Efficacy of Chitosan-Carboxylic Acid Hydrogels in Reducing and Chelating Iron for the Removal of Rust from Stone Surface
Abstract
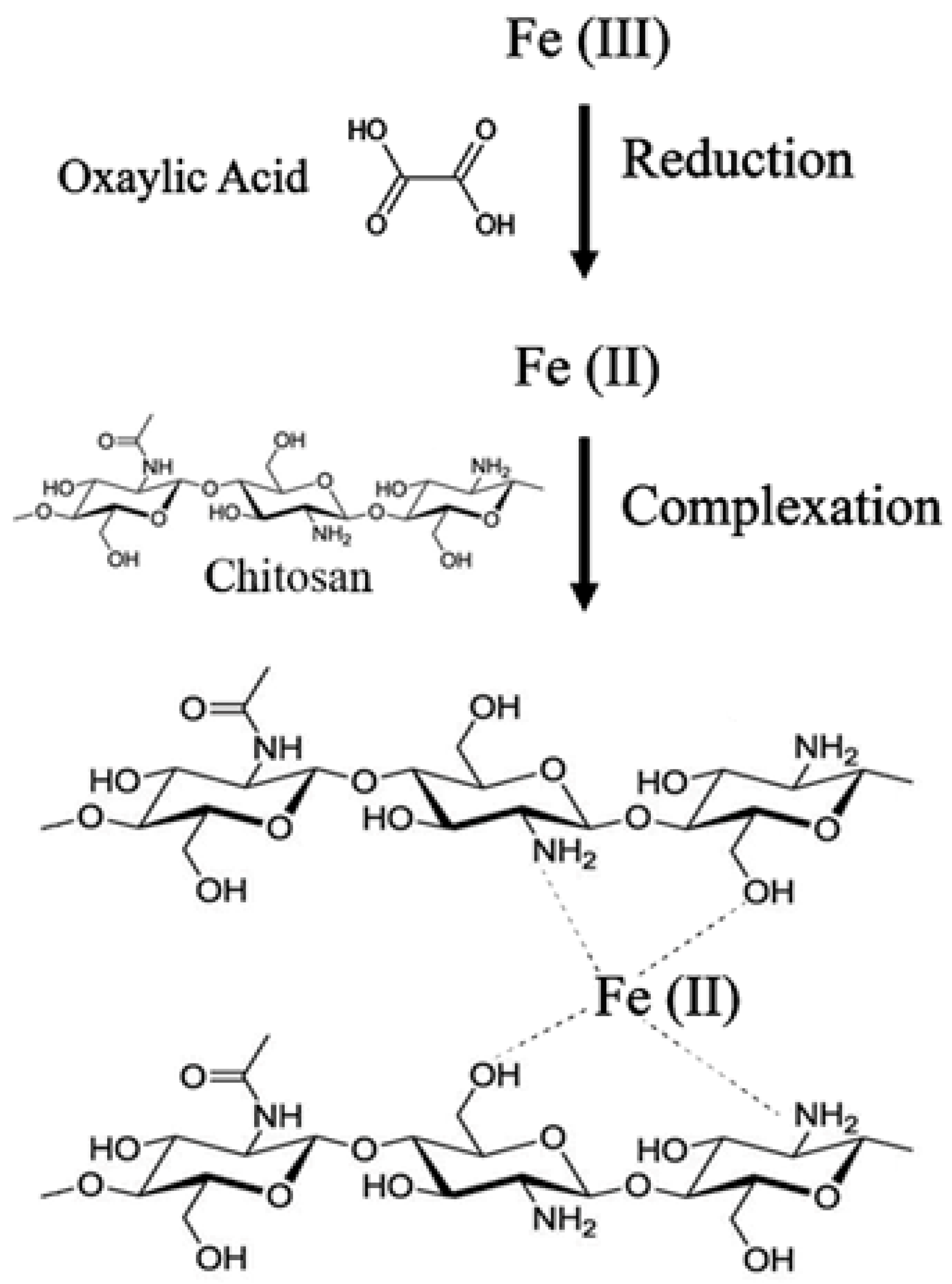
:1. Introduction
2. Results and Discussion
2.1. Cleaning of Lithotypes Stained with Rust Dispersion
2.1.1. Photos and Colorimetry
2.1.2. 1H-NMR Relaxometry
2.1.3. SEM-EDS Analysis
2.2. Cleaning of Lithotypes Stained with Iron Grid
2.2.1. Photos and Colorimetry
2.2.2. Stereomicroscopy
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Staining of Stone Surfaces
4.2.2. Hydrogel Preparation
4.2.3. Colorimetry
4.2.4. 1H-NMR Relaxometry
4.2.5. Microscopy Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinna, D.; Galeotti, M.; Rizzo, A. Brownish alterations on the marble statues in the church of Orsanmichele in Florence: What is their origin? Herit. Sci. 2015, 3, 7. [Google Scholar] [CrossRef]
- Bams, V.; Dewaele, S. Staining of white marble. Mater. Charact. 2007, 58, 1052–1062. [Google Scholar] [CrossRef]
- Henry, A. Stone Conservation: Principles and Practice, 1st ed.; Donhead: Shaftesbury, UK, 2006. [Google Scholar]
- Winkler, E.M. Iron in minerals and the formation of rust in stone. In Stone in Architecture; Winkler, E.M., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 233–240. [Google Scholar] [CrossRef]
- Spile, S.; Suzuki, T.; Bendix, J.; Simonsen, K.P. Effective cleaning of rust stained marble. Herit. Sci. 2016, 4, 12. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C.; Molina, F.J. Cover cracking as a function of bar corrosion: Part I-Experimental test. Mater. Struct. 1993, 26, 453–464. [Google Scholar] [CrossRef]
- Caré, S.; Nguyen, Q.T.; L’Hostis, V.; Berthaud, Y. Mechanical properties of the rust layer induced by impressed current method in reinforced mortar. Cem. Concr. Res. 2008, 38, 1079–1091. [Google Scholar] [CrossRef]
- Beltran, M.; Playà, E.; Artigau, M.; Arroyo, P.; Guinea, A. Iron patinas on alabaster surfaces (Santa Maria de Poblet Monastery, Tarragona, NE Spain). J. Cult. Herit. 2016, 18, 370–374. [Google Scholar] [CrossRef]
- Reale, R.; Andreozzi, G.B.; Sammartino, M.P.; Salvi, A.M. Analytical investigation of iron-based stains on carbonate stones: Rust formation, diffusion mechanisms, and speciation. Molecules 2023, 28, 1582. [Google Scholar] [CrossRef]
- Dillmann, P.; Mazaudier, F.; Hœrlé, S. Advances in understanding atmospheric corrosion of iron. I. Rust characterisation of ancient ferrous artefacts exposed to indoor atmospheric corrosion. Corros. Sci. 2004, 46, 1401–1429. [Google Scholar] [CrossRef]
- Hœrlé, S.; Mazaudier, F.; Dillmann, P.; Santarini, G. Advances in understanding atmospheric corrosion of iron. II. Mechanistic modelling of wet–dry cycles. Corros. Sci. 2004, 46, 1431–1465. [Google Scholar] [CrossRef]
- Brand, J.; Wain, A.; Rode, A.V.; Madden, S.; Rapp, L. Towards safe and effective femtosecond laser cleaning for the preservation of historic monuments. Appl. Phys. A 2023, 129, 246. [Google Scholar] [CrossRef]
- Matero, F.G.; Tagle, A.A. Cleaning, iron stain removal, and surface repair of architectural marble and crystalline limestone: The Metropolitan Club. J. Am. Inst. Conserv. 1995, 34, 49–68. [Google Scholar] [CrossRef]
- Franzen, C.; Fischer, T. Removal of iron crusts from sandstone sculptures in a fountain. Environ. Earth Sci. 2022, 81, 216. [Google Scholar] [CrossRef]
- Selwyn, L.; Tse, S. The chemistry of sodium dithionite and its use in conservation. Stud. Conserv. 2014, 53, 61–73. [Google Scholar] [CrossRef]
- Stambolov, T.; Van Rheeden, B. Note on the removal of rust from old iron with thioglycolic acid. Stud. Conserv. 1968, 13, 142–144. [Google Scholar] [CrossRef]
- Vergès-Belmin, V.; Heritage, A.; Bourgès, A. Powdered cellulose poultices in stone and wall painting conservation—Myths and realities. Stud. Conserv. 2011, 56, 281–297. [Google Scholar] [CrossRef]
- Lauffenburger, J.A.; Grissom, C.A.; Charola, A.E. Changes in gloss of marble surfaces as a result of methylcellulose poulticing. Stud. Conserv. 1992, 37, 155–164. [Google Scholar] [CrossRef]
- Gervais, C.; Grissom, C.A.; Little, N.; Wachowiak, M.J. Cleaning marble with ammonium citrate. Stud. Conserv. 2010, 55, 164–176. [Google Scholar] [CrossRef]
- Campanella, L.; Cardellicchio, F.; Dell’Aglio, E.; Reale, R.; Salvi, A.M. A green approach to clean iron stains from marble surfaces. Herit. Sci. 2022, 10, 79. [Google Scholar] [CrossRef]
- Gabriele, F.; Casieri, C.; Spreti, N. Natural deep eutectic solvents as rust removal agents from lithic and cellulosic substrates. Molecules 2024, 29, 624. [Google Scholar] [CrossRef]
- Macchia, A.; Ruffolo, S.A.; Rivaroli, L.; La Russa, M.F. The treatment of iron-stained marble: Toward a “green” solution. Int. J. Conserv. Sci. 2016, 7, 323–332. [Google Scholar]
- Cushman, M.; Wolbers, R. A new approach to cleaning iron-stained marble surfaces. WAAC Newslett. 2007, 29, 23–28. [Google Scholar]
- Nakamura, T.; Tsukizawa, T.; Oya, M. Combined use of reducing agents and biodegradable chelating agent for iron rust removal. J. Oleo Sci. 2022, 71, 493–504. [Google Scholar] [CrossRef]
- Chelazzi, D.; Baglioni, P. From nanoparticles to gels: A breakthrough in art conservation science. Langmuir 2023, 39, 10744–10755. [Google Scholar] [CrossRef] [PubMed]
- Chelazzi, D.; Bordes, R.; Giorgi, R.; Holmberg, K.; Baglioni, P. The use of surfactants in the cleaning of works of art. Curr. Opin. Colloid Interface Sci. 2020, 45, 108–123. [Google Scholar] [CrossRef]
- Chelazzi, D.; Giorgi, R.; Baglioni, P. Microemulsions, micelles, and functional gels: How colloids and soft matter preserve works of art. Angew. Chem. Int. Ed. 2018, 57, 7296–7303. [Google Scholar] [CrossRef]
- Gabriele, F.; Vetrano, A.; Bruno, L.; Casieri, C.; Germani, R.; Rugnini, L.; Spreti, N. New oxidative alginate-biocide hydrogels against stone biodeterioration. Int. Biodeter. Biodegr. 2021, 163, 105281. [Google Scholar] [CrossRef]
- Ranaldi, R.; Rugnini, L.; Gabriele, F.; Spreti, N.; Casieri, C.; Di Marco, G.; Gismondi, A.; Bruno, L. Plant essential oils suspended into hydrogel: Development of an easy-to-use protocol for the restoration of stone cultural heritage. Int. Biodeter. Biodegr. 2022, 172, 105436. [Google Scholar] [CrossRef]
- Gabriele, F.; Bruno, L.; Casieri, C.; Ranaldi, R.; Rugnini, L.; Spreti, N. Application and monitoring of oxidative alginate-biocide hydrogels for two case studies in “the Sassi and the Park of the Rupestrian Churches of Matera”. Coatings 2022, 12, 462. [Google Scholar] [CrossRef]
- Sansonetti, A.; Bertasa, M.; Canevali, C.; Rabbolini, A.; Anzani, M.; Scalarone, D. A review in using agar gels for cleaning art surfaces. J. Cult. Herit. 2020, 44, 285–296. [Google Scholar] [CrossRef]
- Canevali, C.; Fasoli, M.; Bertasa, M.; Botteon, A.; Colombo, A.; Di Tullio, V.; Capitani, D.; Proietti, N.; Scalarone, D.; Sansonetti, A. A multi-analytical approach for the study of copper stain removal by agar gels. Microchem. J. 2016, 129, 249–258. [Google Scholar] [CrossRef]
- Irizar, P.; Gomez-Laserna, O.; Arana, G.; Madariaga, J.M.; Martínez-Arkarazo, I. Ionic liquids (ILs)-loaded hydrogels as a potential cleaning method of metallic stains for stone conservation. J. Cult. Herit. 2023, 64, 12–22. [Google Scholar] [CrossRef]
- Sonaglia, E.; Schifano, E.; Sharbaf, M.; Uccelletti, D.; Felici, A.C.; Santarelli, M.L. Bacterial nanocellulose hydrogel for the green cleaning of copper stains from marble. Gels 2024, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Sacco, P.; Furlani, F.; De Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for developing physical gels of chitosan and of chitosan derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, D. Energy dissipative and soften resistant hydrogels based on chitosan physical network: From construction to application. Chin. J. Chem. 2022, 40, 2118–2134. [Google Scholar] [CrossRef]
- Cuvillier, L.; Passaretti, A.; Guilminot, E.; Joseph, E. Agar and chitosan hydrogels’ design for metal-uptaking treatments. Gels 2024, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Campos, B.; Marco, A.; Cadeco, G.; Freire-Lista, D.M.; Silvestre-Albero, J.; Algarra, M.; Vieira, E.; Pintado, M.; Moreira, P. Green chitosan: Thiourea dioxide cleaning gel for manganese stains on granite and glass substrates. Herit. Sci. 2021, 9, 160. [Google Scholar] [CrossRef]
- Hernández, R.B.; Franco, A.P.; Yola, O.R.; López-Delgado, A.; Felcman, J.; Recio, M.A.L.; Mercê, A.L.R. Coordination study of chitosan and Fe3+. J. Mol. Struct. 2008, 877, 89–99. [Google Scholar] [CrossRef]
- Burke, A.; Yilmaz, E.; Hasirci, N.; Yilmaz, O. Iron(III) ion removal from solution through adsorption on chitosan. J. Appl. Polym. Sci. 2002, 84, 1185–1192. [Google Scholar] [CrossRef]
- Radnia, H.; Ghoreyshi, A.A.; Younesi, H.; Najafpour, G.D. Adsorption of Fe(II) ions from aqueous phase by chitosan adsorbent: Equilibrium, kinetic, and thermodynamic studies. Desalin. Water Treat. 2012, 50, 348–359. [Google Scholar] [CrossRef]
- Bellamy, L.J.; Pace, R.J. Hydrogen bonding in carboxylic acids—I. Oxalic acids. Spectrochim. Acta 1963, 19, 435–442. [Google Scholar] [CrossRef]
- Chatzigrigoriou, A.; Karapanagiotis, I.; Poulios, I. Superhydrophobic coatings based on siloxane resin and calcium hydroxide nanoparticles for marble protection. Coatings 2020, 10, 334. [Google Scholar] [CrossRef]
- Zia, J.; Pandey, J. Preparation and characterization of iron-metal nanocomposite of chitosan: Towards heterogeneous biocatalysts for organic reactions. JBAER 2018, 5, 101–105. [Google Scholar]
- Brownstein, K.R.; Tarr, C.E. Importance of classical diffusion in NMR studies of water in biological cells. Phys. Rev. A 1979, 19, 2446–2453. [Google Scholar] [CrossRef]
- Meyer, M.; Buchmann, C.; Schaumann, G.E. Determination of quantitative pore-size distribution of soils with 1H NMR relaxometry. Eur. J. Soil Sci. 2018, 69, 393–406. [Google Scholar] [CrossRef]
- Camaiti, M.; Bortolotti, V.; Fantazzini, P. Stone porosity, wettability changes and other features detected by MRI and NMR relaxometry: A more than 15-year study. Magn. Reson. Chem. 2015, 53, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Tortora, M.; Chiarini, M.; Spreti, N.; Casieri, C. 1H-NMR-relaxation and colorimetry for evaluating nanopolymeric dispersions as stone protective coatings. J. Cult. Herit. 2020, 44, 204–210. [Google Scholar] [CrossRef]
- Bortolotti, V.; Camaiti, M.; Casieri, C.; De Luca, F.; Fantazzini, P.; Terenzi, C. Water absorption kinetics in different wettability conditions studied at pore and sample scale in porous media by portable single-sided and laboratory imaging devices. J. Magn. Reson. 2006, 181, 287–295. [Google Scholar] [CrossRef]
- Blümich, B.; Perlo, J.; Casanova, F. Mobile single-sided NMR. Prog. Nucl. Magn. Reason. Spectrosc. 2008, 52, 197–269. [Google Scholar] [CrossRef]
- Careddu, N.; Grillo, S. Rosa Beta granite (Sardinian Pink Granite): A heritage stone of international significance from Italy. Geol. Soc. Spec. Publ. 2015, 407, 155–172. [Google Scholar] [CrossRef]
- Cuccuru, S.; Puccini, A. Petrographic, physical–mechanical and radiological characterisation of the rosa beta granite (Corsica-sardinia batholith). In Engineering Geology for Society and Territory; Lollino, G., Manconi, A., Guzzetti, F., Culshaw, M., Bobrowsky, P., Luino, F., Eds.; Springer: Cham, Switzerland, 2015; Volume 5, pp. 233–236. [Google Scholar] [CrossRef]
- Mancini, A.; Frondini, F.; Capezzuoli, E.; Galvez Mejia, E.; Lezzi, G.; Matarazzi, D.; Brogi, A.; Swennen, R. Porosity, bulk density and CaCO3 content of travertines. A new dataset from Rapolano, Canino and Tivoli travertines (Italy). Data Br. 2019, 25, 104158. [Google Scholar] [CrossRef]
- Mancini, A.; Frondini, F.; Capezzuoli, E.; Galvez Mejia, E.; Lezzi, G.; Matarazzi, D.; Brogi, A.; Swennen, R. Evaluating the geogenic CO2 flux from geothermal areas by analysing quaternary travertine masses. New data from western central Italy and review of previous CO2 flux data. Quat. Sci. Rev. 2019, 215, 132–143. [Google Scholar] [CrossRef]
- Alber, M.; Hauptfleisch, U. Generation and visualization of microfractures in Carrara marble for estimating fracture toughness, fracture shear and fracture normal stiffness. Int. J. Rock Mech. Min. Sci. 1999, 8, 1065–1071. [Google Scholar] [CrossRef]
- Meccheri, M.; Molli, G.; Conti, P.; Blasi, P.; Vaselli, L. The Carrara Marbles (Alpi Apuane, Italy): A geological and economical updated review. Z. Ges. Geowiss. 2007, 158, 719–736. [Google Scholar] [CrossRef]
- Gabriele, F.; Donnadio, A.; Casciola, M.; Germani, R.; Spreti, N. Ionic and covalent crosslinking in chitosan-succinic acid membranes: Effect on physicochemical properties. Carbohydr. Polym. 2021, 251, 117106. [Google Scholar] [CrossRef]
- UNI EN 15886:2010; Conservation of Cultural Property—Test Methods—Color Measurement of Surfaces. Ente Nazionale Italiano di Unificazione (UNI): Roma, Italy, 2010.
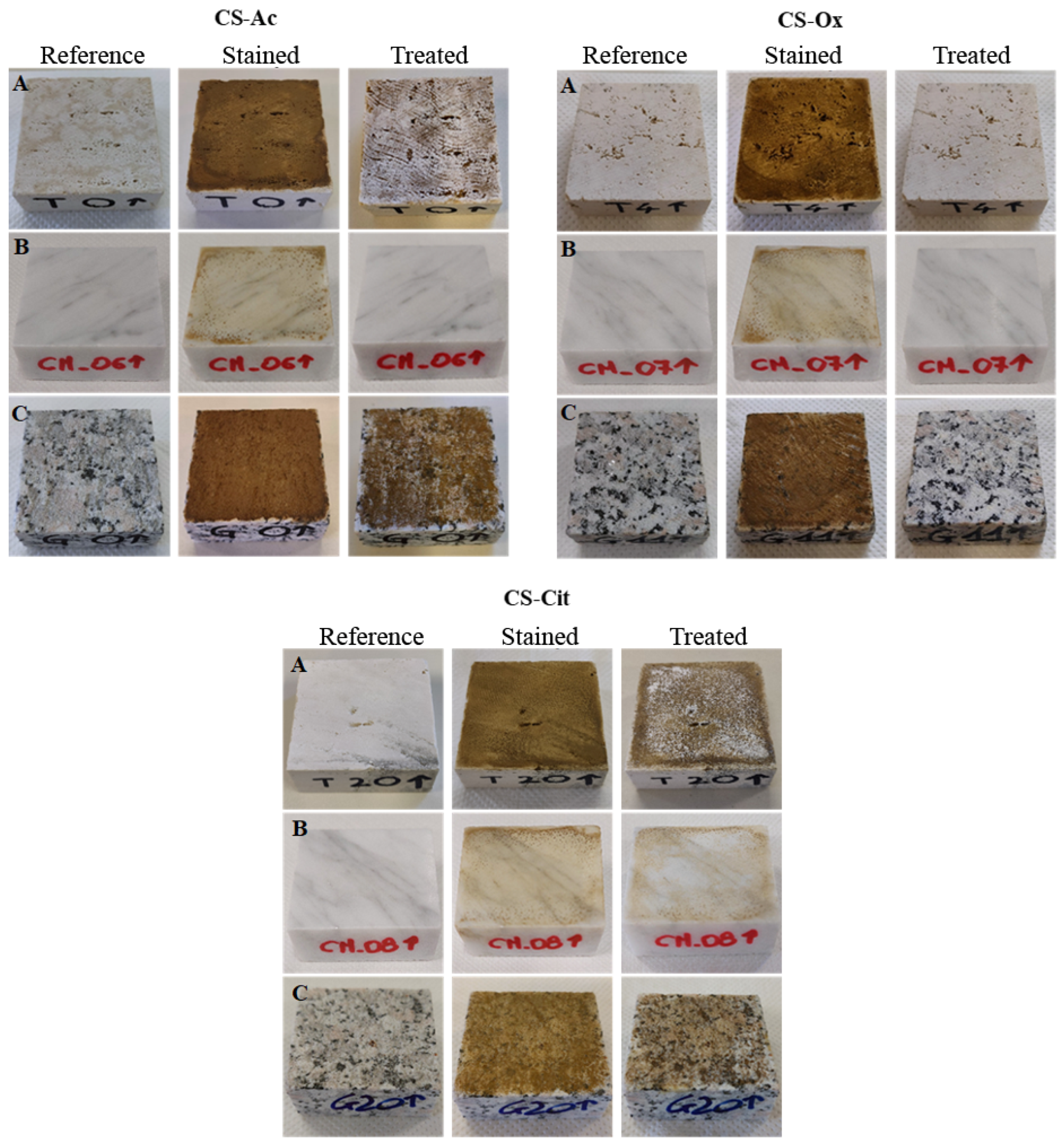
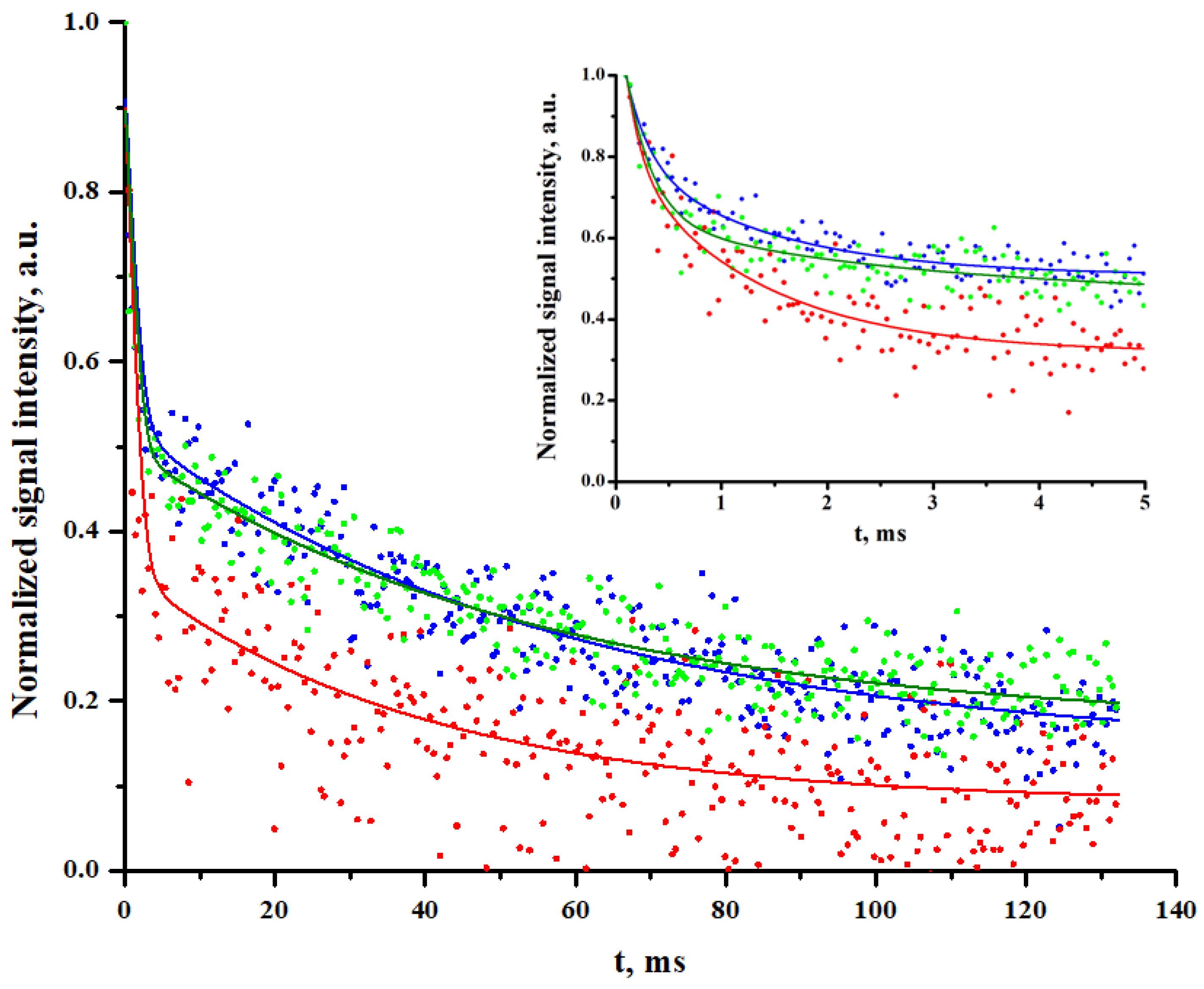
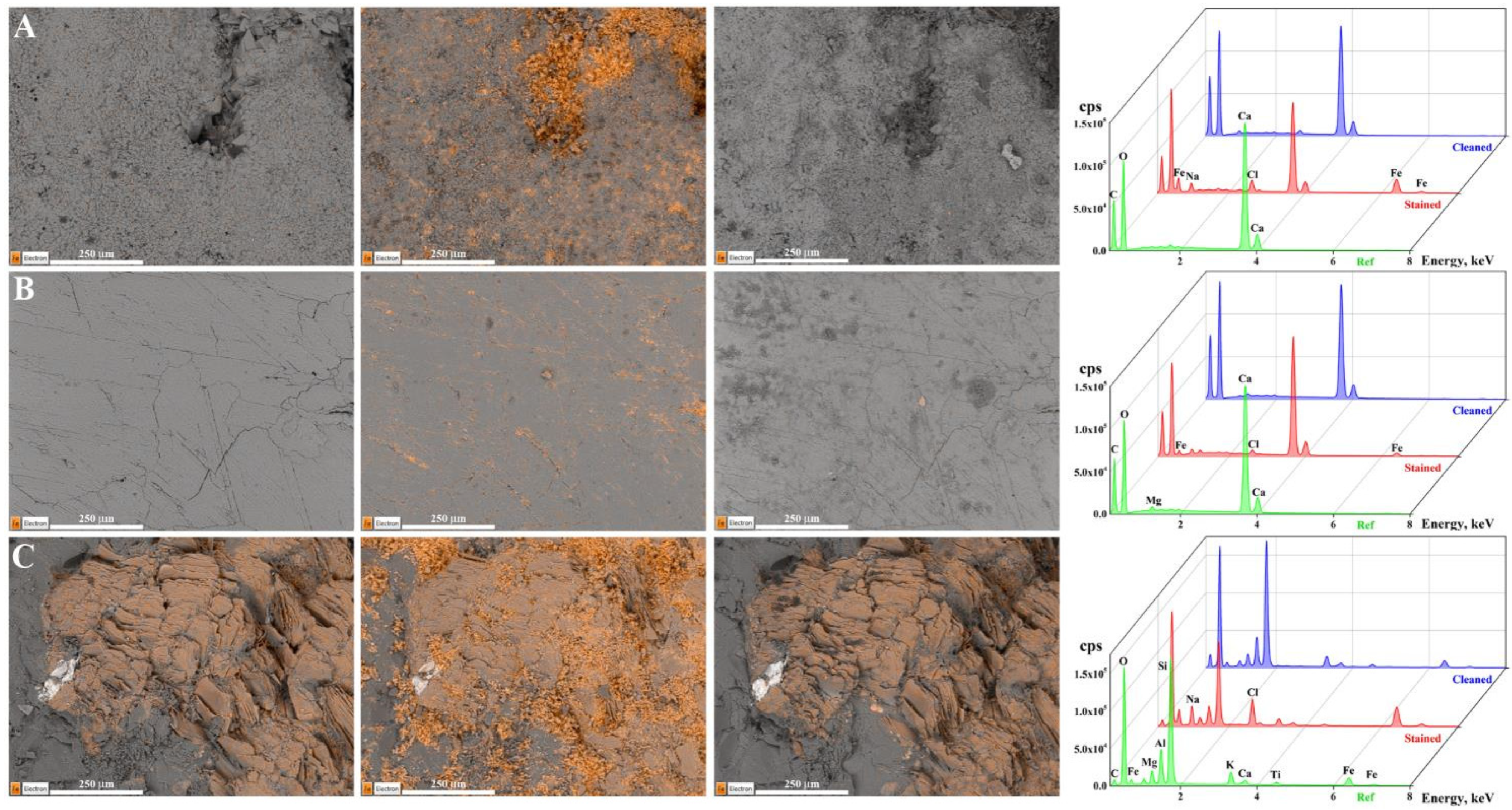


| CS-Ac | Travertine | L* | a* | b* | ||
| Ref | 77 ± 2 | 3.9 ± 0.4 | 10 ± 1 | |||
| ΔL* | Δa* | Δb* | ΔE* | |||
| Stained | −33 ± 3 | 5 ± 1 | 16 ± 2 | 37 | ||
| Treated | −11 ± 2 | 1.1 ± 0.5 | 2 ± 1 | 11 | ||
| Marble | L* | a* | b* | |||
| Ref | 76 ± 2 | −1.4 ± 0.1 | −2.1 ± 0.3 | |||
| ΔL* | Δa* | Δb* | ΔE* | |||
| Stained | −9 ± 3 | 2.4 ± 0.9 | 11 ± 2 | 15 | ||
| Treated | −1 ± 2 | 0.1 ± 0.1 | 0.4 ± 0.4 | 1 | ||
| Granite | L* | a* | b* | |||
| Ref | 65 ± 3 | 0.6 ± 0.6 | 2.4± 0.9 | |||
| ΔL* | Δa* | Δb* | ΔE* | |||
| Stained | −23 ± 3 | 8.2 ± 0.8 | 23 ± 1 | 34 | ||
| Treated | −19 ± 2 | 5.3 ± 0.7 | 14 ± 2 | 24 | ||
| CS-Ox | Travertine | L* | a* | b* | ||
| Ref | 77 ± 1 | 3.3 ± 0.2 | 7.5 ± 0.5 | |||
| ΔL* | Δa* | Δb* | ΔE* | |||
| Stained | −27 ± 3 | 7.3 ± 0.6 | 21 ± 1 | 35 | ||
| Treated | −1 ± 1 | −0.2 ± 0.3 | 0.6 ± 0.7 | 1 | ||
| Marble | L* | a* | b* | |||
| Ref | 75 ± 2 | −1.3 ± 0.2 | −1.7 ± 0.3 | |||
| ΔL* | Δa* | Δb* | ΔE* | |||
| Stained | −11 ± 4 | 3 ± 1 | 13 ± 2 | 17 | ||
| Treated | −1 ± 2 | 0.0 ± 0.2 | 0.5 ± 0.4 | 1 | ||
| Granite | L* | a* | b* | |||
| Ref | 69 ± 3 | 0.2 ± 0.8 | 1 ± 1 | |||
| ΔL* | Δa* | Δb* | ΔE* | |||
| Stained | −25 ± 3 | 8 ± 1 | 22 ± 2 | 34 | ||
| Treated | −1 ± 2 | 0.1 ± 0.6 | 0.1 ± 0.7 | 1 | ||
| CS-Cit | Travertine | L* | a* | b* | ||
| Ref | 81 ± 3 | 3.1 ± 0.3 | 8 ± 1 | |||
| ΔL* | Δa* | Δb* | ΔE* | |||
| Stained | −34 ± 3 | 6.7 ± 0.6 | 19 ± 1 | 40 | ||
| Treated | −15 ± 6 | 2 ± 1 | 4 ± 2 | 16 | ||
| Marble | L* | a* | b* | |||
| Ref | 74 ± 3 | −1.4 ± 0.2 | −1.9 ± 0.3 | |||
| ΔL* | Δa* | Δb* | ΔE* | |||
| Stained | −11 ± 3 | 3 ± 1 | 13 ± 2 | 17 | ||
| Treated | −4 ± 4 | 2 ± 1 | 9 ± 3 | 10 | ||
| Granite | L* | a* | b* | |||
| Ref | 67 ± 4 | 0.3 ± 0.8 | 2 ± 1 | |||
| ΔL* | Δa* | Δb* | ΔE* | |||
| Stained | −17 ± 2 | 6.2 ± 0.7 | 18 ± 2 | 26 | ||
| Treated | −11 ± 3 | 2.5 ± 0.9 | 7± 2 | 13 |
| Travertine | ||||||
| Element | Area 1 | Area 2 | Area 3 | Area 4 | Mean | |
| Reference | Ca | 40.2 | 40.1 | 39.0 | 40.1 | 39.9 ± 0.6 |
| C | 13.7 | 13.7 | 14.3 | 13.9 | 13.9 ± 0.3 | |
| Fe | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 ± 0.0 | |
| Stained | Ca | 32.3 | 30.5 | 26.3 | 30.8 | 30 ± 3 |
| C | 13.2 | 13.5 | 14.0 | 16.8 | 14 ± 2 | |
| Fe | 9.0 | 11.1 | 15.9 | 6.2 | 11 ± 4 | |
| Treated | Ca | 34.8 | 36.9 | 33.1 | 35.5 | 35 ± 2 |
| C | 16.7 | 15.3 | 17.2 | 15.8 | 16.3 ± 0.9 | |
| Fe | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 ± 0.1 | |
| Marble | ||||||
| Element | Area 1 | Area 2 | Area 3 | Area 4 | Mean | |
| Reference | Ca | 37.2 | 38.3 | 37.6 | 38.0 | 37.8 ± 0.5 |
| C | 16.2 | 15.0 | 15.8 | 15.3 | 15.6 ± 0.5 | |
| Fe | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Stained | Ca | 36.9 | 35.9 | 36.1 | 37.0 | 36.5 ± 0.6 |
| C | 14.4 | 13.9 | 13.9 | 13.5 | 13.9 ± 0.4 | |
| Fe | 2.0 | 3.6 | 2.9 | 2.4 | 2.7 ± 0.7 | |
| Treated | Ca | 31.5 | 32.6 | 32.3 | 32.8 | 32.3 ± 0.6 |
| C | 17.5 | 16.7 | 16.5 | 16.8 | 16.9 ± 0.4 | |
| Fe | 0.1 | 0.2 | 0.2 | 0.4 | 0.2 ± 0.1 | |
| Granite | ||||||
| Element | Area 1 | Area 2 | Area 3 | Area 4 | Mean | |
| Reference | Si | 32.4 | 23.5 | 26.6 | 30.8 | 28 ± 4 |
| Al | 5.3 | 6.0 | 9.7 | 3.0 | 6 ± 3 | |
| Fe | 0.9 | 12.0 | 0.6 | 6.7 | 5 ± 5 | |
| Stained | Si | 24.8 | 14.3 | 17.5 | 24.7 | 20 ± 5 |
| Al | 4.3 | 3.2 | 6.4 | 2.3 | 4 ± 2 | |
| Fe | 14.0 | 27.3 | 16.0 | 15.3 | 18 ± 6 | |
| Treated | Si | 31.5 | 21.6 | 24.8 | 28.5 | 27 ± 4 |
| Al | 4.9 | 4.6 | 9.2 | 2.2 | 5 ± 3 | |
| Fe | 0.8 | 10.2 | 0.5 | 7.1 | 5 ± 5 | |
| Travertine | L* | a* | b* | ||
| Ref | 78 ± 4 | 3.6 ± 0.3 | 9 ± 1 | ||
| ΔL* | Δa* | Δb* | ΔE* | ||
| Stained | −7 ± 3 | 6 ± 3 | 16 ± 5 | 19 | |
| Treated | 0 ± 2 | 0.8 ± 0.4 | 2 ± 1 | 3 | |
| Marble | L* | a* | b* | ||
| Ref | 79 ± 4 | −0.8 ± 0.2 | −2.1 ± 0.7 | ||
| ΔL* | Δa* | Δb* | ΔE* | ||
| Stained | −5 ± 4 | 3 ± 2 | 11 ± 5 | 13 | |
| Treated | 0 ± 1 | 0.4 ± 0.4 | 3 ± 2 | 3 | |
| Granite | L* | a* | b* | ||
| Ref | 70 ± 5 | −0.2 ± 0.8 | 1 ± 2 | ||
| ΔL* | Δa* | Δb* | ΔE* | ||
| Stained | −11 ± 9 | 6 ± 3 | 16 ± 6 | 21 | |
| Treated | −1 ± 2 | −0.1 ± 0.6 | −0.3 ± 0.6 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabriele, F.; Casieri, C.; Spreti, N. Efficacy of Chitosan-Carboxylic Acid Hydrogels in Reducing and Chelating Iron for the Removal of Rust from Stone Surface. Gels 2024, 10, 359. https://doi.org/10.3390/gels10060359
Gabriele F, Casieri C, Spreti N. Efficacy of Chitosan-Carboxylic Acid Hydrogels in Reducing and Chelating Iron for the Removal of Rust from Stone Surface. Gels. 2024; 10(6):359. https://doi.org/10.3390/gels10060359
Chicago/Turabian StyleGabriele, Francesco, Cinzia Casieri, and Nicoletta Spreti. 2024. "Efficacy of Chitosan-Carboxylic Acid Hydrogels in Reducing and Chelating Iron for the Removal of Rust from Stone Surface" Gels 10, no. 6: 359. https://doi.org/10.3390/gels10060359







