Half-Curcuminoids Encapsulated in Alginate–Glucosamine Hydrogel Matrices as Bioactive Delivery Systems
Abstract
1. Introduction
2. Results and Discussion
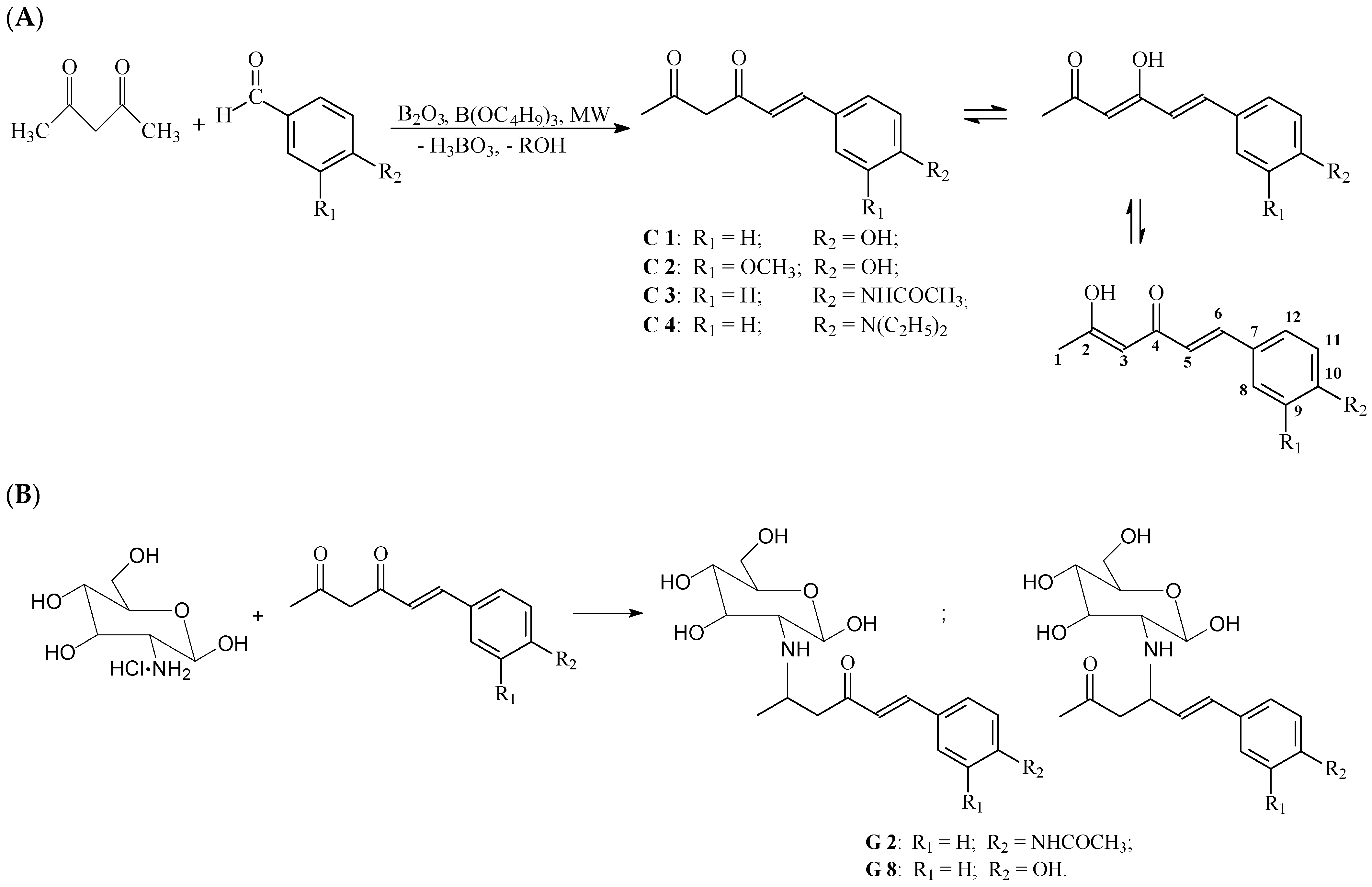
2.1. Synthesis of Asymmetric β-Diketones and Schiff Bases
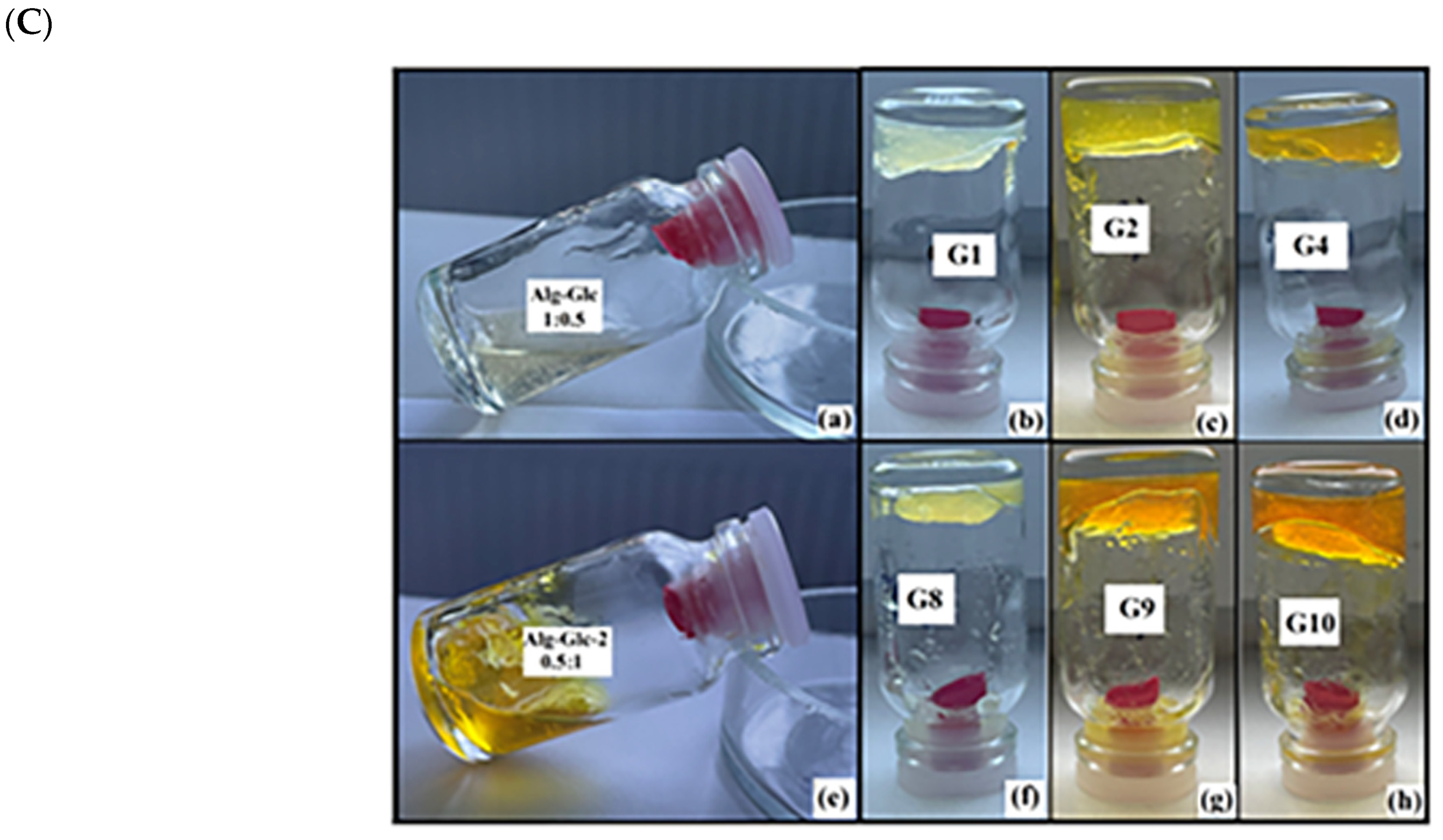
2.2. Obtaining Glucosamine Alginate Hydrogels Loaded with Asymmetric β-Diketones
2.3. Morphostructural Characterization of Half-Curcuminoid Analogs and Hydrogel Matrices by Spectrophotometric Methods
2.3.1. IR Spectroscopy Method
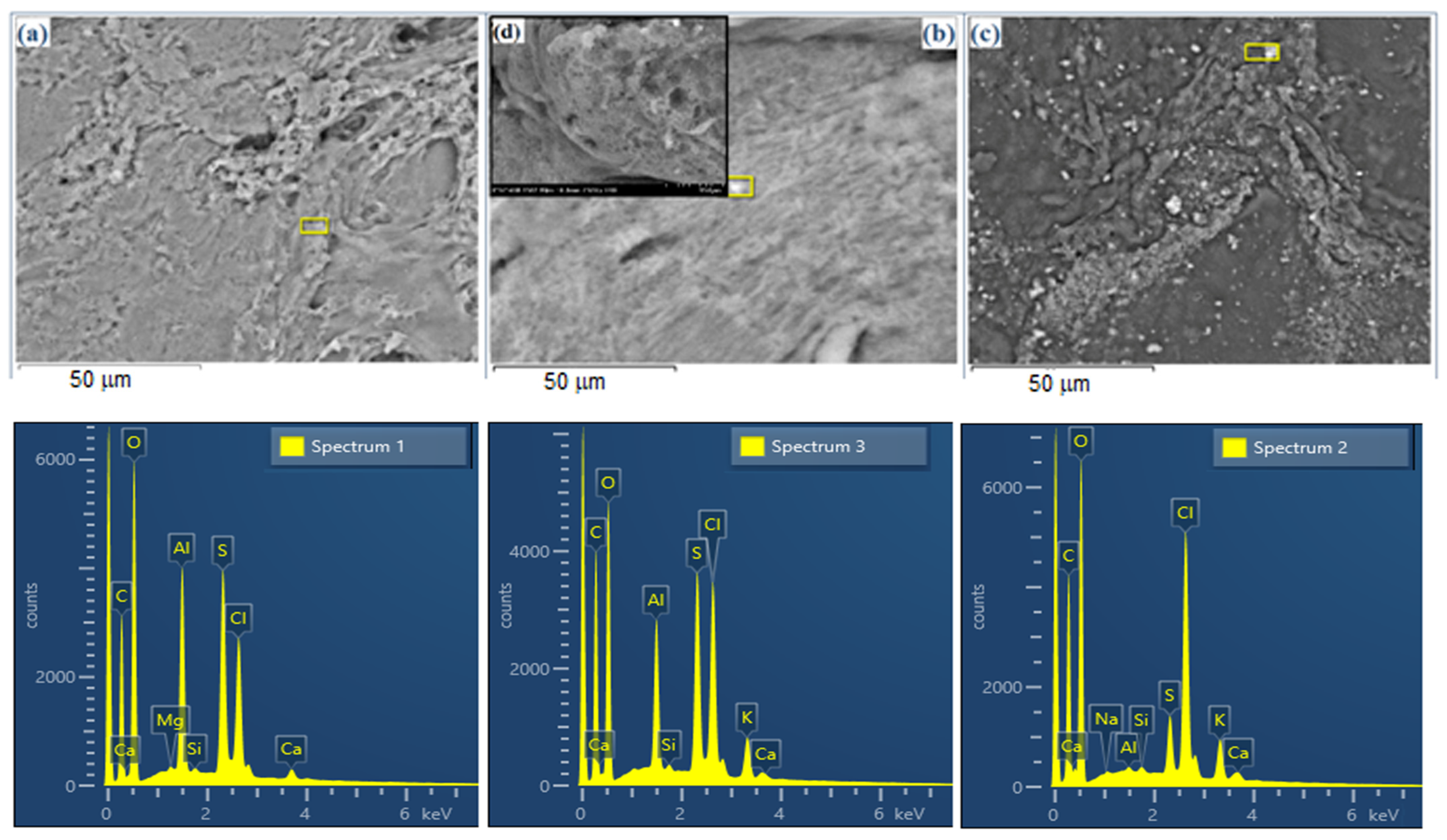
2.3.2. SEM-EDX Analysis
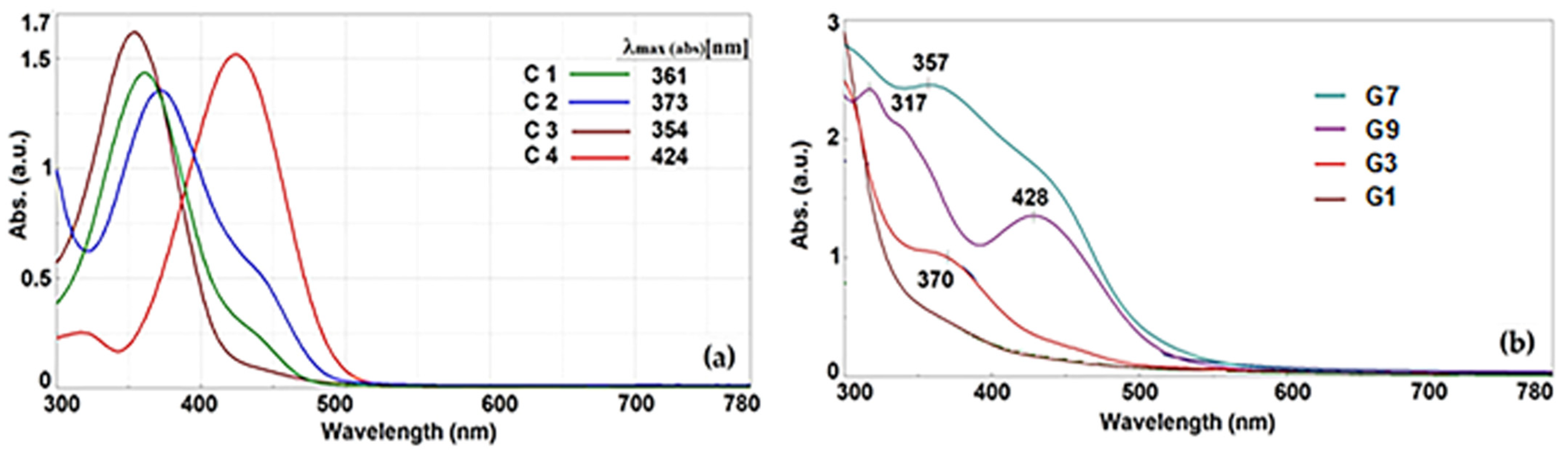
2.3.3. UV Absorption Spectra
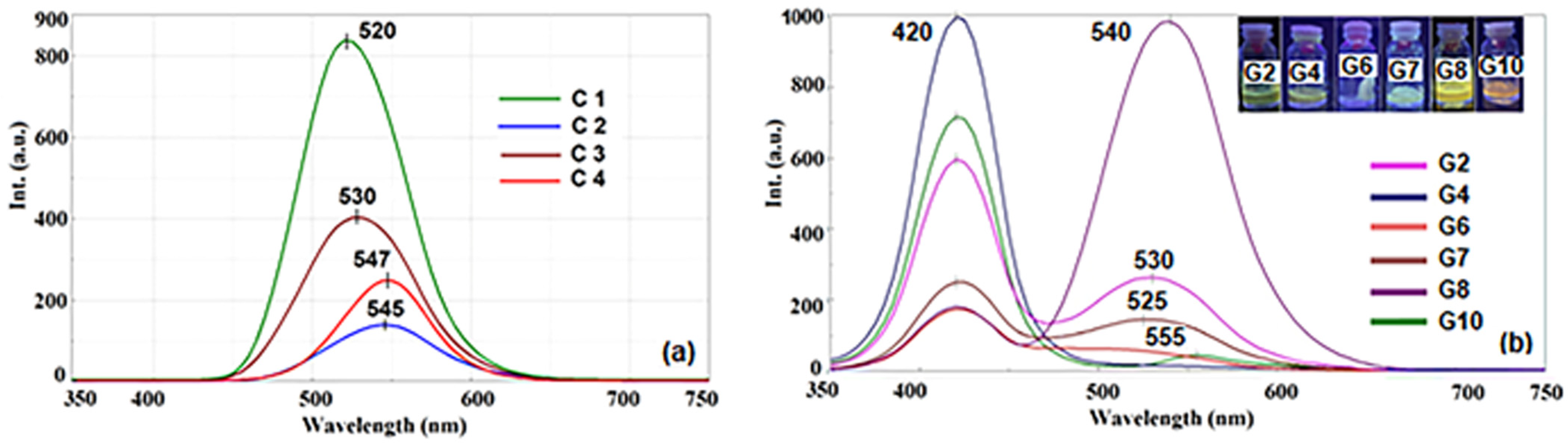
2.3.4. Fluorescence Spectra
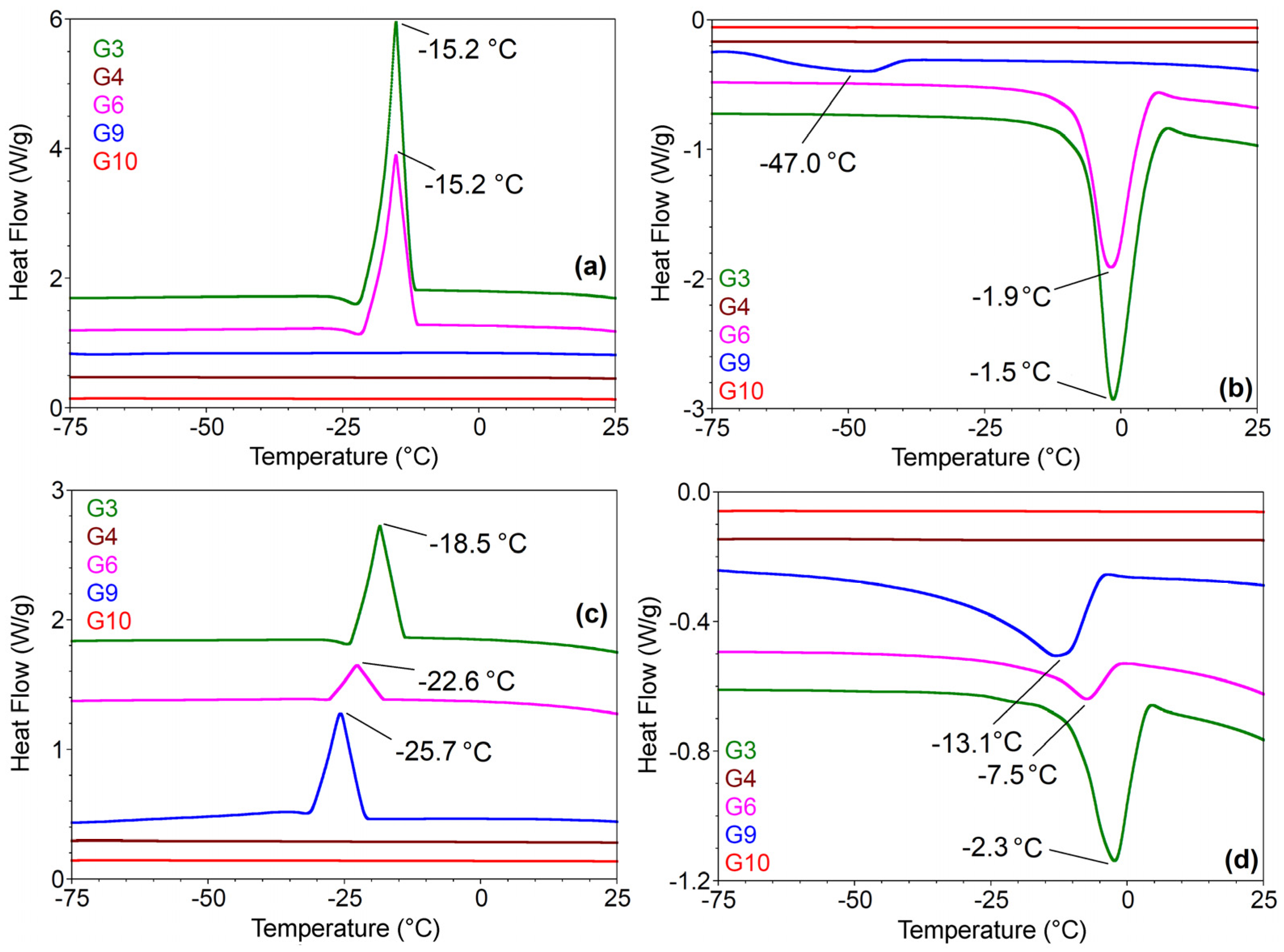
2.3.5. DSC Analyses
2.4. Computational Analysis of Half-Curcuminoid Derivatives
2.4.1. Drug-Likeness Assessment
2.4.2. Predicted Drug Disposition Properties
2.4.3. Predicted Pharmacodynamic Features
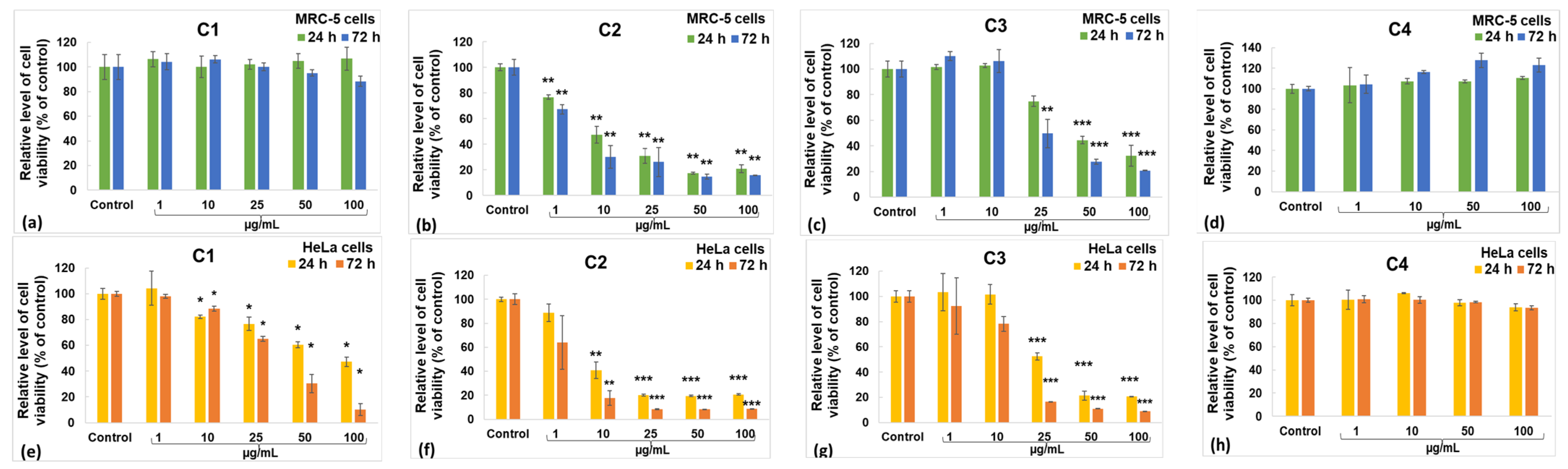
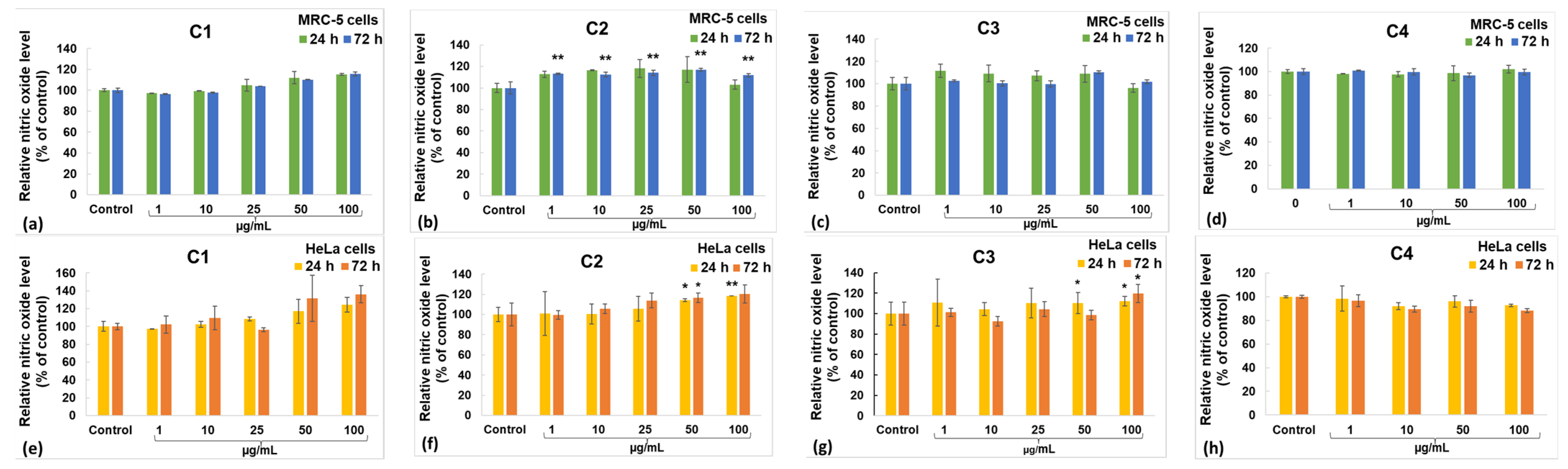
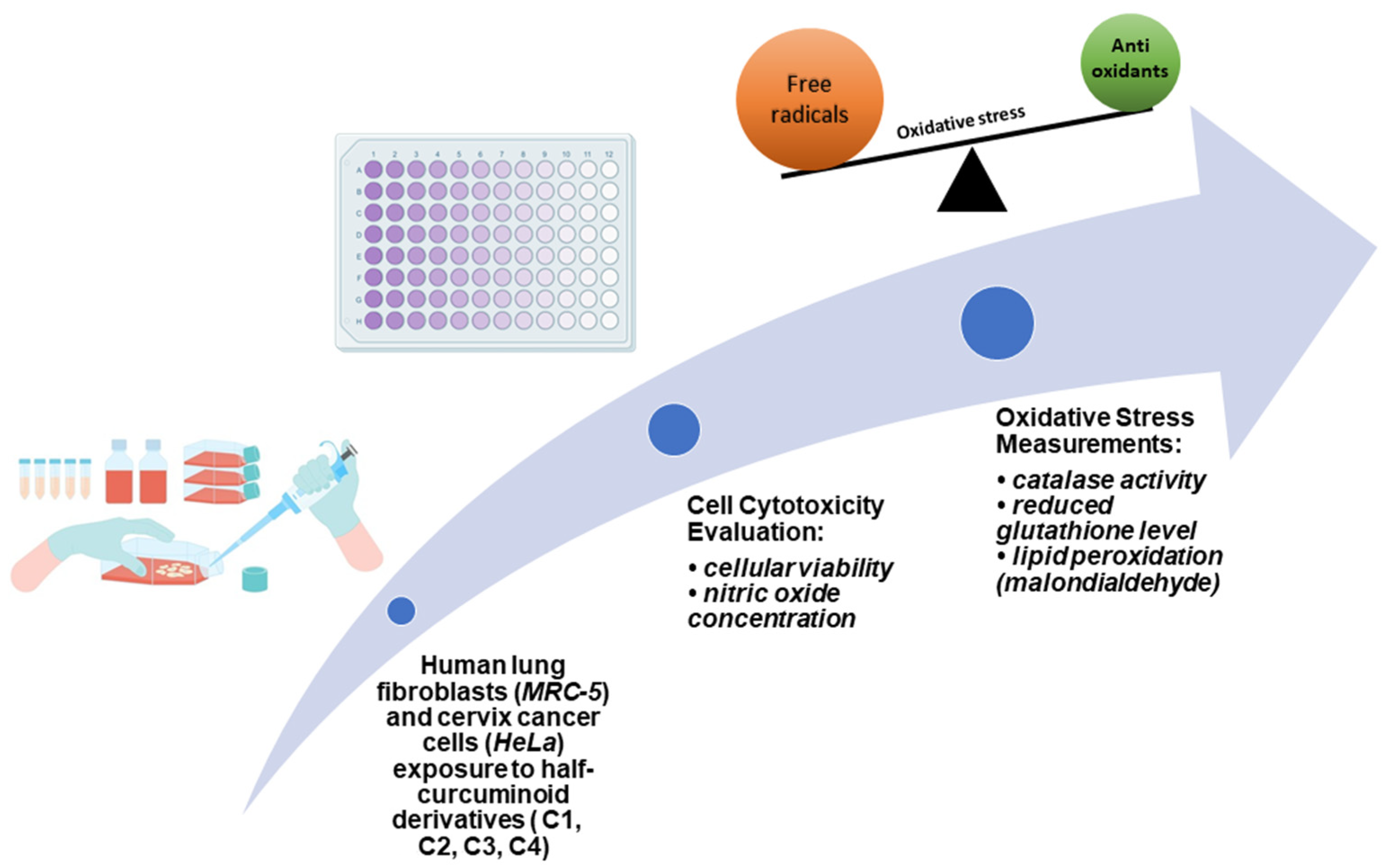
2.5. Biological Evaluation
3. Conclusions
4. Materials and Methods
4.1. Raw Materials for the Synthesis of Beta-Diketone Derivatives and Hybrid Materials
4.2. Methods of Obtaining Biologically Active Compounds and the Carrier Matrix
4.2.1. Synthesis Methods of the Asymmetric β-Diketone Compounds (C1–C4) and Schiff Bases (Glc-C1 and Glc-C3)
4.2.2. Obtaining Composite Gels Loaded with Half-Curcuminoids
4.3. Structural Characterization Methods
4.4. Prediction of the Drug-Likeness and Pharmacokinetic and Pharmacodynamic Features of Compounds
4.5. Biological Characterization Methods
4.5.1. Cell Culture and Exposure to Half-Curcuminoid Derivatives
4.5.2. Cell Cytotoxicity Assays
4.5.3. Biochemical Assays
4.5.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother Res. 2018, 32, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Memarzia, A.; Khazdair, M.R.; Behrouz, S.; Gholamnezhad, Z.; Jafarnezhad, M.; Saadat, S.; Boskabady, M.H. Experimental and clinical reports on anti-inflammatory, antioxidant, and immunomodulatory effects of Curcuma longa and curcumin, an updated and comprehensive review. BioFactors 2021, 47, 311–350. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Dissanayake, K.G.C.; Waliwita, W.A.L.C.; Liyanage, R.P. A Review on Medicinal Uses of Zingiberofficinale (Ginger). Int. J. Health Sci. 2020, 10, 6. [Google Scholar]
- Gál, E.; Nagy, L.C. Photophysical Properties and Electronic Structure of Symmetrical Curcumin Analogues and Their BF2 Complexes, Including a Phenothiazine Substituted Derivative. Symmetry 2021, 13, 2299. [Google Scholar] [CrossRef]
- Galer, P.; Golobič, A.; Koller, J.; Košmrlj, B.; Šket, B. Structures in solid state and solution of dimethoxycurcuminoids: Regioselectivebromination and chlorination. Chem. Cent. J. 2013, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Rege, S.A.; Arya, M.; Momin, S.A. Structure activity relationship of tautomers of curcumin: A review. Ukr. Food J. 2019, 8, 45–60. [Google Scholar] [CrossRef]
- Hansen, P.E. Structural Studies of β-Diketones and Their Implications on Biological Effects. Pharmaceuticals 2021, 14, 1189. [Google Scholar] [CrossRef]
- Michels, L.; Richter, A.; Chellappan, R.K.; Røst, H.I.; Behsen, A.; Wells, K.H.; Leal, L.; Santana, V.; Blawid, R.; da Silva, G.J.; et al. Electronic and structural properties of the natural dyes curcumin, bixin and indigo. RSC Adv. 2021, 11, 14169. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Wang, Z.; Wang, M.; Wilkerson, C.R.; Hall, C.D.; Akhmedov, N.G. Preparation of β-Keto Esters and β-Diketones by C-Acylation/ Deacetylation of Acetoacetic Esters and Acetonyl Ketones with 1-Acylbenzotriazoles. J. Org. Chem. 2004, 69, 6617–6622. [Google Scholar] [CrossRef] [PubMed]
- Nehra, K.; Dalal, A.; Hooda, A.; Bhagwan, S.; Saini, R.K.; Mari, B.; Kumar, S.; Singh, D. Lanthanides β-diketonate complexes as energy-efficient emissive materials: A review. J. Mol. Struct. 2022, 1249, 131531. [Google Scholar] [CrossRef]
- Sheikh, J.; Juneja, H.; Ingle, V.; Ali, P.; Hadda, T.B. Synthesis and in vitro biology of Co(II), Ni(II), Cu(II) and Zinc(II) complexes of functionalized beta-diketone bearing energy buried potential antibacterial and antiviral O, O pharmacophore sites. J. Saudi Chem. Soc. 2013, 17, 269–276. [Google Scholar] [CrossRef]
- Kljun, J.; Turel, I. β-Diketones as Scaffolds for Anticancer Drug Design—From Organic Building Blocks to Natural Products and Metallodrug Components. Eur. J. Inorg. Chem. 2017, 2017, 1655–1666. [Google Scholar] [CrossRef]
- Prasad, S.; DuBourdieu, D.; Srivastava, A.; Kumar, P.; Lall, R. Metal–Curcumin Complexes in Therapeutics: An Approach to Enhance Pharmacological Effects of Curcumin. Int. J. Mol. Sci. 2021, 22, 7094. [Google Scholar] [CrossRef]
- Raduly, F.M.; Raditoiu, V.; Raditoiu, A.; Purcar, V. Curcumin: Modern Applications for a Versatile Additive. Coatings 2021, 11, 519. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef]
- Semlali, A.; Contant, C.; Al-Otaibi, B.; Al-Jammaz, I.; Chandad, F. The curcumin analog (PAC) suppressed cell survival and induced apoptosis and autophagy in oral cancer cells. Sci. Rep. 2021, 11, 11701. [Google Scholar] [CrossRef]
- Khamidullina, L.A.; Puzyrev, I.S.; Burygin, G.L.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Mitrofanova, A.V.; Khrustalev, V.N.; Timofeeva, T.V.; Slepukhin, P.A.; Tobysheva, P.D.; et al. Unsymmetrical TrifluoromethylMethoxyphenyl β-Diketones: Effect of the Position of Methoxy Group and Coordination at Cu(II) on Biological Activity. Molecules 2021, 26, 6466. [Google Scholar] [CrossRef]
- Chou, Y.-T.; Koh, Y.-C.; Nagabhushanam, K.; Ho, C.-T.; Pan, M.-H. A Natural Degradant of Curcumin, Feruloylacetone Inhibits Cell Proliferation via Inducing Cell Cycle Arrest and a Mitochondrial Apoptotic Pathway in HCT116 Colon Cancer Cells. Molecules 2021, 26, 4884. [Google Scholar] [CrossRef]
- Feng, J.Y.; Liu, Z.Q. Feruloylacetone as the model compound of half-curcumin: Synthesis and antioxidant properties. Eur. J. Med. Chem. 2011, 46, 1198–1206. [Google Scholar] [CrossRef]
- de Gonzalo, G.; Alcántara, A.R. Recent Developments in the Synthesis of β-Diketones. Pharmaceuticals 2021, 14, 1043. [Google Scholar] [CrossRef]
- Lin, L.; Shi, Q.; Nyarko, A.K.; Bastow, K.F.; Wu, C.C.; Su, C.-Y.; Shih, C.C.-Y.; Lee, K.-H. Antitumor Agents. 250. Design and Synthesis of New Curcumin Analogues as Potential Anti-Prostate Cancer Agents. J. Med. Chem. 2006, 49, 3963–3972. [Google Scholar] [CrossRef]
- Kim, B.R.; Park, J.-Y.; Jeong, H.J.; Kwon, H.-J.; Park, S.-J.; Lee, I.-C.; Ryu, Y.B.; Lee, W.S. Design, synthesis, and evaluation of curcumin analogues as potential inhibitors of bacterial sialidase. J. Enzym. Inhib. Med. Chem. 2018, 33, 1256–1265. [Google Scholar] [CrossRef]
- Mushtaq, F.; Raza, Z.A.; Batool, S.R.; Zahid, M.; Onder, O.C.; Rafique, A.; Nazeer, M.A. Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. Int. J. Biol. Macromol. 2022, 218, 601–633. [Google Scholar] [CrossRef]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Gomez-Florit, M.; Pardo, A.; Domingues, R.M.A.; Graça, A.L.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Natural-Based Hydrogels for Tissue Engineering Applications. Molecules 2020, 25, 5858. [Google Scholar] [CrossRef]
- Rial-Hermida, M.I.; Rey-Rico, A.; Blanco-Fernandez, B.; Carballo-Pedrares, N.; Byrne, E.M.; Mano, J.F. Recent Progress on Polysaccharide-Based Hydrogels for Controlled Delivery of Therapeutic Biomolecules. ACS Biomater. Sci. Eng. 2021, 7, 4102–4127. [Google Scholar] [CrossRef]
- Hariyadi, D.M.; Islam, N. Current Status of Alginate in Drug Delivery. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 8886095. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Liu, C.; Liu, C.; Yang, L.; Zhang, M.; Liu, W.; Wang, J.; Jian, X. Self-healing alginate hydrogel based on dynamic acylhydrazone and multiple hydrogen bonds. J. Mater. Sci. 2019, 54, 8814–8828. [Google Scholar] [CrossRef]
- Lai, J.; Azad, A.K.; Sulaiman, W.M.A.W.; Kumarasamy, V.; Subramaniyan, V.; Alshehade, S.A. Alginate-Based Encapsulation Fabrication Technique for Drug Delivery: An Updated Review of Particle Type, Formulation Technique, Pharmaceutical Ingredient, and Targeted Delivery System. Pharmaceutics 2024, 16, 370. [Google Scholar] [CrossRef]
- Raduly, M.F.; Raditoiu, V.; Raditoiu, A.; Wagner, L.E.; Amariutei, V.; Ailiesei, G.L. Facile synthesis of curcumin andcurcuminoid-like derivatives at microwaves. Rev. Chim. 2018, 69, 1327–1331. [Google Scholar] [CrossRef]
- Pérez, E.M.S.; Ávalos, M.; Babiano, R.; Cintas, P.; Light, M.E.; Jiménez, J.L.; Palacios, J.C.; Sancho, A. Schiff bases from d-glucosamine and aliphatic ketones. Carbohydr. Res. 2010, 345, 23–32. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: A web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014, 42, W32–W38. [Google Scholar] [CrossRef]
- Aiyelabola, T.; Jordaan, J.; Otto, D.; Akinkunmi, E. Synthesis Characterization and Biological Activities of an Enamine Derivative and Its Coordination Compounds. Adv. Biol. Chem. 2020, 10, 172–189. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, S.; Moulik, S.P. Stability of curcumin in different solvent and solution media: UV–visible and steady-state fluorescence spectral study. J. Photochem. Photobiol. B Biol. 2016, 158, 212–218. [Google Scholar] [CrossRef]
- Raduly, F.M.; Raditoiu, V.; Raditoiu, A.; Frone, A.N.; Nicolae, C.A.; Purcar, V.; Ispas, G.; Constantin, M.; Raut, I. Modeling the Properties of Curcumin Derivatives in Relation to the Architecture of the Siloxane Host Matrices. Materials 2022, 15, 267. [Google Scholar] [CrossRef]
- Gill, P.; Moghadam, T.T.; Ranjbar, B. Differential Scanning Calorimetry Techniques: Applications in Biology and Nanoscience. J. Biomol. Tech. 2010, 21, 167–193. [Google Scholar] [PubMed]
- Lenton, S.; Rhys, N.H.; Towey, J.J.; Soper, A.K.; Dougan, L. Temperature-Dependent Segregation in Alcohol-Water Binary Mixtures Is Driven by Water Clustering. J. Phys. Chem. B 2018, 122, 7884–7894. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, B.; Reiher, M. The Secret of Dimethyl Sulfoxide−Water Mixtures. A Quantum Chemical Study of 1DMSO−nWater Clusters. J. Am. Chem. Soc. 2002, 124, 6206–6215. [Google Scholar] [CrossRef]
- Marvin|ChemAxon. Available online: https://chemaxon.com/products/marvin (accessed on 25 February 2022).
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, druglikeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Nickel, J.; Gohlke, B.O.; Erehman, J.; Banerjee, P.; Rong, W.W.; Goede, A.; Dunkel, M.; Preissner, R. SuperPred: Update on drug classification and target prediction. Nucleic Acids Res. 2014, 42, W26–W31. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings (PII). Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple Selection Criteria for Drug-like Chemical Matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, X.; Xia, W.; Zhang, Y.; Wang, C. Targeting Amyloidogenic Processing of APP in Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 137. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Vassar, R.; De Strooper, B.; Hardy, J.; Willem, M.; Singh, N.; Zhou, J.; Yan, R.; Vanmechelen, E.; De Vos, A.; et al. The β-Secretase BACE1 in Alzheimer’s Disease. Biol. Psychiatry 2021, 15, 745–756. [Google Scholar] [CrossRef]
- Avram, S.; Mernea, M.; Bagci, E.; Hritcu, L.; Borcan, L.C.; Mihailescu, D.F. Advanced Structure-activity Relationships Applied to Menthaspicata L. Subsp. spicata Essential Oil Compounds as AChE and NMDA Ligands, in Comparison with Donepezil, Galantamine and Memantine—New Approach in Brain Disorders Pharmacology. CNS Neurol. Disord. Drug Targets 2017, 16, 800–811. [Google Scholar] [CrossRef]
- Sub Laban, T.; Saadabadi, A. Monoamine Oxidase Inhibitors (MAOI); StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Udrea, A.M.; Puia, A.; Shaposhnikov, S.; Avram, S. Computational Approaches of New Perspectives in The Treatment of Depression During Pregnancy. Farmacia 2018, 66, 680–687. [Google Scholar] [CrossRef]
- Chen, M.; Du, Z.Y.; Zheng, X.; Li, D.L.; Zhou, R.P.; Zhang, K. Use of curcumin in diagnosis, prevention, and treatment of Alzheimer’s disease. Neural Regen. Res. 2018, 13, 742–752. [Google Scholar] [CrossRef]
- Benameur, T.; Giacomucci, G.; Panaro, M.A.; Ruggiero, M.; Trotta, T.; Monda, V.; Pizzolorusso, I.; Lofrumento, D.D.; Porro, C.; Messina, G. New Promising Therapeutic Avenues of Curcumin in Brain Diseases. Molecules 2022, 27, 236. [Google Scholar] [CrossRef]
- Bassiouny, A.R.; Atteya, M.A.; El-Rashidy, F.H.; Neenaa, H.M. Curcumin and EGCG Suppress Apurinic/Apyrimidinic Endonuclease 1 and Induce Complete Remission in B-cell Non-Hodgkin’s lymphoma Patients. Funct. Foods Health Dis. 2011, 1, 525–544. [Google Scholar] [CrossRef]
- Martín-Cordero, C.; López-Lázaro, M.; Gálvez, M.; Ayuso, M.J. Curcumin as a DNA Topoisomerase II Poison. J. Enzym. Inhib. Med. Chem. 2003, 18, 505–509. [Google Scholar] [CrossRef]
- Majd, S.; Power, J.; Majd, Z. Alzheimer’s Disease and Cancer: When Two Monsters Cannot Be Together. Front. Neurosci 2019, 13, 155. [Google Scholar] [CrossRef]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, R.; Chen, G.; Dejean, L.; Chen, Q.H. The effects of curcumin-based compounds on proliferation and cell death in cervical cancer cells. Anticancer Res. 2015, 35, 5293–5298. [Google Scholar] [PubMed]
- Ravindran, F.; Koroth, J.; Manjunath, M.; Narayan, S.; Choudhary, B. Curcumin derivative ST09 modulates the miR-199a-5p/DDR1 axis and regulates proliferation and migration in ovarian cancer cells. Sci. Rep. 2021, 11, 23025. [Google Scholar] [CrossRef]
- Khoubnasabjafari, M.; Ansarin, K.; Jouyban, A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. Bioimpacts 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Mari, M.; Carrozza, D.; Malavasi, G.; Venturi, E.; Avino, G.; Capponi, P.C.; Iori, M.; Rubagotti, S.; Belluti, S.; Asti, M.; et al. Curcu-min-Based β-Diketo Ligands for Ga3+: Thermodynamic Investigation of Potential Metal-Based Drugs. Pharmaceuticals 2022, 15, 854. [Google Scholar] [CrossRef]
- Tunçer, S.; Gurbanov, R.; Sheraj, I.; Solel, E.; Esenturk, O.; Banerjee, S. Low dose dimethyl sulfoxide driven gross molecular changes have the potential to interfere with various cellular processes. Sci. Rep. 2018, 8, 14828. [Google Scholar] [CrossRef]
- Gallardo-Villagrán, M.; Paulus, L.; Leger, D.Y.; Therrien, B.; Liagre, B. Dimethyl Sulfoxide: A Bio-Friendly or Bio-Hazard Chemical? The Effect of DMSO in Human Fibroblast-like Synoviocytes. Molecules 2022, 27, 4472. [Google Scholar] [CrossRef]
- Aebi, H. Isolation, purification, characterization and assay of antioxygenic enzymes: Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]


| Sample | Alginate H2O [mL] | Glucosamine H2O/EtOH [mL] | Glucosamine–Dye H2O/EtOH [mL] | Glucosamine DMSO [mL] | Glucosamine–Dye DMSO [mL] | Dye EtOH [mL] |
|---|---|---|---|---|---|---|
| G0 | 1 | - | - | 1 | - | - |
| C1 | ||||||
| G1 | 1 | 1 | - | - | - | 0.5 |
| G2 | 1 | - | 1.5 | - | - | - |
| C2 | ||||||
| G3 | 1 | 1 | - | - | - | 0.5 |
| G4 | 1 | - | - | - | 1.5 | - |
| G5 | 1 | - | - | 1 | - | 0.5 |
| G6 | 1 | 1 * | - | - | - | 0.5 |
| C3 | ||||||
| G7 | 1 | 1 | - | - | - | 0.5 |
| G8 | 1 | - | 1.5 | - | - | - |
| C4 | ||||||
| G9 | 1 | - | - | 1 | - | 0.5 |
| G10 | 1 | - | - | - | 1.5 | - |
| Element | G0 Weight % | G1 Weight % | G2 Weight % | G3 Weight % | G4 Weight % | G5 Weight % | G6 Weight % | G7 Weight % | G8 Weight % | G9 Weight % |
|---|---|---|---|---|---|---|---|---|---|---|
| C | 47.0 (±0.4) | 84.5 (±0.2) | 51.8 (±0.5) | 56.0 (±0.4) | 53.8 (±0.4) | 50.0 (±0.4) | 37.4 (±0.6) | 37.3 (±0.9) | 30.2 (±1.0) | 25.6 (±1.2) |
| O | 36.3 (±0.3) | 14.1 (±0.2) | 22.4 (±0.3) | 24.7 (±0.3) | 30.5 (±0.3) | 37.7 (±0.3) | 33.0 (±0.4) | 11.0 (±0.3) | 12.4 (±0.3) | 11.9 (±0.4) |
| S | 6.1 (±0.1) | 0.1 (±0.0) | 3.9 (±0.1) | 12.3 (±0.1) | 5.0 (±0.1) | 1.6 (±0.0) | 14.9 (±0.2) | 1.8 (±0.1) | 3.1 (±0.1) | 1.1 (±0.0) |
| Cl | 4.9 (±0.1) | 1.1 (±0.0) | 12.4 (±0.2) | 3.3 (±0.0) | 5.8 (±0.1) | 8.1 (±0.1) | 3.1 (±0.1) | 27.3 (±0.5) | 27.3 (±0.5) | 35.4 (±0.7) |
| N | - | - | - | - | - | - | - | 17.0 (±0.8) | 18.1 (±0.7) | 20.2 (±0.9) |
| Ca | 0.5 (±0.0) | - | 1.2 (±0.0) | 0.5 (±0.0) | 0.6 (±0.1) | 0.4 (±0.0) | 3.6 (±0.1) | 0.6 (±0.0) | - | 0.9 (±0.0) |
| K | 0.1 (±0.1) | 0.2 (±0.0) | 8.0 (±0.1) | 3.0 (±0.0) | 1.7 (±0.0) | 1.9 (±0.0) | 8.0 (±0.1) | 4.4 (±0.1) | 7.9 (±0.1) | 4.5 (±0.1) |
| Na | - | - | 0.2 (±0.0) | 0.1 (±0.0) | - | 0.1 (±0.0) | - | 0.2 (±0.0) | 0.7 (±0.0) | 0.3 (±0.0) |
| Si | 0.1 (±0.0) | - | - | 0.1 (±0.0) | 0.1 (±0.0) | 0.1 (±0.0) | - | 0.2 (±0.0) | 0.1 (±0.0) | 0.1 (±0.0) |
| Al | 4.9 (±0.1) | 0.1 (±0.0) | 0.1 (±0.0) | 0.1 (±0.0) | 3.0 (±0.0) | 0.1 (±0.0) | 0.1 (±0.0) | 0.1 (±0.0) | 0.3 (±0.0) | 0.3 (±0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raduly, F.M.; Raditoiu, V.; Raditoiu, A.; Nicolae, C.A.; Grapin, M.; Stan, M.S.; Voinea, I.C.; Vlasceanu, R.-I.; Nitu, C.D.; Mihailescu, D.F.; et al. Half-Curcuminoids Encapsulated in Alginate–Glucosamine Hydrogel Matrices as Bioactive Delivery Systems. Gels 2024, 10, 376. https://doi.org/10.3390/gels10060376
Raduly FM, Raditoiu V, Raditoiu A, Nicolae CA, Grapin M, Stan MS, Voinea IC, Vlasceanu R-I, Nitu CD, Mihailescu DF, et al. Half-Curcuminoids Encapsulated in Alginate–Glucosamine Hydrogel Matrices as Bioactive Delivery Systems. Gels. 2024; 10(6):376. https://doi.org/10.3390/gels10060376
Chicago/Turabian StyleRaduly, Florentina Monica, Valentin Raditoiu, Alina Raditoiu, Cristian Andi Nicolae, Maria Grapin, Miruna Silvia Stan, Ionela Cristina Voinea, Raluca-Ioana Vlasceanu, Cristina Doina Nitu, Dan F. Mihailescu, and et al. 2024. "Half-Curcuminoids Encapsulated in Alginate–Glucosamine Hydrogel Matrices as Bioactive Delivery Systems" Gels 10, no. 6: 376. https://doi.org/10.3390/gels10060376
APA StyleRaduly, F. M., Raditoiu, V., Raditoiu, A., Nicolae, C. A., Grapin, M., Stan, M. S., Voinea, I. C., Vlasceanu, R.-I., Nitu, C. D., Mihailescu, D. F., Avram, S., & Mernea, M. (2024). Half-Curcuminoids Encapsulated in Alginate–Glucosamine Hydrogel Matrices as Bioactive Delivery Systems. Gels, 10(6), 376. https://doi.org/10.3390/gels10060376










