Effect of Gold Nanoparticle Size on Regulated Catalytic Activity of Temperature-Responsive Polymer−Gold Nanoparticle Hybrid Microgels
Abstract
:1. Introduction
2. Results and Discussion
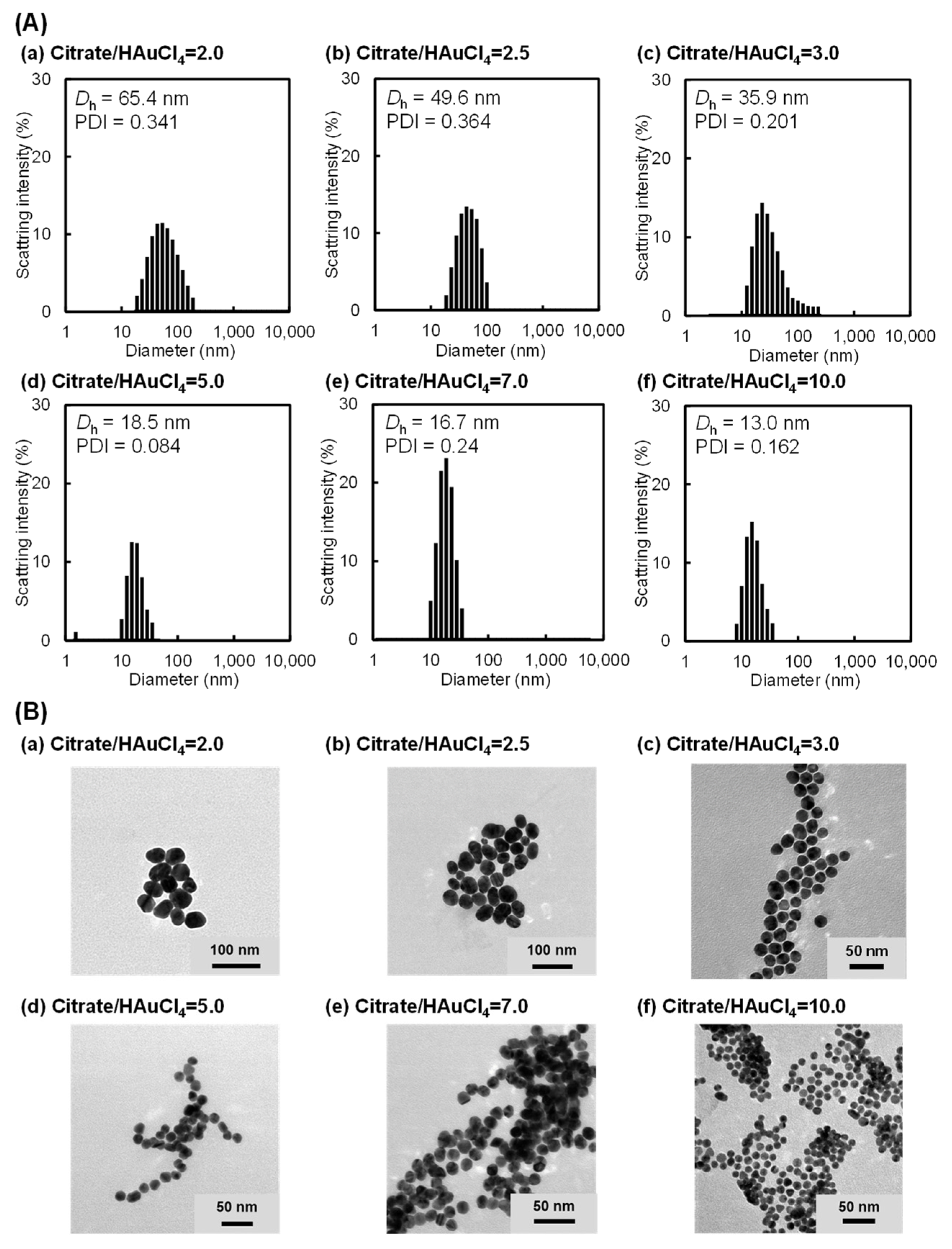
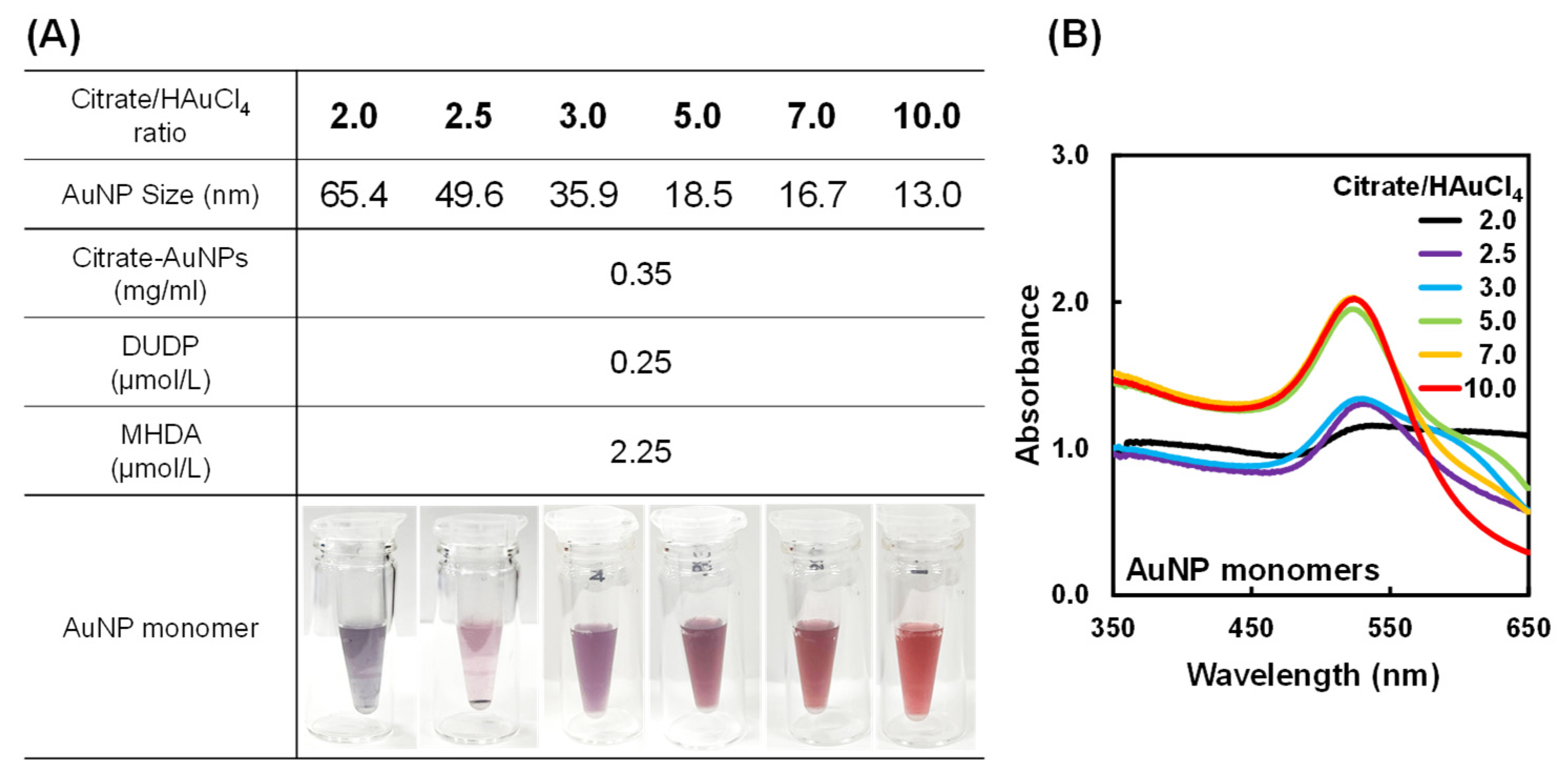
2.1. Preparation of AuNP Monomers with Various Sizes
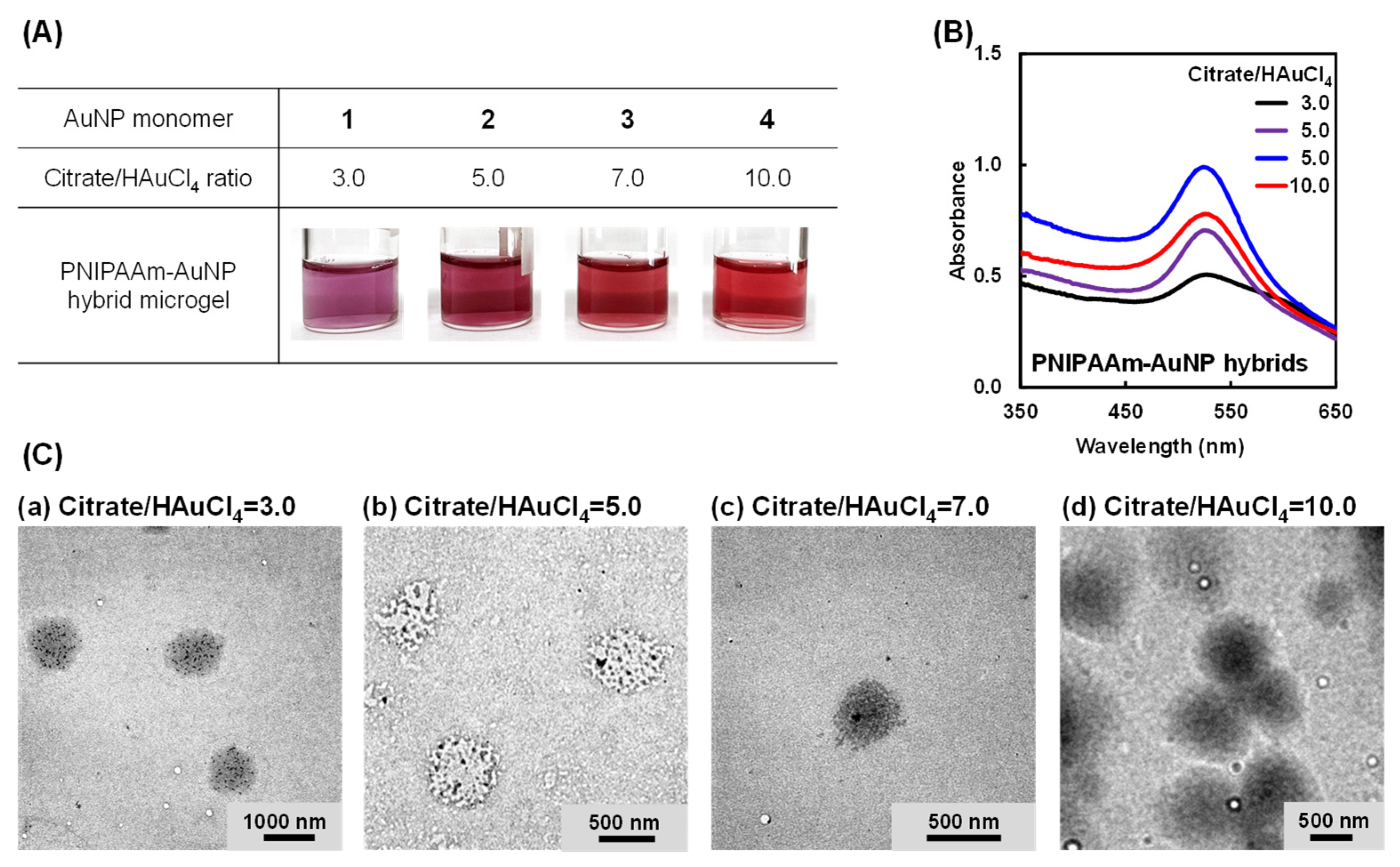
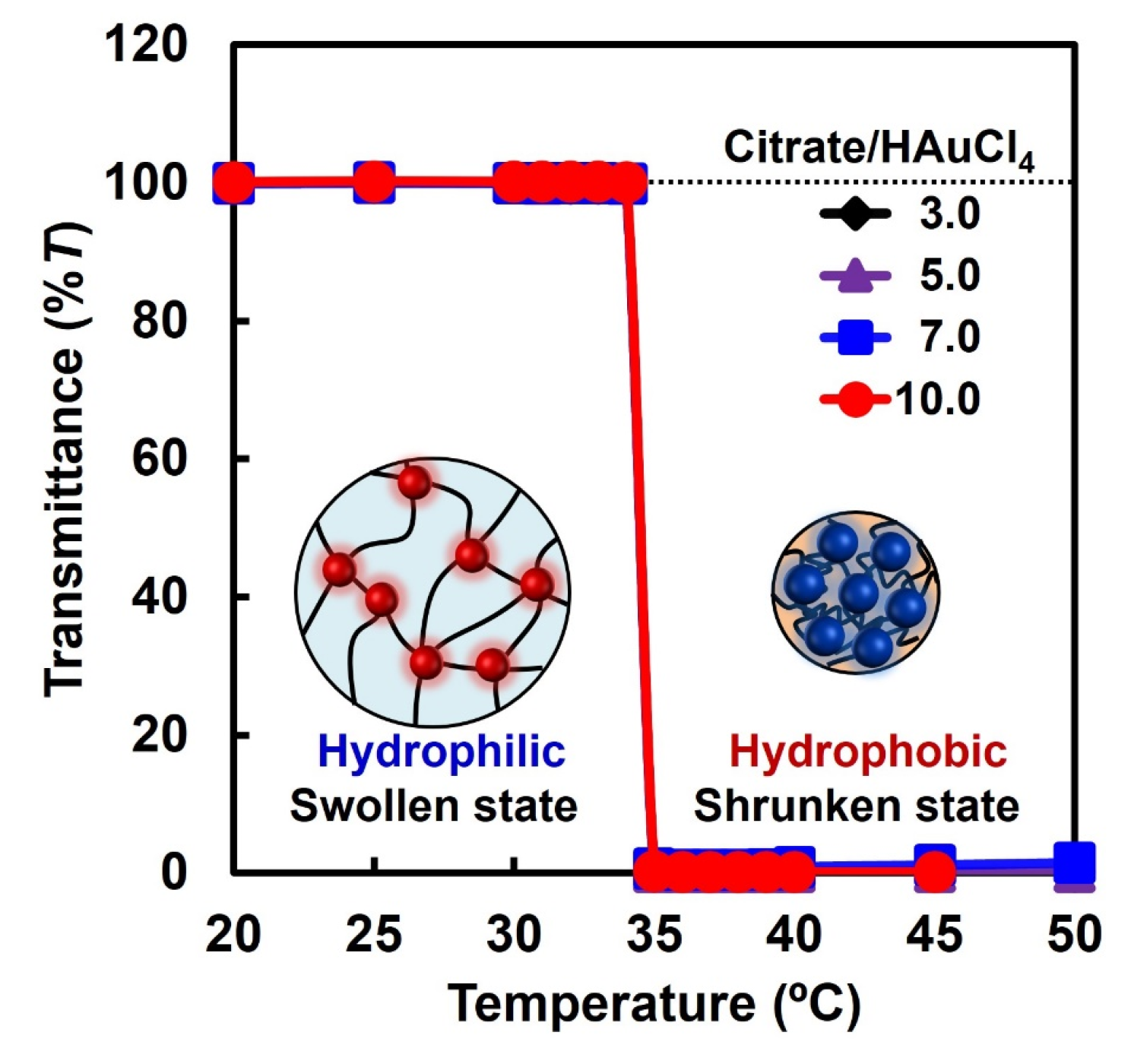
2.2. Preparation of PNIPAAM–AuNP Hybrid Microgels Using AuNP Monomers with Different AuNP Sizes
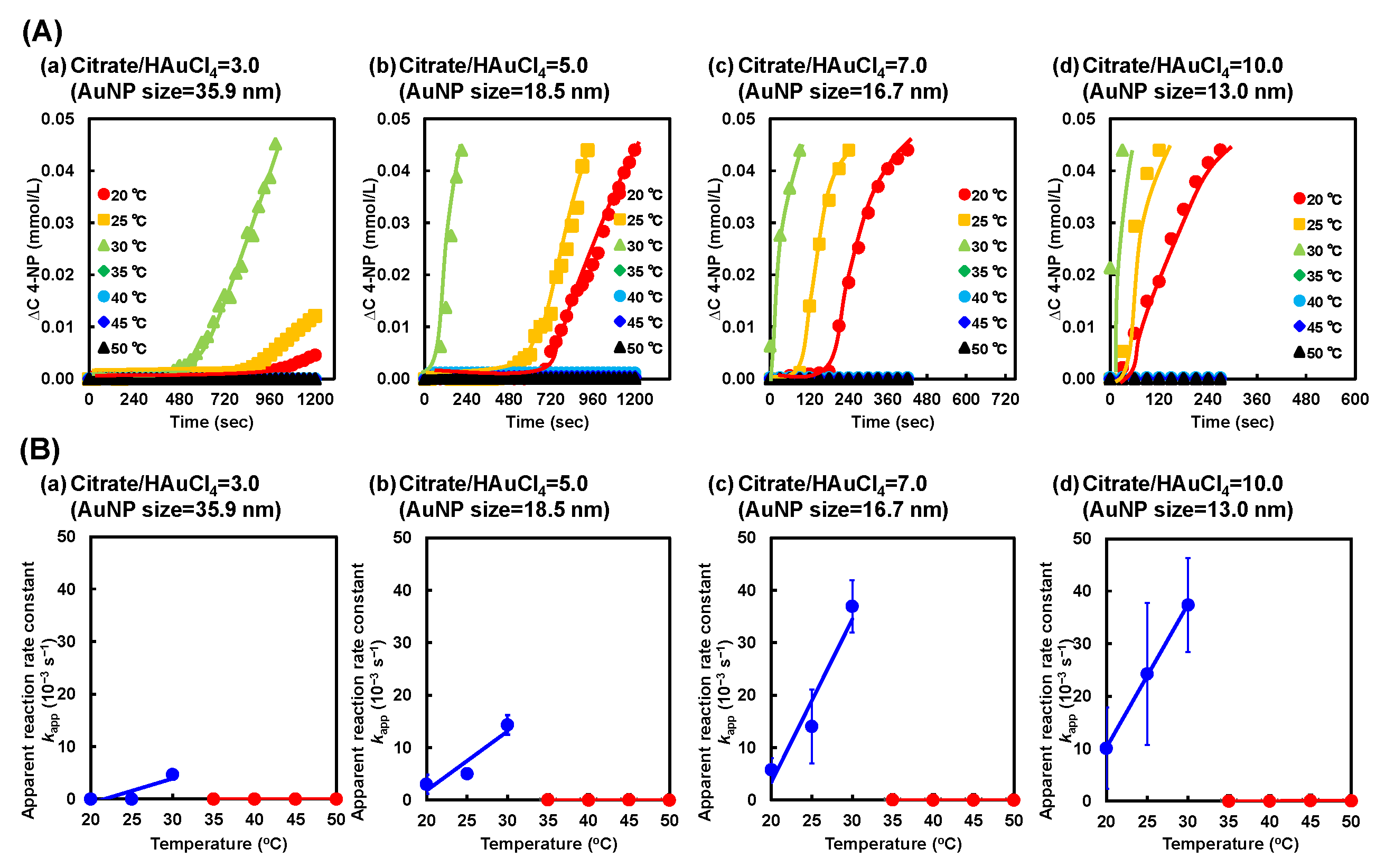
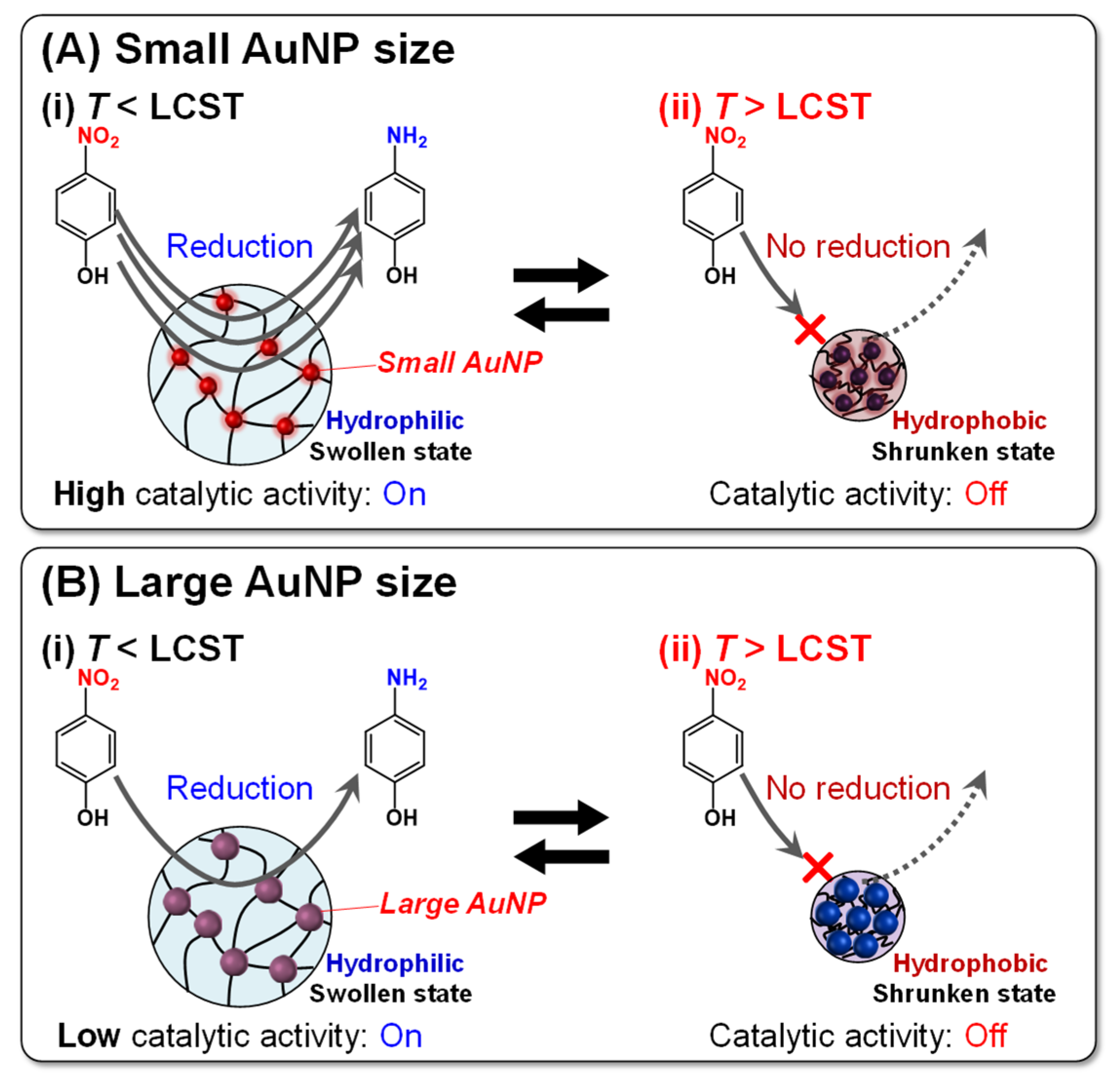
2.3. Catalytic Activity of PNIPAAm–AuNP Hybrid Microgels with Various AuNP Sizes
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of AuNP Monomer and PNIPAAm–AuNP Hybrid Microgels
4.3. Characterization of AuNP Monomers and PNIPAAm–AuNP Hybrid Microgels
4.4. Evaluation of Catalytic Activity of PNIPAAm–AuNP Hybrid Microgels
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Daniel, M.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold Nanoparticles: Preparation, Properties, and Applications in Bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Choi, E.; Ellis, E.; Lee, T. Recent Advances in Gold Nanoparticles for Biomedical Applications: From Hybrid Structures. J. Mater. Chem. B 2019, 7, 3480–3496. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold Nanoparticles in Biomedical Applications: Recent Advances and Perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, N.; Khan, I. Plasmonic Gold Nanoparticles (AuNPs): Properties, Synthesis and Their Advanced Energy, Environmental and Biomedical Applications. Chem. Asian J. 2021, 16, 720–742. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.V.; Anthony, S.P. AuNP Based Selective Colorimetric Sensor for Cysteine at a Wide PH Range: Investigation of Capping Molecule Structure on the Colorimetric Sensing and Catalytic Properties. RSC Adv. 2014, 4, 18467–18472. [Google Scholar] [CrossRef]
- Kusolkamabot, K.; Sae-Ung, P.; Niamnont, N.; Wongravee, K.; Sukwattanasinitt, M.; Hoven, V.P. Poly(N -Isopropylacrylamide)-Stabilized Gold Nanoparticles in Combination with Tricationic Branched Phenylene-Ethynylene Fluorophore for Protein Identification. Langmuir 2013, 29, 12317–12327. [Google Scholar] [CrossRef] [PubMed]
- Her, S.; Jaffray, D.A.; Allen, C. Gold Nanoparticles for Applications in Cancer Radiotherapy: Mechanisms and Recent Advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Nonappa, N. Luminescent Gold Nanoclusters for Bioimaging Applications. Beilstein J. Nanotechnol. 2020, 11, 533–546. [Google Scholar] [CrossRef]
- Gluhoi, A.C.; Bakker, J.W.; Nieuwenhuys, B.E. Gold, Still a Surprising Catalyst: Selective Hydrogenation of Acetylene to Ethylene over Au Nanoparticles. Catal. Today 2010, 154, 13–20. [Google Scholar] [CrossRef]
- Liang, S.; Jasinski, J.; Hammond, G.B.; Xu, B. Supported Gold Nanoparticle-Catalyzed Hydration of Alkynes under Basic Conditions. Org. Lett. 2015, 17, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jin, R. Catalysis by Gold Nanoparticles: Carbon-Carbon Coupling Reactions. Nanotechnol. Rev. 2013, 2, 529–545. [Google Scholar] [CrossRef]
- Sisodiya, S.; Wallenberg, L.R.; Lewin, E.; Wendt, O.F. Applied Catalysis A: General Sonogashira Coupling Reaction over Supported Gold Nanoparticles: Influence of Support and Catalyst Synthesis Route. Appl. Catal. A Gen. 2015, 503, 69–76. [Google Scholar] [CrossRef]
- Tsunoyama, H.; Sakurai, H.; Negishi, Y.; Tsukuda, T. Size-Specific Catalytic Activity of Polymer-Stabilized Gold Nanoclusters for Aerobic Alcohol Oxidation in Water. J. Am. Chem. Soc. 2005, 127, 9374–9375. [Google Scholar] [CrossRef] [PubMed]
- Della Pina, C.; Falletta, E.; Rossi, M. Update on Selective Oxidation Using Gold. Chem. Soc. Rev. 2012, 41, 350–369. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.J.; Liu, B.; Liu, J. Characterization of Glucose Oxidation by Gold Nanoparticles Using Nanoceria. J. Colloid Interface Sci. 2014, 428, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic Concepts and Recent Advances in Nitrophenol Reduction by Gold- and Other Transition Metal Nanoparticles. Coord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Ilunga, A.K.; Mamba, B.B.; Nkambule, T.T.I.I. Ferricyanide Reduction to Elucidate Kinetic and Electrochemical Activities on the Metal Nanocatalysts Surface. Chem. Eng. J. 2020, 398, 125623. [Google Scholar] [CrossRef]
- Corma, A.; Garci, H. Supported Gold Nanoparticles as Catalysts for Organic Reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, S.R.; Heo, J.H. Simultaneous Stabilization and Functionalization of Gold Nanoparticles via Biomolecule Conjugation: Progress and Perspectives. ACS Appl. Mater. Interfaces 2021, 13, 42311–42328. [Google Scholar] [CrossRef]
- Fan, J.; Gao, Y. Nanoparticle-Supported Catalysts and Catalytic Reactions—A Mini-Review. J. Exp. Nanosci. 2006, 1, 457–475. [Google Scholar] [CrossRef]
- Tang, S.; Vongehr, S.; Meng, X. Controllable Incorporation of Ag and Ag–Au Nanoparticles in Carbon Spheres for Tunable Optical and Catalytic Properties. J. Mater. Chem. 2010, 20, 5436–5445. [Google Scholar] [CrossRef]
- Christau, S.; Möller, T.; Brose, F.; Genzer, J.; Soltwedel, O.; von Klitzing, R. Effect of Gold Nanoparticle Hydrophobicity on Thermally Induced Color Change of PNIPAM Brush/Gold Nanoparticle Hybrids. Polymer 2016, 98, 454–463. [Google Scholar] [CrossRef]
- Nguyen, M.; Felidj, N.; Mangeney, C. Looking for Synergies in Molecular Plasmonics through Hybrid Thermoresponsive Nanostructures. Chem. Mater. 2016, 28, 3564–3577. [Google Scholar] [CrossRef]
- Seo, E.; Kim, J.; Hong, Y.; Kim, Y.S.; Lee, D.; Kim, B.S. Double Hydrophilic Block Copolymer Templated Au Nanoparticles with Enhanced Catalytic Activity toward Nitroarene Reduction. J. Phys. Chem. C 2013, 117, 11686–11693. [Google Scholar] [CrossRef]
- Wang, D.; Liu, B.; Lü, J.; Lü, C. Facile Synthesis of Thermo-Responsive Episulfide Group-Containing Diblock Copolymers as Robust Protecting Ligands of Gold Nanoparticles for Catalytic Applications. RSC Adv. 2016, 6, 37487–37499. [Google Scholar] [CrossRef]
- Li, C.; Wang, C.; Ji, Z.; Jiang, N.; Lin, W.; Li, D. Synthesis of Thiol-Terminated Thermoresponsive Polymers and Their Enhancement Effect on Optical Limiting Property of Gold Nanoparticles. Eur. Polym. J. 2019, 113, 404–410. [Google Scholar] [CrossRef]
- Ida, S.; Harada, H.; Sakai, K.; Atsumi, K.; Tani, Y.; Tanimoto, S.; Hirokawa, Y. Shape and Size Regulation of Gold Nanoparticles by Poly(N,N-Diethylacrylamide) Microgels. Chem. Lett. 2017, 46, 760–763. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Y.; Feng, W.; Qi, J.; Zhang, G.; Zhang, F.; Fan, X. Robust and Smart Gold Nanoparticles: One-Step Synthesis, Tunable Optical Property, and Switchable Catalytic Activity. J. Mater. Chem. 2011, 21, 6173–6178. [Google Scholar] [CrossRef]
- Marcelo, G.; López-González, M.; Mendicuti, F.; Tarazona, M.P.; Valiente, M. Poly(N-Isopropylacrylamide)/Gold Hybrid Hydrogels Prepared by Catechol Redox Chemistry. Characterization and Smart Tunable Catalytic Activity. Macromolecules 2014, 47, 6028–6036. [Google Scholar] [CrossRef]
- Mandal, T.K.; Fleming, M.S.; Walt, D.R. Preparation of Polymer Coated Gold Nanoparticles by Surface-Confined Living Radical Polymerization at Ambient Temperature. Nano. Lett. 2002, 2, 3–7. [Google Scholar] [CrossRef]
- Koenig, S.; Chechik, V. Reactivity of Acrylate-Terminated Au Nanoparticles: Suppressed Intramolecular Catalysis and Lack of Cooperative Effect. Langmuir 2003, 19, 9511–9517. [Google Scholar] [CrossRef]
- Yang, Y.; Serrano, L.A.; Guldin, S. A Versatile AuNP Synthetic Platform for Decoupled Control of Size and Surface Composition. Langmuir 2018, 34, 6820–6826. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T. Stimuli-Responsive Polymers and Gels. In Supramolecular Design for Biological Applictions; Yui, N., Ed.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2002; pp. 191–225. [Google Scholar]
- Lanzalaco, S.; Armelin, E. Poly(N-Isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.; Yang, Y.; Gao, J.; Duan, H.; Lü, C. Thermoresponsive Amphiphilic Block Copolymer-Stablilized Gold Nanoparticles: Synthesis and High Catalytic Properties. Langmuir 2018, 34, 8205–8214. [Google Scholar] [CrossRef]
- Li, F.; Wang, C.; Guo, W. Multifunctional Poly-N-Isopropylacrylamide/DNAzyme Microgels as Highly Efficient and Recyclable Catalysts for Biosensing. Adv. Funct. Mater. 2018, 28, 1705876. [Google Scholar] [CrossRef]
- Miyata, T.; Jige, M.; Nakaminami, T.; Uragami, T. Tumor Marker-Responsive Behavior of Gels Prepared by Biomolecular Imprinting. Proc. Natl. Acad. Sci. USA 2006, 103, 1190–1193. [Google Scholar] [CrossRef]
- Toyoshima, Y.; Kawamura, A.; Takashima, Y.; Miyata, T. Design of Molecularly Imprinted Hydrogels with Thermoresponsive Drug Binding Sites. J. Mater. Chem. B 2022, 10, 6644–6654. [Google Scholar] [CrossRef]
- Miyata, T.; Asami, N.; Uragami, T. A Reversibly Antigen-Responsive Hydrogel. Nature 1999, 399, 766–769. [Google Scholar] [CrossRef]
- Shi, S.; Wang, Q.; Wang, T.; Ren, S.; Gao, Y.; Wang, N. Thermo-, PH-, and Light-Responsive Poly(N-Isopropylacrylamide-Co-Methacrylic Acid)-Au Hybrid Microgels Prepared by the in Situ Reduction Method Based on Au-Thiol Chemistry. J. Phys. Chem. B 2014, 118, 7177–7186. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, X.; Liu, Y.; Wu, C.; Ma, R.; An, Y.; Shi, L. Thermosensitive and PH-Sensitive Au-Pd Bimetallic Nanocomposites. J. Colloid Interface Sci. 2009, 331, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Ahiabu, A.; Serpe, M.J. Rapidly Responding PH- and Temperature-Responsive Poly (N—Isopropylacrylamide)-Based Microgels and Assemblies. ACS Omega 2017, 2, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Goldhahn, C.; Schubert, J.; Schlaad, H.; Ferri, J.K.; Fery, A.; Chanana, M. Synthesis of Metal@Protein@Polymer Nanoparticles with Distinct Interfacial and Phase Transfer Behavior. Chem. Mater. 2018, 30, 6717–6727. [Google Scholar] [CrossRef]
- Jones, S.T.; Walsh-korb, Z.; Barrow, S.J.; Henderson, S.L.; Scherman, O.A. The Importance of Excess Poly(N—Isopropylacrylamide) for the Aggregation of Poly(N—Isopropylacrylamide)-Coated Gold Nanoparticles. ACS Nano 2016, 10, 3158–3165. [Google Scholar] [CrossRef] [PubMed]
- Kureha, T.; Nagase, Y.; Suzuki, D. High Reusability of Catalytically Active Gold Nanoparticles Immobilized in Core-Shell Hydrogel Microspheres. ACS Omega 2018, 3, 6158–6165. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Liu, X.; Wang, J.; An, Y.; Zhang, Z.; Shi, L. Thermosensitive Mixed Shell Polymeric Micelles Decorated with Gold Nanoparticles at the Outmost Surface: Tunable Surface Plasmon Resonance and Enhanced Catalytic Properties with Excellent Colloidal Stability. RSC Adv. 2015, 5, 47458–47465. [Google Scholar] [CrossRef]
- Sardar, R.; Park, J.; Shumaker-parry, J.S.; August, R. V Polymer-Induced Synthesis of Stable Gold and Silver Nanoparticles and Subsequent Ligand Exchange in Water. Langmuir 2020, 23, 11883–11889. [Google Scholar] [CrossRef] [PubMed]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle–Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef]
- Wu, G.; Wang, L.; Zhou, P.; Wen, P.; Ma, C.; Huang, X.; Huang, Y. Design and Construction of Hybrid Microcapsules with Higher-Order Structure and Multiple Functions. Adv. Sci. 2018, 5, 1700460. [Google Scholar] [CrossRef]
- Li, D.; Cui, Y.; Wang, K.; He, Q.; Yan, X.; Li, J. Thermosensitive Nanostructures Comprising Gold Nanoparticles Grafted with Block Copolymers. Adv. Funct. Mater. 2007, 17, 3134–3140. [Google Scholar] [CrossRef]
- He, Z.; Zhong, A.; Zhang, H.; Xiong, L.; Xu, Y.; Wang, T.; Zhou, M.; Huang, K. In Situ Formation of Dual-Phase Thermosensitive Ultrasmall Gold Nanoparticles. Chem. Eur. J. 2015, 21, 10220–10225. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, D.; Zhou, F.; Liu, W. Electrostatic Self-Assembly of Au Nanoparticles onto Thermosensitive Magnetic Core-Shell Microgels for Thermally Tunable and Magnetically Recyclable Catalysis. Small 2015, 11, 2807–2816. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhang, L.; Wang, T.; Wang, Q.; Gao, Y.; Wang, N. Poly(N-Isopropylacrylamide)-Au Hybrid Microgels: Synthesis, Characterization, Thermally Tunable Optical and Catalytic Properties. Soft Matter 2013, 9, 10966–10970. [Google Scholar] [CrossRef]
- Pongsanon, P.; Oota, Y.; Kawamura, A.; Kawasaki, H.; Miyata, T. Controllable Catalytic Activity of Temperature-Responsive Polymer Hybrid Microgels Designed Using a Gold Nanoparticle Monomer with Polymerizable Groups. Macromolecules 2023, 56, 9853–9865. [Google Scholar] [CrossRef]
- Narayanan, R.; El-Sayed, M.A. Catalysis with Transition Metal Nanoparticles in Colloidal Solution: Nanoparticle Shape and Stability. J. Phys. Chem. B 2005, 109, 12663–12676. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, L.; Doña, M.; Lopez-Romero, J.M.; Fery, A.; Contreras-Caceres, R. Temperature-Controlled Catalysis by Core-Shell-Satellite AuAg@pNIPAM@Ag Hybrid Microgels: A Highly Efficient Catalytic Thermoresponsive Nanoreactor. ACS Appl. Mater. Interfaces 2019, 11, 29360–29372. [Google Scholar] [CrossRef] [PubMed]
- Donoeva, B.G.; Ovoshchnikov, D.S.; Golovko, V.B. Establishing a Au Nanoparticle Size Effect in the Oxidation of Cyclohexene Using Gradually Changing Au Catalysts. ACS Catal. 2013, 3, 2986–2991. [Google Scholar] [CrossRef]
- Fenger, R.; Fertitta, E.; Kirmse, H.; Thünemann, A.F.; Rademann, K. Size Dependent Catalysis with CTAB-Stabilized Gold Nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9343–9349. [Google Scholar] [CrossRef]
- Lin, C.; Tao, K.; Hua, D.; Ma, Z.; Zhou, S. Size Effect of Gold Nanoparticles in Catalytic Reduction Of-Nitrophenol with NaBH4. Molecules 2013, 18, 12609–12620. [Google Scholar] [CrossRef]
- Kapil, N.; Cardinale, F.; Weissenberger, T.; Trogadas, P.; Nijhuis, T.A.; Nigra, M.M.; Coppens, M.O. Gold Nanoparticles with Tailored Size through Ligand Modification for Catalytic Applications. Chem. Commun. 2021, 57, 10775–10778. [Google Scholar] [CrossRef]
- Liang, C.; Cheong, J.Y.; Sitaru, G.; Rosenfeldt, S.; Schenk, A.S.; Gekle, S.; Kim, I.D.; Greiner, A. Size-Dependent Catalytic Behavior of Gold Nanoparticles. Adv. Mater. Interfaces 2022, 9, 2100867. [Google Scholar] [CrossRef]
- Suchomel, P.; Kvitek, L.; Prucek, R.; Panacek, A.; Halder, A.; Vajda, S.; Zboril, R. Simple Size-Controlled Synthesis of Au Nanoparticles and Their Size-Dependent Catalytic Activity. Sci. Rep. 2018, 8, 4589. [Google Scholar] [CrossRef] [PubMed]
- Seoudi, R.; Said, D.A. Studies on the Effect of the Capping Materials on the Spherical Gold Nanoparticles Catalytic Activity. World J. Nano Sci. Eng. 2011, 1, 51–61. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, W.; Liu, G.; Panda, D.; Chen, P. Size-Dependent Catalytic Activity and Dynamics of Gold at the Single-Molecule Level. J. Am. Chem. Soc. 2010, 132, 138–146. [Google Scholar] [CrossRef]
- Bano, A.; Dawood, A.; Rida; Saira, F.; Malik, A.; Alkholief, M.; Ahmad, H.; Khan, M.A.; Ahmad, Z.; Bazighifan, O. Enhancing Catalytic activity of Gold Nanoparticles in a Standard Reaction by investigating the Impact of AuNPs Size, Temperature and Reductant. Sci. Rep. 2023, 13, 12359. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Tao, F.F.; Tang, Y.; Li, Y.; Yu, J. Size- and Shape-Dependent Catalytic Performances of Oxidation and Reduction Reactions on Nanocatalysts. Chem. Soc. Rev. 2016, 45, 4747–4765. [Google Scholar] [CrossRef] [PubMed]
- Valden, M.; Pak, S.; Lai, X.; Goodman, D.W. Structure Sensitivity of CO Oxidation over Model Au/TiO2 Catalysts. Catal. Lett. 1998, 56, 7–10. [Google Scholar] [CrossRef]
- Laoufi, I.; Saint-Lager, M.C.; Lazzari, R.; Jupille, J.; Robach, O.; Garaudée, S.; Cabailh, G.; Dolle, P.; Cruguel, H.; Bailly, A. Size and Catalytic Activity of Supported Gold Nanoparticles: An in Operando Study during CO Oxidation. J. Phys. Chem. C 2011, 115, 4673–4679. [Google Scholar] [CrossRef]
- Bokhimi, X.; Zanella, R.; Morales, A. Au/Rutile Catalysts: Effect of Support Dimensions on the Gold Crystallite Size and the Catalytic Activity for CO Oxidation. J. Phys. Chem. C 2007, 111, 15210–15216. [Google Scholar] [CrossRef]
- Doyen, M.; Bartik, K.; Bruylants, G. UV-Vis and NMR Study of the Formation of Gold Nanoparticles by Citrate Reduction: Observation of Gold-Citrate Aggregates. J. Colloid Interface Sci. 2013, 399, 1–5. [Google Scholar] [CrossRef]
- Park, J.W.; Shumaker-Parry, J.S. Structural Study of Citrate Layers on Gold Nanoparticles: Role of Intermolecular Interactions in Stabilizing Nanoparticles. J. Am. Chem. Soc. 2014, 136, 1907–1921. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Carpinone, P.L.; Pyrgiotakis, G.; Demokritou, P.; Moudgil, B.M. Synthesis of Precision Gold Nanoparticles Using Turkevich Method. Physiol. Behav. 2016, 176, 100–106. [Google Scholar] [CrossRef]
- Panigrahi, S.; Basu, S.; Praharaj, S.; Pande, S.; Jana, S.; Pal, A.; Ghosh, S.K.; Pal, T. Synthesis and Size-Selective Catalysis by Supported Gold Nanoparticles: Study on Heterogeneous and Homogeneous Catalytic Process. J. Phys. Chem. C 2007, 111, 4596–4605. [Google Scholar] [CrossRef]
- Mei, Y.; Lu, Y.; Polzer, F.; Ballauff, M.; Drechsler, M. Catalytic Activity of Palladium Nanoparticles Encapsulated in Spherical Poly Electrolyte Brushes and Core-Shell Microgels. Chem. Mater. 2007, 19, 1062–1069. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, X.; Xu, C.; Dai, Z.; Meng, Z. AuNPs-Based Thermoresponsive Nanoreactor as an Efficient Catalyst for the Reduction of 4-Nitrophenol. Nanomaterials 2018, 8, 963. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pongsanon, P.; Kawamura, A.; Kawasaki, H.; Miyata, T. Effect of Gold Nanoparticle Size on Regulated Catalytic Activity of Temperature-Responsive Polymer−Gold Nanoparticle Hybrid Microgels. Gels 2024, 10, 357. https://doi.org/10.3390/gels10060357
Pongsanon P, Kawamura A, Kawasaki H, Miyata T. Effect of Gold Nanoparticle Size on Regulated Catalytic Activity of Temperature-Responsive Polymer−Gold Nanoparticle Hybrid Microgels. Gels. 2024; 10(6):357. https://doi.org/10.3390/gels10060357
Chicago/Turabian StylePongsanon, Palida, Akifumi Kawamura, Hideya Kawasaki, and Takashi Miyata. 2024. "Effect of Gold Nanoparticle Size on Regulated Catalytic Activity of Temperature-Responsive Polymer−Gold Nanoparticle Hybrid Microgels" Gels 10, no. 6: 357. https://doi.org/10.3390/gels10060357
APA StylePongsanon, P., Kawamura, A., Kawasaki, H., & Miyata, T. (2024). Effect of Gold Nanoparticle Size on Regulated Catalytic Activity of Temperature-Responsive Polymer−Gold Nanoparticle Hybrid Microgels. Gels, 10(6), 357. https://doi.org/10.3390/gels10060357








