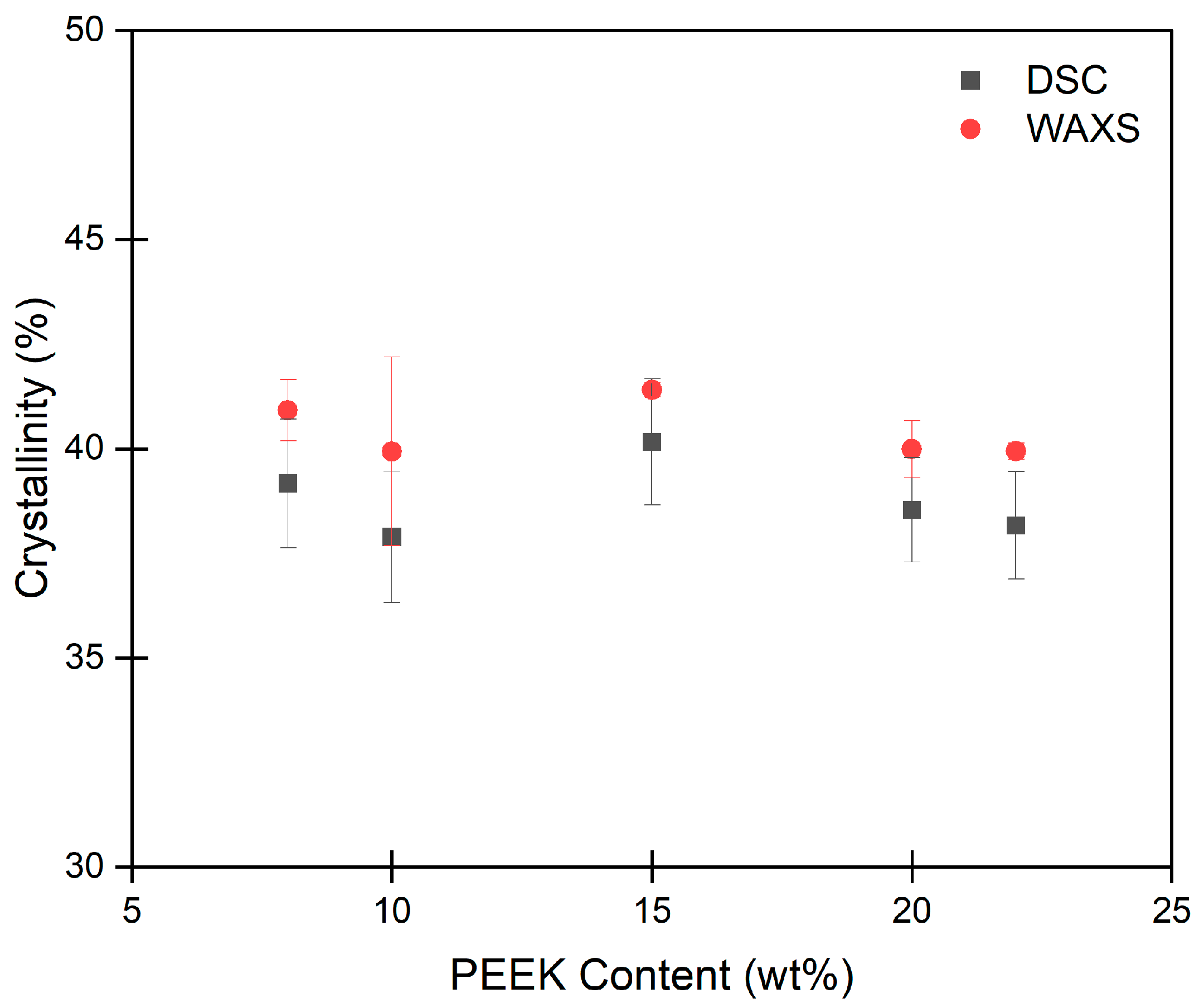

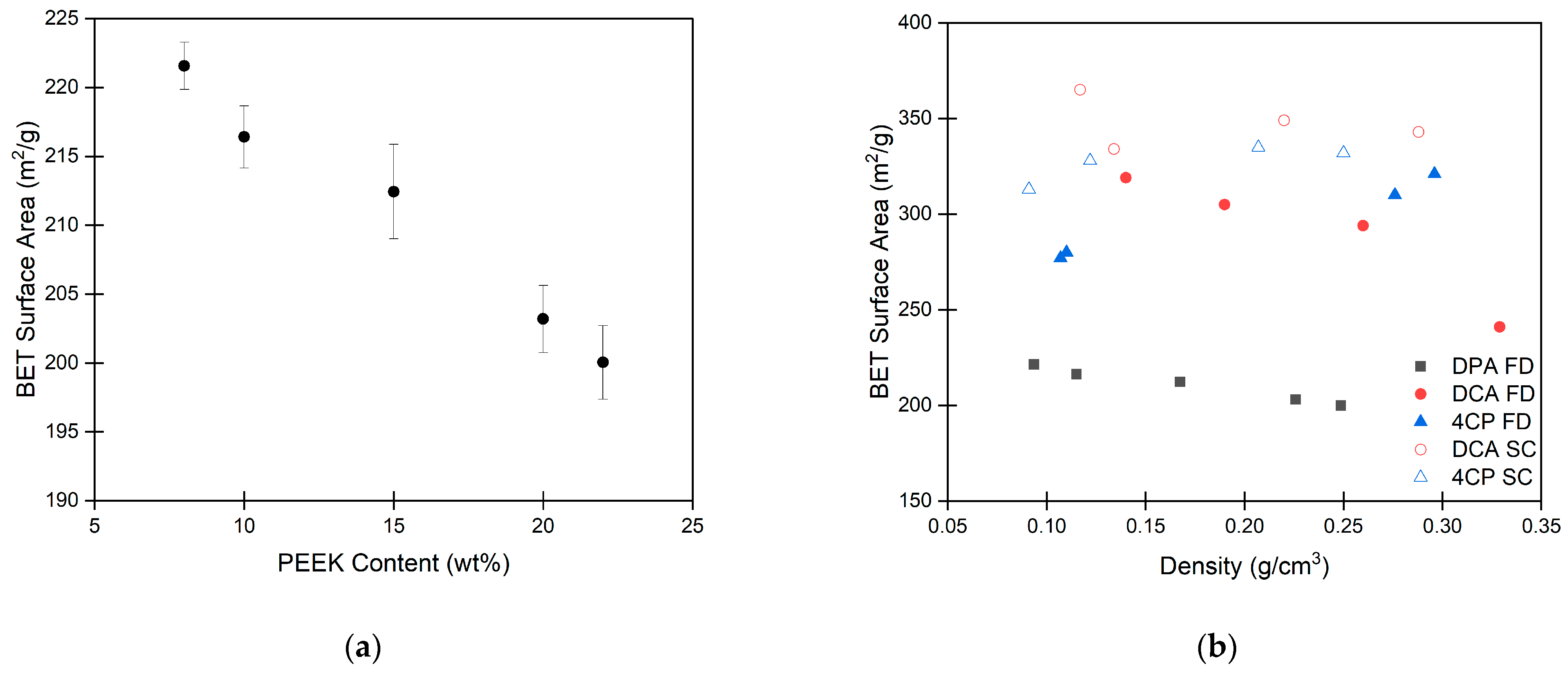


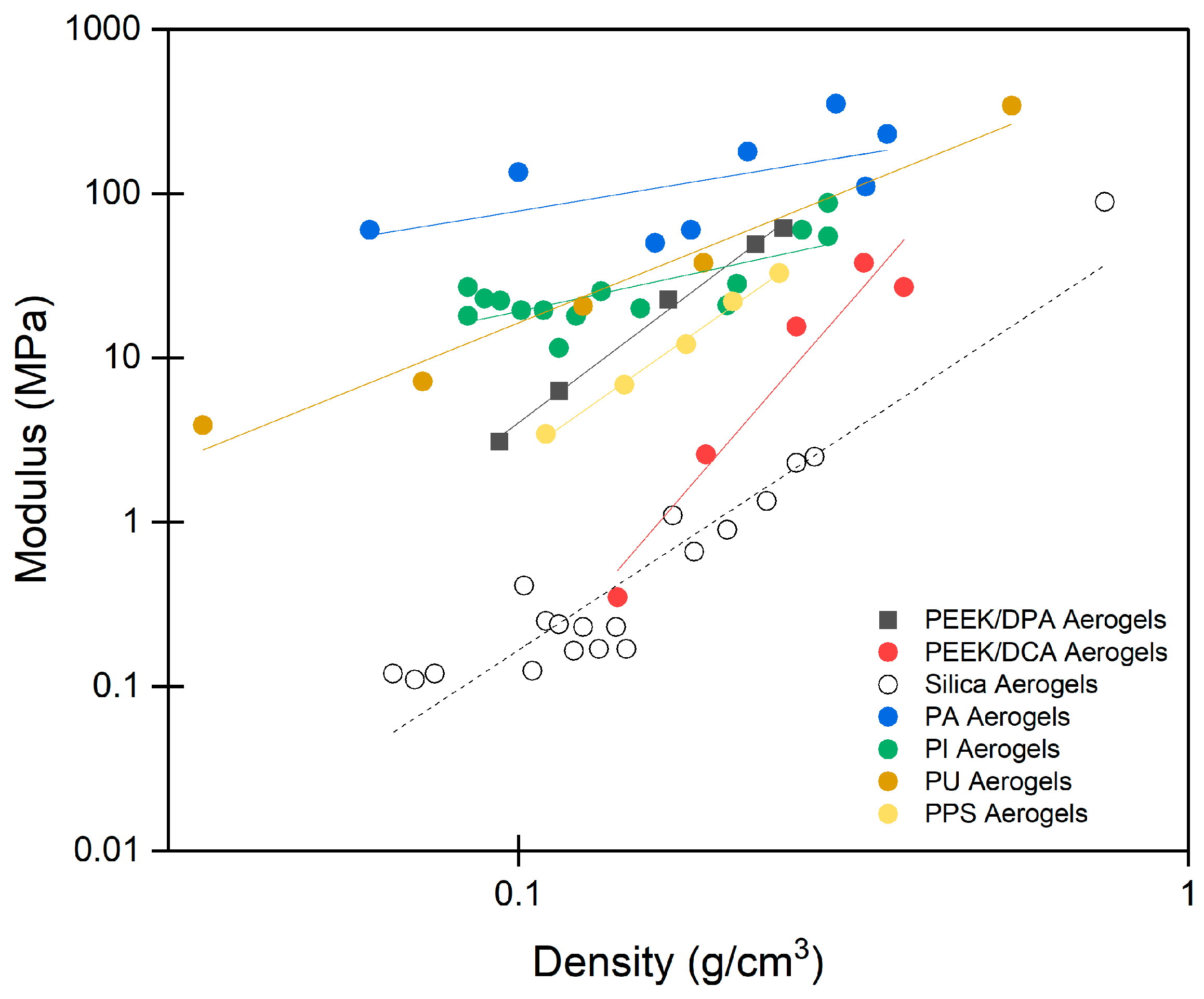
| PEEK Concentration (wt.%) | Average Strut Thickness (μm) | Average Strut Length (μm) |
|---|---|---|
| 8 | 0.471 ± 0.094 | 2.57 ± 0.38 |
| 10 | 0.499 ± 0.110 | 3.12 ± 0.49 |
| 15 | 0.418 ± 0.083 | 2.39 ± 0.31 |
| 20 | 0.470 ± 0.111 | 3.07 ± 0.59 |
| 22 | 0.476 ± 0.089 | 3.48 ± 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiering, G.A.; Godshall, G.F.; Moore, R.B. High Modulus, Strut-like poly(ether ether ketone) Aerogels Produced from a Benign Solvent. Gels 2024, 10, 283. https://doi.org/10.3390/gels10040283
Spiering GA, Godshall GF, Moore RB. High Modulus, Strut-like poly(ether ether ketone) Aerogels Produced from a Benign Solvent. Gels. 2024; 10(4):283. https://doi.org/10.3390/gels10040283
Chicago/Turabian StyleSpiering, Glenn A., Garrett F. Godshall, and Robert B. Moore. 2024. "High Modulus, Strut-like poly(ether ether ketone) Aerogels Produced from a Benign Solvent" Gels 10, no. 4: 283. https://doi.org/10.3390/gels10040283
APA StyleSpiering, G. A., Godshall, G. F., & Moore, R. B. (2024). High Modulus, Strut-like poly(ether ether ketone) Aerogels Produced from a Benign Solvent. Gels, 10(4), 283. https://doi.org/10.3390/gels10040283







