Exploring Applications and Preparation Techniques for Cellulose Hydrogels: A Comprehensive Review
Abstract
1. Introduction
2. Basic Properties and Chemical Structure of Cellulose
3. Factors Effecting the Properties of Cellulose Hydrogels
3.1. Solvent Selection and Cellulose Dissolution
3.2. Swelling Kinetics, Temperature, and pH Effects
3.3. Crosslinking Methods
3.4. Chemical Cross-Linking Methods
3.5. Physical Cross-Linking Methods
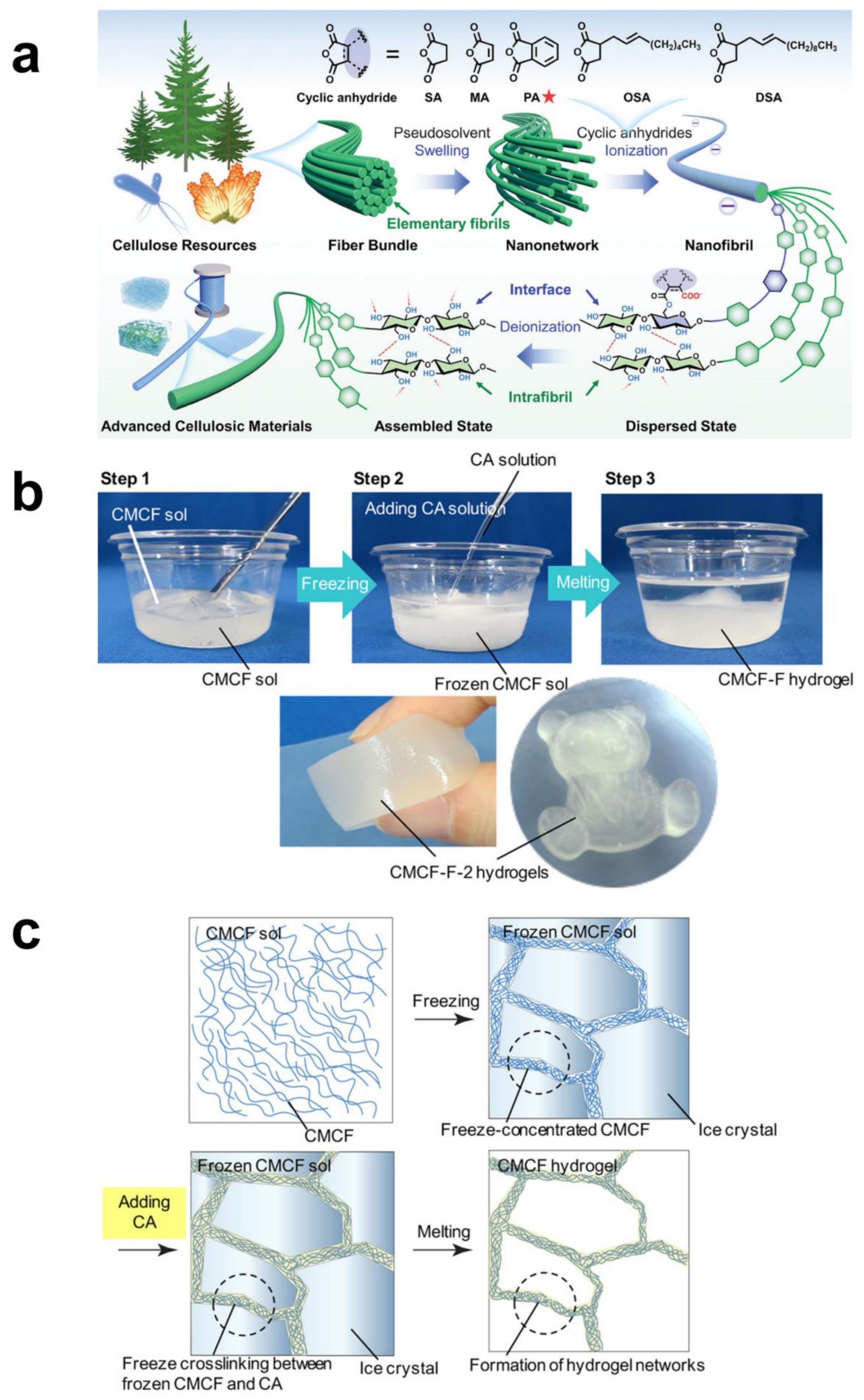
3.6. Radiation Cross-Linking Methods
4. Performance Evaluation of Cellulose Hydrogels
4.1. Mechanical Properties of Cellulose Hydrogels
4.2. Water Absorption Performance
4.3. Biocompatibility and Biodegradability
4.4. Thermal Properties
5. Application Fields of Cellulose Hydrogels
5.1. Applications of Cellulose Hydrogels in Medical and Drug Delivery Fields
5.2. Applications of Cellulose Hydrogels in Environmental Engineering
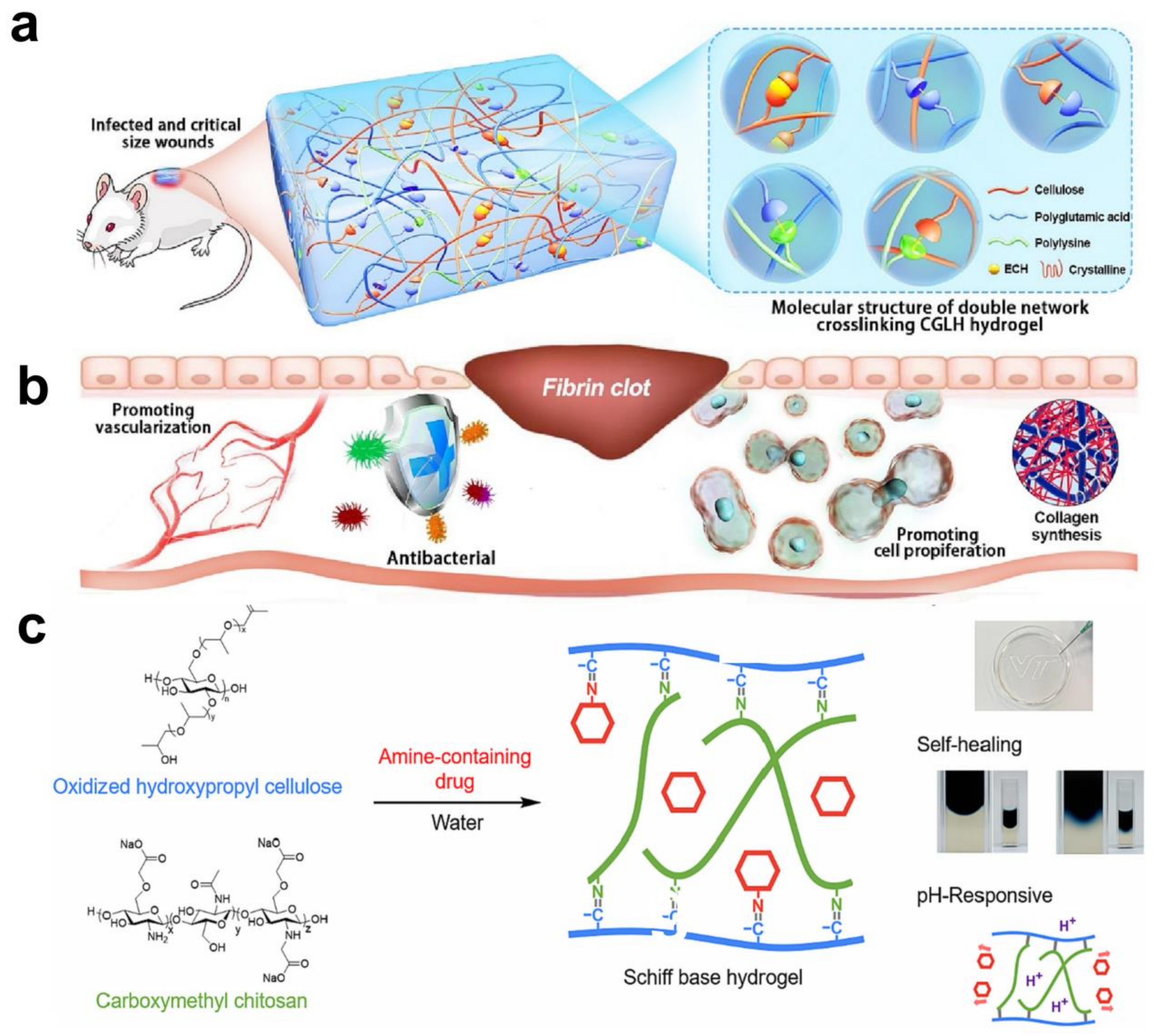

5.3. Applications of Cellulose Hydrogels in Food Industry
5.4. Personal Care Products
6. Challenges and Future Directions
6.1. In Vivo Performance and Long-Term Stability
6.2. Scalability and Cost-Effectiveness
6.3. Mechanical Properties
6.4. Environmental Impact
6.5. Functionalization and Customization
6.6. Regulatory and Market Acceptance
6.7. Eco-Friendly Solvents and Green Preparation Technologies
6.8. Enhancing Mechanical Performance and Stability
6.9. Smart and Responsive Hydrogels
6.10. Precise Control of Biocompatibility and Biodegradability
6.11. Sustainable Production and Application
6.12. Multifunctional Integrated Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anastas:, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.B.; Anastas, P.T.; Erythropel, H.C.; Leitner, W. Designing for a green chemistry future. Science 2020, 367, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, N.; Habeebsheriff, H.S.; Dhanabalan, K.; Cong, V.H.; Wong, L.S.; Rajamani, R.; Dhar, B.K. Mitigating Global Challenges: Harnessing Green Synthesized Nanomaterials for Sustainable Crop Production Systems. Glob. Chall. 2024, 8, 2300187. [Google Scholar] [CrossRef] [PubMed]
- Kundu, R.; Mahada, P.; Chhirang, B.; Das, B. Cellulose hydrogels: Green and sustainable soft biomaterials. Curr. Res. Green Sustain. Chem. 2022, 5, 100252. [Google Scholar] [CrossRef]
- Sonaglia, E.; Schifano, E.; Sharbaf, M.; Uccelletti, D.; Felici, A.C.; Santarelli, M.L. Bacterial Nanocellulose Hydrogel for the Green Cleaning of Copper Stains from Marble. Gels 2024, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, J.; You, T.; Wang, K.; Xu, F. Effects of polymorphs on dissolution of cellulose in NaOH/urea aqueous solution. Carbohydr. Polym. 2015, 125, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Karaaslan, M.A.; Tshabalala, M.A.; Yelle, D.J.; Buschle-Diller, G. Nanoreinforced biocompatible hydrogels from wood hemicelluloses and cellulose whiskers. Carbohydr. Polym. 2011, 86, 192–201. [Google Scholar] [CrossRef]
- Cywar, R.M.; Rorrer, N.A.; Hoyt, C.B.; Beckham, G.T.; Chen, E.Y.-X. Bio-based polymers with performance-advantaged properties. Nat. Rev. Mater. 2022, 7, 83–103. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, Y.; Cheng, W.; Chen, W.; Wu, Y.; Yu, H. Cellulose-based flexible functional materials for emerging intelligent electronics. Adv. Mater. 2021, 33, 2000619. [Google Scholar] [CrossRef]
- Gupta, P.K.; Raghunath, S.S.; Prasanna, D.V.; Venkat, P.; Shree, V.; Chithananthan, C.; Choudhary, S.; Surender, K.; Geetha, K. An update on overview of cellulose, its structure and applications. Cellulose 2019, 201, 84727. [Google Scholar]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Barud, H.G.; Da Silva, R.R.; da Silva Barud, H.; Tercjak, A.; Gutierrez, J.; Lustri, W.R.; de Oliveira Junior, O.B.; Ribeiro, S.J. A multipurpose natural and renewable polymer in medical applications: Bacterial cellulose. Carbohydr. Polym. 2016, 153, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Bhaladhare, S.; Das, D. Cellulose: A fascinating biopolymer for hydrogel synthesis. J. Mater. Chem. B 2022, 10, 1923–1945. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.D.; Yu, X.J.; Fang, Y.J.; Wang, L.; Zhang, H.; Zhu, C.Z.; Cai, J. Strong and Tough Cellulose Hydrogels via Solution Annealing and Dual Cross-Linking. Small 2023, 19, 2301204. [Google Scholar] [CrossRef] [PubMed]
- Sahar, F.; Riaz, A.; Malik, N.S.; Gohar, N.; Rasheed, A.; Tulain, U.R.; Erum, A.; Barkat, K.; Badshah, S.F.; Shah, S.I. Design, characterization and evaluation of gelatin/carboxymethyl cellulose hydrogels for effective delivery of ciprofloxacin. Polym. Bull. 2023, 80, 12271–12299. [Google Scholar] [CrossRef]
- Zhang, D.H.; Jian, J.Y.; Xie, Y.T.; Gao, S.S.; Ling, Z.; Lai, C.H.; Wang, J.F.; Wang, C.P.; Chu, F.X.; Dumont, M.J. Mimicking skin cellulose hydrogels for sensor applications. Chem. Eng. J. 2022, 427, 130921. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Guo, J.H.; Wang, Y.X.; Chen, L.Y.; Cai, J.; Zhang, L.N. Creation of the tunable color light emission of cellulose hydrogels consisting of primary rare-earth compounds. Carbohydr. Polym. 2017, 161, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.B.; Wang, J.Q.; Dai, X.F.; Shao, Z.Q.; Huang, X.N. Dual physically crosslinked healable polyacrylamide/cellulose nanofibers nanocomposite hydrogels with excellent mechanical properties. Carbohydr. Polym. 2018, 193, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Kaneko, K.; Fukaya, R.; Seonju, J.; Yamada, M.; Ishii, H.; Inoue, T.; Shimizu, A. Influence of hydrostatic pressure during gelation on physicochemical properties of cellulose hydrogels prepared from ionic liquid/DMSO solution. J. Mol. Liq. 2023, 381, 121810. [Google Scholar] [CrossRef]
- Yi, X.T.; Cheng, F.; Wei, X.J.; Li, H.B.; Qian, J.T.; He, J.M. Bioinspired adhesive and self-healing bacterial cellulose hydrogels formed by a multiple dynamic crosslinking strategy for sealing hemostasis. Cellulose 2023, 30, 397–411. [Google Scholar] [CrossRef]
- Wu, C.L.; McClements, D.J.; He, M.Y.; Fan, Z.J.; Li, Y.; Teng, F. Preparation of okara cellulose hydrogels using ionic liquids: Structure, properties, and performance. J. Mol. Liq. 2021, 331, 115744. [Google Scholar]
- Adedeji, O.E.; Min, J.H.; Park, G.E.; Kang, H.J.; Choi, J.Y.; Aminu, M.O.; Ocheme, O.B.; Joo, S.T.; Moon, K.D.; Jung, Y.H. Development of a 3D-printable matrix using cellulose microfibrils/guar gum-based hydrogels and its post-printing antioxidant activity. Int. J. Bioprint. 2024, 10, 242–256. [Google Scholar]
- Zinge, C.; Kandasubramanian, B. Nanocellulose based biodegradable polymers. Eur. Polym. J. 2020, 133, 109758. [Google Scholar] [CrossRef]
- Zhang, Y.; Nypelö, T.; Salas, C.; Arboleda, J.; Hoeger, I.C.; Rojas, O.J. Cellulose nanofibrils. J. Renew. Mater. 2013, 1, 195–211. [Google Scholar] [CrossRef]
- Mihranyan, A.; Edsman, K.; Stromme, M. Rheological properties of cellulose hydrogels prepared from Cladophora cellulose powder. Food Hydrocoll. 2007, 21, 267–272. [Google Scholar] [CrossRef]
- Chang, C.Y.; Zhang, L.Z.; Zhou, J.P.; Zhang, L.N.; Kennedy, J.F. Structure and properties of hydrogels prepared from cellulose in NaOH/urea aqueous solutions. Carbohydr. Polym. 2010, 82, 122–127. [Google Scholar] [CrossRef]
- Abe, K.; Yano, H. Cellulose nanofiber-based hydrogels with high mechanical strength. Cellulose 2012, 19, 1907–1912. [Google Scholar] [CrossRef]
- Mo, K.W.; Zhang, T.T.; Yan, W.; Chang, C.Y. Tunicate cellulose nanocrystal reinforced polyacrylamide hydrogels with tunable mechanical performance. Cellulose 2018, 25, 6561–6570. [Google Scholar] [CrossRef]
- Lin, Z.X.; Huang, R.L.; Wu, J.J.X.; Penkova, A.; Qi, W.; He, Z.M.; Su, R.X. Injectable self-healing nanocellulose hydrogels crosslinked by aluminum: Cellulose nanocrystals vs. cellulose nanofibrils. Chin. J. Chem. Eng. 2022, 50, 389–397. [Google Scholar] [CrossRef]
- Bao, Y.H.; He, J.; Song, K.; Guo, J.; Zhou, X.W.; Liu, S.M. Functionalization and Antibacterial Applications of Cellulose-Based Composite Hydrogels. Polymers 2022, 14, 769. [Google Scholar] [CrossRef]
- Bashari, A.; Rouhani Shirvan, A.; Shakeri, M. Cellulose-based hydrogels for personal care products. Polym. Adv. Technol. 2018, 29, 2853–2867. [Google Scholar] [CrossRef]
- Peng, N.; Hu, D.N.; Zeng, J.; Li, Y.; Liang, L.; Chang, C.Y. Superabsorbent Cellulose-Clay Nanocomposite Hydrogels for Highly Efficient Removal of Dye in Water. Acs Sustain. Chem. Eng. 2016, 4, 7217–7224. [Google Scholar] [CrossRef]
- Wu, L.L.; Fan, B.J.; Yan, B.B.; Liu, Y.; Yu, Y.Y.; Cui, L.; Zhou, M.; Wang, Q.; Wang, P. Construction of durable antibacterial cellulose textiles through grafting dynamic disulfide-containing amino-compound and nanosilver deposition. Int. J. Biol. Macromol. 2024, 259, 129085. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Yuan, R. Applications of natural polymer-based hydrogels in the food industry. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 357–410. [Google Scholar]
- Wang, Y.F.; Liu, H.Y.; Yu, J.C.; Liao, H.J.; Yang, L.; Ren, E.R.; Lin, S.J.; Lan, J.W. Ionic Conductive Cellulose-Based Hydrogels with Superior Long-Lasting Moisture and Antifreezing Features for Flexible Strain Sensor Applications. Biomacromolecules 2024, 25, 838–852. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.Y.; Liu, F.F.; Abdiryim, T.; Chen, J.Y.; Liu, X. Sodium carboxymethyl cellulose and MXene reinforced multifunctional conductive hydrogels for multimodal sensors and flexible supercapacitors. Carbohydr. Polym. 2024, 327, 121677. [Google Scholar] [CrossRef] [PubMed]
- Rahmadiawan, D.; Shi, S.-C.; Abral, H.; Ilham, M.K.; Sugiarti, E.; Muslimin, A.N.; Ilyas, R.A.; Lapisa, R.; Putra, N.S.D. Comparative Analysis of the Influence of Different Preparation Methods on the Properties of TEMPO-Oxidized Bacterial Cellulose Powder Films. J. Nat. Fibers 2024, 21, 2301386. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, C.F.; Liao, X.Y.; Luo, A.X.; Jing, Y.D.; Yu, N.Y.; Su, S.P.; Zhang, X.M.; Zhu, J.; Deng, G.B. Preparation of PVA/cellulose composite hydrogel electrolytes based on zinc chloride-dissolved cellulose for flexible solid-state capacitors. J. Mater. Chem. C 2024, 12, 2063–2072. [Google Scholar] [CrossRef]
- Fu, L.-H.; Qi, C.; Ma, M.-G.; Wan, P. Multifunctional cellulose-based hydrogels for biomedical applications. J. Mater. Chem. B 2019, 7, 1541–1562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.X.; Zhao, S.B.; Guan, Q.F.; Li, P.; Fan, Y.B. Enhancing Chronic Wound Healing through Engineering Mg2+-Coordinated Asiatic Acid/Bacterial Cellulose Hybrid Hydrogels. Acs Appl. Mater. Interfaces 2024, 16, 8238–8249. [Google Scholar] [CrossRef]
- You, C.Q.; Ji, X.Y.; Lin, H.C.; Ma, N.; Wei, W.; Long, L.F.; Ning, L.K.; Wang, F. Preparation of UV-responsive hydrogels based on nanocellulose and their utilization in fungicide delivery. Environ. Sci.-Nano 2024, 11, 1442–1451. [Google Scholar] [CrossRef]
- Wang, X.; Ning, L.K.; Lin, H.C.; Ma, N.; Li, X.; Wang, F.; Zhang, R.; You, C.Q. Efficient tumor treatment by triphenylphosphine conjugated nanocellulose composite hydrogels for enhanced mitochondria targeting. J. Drug Deliv. Sci. Technol. 2024, 92, 105286. [Google Scholar] [CrossRef]
- Akter, M.; Bhattacharjee, M.; Dhar, A.K.; Rahman, F.B.A.; Haque, S.; Rashid, T.U.; Kabir, S.F. Cellulose-based hydrogels for wastewater treatment: A concise review. Gels 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.-F.; Yang, H.-B.; Han, Z.-M.; Ling, Z.-C.; Yin, C.-H.; Yang, K.-P.; Zhao, Y.-X.; Yu, S.-H. Sustainable cellulose-nanofiber-based hydrogels. ACS Nano 2021, 15, 7889–7898. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Zhu, M.; Duan, B.; Zhang, L. Recent progress in high-strength and robust regenerated cellulose materials. Adv. Mater. 2021, 33, 2000682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Abidi, N.; Lucia, L.; Chabi, S.; Denny, C.T.; Parajuli, P.; Rumi, S.S. Cellulose/nanocellulose superabsorbent hydrogels as a sustainable platform for materials applications: A mini-review and perspective. Carbohydr. Polym. 2023, 299, 120140. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Chen, H.; Zhang, X.J.; Wang, C.S.; Xu, C.; Xue, Y.T.; Wang, J.S.; Zhou, P.H.; Zhao, Q.X. Construction of novel cellulose/chitosan composite hydrogels and films and their applications. Cellulose 2018, 25, 1987–1996. [Google Scholar] [CrossRef]
- Ghilan, A.; Nita, L.E.; Pamfil, D.; Simionescu, N.; Tudorachi, N.; Rusu, D.; Rusu, A.G.; Bercea, M.; Rosca, I.; Ciolacu, D.E.; et al. One-Step Preparation of Carboxymethyl Cellulose-Phytic Acid Hydrogels with Potential for Biomedical Applications. Gels 2022, 8, 647. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; McClements, D.J.; Ma, B.H.; He, Z.P.; Wu, F.H.; Zhang, Y.Z.; Liu, X.Q.; Wang, P. Fabrication of composite hydrogels by sonication-assisted assembly of okara cellulose nanofibers and chitosan: Structure and properties. J. Sci. Food Agric. 2024, 104, 3458–3467. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.P.; Biswas, S.K.; Yano, H.; Abe, K. Fabrication of ultrastiff and strong hydrogels by in situ polymerization in layered cellulose nanofibers. Cellulose 2020, 27, 693–702. [Google Scholar] [CrossRef]
- Cao, L.L.; Tian, D.; Lin, B.C.; Wang, W.X.; Bai, L.J.; Chen, H.; Yang, L.X.; Yang, H.W.; Wei, D.L. Fabrication of self-healing nanocomposite hydrogels with the cellulose nanocrystals-based Janus hybrid nanomaterials. Int. J. Biol. Macromol. 2021, 184, 259–270. [Google Scholar] [CrossRef]
- Habib, A.; Quigley, C.; Sarah, R.; Hurd, W.; Clark, S. Design and Fabrication of In-House Nozzle System to Extrude Multi-Hydrogels for 3D Bioprinting Process. J. Manuf. Sci. Eng.-Trans. ASME 2024, 146, 021003. [Google Scholar] [CrossRef]
- Sugawara, A.; Asoh, T.A.; Takashima, Y.; Harada, A.; Uyama, H. Thermoresponsive Hydrogels Reinforced with Supramolecular Cellulose Filler. Chem. Lett. 2022, 51, 145–148. [Google Scholar] [CrossRef]
- Lu, Q.L.; Zhang, S.H.; Xiong, M.C.; Lin, F.C.; Tang, L.R.; Huang, B.; Chen, Y.D. One-pot construction of cellulose-gelatin supramolecular hydrogels with high strength and pH-responsive properties. Carbohydr. Polym. 2018, 196, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.C.; Zhu, J.; Jin, S.S.; Zheng, Y.S.; Gao, W.H.; Wu, D.Q.; Yu, J.Y.; Dai, Z.J. Cellulose-nanofibril-reinforced hydrogels with pH sensitivity and mechanical stability for wound healing. Mater. Lett. 2022, 323, 132596. [Google Scholar] [CrossRef]
- Chen, C.; Song, J.; Cheng, J.; Pang, Z.; Gan, W.; Chen, G.; Kuang, Y.; Huang, H.; Ray, U.; Li, T. Highly elastic hydrated cellulosic materials with durable compressibility and tunable conductivity. ACS Nano 2020, 14, 16723–16734. [Google Scholar] [CrossRef] [PubMed]
- Srirachya, N.; Boonkerd, K.; Kobayashi, T. Effective elongation properties of cellulose-natural rubber composite hydrogels having interconnected domain. J. Elastomers Plast. 2020, 52, 337–355. [Google Scholar] [CrossRef]
- Sui, B.W.; Zhang, Y.P.; Huang, L.; Chen, Y.X.; Li, D.W.; Li, Y.F.; Yang, B. Fluorescent Nanofibrillar Hydrogels of Carbon Dots and Cellulose Nanocrystals and Their Biocompatibility. ACS Sustain. Chem. Eng. 2020, 8, 18492–18499. [Google Scholar] [CrossRef]
- Le, V.T.; Joo, S.W.; Berkani, M.; Mashifana, T.; Kamyab, H.; Wang, C.Q.; Vasseghian, Y. Sustainable cellulose-based hydrogels for water treatment and purification. Ind. Crops Prod. 2023, 205, 117525. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.J.; Zhang, L.; Miao, D.G.; Sui, S.Y.; Deng, F.L.; Zhu, P. Preparation and properties of carboxymethyl cellulose hydrogels. Ferroelectrics 2019, 547, 37–43. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Li, M.; Yu, N.Y.; Su, S.P.; Zhang, X.M. Fabrication of AgCl@tannic acid-cellulose hydrogels for NaBH4/-mediated reduction of 4-nitrophenol. Cellulose 2021, 28, 3515–3529. [Google Scholar] [CrossRef]
- Li, Y.Q.; Chen, L.; Dai, Y.M.; Lu, Q.; Fang, C.Q.; Wang, Z.H.; Cai, L.; Liu, B.; Zhang, Y.F.; Li, Y.; et al. Fabrication of ionic liquids-based magnetite-cellulose-sepiolite nanocomposite for the removal of Congo red. J. Mater. Sci.-Mater. Electron. 2024, 35, 215. [Google Scholar]
- Xu, M.M.; Huang, Q.B.; Wang, X.H.; Sun, R.C. Highly tough cellulose/graphene composite hydrogels prepared from ionic liquids. Ind. Crops Prod. 2015, 70, 56–63. [Google Scholar] [CrossRef]
- Yu, X.Q.; Ma, X.J.; Pan, Z.M.; Ma, X.Y.; Ji, X.L.; Lv, Y.; Wei, Z. Preparation of 3D Cellulose-Carbon Quantum Dots Hydrogels for Adsorption of Mercury from Aqueous Solution. J. Polym. Environ. 2024. [Google Scholar] [CrossRef]
- Yin, X.C.; Xu, P.; Wang, H.Y. Efficient and Selective Removal of Heavy Metals and Dyes from Aqueous Solutions Using Guipi Residue-Based Hydrogel. Gels 2024, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Jayaramudu, T.; Ko, H.U.; Kim, H.C.; Kim, J.W.; Kim, J. Swelling Behavior of Polyacrylamide-Cellulose Nanocrystal Hydrogels: Swelling Kinetics, Temperature, and pH Effects. Materials 2019, 12, 2080. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.M.; Zhang, B.L.; Zhao, H.B.; Liao, X.L.; Xu, H.Q. Preparation and performance of poly(AM/NVCL) temperature-sensitive composite hydrogels enhanced by laponite. Iran. Polym. J. 2024, 33, 555–565. [Google Scholar] [CrossRef]
- Fabian, D.R.C.; Durpekova, S.; Dusankova, M.; Hanusova, D.; Bergerova, E.D.; Sedlacik, M.; Skoda, D.; Sedlarik, V. Renewable whey-based hydrogel with polysaccharides and polyvinyl alcohol as a soil amendment for sustainable agricultural application. Int. J. Biol. Macromol. 2024, 259, 129056. [Google Scholar] [CrossRef] [PubMed]
- Aswathy, S.H.; NarendraKumar, U.; Manjubala, I. The influence of molecular weight of cellulose on the properties of carboxylic acid crosslinked cellulose hydrogels for biomedical and environmental applications. Int. J. Biol. Macromol. 2023, 239, 124282. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yang, F.; Guo, Z. The chitosan hydrogels: From structure to function. New J. Chem. 2018, 42, 17162–17180. [Google Scholar] [CrossRef]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Kolahchi, A.R.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef]
- Sivashanmugam, A.; Kumar, R.A.; Priya, M.V.; Nair, S.V.; Jayakumar, R. An overview of injectable polymeric hydrogels for tissue engineering. Eur. Polym. J. 2015, 72, 543–565. [Google Scholar] [CrossRef]
- Sekine, Y.; Nankawa, T.; Yunoki, S.; Sugita, T.; Nakagawa, H.; Yamada, T. Eco-friendly carboxymethyl cellulose nanofiber hydrogels prepared via freeze cross-linking and their applications. ACS Appl. Polym. Mater. 2020, 2, 5482–5491. [Google Scholar] [CrossRef]
- Ortega, A.; Valencia, S.; Rivera, E.; Segura, T.; Burillo, G. Reinforcement of Acrylamide Hydrogels with Cellulose Nanocrystals Using Gamma Radiation for Antibiotic Drug Delivery. Gels 2023, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Ye, S.; Zhang, Y.; Chen, J.; Chen, F.; Weng, H.; Xiao, A. Gel properties transition from mono-succinylation to cross-linking of agar by attemperation with succinic anhydride. Food Chem. 2022, 381, 132164. [Google Scholar] [CrossRef] [PubMed]
- Shariatzadeh, F.J.; Solouk, A.; Mirzadeh, H.; Bonakdar, S.; Sadeghi, D.; Khoulenjani, S.B. Cellulose nanocrystals-reinforced dual crosslinked double network GelMA/hyaluronic acid injectable nanocomposite cryogels with improved mechanical properties for cartilage tissue regeneration. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2024, 112, e35346. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Li, J.; Fan, L. Tannic acid-enriched nanocellulose hydrogels improve physical and oxidative stability of high-internal-phase Pickering emulsions. Int. J. Biol. Macromol. 2024, 259, 128796. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, F.; Han, Z.; Qu, X.; Li, J.; Zhou, Z.; Chen, S.; Wang, H.; Lv, X. Bacterial cellulose-based hydrogel with regulated rehydration and enhanced antibacterial activity for wound healing. Int. J. Biol. Macromol. 2024, 267, 131291. [Google Scholar] [CrossRef] [PubMed]
- Swilem, A.E.; Oyama, T.G.; Oyama, K.; Kimura, A.; Taguchi, M. Development of carboxymethyl cellulose/gelatin hybrid hydrogels via radiation-induced cross-linking as novel anti-adhesion barriers. Polym. Degrad. Stab. 2022, 197, 109856. [Google Scholar] [CrossRef]
- Pekel, N.; Yoshii, F.; Kume, T.; Güven, O. Radiation crosslinking of biodegradable hydroxypropylmethylcellulose. Carbohydr. Polym. 2004, 55, 139–147. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, D.; Chen, Q.; Chen, P.; Song, G.; Chang, C. Reversible Surface Engineering of Cellulose Elementary Fibrils: From Ultralong Nanocelluloses to Advanced Cellulosic Materials. Adv. Mater. 2024, 36, 2312220. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Boufi, S. Poly (acrylic acid-co-acrylamide)/cellulose nanofibrils nanocomposite hydrogels: Effects of CNFs content on the hydrogel properties. Cellulose 2016, 23, 3691–3701. [Google Scholar] [CrossRef]
- García-Astrain, C.; González, K.; Gurrea, T.; Guaresti, O.; Algar, I.; Eceiza, A.; Gabilondo, N. Maleimide-grafted cellulose nanocrystals as cross-linkers for bionanocomposite hydrogels. Carbohydr. Polym. 2016, 149, 94–101. [Google Scholar] [CrossRef]
- Laurén, I.; Farzan, A.; Teotia, A.; Lindfors, N.C.; Seppälaä, J. Direct ink writing of biocompatible chitosan/non-isocyanate polyurethane/cellulose nanofiber hydrogels for wound-healing applications. Int. J. Biol. Macromol. 2024, 259, 129321. [Google Scholar] [CrossRef]
- Lu, S.C.; Bian, S.; Jia, Y.; Guo, Y.; Xiao, H.; Zhang, M.; Liu, K.; Huang, L.L.; Chen, L.H.; Ni, Y.H.; et al. Catechol-functionalised dialdehyde cellulose-containing hydrogels with tissue adhesion, sensing and haemostatic properties for wound healing. Cellulose 2024, 31, 2355–2377. [Google Scholar] [CrossRef]
- Wu, T.; Liu, H.Y.; Wang, H.C.; Bu, Y.N.; Liu, J.Y.; Chen, X.Q.; Yan, H.Q.; Lin, Q. Fabrication of alginate/sericin/cellulose nanocrystals interpenetrating network composite hydrogels with enhanced physicochemical properties and biological activity. J. Appl. Polym. Sci. 2023, 141, e55052. [Google Scholar] [CrossRef]
- Morrison, T.X.; Gramlich, W.M. Tunable, thiol-ene, interpenetrating network hydrogels of norbornene-modified carboxymethyl cellulose and cellulose nanofibrils. Carbohydr. Polym. 2023, 319, 121173. [Google Scholar] [CrossRef]
- Wang, B.X.; Peng, Q.; Yan, Y.X.; Ding, Y.L.; Wang, Z.K. Biomimetic, strong, and tough hydrogels by integrating cellulose nanocrystals into polymer networks. Ind. Crops Prod. 2020, 158, 112973. [Google Scholar] [CrossRef]
- Niu, L.; Zhang, D.H.; Liu, Y.P.; Zhou, X.; Wang, J.F.; Wang, C.P.; Chu, F.X. Combination of acid treatment and dual network fabrication to stretchable cellulose based hydrogels with tunable properties. Int. J. Biol. Macromol. 2020, 147, 1–9. [Google Scholar] [CrossRef]
- Della Sala, F.; di Gennaro, M.; Makvandi, P.; Borzacchiello, A. A Covalently Cross-Linked Hyaluronic Acid/Carboxymethyl Cellulose Composite Hydrogel as a Potential Filler for Soft Tissue Augmentation. Gels 2024, 10, 67. [Google Scholar] [CrossRef]
- Hasan, M.S.; Al Foisal, J.; Khan, G.M.A.; Jahan, R.; Hasanuzzaman, M.; Alam, M.S.; Karim, M.M.; Gafur, M.A.; Khan, M.A.; Sabur, M.A. Microfibrillated Cellulose-Silver Nanocomposite Based PVA Hydrogels and Their Enhanced Physical, Mechanical and Antibacterial Properties. J. Polym. Environ. 2022, 30, 2875–2887. [Google Scholar] [CrossRef]
- Chen, L.Z.; Rong, X.H.; Liu, Z.Q.; Ding, Q.J.; Li, X.; Jiang, Y.F.; Han, W.J.; Lou, J. Negative thermopower anisotropic ionic thermoelectric hydrogels based on synergistic coordination and hydration for low-grade heat harvesting. Chem. Eng. J. 2024, 481, 148797. [Google Scholar] [CrossRef]
- Zhou, F.; Wu, S.H.; Rader, C.; Ma, J.W.; Chen, S.J.; Yuan, X.Y.; Foster, E.J. Crosslinked Ionic Alginate and Cellulose-based Hydrogels for Photoresponsive Drug Release Systems. Fibers Polym. 2020, 21, 45–54. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Otoni, C.G.; Kevin, J.; Barud, H.S.; Lona, L.M.; Cranston, E.D.; Rojas, O.J. Porous nanocellulose gels and foams: Breakthrough status in the development of scaffolds for tissue engineering. Mater. Today 2020, 37, 126–141. [Google Scholar] [CrossRef]
- Padzil, F.N.M.; Gan, S.; Zakaria, S.; Mohamad, S.F.; Mohamed, N.H.; Seo, Y.B.; Ellis, A.V. Increased solubility of plant core pulp cellulose for regenerated hydrogels through electron beam irradiation. Cellulose 2018, 25, 4993–5006. [Google Scholar] [CrossRef]
- Cafiso, D.; Septevani, A.A.; Noe, C.; Schiller, T.; Pirri, C.F.; Roppolo, I.; Chiappone, A. 3D printing of fully cellulose-based hydrogels by digital light processing. Sustain. Mater. Technol. 2022, 32, e00444. [Google Scholar] [CrossRef]
- Lai, P.C.; Yu, S.S. Cationic Cellulose Nanocrystals-Based Nanocomposite Hydrogels: Achieving 3D Printable Capacitive Sensors with High Transparency and Mechanical Strength. Polymers 2021, 13, 688. [Google Scholar] [CrossRef]
- Arpa, M.D.; Kesmen, E.E.; Arslan, T.; Karadag, A.E.; Biltekin, S.N.; Demirci, F. Ginger essential oil-loaded hydrogels: Preparation, characterization, cytotoxicity, antimicrobial and anti-inflammatory activity. J. Essent. Oil Res. 2024, 36, 16–29. [Google Scholar] [CrossRef]
- Kassem, I.; Kassab, Z.; Khouloud, M.; Sehaqui, H.; Bouhfid, R.; Jacquemin, J.; Qaiss, A.; El Achably, M. Phosphoric acid-mediated green preparation of regenerated cellulose spheres and their use for all-cellulose cross-linked superabsorbent hydrogels. Int. J. Biol. Macromol. 2020, 162, 136–149. [Google Scholar] [CrossRef] [PubMed]
- McLean, B.; Ratcliffe, J.; Parker, B.J.; Field, E.H.; Hughes, S.J.; Cutter, S.W.; Iseppi, K.J.; Cameron, N.R.; Binger, K.J.; Reynolds, N.P. Composite Bioprinted Hydrogels Containing Porous Polymer Microparticles Provide Tailorable Mechanical Properties for 3D Cell Culture. Biomacromolecules 2024, 25, 829–837. [Google Scholar] [CrossRef]
- Fan, J.Y.; He, X.L.; Zhou, X.P.; Li, S.S.; Yang, Y.Q. Effect of Amino Acid Types on the Mechanical and Antimicrobial Properties of Amino Acid-Based Polyionic Liquid Hydrogels. Macromol. Rapid Commun. 2024, 45, e2300689. [Google Scholar] [CrossRef]
- Martínez-Salcedo, S.L.; Torres-Rendón, J.G.; García-Enriquez, S.; Anzaldo-Hernández, J.; Silva-Guzmán, J.A.; de Muniz, G.I.B.; Lomelí-Ramírez, M.G. Physicomechanical Characterization of Poly(acrylic acid-co-acrylamide) Hydrogels Reinforced with TEMPO-oxidized Blue Agave Cellulose Nanofibers. Fibers Polym. 2022, 23, 1161–1170. [Google Scholar] [CrossRef]
- Enoch, K.; Somasundaram, A.A. Tailoring the rheological properties of biosynthesized Copper oxide nanoparticles decorated Carboxymethyl cellulose hydrogels for biomedical applications. Colloids Surf. A-Physicochem. Eng. Asp. 2024, 682, 132890. [Google Scholar] [CrossRef]
- Qiu, X.Y.; Wang, S.Y.; Chen, S.X. The self-assembly of dialdehyde-cellulose-nanofiber-based hydrogels with high compression resilience. Cellulose 2022, 29, 5645–5658. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Lin, X.Y.; Wang, Z.M.; Zhang, L.; Wang, S.H.; Huang, Z.; Liu, H.; Xu, X. Multiple hydrogen bonds enable high strength and anti-swelling cellulose-based ionic conductive hydrogels for flexible sensors. Chem. Eng. J. 2024, 480, 148318. [Google Scholar] [CrossRef]
- Syverud, K.; Pettersen, S.R.; Draget, K.; Chinga-Carrasco, G. Controlling the elastic modulus of cellulose nanofibril hydrogels-scaffolds with potential in tissue engineering. Cellulose 2015, 22, 473–481. [Google Scholar] [CrossRef]
- Sugawara, A.; Asoh, T.A.; Takashima, Y.; Harada, A.; Uyama, H. Composite hydrogels reinforced by cellulose-based supramolecular filler. Polym. Degrad. Stab. 2020, 177, 109157. [Google Scholar] [CrossRef]
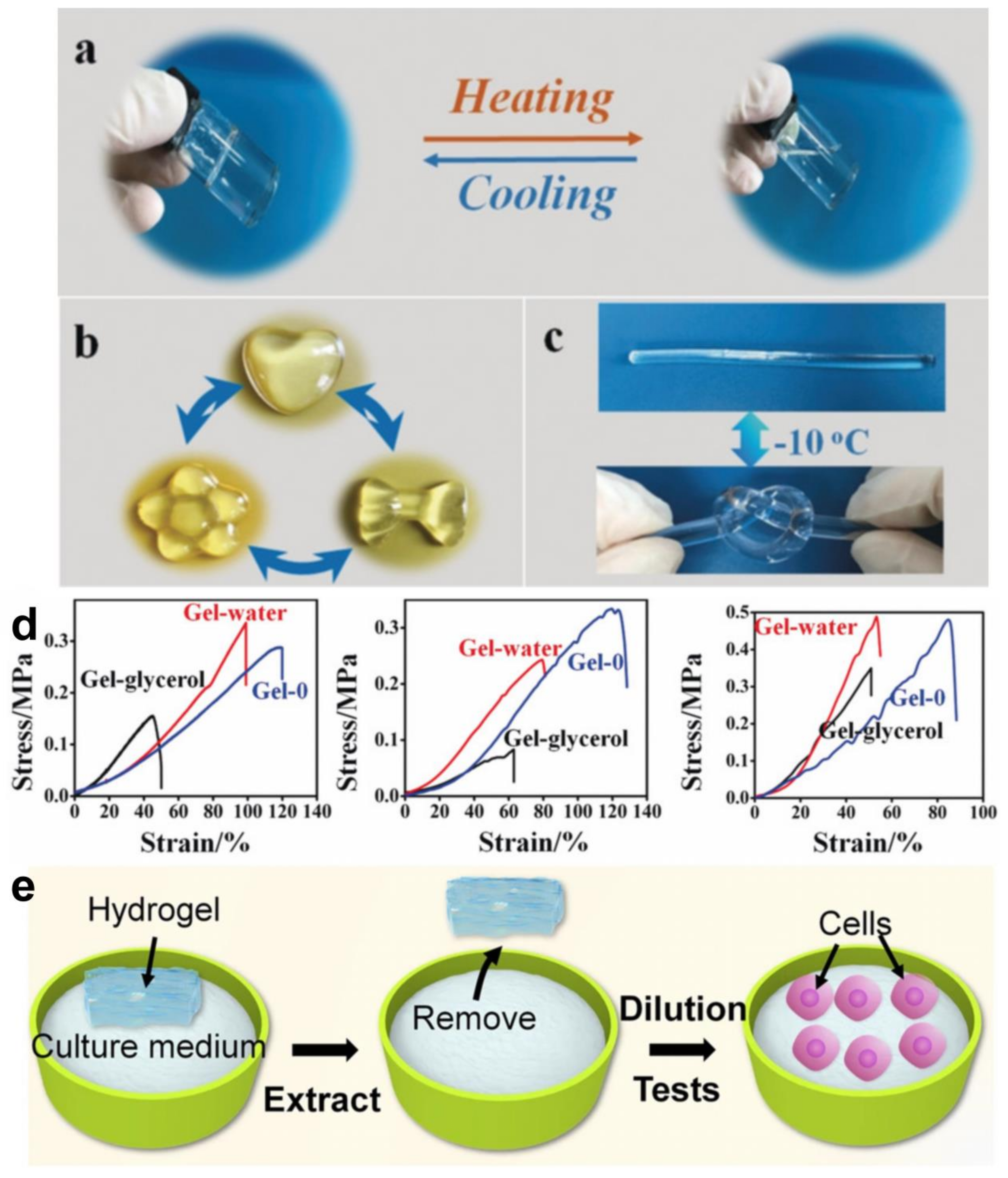
- Zhang, X.F.; Ma, X.; Hou, T.; Guo, K.; Yin, J.; Wang, Z.; Shu, L.; He, M.; Yao, J. Inorganic salts induce thermally reversible and anti-freezing cellulose hydrogels. Angew. Chem. Int. Ed. 2019, 58, 7366–7370. [Google Scholar] [CrossRef]
- Cui, X.; Lee, J.J.; Chen, W.N. Eco-friendly and biodegradable cellulose hydrogels produced from low cost okara: Towards non-toxic flexible electronics. Sci. Rep. 2019, 9, 18166. [Google Scholar] [CrossRef]
- Yunus, R.A.M.; Koch, M.; Dieudonné-George, P.; Truzzolillo, D.; Colby, R.H.; Parisi, D. Water-Driven Sol-Gel Transition in Native Cellulose/1-Ethyl-3-methylimidazolium Acetate Solutions. Acs Macro Lett. 2024, 13, 219–226. [Google Scholar] [CrossRef]
- Yu, W.; Xiong, L.P.; Teng, J.H.; Chen, C.; Li, B.S.; Zhao, L.H.; Lin, H.J.; Shen, L.G. Advances in synthesis and application of amphoteric polymer-based water treatment agents. Desalination 2024, 574, 117280. [Google Scholar] [CrossRef]
- Wang, H.H.; Zhang, H.; Yang, C.R.; Fei, G.Q.; Xie, P. Preparation and properties of transparent, waterproof, and antibacterial composite film materials. J. Appl. Polym. Sci. 2024, 141, e55329. [Google Scholar] [CrossRef]
- Huynh, N.; Valle-Delgado, J.J.; Fang, W.W.; Arola, S.; Österberg, M. Tuning the water interactions of cellulose nanofibril hydrogels using willow bark extract. Carbohydr. Polym. 2023, 317, 121095. [Google Scholar] [CrossRef] [PubMed]
- Goncharuk, V.V.; Dubrovina, L.V. Rheological Properties and Water-Retaining Power of Agar Hydrogels with Carboxymethyl Cellulose. Russ. J. Appl. Chem. 2020, 93, 1019–1026. [Google Scholar] [CrossRef]
- Li, T.L.; Kang, Y.C.; Liu, X.M.; Yang, C.L.; Li, L.; Liu, P.P.; Lei, Z.Q. Synthesis and performance of water absorbing polymer with salt isolation and anti-leakage functions. J. Environ. Chem. Eng. 2024, 12, 111682. [Google Scholar] [CrossRef]
- dos Santos, F.B.; Perez, I.D.; McMichael, P.S.; Fregolente, L.V.; Maciel, M.R.W.; Tam, K.C. Synthesis of a Novel Cellulose Nanofiber-Based Composite Hydrogel with Poly(methyl methacrylate-co-methacrylic Acid) for Effective Water Removal from Liquid Fuels. Ind. Eng. Chem. Res. 2024, 63, 2210–2222. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Z.Y.; Hua, S.; Kan, Z.; Yang, M.B. States of Water in Cellulose Hydrogels and Influence on the Crystallization of Cellulose. Chem. J. Chin. Univ.-Chin. 2015, 36, 2034–2039. [Google Scholar]
- Ingverud, T.; Larsson, E.; Hemmer, G.; Rojas, R.; Malkoch, M.; Carlmark, A. High Water-Content Thermoresponsive Hydrogels via Electrostatic Macrocrosslinking of Cellulose Nanofibrils. J. Polym. Sci. Part A-Polym. Chem. 2016, 54, 3415–3424. [Google Scholar] [CrossRef]
- Rao, K.M.; Kumar, A.; Han, S.S. Polysaccharide based bionanocomposite hydrogels reinforced with cellulose nanocrystals: Drug release and biocompatibility analyses. Int. J. Biol. Macromol. 2017, 101, 165–171. [Google Scholar]
- Nath, P.C.; Sharma, R.; Debnath, S.; Nayak, P.K.; Roy, R.; Sharma, M.; Inbaraj, B.S.; Sridhar, K. Recent advances in production of sustainable and biodegradable polymers from agro-food waste: Applications in tissue engineering and regenerative medicines. Int. J. Biol. Macromol. 2024, 259, 129129. [Google Scholar] [CrossRef]
- Xie, Y.T.; Shi, X.Y.; Gao, S.S.; Lai, C.H.; Lu, C.W.; Huang, Y.X.; Zhang, D.H.; Nie, S.X.; Xu, F.; Chu, F.X. Biomimicking natural wood to fabricate isotropically super-strong, tough, and transparent hydrogels for strain sensor and triboelectric nanogenerator applications. J. Mater. Chem. A 2024, 12, 5124–5132. [Google Scholar] [CrossRef]
- Hu, X.T.; Wang, J.H.; Song, S.Q.; Gan, W.J.; Li, W.Z.; Qi, H.C.; Zhang, Y. Ionic conductive konjac glucomannan/liquid crystal cellulose composite hydrogels with dual sensing of photo-and electro-signals capacities as wearable strain sensors. Int. J. Biol. Macromol. 2024, 258, 129038. [Google Scholar] [CrossRef]
- Zhao, Z.R.; Gao, J.; Cai, W.R.; Li, J.Y.; Kong, Y.; Zhou, M. Synthesis of oxidized carboxymethyl cellulose/chitosan hydrogels doped with graphene oxide for pH- and NIR-responsive drug delivery. Eur. Polym. J. 2023, 199, 112437. [Google Scholar] [CrossRef]
- Onofrei, M.; Filimon, A. Cellulose-based hydrogels: Designing concepts, properties, and perspectives for biomedical and environmental applications. In Polymer Science: Research Advances, Practical Applications and Educational Aspects; Formatex Research Center: Badajoz, Spain, 2016; pp. 108–120. [Google Scholar]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Cellulose-based hydrogels as sustained drug-delivery systems. Materials 2020, 13, 5270. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Aderibigbe, B.A. Chitosan and cellulose-based hydrogels for wound management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef] [PubMed]
- Rodrŕguez, R.A.; Alvarez-Lorenzo, C.; Concheiro, A. Cationic cellulose hydrogels: Kinetics of the cross-linking process and characterization as pH-/ion-sensitive drug delivery systems. J. Control. Release 2003, 86, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Ahmad, N.; Pandey, M.; Xin, C.J. Stimuli-responsive bacterial cellulose-g-poly(acrylic acid-co-acrylamide) hydrogels for oral controlled release drug delivery. Drug Dev. Ind. Pharm. 2014, 40, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Oprea, A.M.; Profire, L.; Lupusoru, C.E.; Ghiciuc, C.M.; Ciolacu, D.; Vasile, C. Synthesis and characterization of some cellulose/chondroitin sulphate hydrogels and their evaluation as carriers for drug delivery. Carbohydr. Polym. 2012, 87, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, Z.; Chen, X.; Feng, K.; Hu, T.; Huang, B.; Tang, J.; Wang, G.; Liu, S.; Yang, G. Double-network cellulose-based hybrid hydrogels with favourable biocompatibility and antibacterial activity for wound healing. Carbohydr. Polym. 2023, 319, 121193. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhai, Z.H.; Yao, Y.M.; Stant, J.C.; Landrum, S.L.; Bortner, M.J.; Frazier, C.E.; Edgar, K.J. Oxidized hydroxypropyl cellulose/carboxymethyl chitosan hydrogels permit pH-responsive, targeted drug release. Carbohydr. Polym. 2023, 300, 120213. [Google Scholar] [CrossRef]
- Zhou YiMing, Z.Y.; Zhang LiangLiang, Z.L.; Fu ShiYu, F.S.; Zheng LiMing, Z.L.; Zhan HuaiYu, Z.H. Adsorption behavior of Cd2+, Pb2+, and Ni2+ from aqueous solutions on cellulose-based hydrogels. BioResources 2012, 7, 2752–2765. [Google Scholar] [CrossRef]
- Xiong, Y.; Xu, L.; Jin, C.; Sun, Q. Cellulose hydrogel functionalized titanate microspheres with self-cleaning for efficient purification of heavy metals in oily wastewater. Cellulose 2020, 27, 7751–7763. [Google Scholar] [CrossRef]
- Yang, S.; Fu, S.; Liu, H.; Zhou, Y.; Li, X. Hydrogel beads based on carboxymethyl cellulose for removal heavy metal ions. J. Appl. Polym. Sci. 2011, 119, 1204–1210. [Google Scholar] [CrossRef]
- Lin, Y.; Fang, G.; Deng, Y.; Shen, K.; Wu, T.; Li, M. Highly effective removal of methylene blue using a chemi-mechanical pretreated cellulose-based superabsorbent hydrogel. Bioresources 2018, 13, 8709–8722. [Google Scholar] [CrossRef]
- Benhalima, T.; Ferfera-Harrar, H. Eco-friendly porous carboxymethyl cellulose/dextran sulfate composite beads as reusable and efficient adsorbents of cationic dye methylene blue. Int. J. Biol. Macromol. 2019, 132, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Yu, Y.; Chen, J.; Shi, X.; Zhou, J.; Deng, H.; Du, Y. Highly cost-effective and high-strength hydrogels as dye adsorbents from natural polymers: Chitosan and cellulose. Polym. Chem. 2017, 8, 2913–2921. [Google Scholar] [CrossRef]
- Hu, Z.-H.; Omer, A.M.; Ouyang, X.k.; Yu, D. Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb (II) from aqueous solution. Int. J. Biol. Macromol. 2018, 108, 149–157. [Google Scholar] [CrossRef]
- Fang, R.; He, W.; Xue, H.; Chen, W. Synthesis and characterization of a high-capacity cationic hydrogel adsorbent and its application in the removal of Acid Black 1 from aqueous solution. React. Funct. Polym. 2016, 102, 1–10. [Google Scholar] [CrossRef]
- Tang, Y.; Lai, Y.; Gao, R.; Chen, Y.; Xiong, K.; Ye, J.; Zheng, Q.; Fang, Z.; Pang, G.; Lee, H.-J. Functional Aerogels Composed of Regenerated Cellulose and Tungsten Oxide for UV Detection and Seawater Desalination. Gels 2022, 9, 10. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, S.; Liu, H.; Yang, S.; Zhan, H. Removal of methylene blue dyes from wastewater using cellulose-based superadsorbent hydrogels. Polym. Eng. Sci. 2011, 51, 2417–2424. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Chen, L. A green composite hydrogel based on cellulose and clay as efficient absorbent of colored organic effluent. Carbohydr. Polym. 2019, 210, 314–321. [Google Scholar] [CrossRef]
- Hosseini, H.; Zirakjou, A.; McClements, D.J.; Goodarzi, V.; Chen, W.-H. Removal of methylene blue from wastewater using ternary nanocomposite aerogel systems: Carboxymethyl cellulose grafted by polyacrylic acid and decorated with graphene oxide. J. Hazard. Mater. 2022, 421, 126752. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Liu, Q.; Zhu, S. Selective biosorption mechanism of methylene blue by a novel and reusable sugar beet pulp cellulose/sodium alginate/iron hydroxide composite hydrogel. Int. J. Biol. Macromol. 2021, 188, 993–1002. [Google Scholar] [CrossRef]
- Poornachandhra, C.; Jayabalakrishnan, R.M.; Prasanthrajan, M.; Balasubramanian, G.; Lakshmanan, A.; Selvakumar, S.; John, J.E. Cellulose-based hydrogel for adsorptive removal of cationic dyes from aqueous solution: Isotherms and kinetics. RSC Adv. 2023, 13, 4757–4774. [Google Scholar] [CrossRef] [PubMed]
- Ning, F.; Zhang, J.; Kang, M.; Ma, C.; Li, H.; Qiu, Z. Hydroxyethyl cellulose hydrogel modified with tannic acid as methylene blue adsorbent. J. Appl. Polym. Sci. 2021, 138, 49880. [Google Scholar] [CrossRef]
- Hu, D.N.; Sun, Y.F.; Tao, L.; Yuan, J.Y.; Sui, X.F.; Wei, Y. Environmentally Responsive Hydrogels Based on Cellulose. Acta Polym. Sin. 2020, 51, 880–889. [Google Scholar]
- Bauli, C.R.; Lima, G.F.; de Souza, A.G.; Ferreira, R.R.; Rosa, D.S. Eco-friendly carboxymethyl cellulose hydrogels filled with nanocellulose or nanoclays for agriculture applications as soil conditioning and nutrient carrier and their impact on cucumber growing. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126771. [Google Scholar] [CrossRef]
- Yue, Y.; Gu, J.; Han, J.; Wu, Q.; Jiang, J. Effects of cellulose/salicylaldehyde thiosemicarbazone complexes on PVA based hydrogels: Portable, reusable, and high-precision luminescence sensing of Cu2+. J. Hazard. Mater. 2021, 401, 123798. [Google Scholar] [CrossRef]
- Qin, C.-C.; Abdalkarim, S.Y.H.; Zhou, Y.; Yu, H.-Y.; He, X. Ultrahigh water-retention cellulose hydrogels as soil amendments for early seed germination under harsh conditions. J. Clean. Prod. 2022, 370, 133602. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, R.; Shi, X.; Lian, S.; Zhou, Q.; Chen, Y.; Liu, W.; Li, W. Synthesis of cellulose-based superabsorbent hydrogel with high salt tolerance for soil conditioning. Int. J. Biol. Macromol. 2022, 209, 1169–1178. [Google Scholar] [CrossRef]
- Nie, J.; Xie, H.; Zhang, M.; Liang, J.; Nie, S.; Han, W. Effective and facile fabrication of MOFs/cellulose composite paper for air hazards removal by virtue of in situ synthesis of MOFs/chitosan hydrogel. Carbohydr. Polym. 2020, 250, 116955. [Google Scholar] [CrossRef]
- Cui, C.; Li, D.; Wang, L.-J.; Wang, Y. Curdlan/sodium carboxymethylcellulose composite adsorbents: A biodegradable solution for organic dye removal from water. Carbohydr. Polym. 2024, 328, 121737. [Google Scholar] [CrossRef] [PubMed]
- Peydayesh, M. Sustainable Materials via the Assembly of Biopolymeric Nanobuilding Blocks Valorized from Agri-Food Waste. Sustainability 2024, 16, 1286. [Google Scholar] [CrossRef]
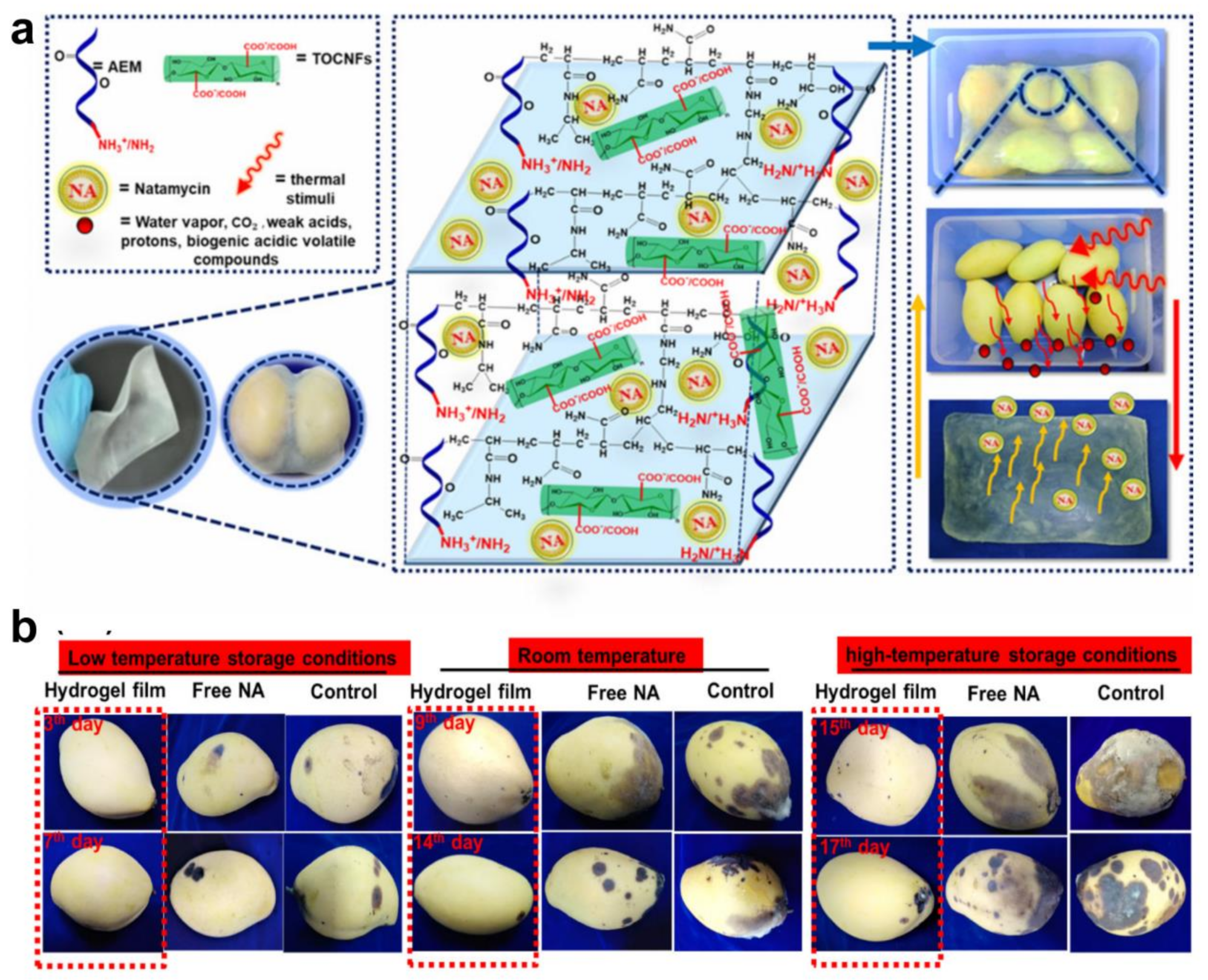
- Shaghaleh, H.; Hamoud, Y.A.; Xu, X.; Liu, H.; Wang, S.; Sheteiwy, M.; Dong, F.; Guo, L.; Qian, Y.; Li, P. Thermo-/pH-responsive preservative delivery based on TEMPO cellulose nanofiber/cationic copolymer hydrogel film in fruit packaging. Int. J. Biol. Macromol. 2021, 183, 1911–1924. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Zhang, X.-F.; Wang, Z.; Yao, J. Structure reorganization of cellulose hydrogel by green solvent exchange for potential plastic replacement. Carbohydr. Polym. 2022, 275, 118695. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, X.; Hu, L.; Zhang, Y.; Jiang, Y.; Mao, Z.; Xu, H.; Wang, B.; Feng, X.; Sui, X. A naked-eye detection polyvinyl alcohol/cellulose-based pH sensor for intelligent packaging. Carbohydr. Polym. 2020, 233, 115859. [Google Scholar] [CrossRef] [PubMed]
- Ismaeilimoghadam, S.; Jonoobi, M.; Ashori, A.; Shahraki, A.; Azimi, B.; Danti, S. Interpenetrating and semi-interpenetrating network superabsorbent hydrogels based on sodium alginate and cellulose nanocrystals: A biodegradable and high-performance solution for adult incontinence pads. Int. J. Biol. Macromol. 2023, 253, 127118. [Google Scholar] [CrossRef] [PubMed]
- Reshma, G.; Reshmi, C.; Nair, S.V.; Menon, D. Superabsorbent sodium carboxymethyl cellulose membranes based on a new cross-linker combination for female sanitary napkin applications. Carbohydr. Polym. 2020, 248, 116763. [Google Scholar]
- Wang, F.Q.; Xu, Z.P.; Chen, L.; Qiao, Z.Y.; Hu, Y.Y.; Fan, X.J.; Liu, Y.P.; Kang, Z.L.; Huang, F.; Han, M.Y.; et al. Super absorbent resilience antibacterial aerogel with curcumin for fresh pork preservation. Food Control 2024, 159, 110289. [Google Scholar] [CrossRef]
- Tanpichai, S.; Phoothong, F.; Boonmahitthisud, A. Superabsorbent cellulose-based hydrogels cross-liked with borax. Sci. Rep. 2022, 12, 8920. [Google Scholar] [CrossRef]
- Wei, P.; Chen, W.W.; Song, Q.H.; Wu, Y.B.; Xu, Y.J. Superabsorbent hydrogels enhanced by quaternized tunicate cellulose nanocrystals with adjustable strength and swelling ratio. Cellulose 2021, 28, 3723–3732. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Z.; Liu, K.; Ji, X.; Fatehi, P.; Chen, J. A cellulose nanofibril-reinforced hydrogel with robust mechanical, self-healing, pH-responsive and antibacterial characteristics for wound dressing applications. J. Nanobiotechnol. 2022, 20, 312. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Zheng, K.; Nan, J.Y.; Tang, C.; Chen, Y.; Hu, Y. Synthesis and characterization of lignosulfonate-graft-poly (acrylic acid)/hydroxyethyl cellulose semi-interpenetrating hydrogels. React. Funct. Polym. 2017, 115, 28–35. [Google Scholar] [CrossRef]


| Method Category | Cross-Linking Mechanism | Specific Method | Physico-Chemical Properties | Ref. |
|---|---|---|---|---|
| Chemical Cross-Linking Methods | Microcrystalline | Hydrate Epichlorohydrin (ECH) | Water content: 76–84%, Mechanical strength: 21 ± 3 MPa, Fracture energy: 2.6 ± 0.4 MJ m−3 | [14] |
| Agarose Transition from Mono-Succinylation to Cross-Linking | Succinic Anhydride (SA) | Transparency: 89%, Strength: 815 g/cm2 Water content: 94.7% | [75] | |
| Formation of Ester Bonds between Two Polymer Chains | Citric Acid (CA) | Water content: 13.5–38.4%, Mechanical strength: 1.09 ± 0.11 MPa, Cell compatibility, Blood compatibility, and pH sensitivity | [69] | |
| Dual Cross-Linking of Nanocrystals | Gelatin Methacrylate (GelMA) and Ionically Cross-Linked Hyaluronic Acid (HA) | Porosity (>90%) and Average Pore Size: 130–296 μm Mechanical strength: 10 kPa, Enhancing tissue regeneration | [76] | |
| Physical Cross-Linking Methods | Cellulose Nanocrystal Interface Adsorption and Hydrogen Bonding | Ultrasonication | Viscosity: 998.46 Pa.s Antioxidant | [77] |
| Strong Hydrogen Bond Interaction | Freeze-Casting Method | Immobilized Papain pH, Thermal Stability, and Storage Stability | [78] | |
| Radiation Cross-Linking Methods | CMC and Gelatin Cross-Linking | γ-Ray Radiation | Mechanical strength: 20–100 kPa Cell viability | [79] |
| Glycosidic Bond Cleavage in Hydroxypropyl Methylcellulose Main Chain | Electron Beam Radiation | Temperature Sensitivity, Biodegradability | [80] |
| Types of Cellulose | Additives | Application | Characteristics | Ref. |
|---|---|---|---|---|
| Natural Cellulose | Magnesium Ion | Medical and Drug Delivery | Biocompatibility, antimicrobial efficacy, accelerated wound healing | [40] |
| Carboxymethyl Cellulose (CMC) | MXene | Environmental Engineering | Multifunctional conductive cellulose hydrogel | [36] |
| Nanocellulose | Alginate | Environmental Engineering | Enhanced moisture retention, antibacterial properties | [126] |
| Bacterial Cellulose (BC) | Silver Nanoparticles | Personal Care Products | Antibacterial effect, fast-reducing, anti-wrinkle and UV protection | [33] |
| Hydroxyethyl Cellulose (HEC) | Lignosulfonate | Environmental Engineering | High toughness and ductility, porous structure, dye absorption and removal | [164] |
| Exfoliated Fibrils | Proteins and polysaccharides | Food Industry | Recyclable, sustainable, economical | [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Fang, Z.; Lee, H.-J. Exploring Applications and Preparation Techniques for Cellulose Hydrogels: A Comprehensive Review. Gels 2024, 10, 365. https://doi.org/10.3390/gels10060365
Tang Y, Fang Z, Lee H-J. Exploring Applications and Preparation Techniques for Cellulose Hydrogels: A Comprehensive Review. Gels. 2024; 10(6):365. https://doi.org/10.3390/gels10060365
Chicago/Turabian StyleTang, Yanjin, Zhenxing Fang, and Hoo-Jeong Lee. 2024. "Exploring Applications and Preparation Techniques for Cellulose Hydrogels: A Comprehensive Review" Gels 10, no. 6: 365. https://doi.org/10.3390/gels10060365
APA StyleTang, Y., Fang, Z., & Lee, H.-J. (2024). Exploring Applications and Preparation Techniques for Cellulose Hydrogels: A Comprehensive Review. Gels, 10(6), 365. https://doi.org/10.3390/gels10060365










