Development and Characterization of Ethylcellulose Oleogels Based on Pumpkin Seed Oil and Rapeseed Oil
Abstract
:1. Introduction
2. Results and Discussion
2.1. Visual Appearance
2.2. Gel Formation Time
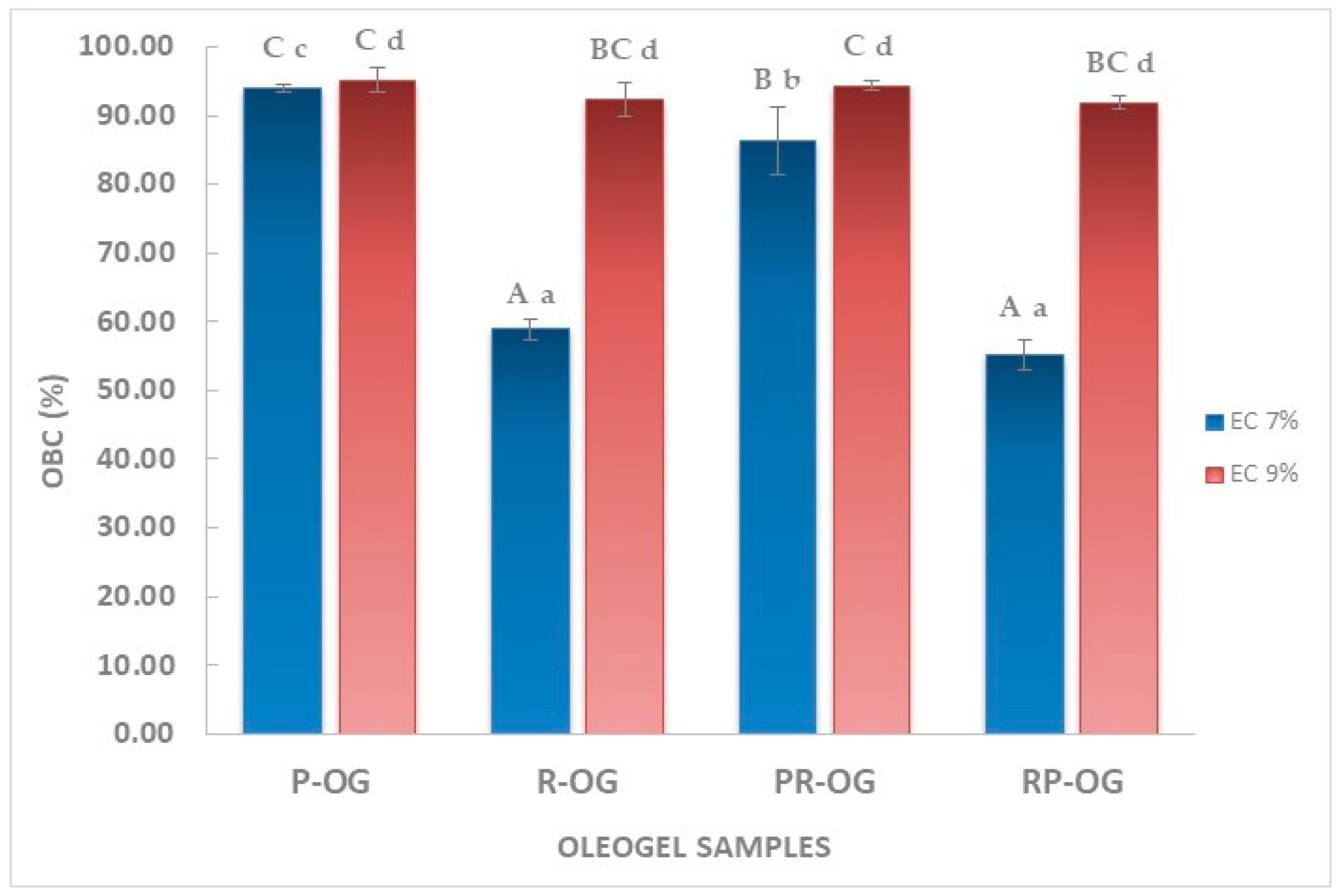
2.3. Oil-Binding Capacity
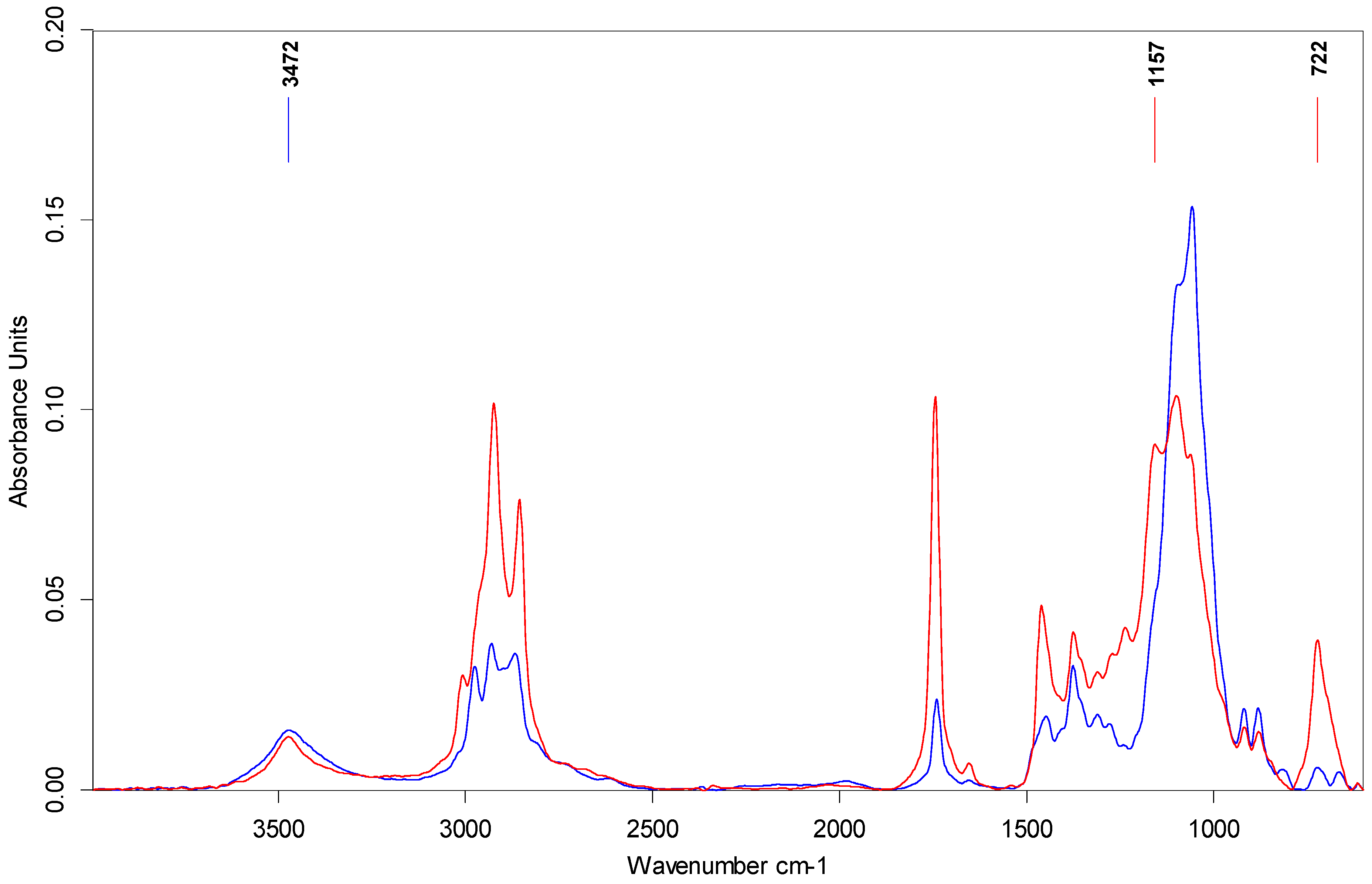
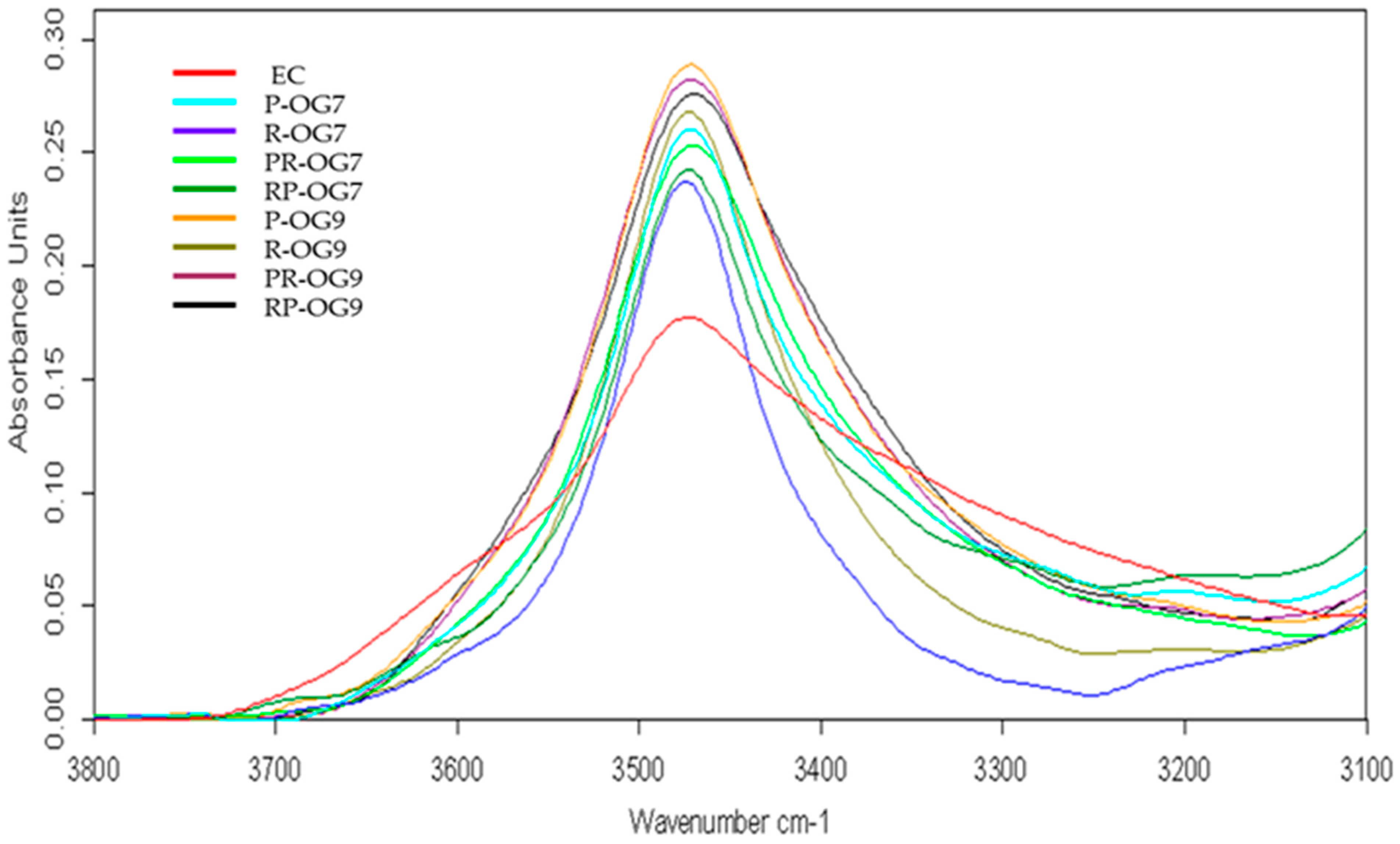
2.4. Attenuated Total Reflectance–Fourier Transform Infrared
2.5. Oxidative Stability
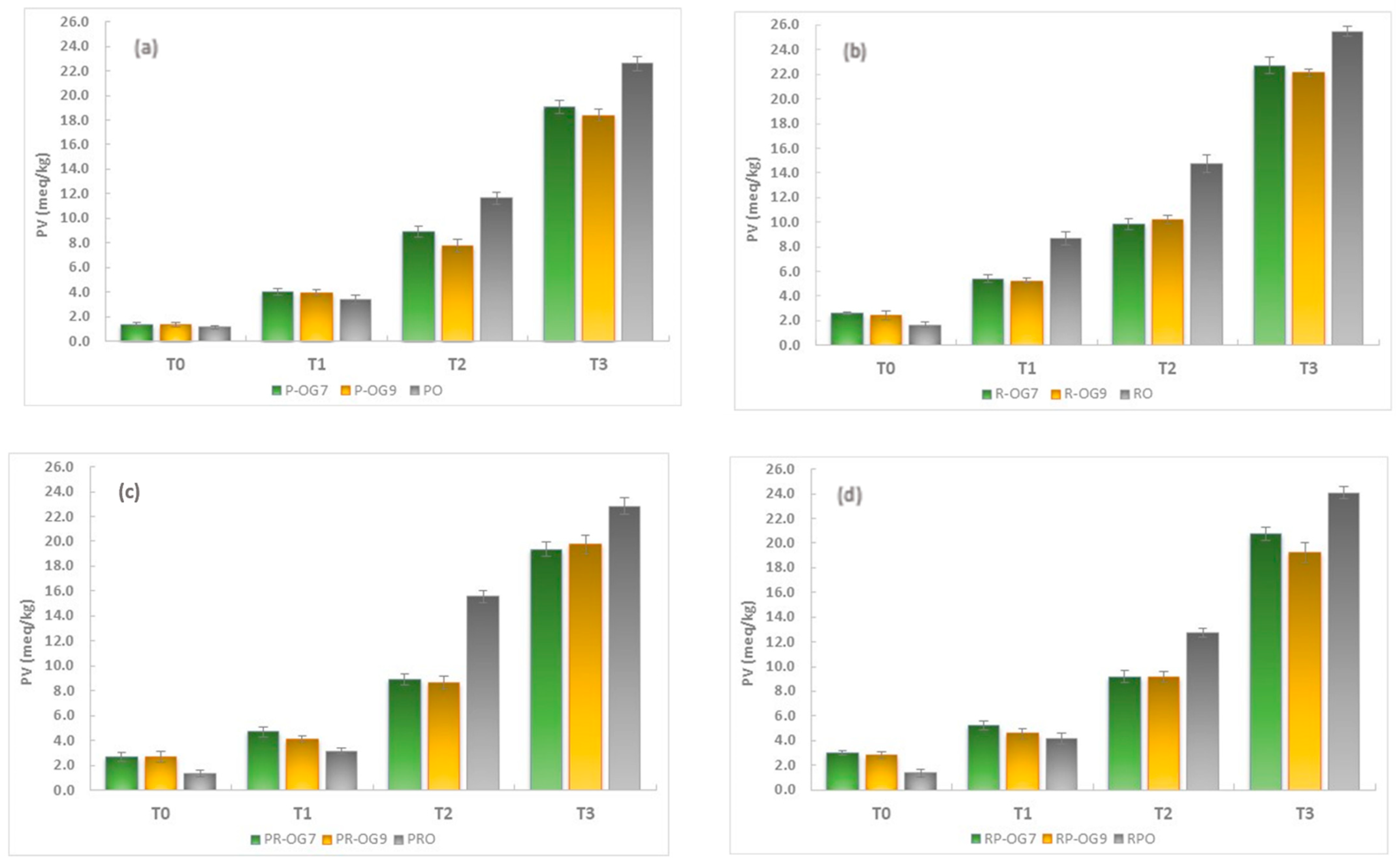
2.5.1. Peroxide Value
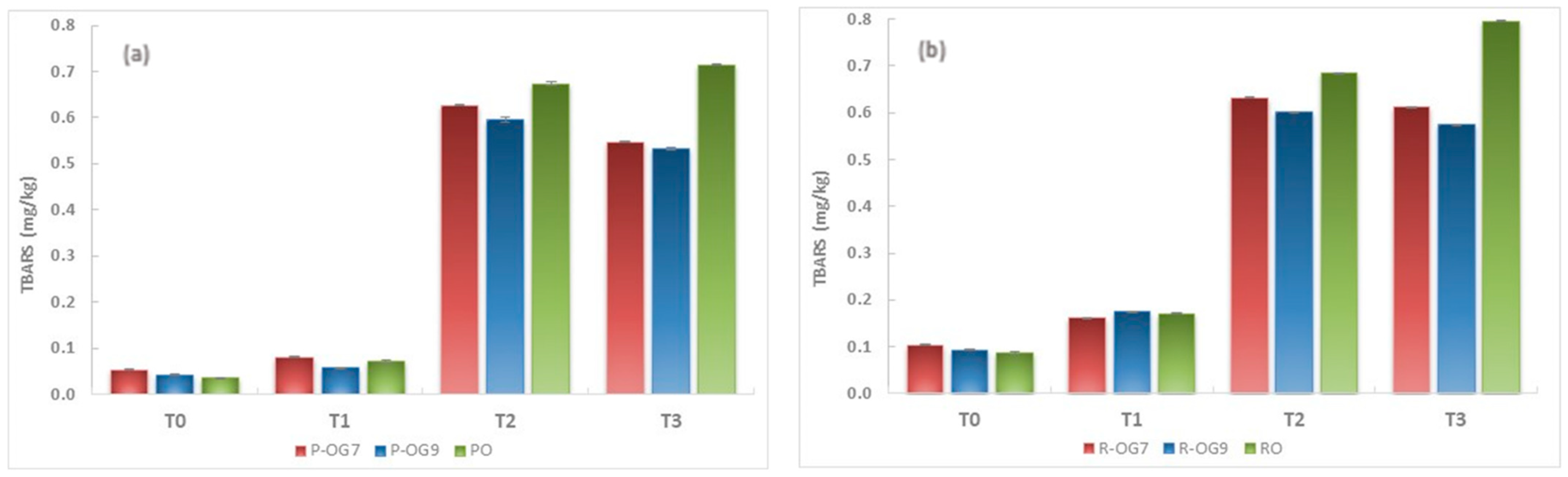
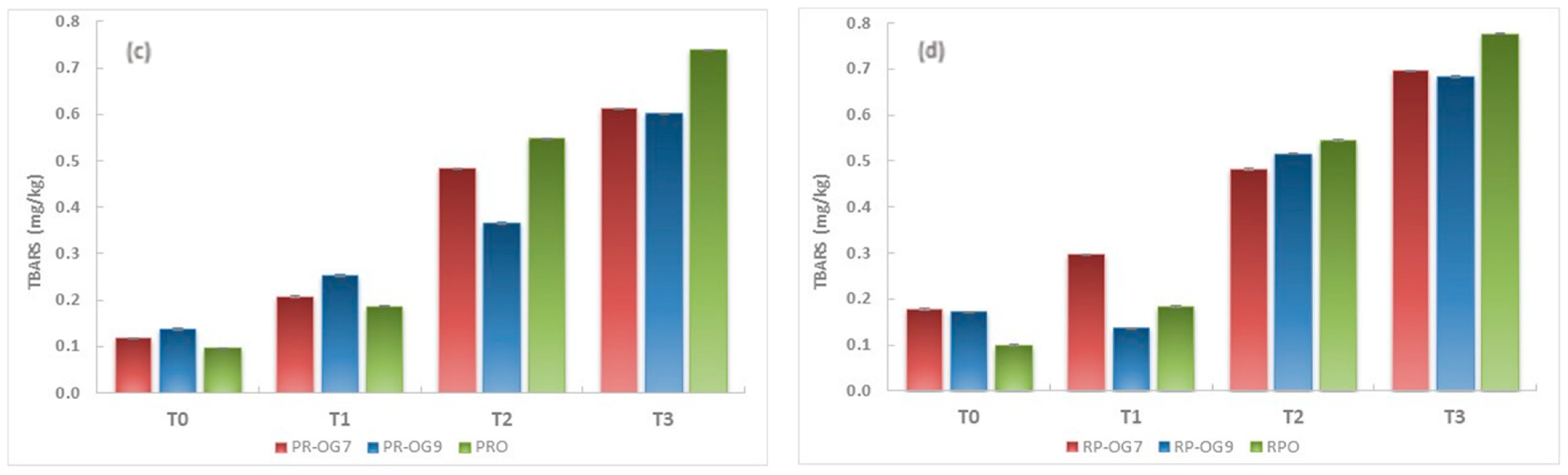
2.5.2. TBARS
2.6. Texture Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Oleogels Preparation
4.3. Visual Appearance
4.4. Gel Formation Time
4.5. Oil-Binding Capacity
4.6. Attenuated Total Reflectance-Fourier Transform Infrared
4.7. Oxidative Stability
4.7.1. Peroxide Value
4.7.2. TBARS
4.8. Texture Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perța-Crișan, S.; Ursachi, C.-Ș.; Chereji, B.-D.; Tolan, I.; Munteanu, F.-D. Food-Grade Oleogels: Trends in Analysis, Characterization, and Applicability. Gels 2023, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- García-Ortega, M.L.; Toro-Vazquez, J.F.; Ghosh, S. Development and characterization of structured water-in-oil emulsions with ethyl cellulose oleogels. Food Res. Int. 2021, 150, 110763. [Google Scholar] [CrossRef] [PubMed]
- Naeli, M.H.; Milani, J.M.; Farmani, J.; Zargaraan, A. Developing and optimizing low-saturated oleogel shortening based on ethyl cellulose and hydroxypropyl methyl cellulose biopolymers. Food Chem. 2022, 369, 130963. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhong, J.; Liu, X.; Wang, Q.; Qin, X. Physicochemical properties and intermolecular interactions of a novel diacylglycerol oil oleogel made with ethyl cellulose as affected by γ-oryzanol. Food Hydrocoll. 2023, 138, 108484. [Google Scholar] [CrossRef]
- Millao, S.; Iturra, N.; Contardo, I.; Morales, E.; Quilaqueo, M.; Rubilar, M. Structuring of oils with high PUFA content: Evaluation of the formulation conditions on the oxidative stability and structural properties of ethylcellulose oleogels. Food Chem. 2023, 405, 134772. [Google Scholar] [CrossRef] [PubMed]
- Zetzl, A.K.; Gravelle, A.J.; Kurylowicz, M.; Dutcher, J.; Barbut, S.; Marangoni, A.G. Microstructure of ethylcellulose oleogels and its relationship to mechanical properties. Food Struct. 2014, 2, 27–40. [Google Scholar] [CrossRef]
- Borriello, A.; Masi, P.; Cavella, S. Novel pumpkin seed oil-based oleogels: Development and physical characterization. LWT 2021, 152, 112165. [Google Scholar] [CrossRef]
- Fu, H.; Lo, Y.M.; Yan, M.; Li, P.; Cao, Y. Characterization of thermo-oxidative behavior of ethylcellulose oleogels. Food Chem. 2020, 305, 125470. [Google Scholar] [CrossRef] [PubMed]
- Soleimanian, Y.; Ghazani, S.M.; Marangoni, A.G. Ethylcellulose oleogels of oil glycerolysis products as functional adipose tissue mimetics. Food Hydrocoll. 2024, 151, 109756. [Google Scholar] [CrossRef]
- Qiu, H.; Qu, K.; Eun, J.-B.; Zhang, H. Analysis of thermal oxidation of different multi-element oleogels based on carnauba wax, β-sitosterol/lecithin, and ethyl cellulose by classical oxidation determination method combined with the electronic nose. Food Chem. 2023, 405, 134970. [Google Scholar] [CrossRef]
- Santos, P.D.D.F.; Keshanidokht, S.; Kumar, S.; Clausen, M.P.; Via, M.A.; Favaro-Trindade, C.S.; Andersen, M.L.; Risbo, J. A novel low-temperature procedure for oleogelation of heat-sensitive oils: Oleogels based on tucumã oil and ethyl cellulose. LWT 2024, 193, 115776. [Google Scholar] [CrossRef]
- Qu, K.; Ma, J.; Zhang, H.; Li, X. Characterization of construction and physical properties of composite oleogel based on single low molecular weight wax and polymer ethyl cellulose. LWT 2024, 192, 115722. [Google Scholar] [CrossRef]
- Ursachi, C.-Ș.; Perța-Crișan, S.; Tolan, I.; Munteanu, F.-D. Preliminary studies about some factors influencing the properties of oleogels. Sci. Tech. Bull.-Chem. Food Sci. Eng. 2022, 19, 34–42. [Google Scholar]
- Singh, A.; Kumar, V. Phyto-chemical and bioactive compounds of pumpkin seed oil as affected by different extraction methods. Food Chem. Adv. 2023, 2, 100211. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Wu, X.; Zhao, Y.; Wu, L.; Lu, B. Quantitative determination of free and esterified phytosterol profile in nuts and seeds commonly consumed in China by SPE/GC–MS. LWT 2019, 100, 355–361. [Google Scholar] [CrossRef]
- Hu, T.; Zhou, L.; Kong, F.; Wang, S.; Hong, K.; Lei, F.; He, D. Effects of Extraction Strategies on Yield, Physicochemical and Antioxidant Properties of Pumpkin Seed Oil. Foods 2023, 12, 3351. [Google Scholar] [CrossRef]
- Banaś, K.; Piwowar, A.; Harasym, J. The potential of rapeseed (canola) oil nutritional benefits wide spreading via oleogelation. Food Biosci. 2023, 56, 103162. [Google Scholar] [CrossRef]
- Chew, S.C. Cold-pressed rapeseed (Brassica napus) oil: Chemistry and functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [CrossRef]
- Amiri, M.; Raeisi-Dehkordi, H.; Sarrafzadegan, N.; Forbes, S.C.; Salehi-Abargouei, A. The effects of Canola oil on cardiovascular risk factors: A systematic review and meta-analysis with dose-response analysis of controlled clinical trials. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2133–2145. [Google Scholar] [CrossRef]
- Pușcaș, A.; Mureșan, A.; Ranga, F.; Fetea, F.; Muste, S.; Socaciu, C.; Mureșan, V. Phenolics Dynamics and Infrared Fingerprints during the Storage of Pumpkin Seed Oil and Thereof Oleogel. Processes 2020, 8, 1412. [Google Scholar] [CrossRef]
- Borriello, A.; Miele, N.A.; Masi, P.; Cavella, S. Rheological Properties, Particle Size Distribution and Physical Stability of Novel Refined Pumpkin Seed Oil Creams with Oleogel and Lucuma Powder. Foods 2022, 11, 1844. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Singh, M.; Winkler-Moser, J.K.; Bakota, E.L.; Liu, S.X. Preparation of Margarines from Organogels of Sunflower Wax and Vegetable Oils. J. Food Sci. 2014, 79, C1926–C1932. [Google Scholar] [CrossRef] [PubMed]
- David, A.; David, M.; Lesniarek, P.; Corfias, E.; Pululu, Y.; Delample, M.; Snabre, P. Oleogelation of rapeseed oil with cellulose fibers as an innovative strategy for palm oil substitution in chocolate spreads. J. Food Eng. 2021, 292, 110315. [Google Scholar] [CrossRef]
- Gao, Y.; Li, M.; Zhang, L.; Wang, Z.; Yu, Q.; Han, L. Preparation of rapeseed oil oleogels based on beeswax and its application in beef heart patties to replace animal fat. LWT 2021, 149, 111986. [Google Scholar] [CrossRef]
- Patel, A.R.; Rajarethinem, P.S.; Grędowska, A.; Turhan, O.; Lesaffer, A.; De Vos, W.H.; Van De Walle, D.; Dewettinck, K. Edible applications of shellac oleogels: Spreads, chocolate paste and cakes. Food Funct. 2014, 5, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Erlenbusch, N.; Wittland, S.; Nikolay, S.; Hetzer, B.; Matthäus, B. Rapeseed Oil Based Oleogels for the Improvement of the Fatty Acid Profile Using Cookies as an Example. Eur. J. Lipid Sci. Technol. 2022, 124, 2200033. [Google Scholar] [CrossRef]
- Shao, L.; Bi, J.; Li, X.; Dai, R. Effects of vegetable oil and ethylcellulose on the oleogel properties and its application in Harbin red sausage. Int. J. Biol. Macromol. 2023, 239, 124299. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Tang, C.; Li, Y. Crystallization Behavior and Physical Properties of Monoglycerides-Based Oleogels as Function of Oleogelator Concentration. Foods 2023, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Gravelle, A.J.; Marangoni, A.G. Ethylcellulose Oleogels: Structure, Functionality, and Food Applications. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2018; Volume 84, pp. 1–56. [Google Scholar]
- Gravelle, A.J.; Davidovich-Pinhas, M.; Zetzl, A.K.; Barbut, S.; Marangoni, A.G. Influence of solvent quality on the mechanical strength of ethylcellulose oleogels. Carbohydr. Polym. 2016, 135, 169–179. [Google Scholar] [CrossRef]
- Bardaa, S.; Ben Halima, N.; Aloui, F.; Ben Mansour, R.; Jabeur, H.; Bouaziz, M.; Sahnoun, Z. Oil from pumpkin (Cucurbita pepo L.) seeds: Evaluation of its functional properties on wound healing in rats. Lipids Health Dis. 2016, 15, 73. [Google Scholar] [CrossRef]
- Malvano, F.; Albanese, D.; Cinquanta, L.; Liparoti, S.; Marra, F. A Comparative Study between Beeswax and Glycerol Monostearate for Food-Grade Oleogels. Gels 2024, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Rodrigues, L.C.; Fernandes, E.M.; Lobo, F.C.M.; Gomes, J.M.; Reis, R.L. Tailoring Natural-Based Oleogels Combining Ethylcellulose and Virgin Coconut Oil. Polymers 2022, 14, 2473. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, I.; Żbikowska, A.; Kowalska, M. Physical stability of model emulsions based on ethyl cellulose oleogels. Int. Agrophysics 2020, 34, 289–300. [Google Scholar] [CrossRef]
- Rohman, A.; Che Man, Y.B.; Nurrulhidayah, A.F. Fourier-Transform Infrared Spectra Combined with Chemometrics and Fatty Acid Composition for Analysis of Pumpkin Seed Oil Blended Into Olive Oil. Int. J. Food Prop. 2015, 18, 1086–1096. [Google Scholar] [CrossRef]
- Saucedo-Hernández, Y.; Lerma-García, M.J.; Herrero-Martínez, J.M.; Ramis-Ramos, G.; Jorge-Rodríguez, E.; Simó-Alfonso, E.F. Classification of Pumpkin Seed Oils According to Their Species and Genetic Variety by Attenuated Total Reflection Fourier-Transform Infrared Spectroscopy. J. Agric. Food Chem. 2011, 59, 4125–4129. [Google Scholar] [CrossRef] [PubMed]
- Yuzhen, L.; Changwen, D.; Yanqiu, S.; Jianmin, Z. Characterization of Rapeseed Oil Using FTIR-ATR Spectroscopy. J. Food Sci. Eng. 2014, 4, 244–249. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Barbut, S.; Marangoni, A.G. Development, Characterization, and Utilization of Food-Grade Polymer Oleogels. Annu. Rev. Food Sci. Technol. 2016, 7, 65–91. [Google Scholar] [CrossRef] [PubMed]
- Totosaus, A.; Gonzaléz-Gonzaléz, R.; Fragoso, M. Influence of the type of cellulosic derivatives on the texture, and oxidative and thermal stability of soybean oil oleogel. Grasas Aceites 2016, 67, e152. [Google Scholar] [CrossRef]
- Rohman, A.; Che Man, Y.B.; Ismail, A.; Hashim, P. Application of FTIR Spectroscopy for the Determination of Virgin Coconut Oil in Binary Mixtures with Olive Oil and Palm Oil. J. Am. Oil Chem. Soc. 2010, 87, 601–606. [Google Scholar] [CrossRef]
- Lin, Y.; Asante, F.O.; Xu, X.; Li, S.; Ding, H.; Xu, L.; Yang, X.; Xia, J.; Li, M. A naturally tailored small molecule for the preparation of ethyl cellulose supramolecular composite film. Cellulose 2021, 28, 289–300. [Google Scholar] [CrossRef]
- Nikonenko, N.A.; Buslov, D.K.; Sushko, N.I.; Zhbankov, R.G. Spectroscopic manifestation of stretching vibrations of glycosidic linkage in polysaccharides. J. Mol. Struct. 2005, 752, 20–24. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry, 1st ed.; Meyers, R.A., Ed.; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Ahmadi, P.; Jahanban-Esfahlan, A.; Ahmadi, A.; Tabibiazar, M.; Mohammadifar, M. Development of Ethyl Cellulose-based Formulations: A Perspective on the Novel Technical Methods. Food Rev. Int. 2022, 38, 685–732. [Google Scholar] [CrossRef]
- O’Sullivan, C.M.; Barbut, S.; Marangoni, A.G. Edible oleogels for the oral delivery of lipid soluble molecules: Composition and structural design considerations. Trends Food Sci. Technol. 2016, 57, 59–73. [Google Scholar] [CrossRef]
- Rosen-Kligvasser, J.; Davidovich-Pinhas, M. The role of hydrogen bonds in TAG derivative-based oleogel structure and properties. Food Chem. 2021, 334, 127585. [Google Scholar] [CrossRef] [PubMed]
- Lupi, F.R.; Shakeel, A.; Greco, V.; Baldino, N.; Calabrò, V.; Gabriele, D. Organogelation of extra virgin olive oil with fatty alcohols, glyceryl stearate and their mixture. LWT 2017, 77, 422–429. [Google Scholar] [CrossRef]
- Barriuso, B.; Astiasarán, I.; Ansorena, D. A review of analytical methods measuring lipid oxidation status in foods: A challenging task. Eur. Food Res. Technol. 2013, 236, 1–15. [Google Scholar] [CrossRef]
- Bekdeşer, B.; Esin Çelik, S.; Bener, M.; Dondurmacıoğlu, F.; Yıldırım, E.; Nida Yavuz, E.; Apak, R. Determination of primary and secondary oxidation products in vegetable oils with gold nanoparticle based fluorometric turn-on nanosensor: A new total oxidation value. Food Chem. 2024, 434, 137426. [Google Scholar] [CrossRef] [PubMed]
- Pignitter, M.; Hernler, N.; Zaunschirm, M.; Kienesberger, J.; Somoza, M.; Kraemer, K.; Somoza, V. Evaluation of Palm Oil as a Suitable Vegetable Oil for Vitamin A Fortification Programs. Nutrients 2016, 8, 378. [Google Scholar] [CrossRef]
- Grajzer, M.; Szmalcel, K.; Kuźmiński, Ł.; Witkowski, M.; Kulma, A.; Prescha, A. Characteristics and Antioxidant Potential of Cold-Pressed Oils—Possible Strategies to Improve Oil Stability. Foods 2020, 9, 1630. [Google Scholar] [CrossRef]
- Poiana, M.-A.; Alexa, E.; Moigradean, D.; Popa, M. The influence of the storage conditions on the oxidative stability and antioxidant properties of sunflower and pumpkin oil. In Proceedings of the 44th Croatian and 4th International Symposium on Agriculture, Opatija, Croatia, 16–20 February 2009. [Google Scholar]
- Symoniuk, E.; Wroniak, M.; Napiórkowska, K.; Brzezińska, R.; Ratusz, K. Oxidative Stability and Antioxidant Activity of Selected Cold-Pressed Oils and Oils Mixtures. Foods 2022, 11, 1597. [Google Scholar] [CrossRef]
- Kampa, J.; Frazier, R.; Rodriguez-Garcia, J. Physical and Chemical Characterisation of Conventional and Nano/Emulsions: Influence of Vegetable Oils from Different Origins. Foods 2022, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Qiu, H.; Zhang, H.; Yin, X. Characterization of physically stable oleogels transporting active substances rich in resveratrol. Food Biosci. 2022, 49, 101830. [Google Scholar] [CrossRef]
- Encinar, J.; Pardal, A.; Sánchez, N.; Nogales, S. Biodiesel by Transesterification of Rapeseed Oil Using Ultrasound: A Kinetic Study of Base-Catalysed Reactions. Energies 2018, 11, 2229. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, R.; Wang, Z.; Wang, B.; Yang, Y.; Ju, X.; He, R. The effect of refining process on the physicochemical properties and micronutrients of rapeseed oils. PLoS ONE 2019, 14, e0212879. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.; Lee, J.; Lee, H.G.; Lee, S. Feasibility of hydroxypropyl methylcellulose oleogel as an animal fat replacer for meat patties. Food Res. Int. 2019, 122, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Frolova, Y.V.; Sobolev, R.V.; Sarkisyan, V.A.; Kochetkova, A.A. Approaches to study the oxidative stability of oleogels. IOP Conf. Ser. Earth Environ. Sci. 2021, 677, 032045. [Google Scholar] [CrossRef]
- Neđeral, S.; Petrović, M.; Vincek, D.; Pukec, D.; Škevin, D.; Kraljić, K.; Obranović, M. Variance of quality parameters and fatty acid composition in pumpkin seed oil during three crop seasons. Ind. Crops Prod. 2014, 60, 15–21. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Calhelha, R.C.; Rouphael, Y.; Petrović, J.; Soković, M.; Ferreira, I.C.F.R.; Barros, L. Antimicrobial Properties, Cytotoxic Effects, and Fatty Acids Composition of Vegetable Oils from Purslane, Linseed, Luffa, and Pumpkin Seeds. Appl. Sci. 2021, 11, 5738. [Google Scholar] [CrossRef]
- Shen, J.; Liu, Y.; Wang, X.; Bai, J.; Lin, L.; Luo, F.; Zhong, H. A Comprehensive Review of Health-Benefiting Components in Rapeseed Oil. Nutrients 2023, 15, 999. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, G.; Calligaris, S.; Nicoli, M.C. Comparative Study on the Ability of Different Oleogelators to Structure Sunflower Oil. Food Biophys. 2020, 15, 42–49. [Google Scholar] [CrossRef]
- Keskin Uslu, E.; Yılmaz, E. Preparation and characterization of oleogels and emulgels with glycerol monooleate–cholesterol mixtures. Chem. Pap. 2021, 75, 2075–2085. [Google Scholar] [CrossRef]
- Lim, J.; Hwang, H.-S.; Lee, S. Oil-structuring characterization of natural waxes in canola oil oleogels: Rheological, thermal, and oxidative properties. Appl. Biol. Chem. 2017, 60, 17–22. [Google Scholar] [CrossRef]
- Dimakopoulou-Papazoglou, D.; Zampouni, K.; Prodromidis, P.; Moschakis, T.; Katsanidis, E. Microstructure, Physical Properties, and Oxidative Stability of Olive Oil Oleogels Composed of Sunflower Wax and Monoglycerides. Gels 2024, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Tang, L.; Dong, Q.; Li, Y.; Zhang, H. Effect of oleogelation on physical properties and oxidative stability of camellia oil-based oleogels and oleogel emulsions. Food Res. Int. 2021, 140, 110057. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Estaca, J.; Herrero, A.M.; Herranz, B.; Álvarez, M.D.; Jiménez-Colmenero, F.; Cofrades, S. Characterization of ethyl cellulose and beeswax oleogels and their suitability as fat replacers in healthier lipid pâtés development. Food Hydrocoll. 2019, 87, 960–969. [Google Scholar] [CrossRef]
- Pang, M.; Shi, Z.; Lei, Z.; Ge, Y.; Jiang, S.; Cao, L. Structure and thermal properties of beeswax-based oleogels with different types of vegetable oil. Grasas Aceites 2020, 71, 380. [Google Scholar] [CrossRef]
- Sarkisyan, V.; Frolova, Y.; Sobolev, R.; Kochetkova, A. On the Role of Beeswax Components in the Regulation of Sunflower Oil Oleogel Properties. Food Biophys. 2023, 18, 262–272. [Google Scholar] [CrossRef]
- Palla, C.; Giacomozzi, A.; Genovese, D.B.; Carrín, M.E. Multi–objective optimization of high oleic sunflower oil and monoglycerides oleogels: Searching for rheological and textural properties similar to margarine. Food Struct. 2017, 12, 1–14. [Google Scholar] [CrossRef]



| Samples | GFT (min) |
|---|---|
| P-OG7 | |
| PR-OG7 | |
| RP-OG7 | no gel formation |
| R-OG7 | no gel formation |
| P-OG9 | |
| PR-OG9 | |
| RP-OG9 | |
| R-OG9 |
| Samples | Firmness (N) | Adhesiveness (N) |
|---|---|---|
| P-OG7 | 11.45 ± 0.09 | −0.72 ± 0.01 |
| PR-OG7 | 1.63 ± 0.04 | −0.37 ± 0.02 |
| RP-OG7 | 0.13 ± 0.04 | −0.01 ± 0.00 |
| R-OG7 | 0.20 ± 0.04 | −0.06 ± 0.01 |
| P-OG9 | 14.02 ± 0.18 | −0.95 ± 0.03 |
| PR-OG9 | 5.46 ± 0.10 | −0.48 ± 0.01 |
| RP-OG9 | 1.37 ± 0.02 | −0.18 ± 0.01 |
| R-OG9 | 3.53 ± 0.25 | −0.15 ± 0.02 |
| Sample Codification | EC (%) | PO (%) | RO (%) |
|---|---|---|---|
| P-OG7 | 7 | 100 | 0 |
| PR-OG7 | 7 | 75 | 25 |
| RP-OG7 | 7 | 50 | 50 |
| R-OG7 | 7 | 0 | 100 |
| P-OG9 | 9 | 100 | 0 |
| PR-OG9 | 9 | 75 | 25 |
| RP-OG9 | 9 | 50 | 50 |
| R-OG9 | 9 | 0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ursachi, C.-Ș.; Perța-Crișan, S.; Tolan, I.; Chambre, D.R.; Chereji, B.-D.; Condrat, D.; Munteanu, F.-D. Development and Characterization of Ethylcellulose Oleogels Based on Pumpkin Seed Oil and Rapeseed Oil. Gels 2024, 10, 384. https://doi.org/10.3390/gels10060384
Ursachi C-Ș, Perța-Crișan S, Tolan I, Chambre DR, Chereji B-D, Condrat D, Munteanu F-D. Development and Characterization of Ethylcellulose Oleogels Based on Pumpkin Seed Oil and Rapeseed Oil. Gels. 2024; 10(6):384. https://doi.org/10.3390/gels10060384
Chicago/Turabian StyleUrsachi, Claudiu-Ștefan, Simona Perța-Crișan, Iolanda Tolan, Dorina Rodica Chambre, Bianca-Denisa Chereji, Dumitru Condrat, and Florentina-Daniela Munteanu. 2024. "Development and Characterization of Ethylcellulose Oleogels Based on Pumpkin Seed Oil and Rapeseed Oil" Gels 10, no. 6: 384. https://doi.org/10.3390/gels10060384
APA StyleUrsachi, C.-Ș., Perța-Crișan, S., Tolan, I., Chambre, D. R., Chereji, B.-D., Condrat, D., & Munteanu, F.-D. (2024). Development and Characterization of Ethylcellulose Oleogels Based on Pumpkin Seed Oil and Rapeseed Oil. Gels, 10(6), 384. https://doi.org/10.3390/gels10060384








