Molten Alkali-Assisted Formation of Silicate Gels and Its Application for Preparing Zeolites
Abstract
:1. Introduction
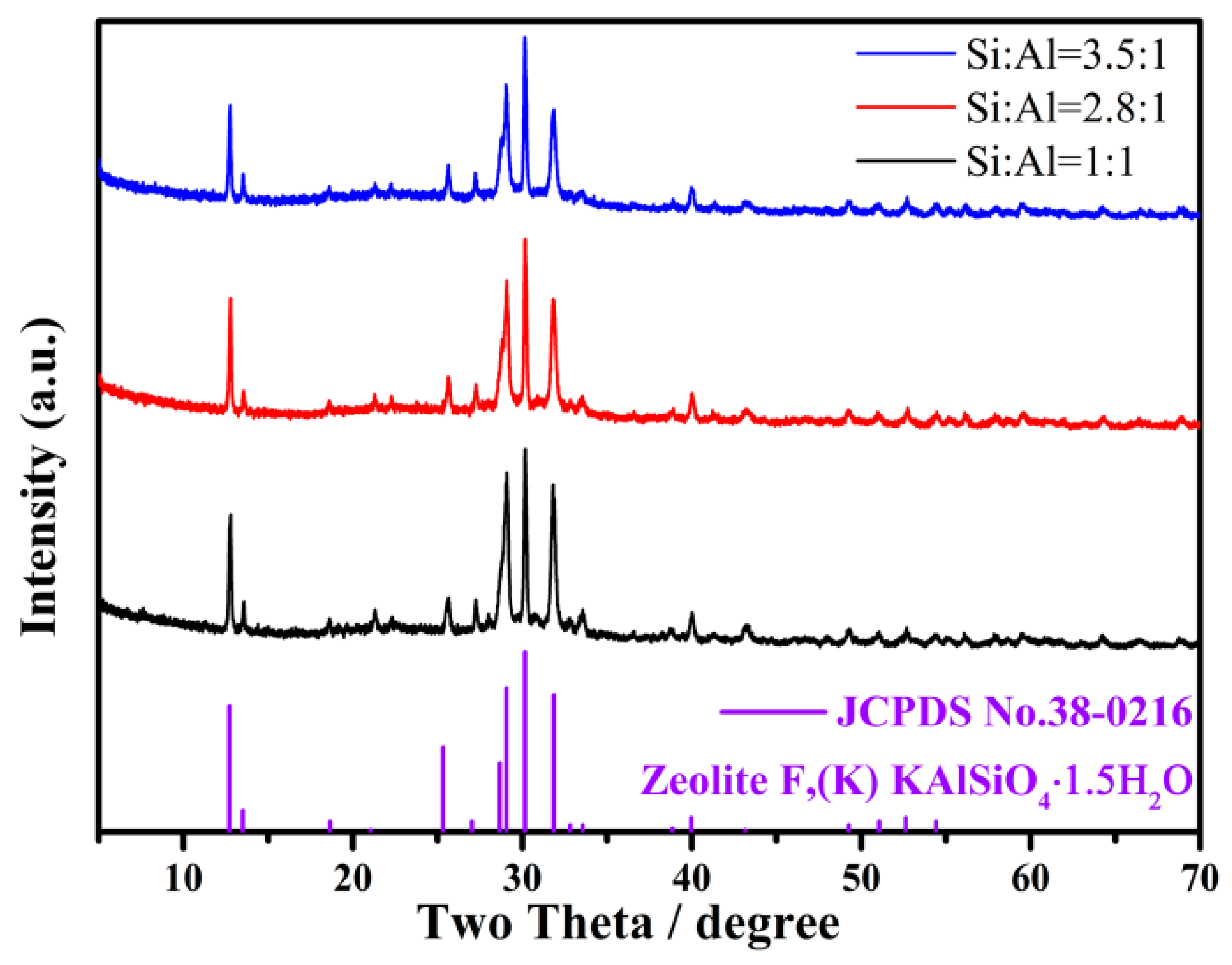
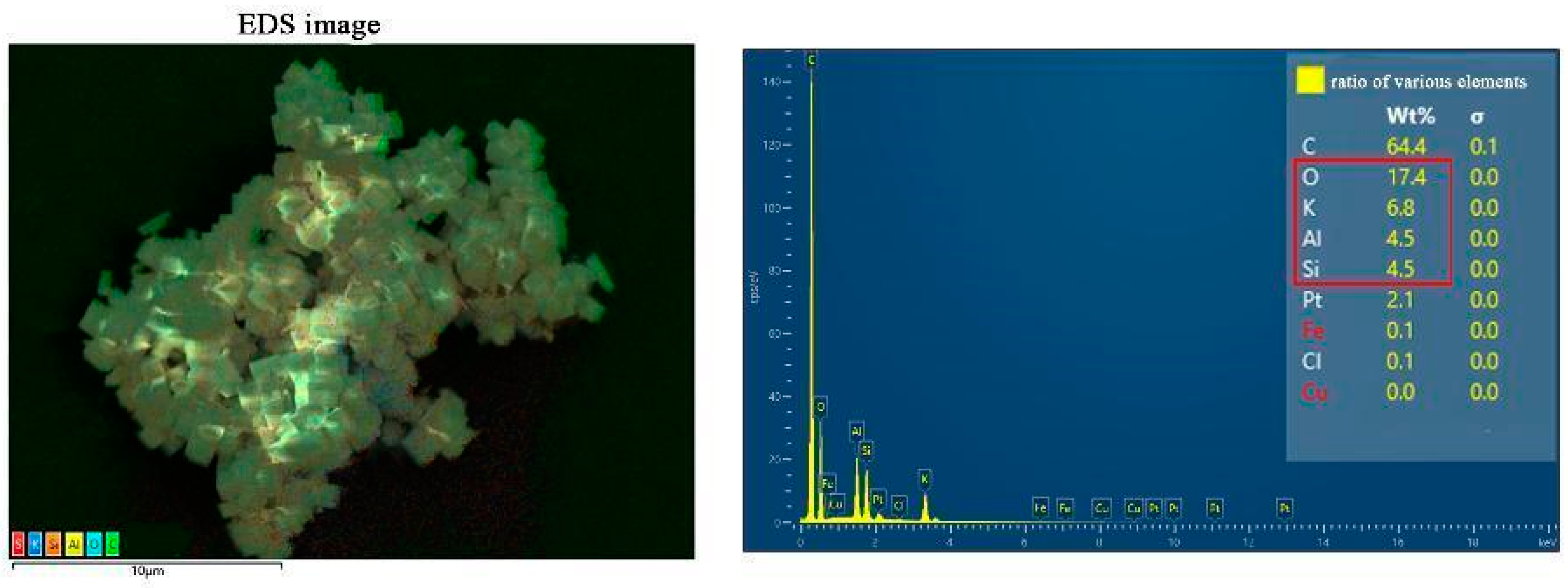
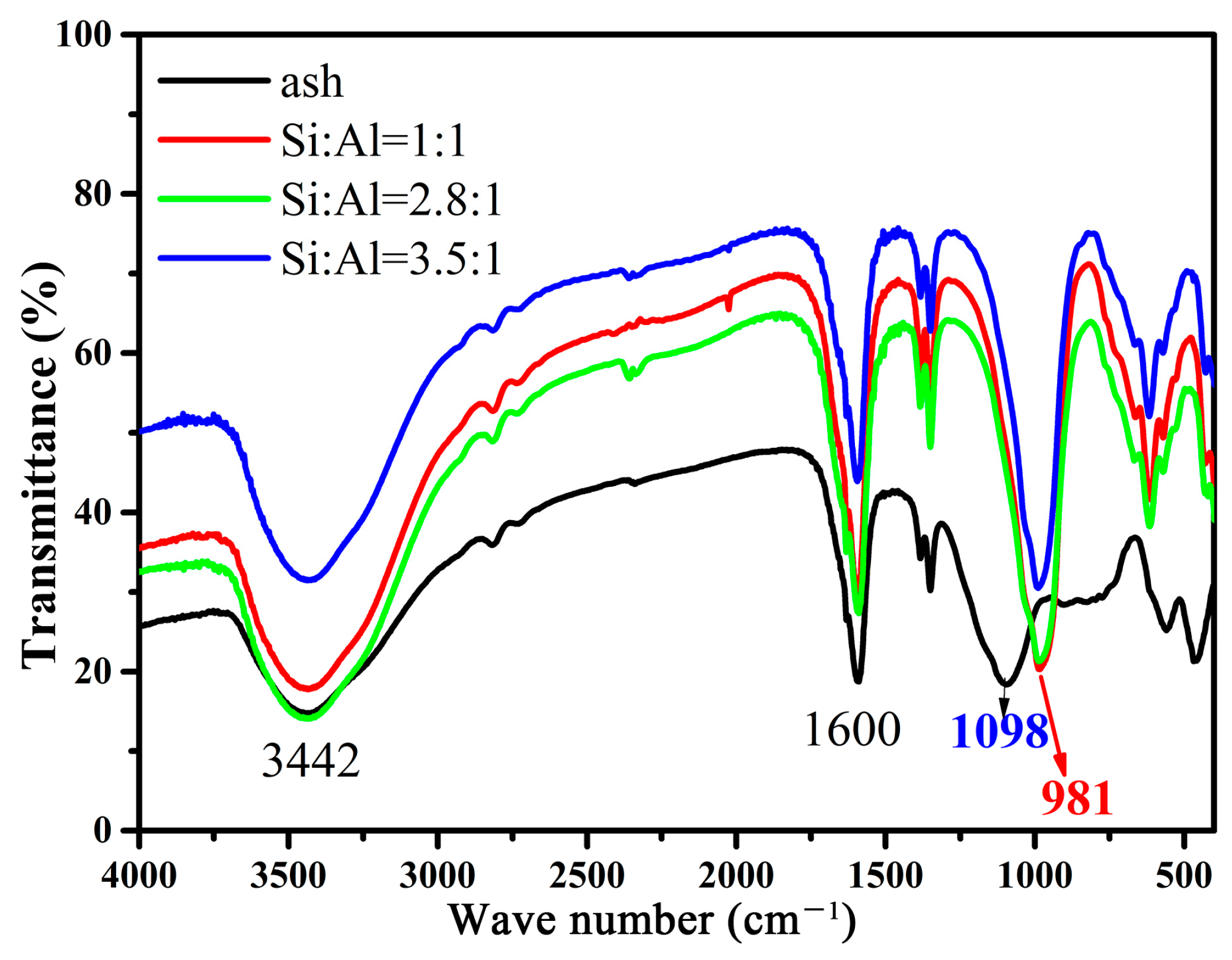
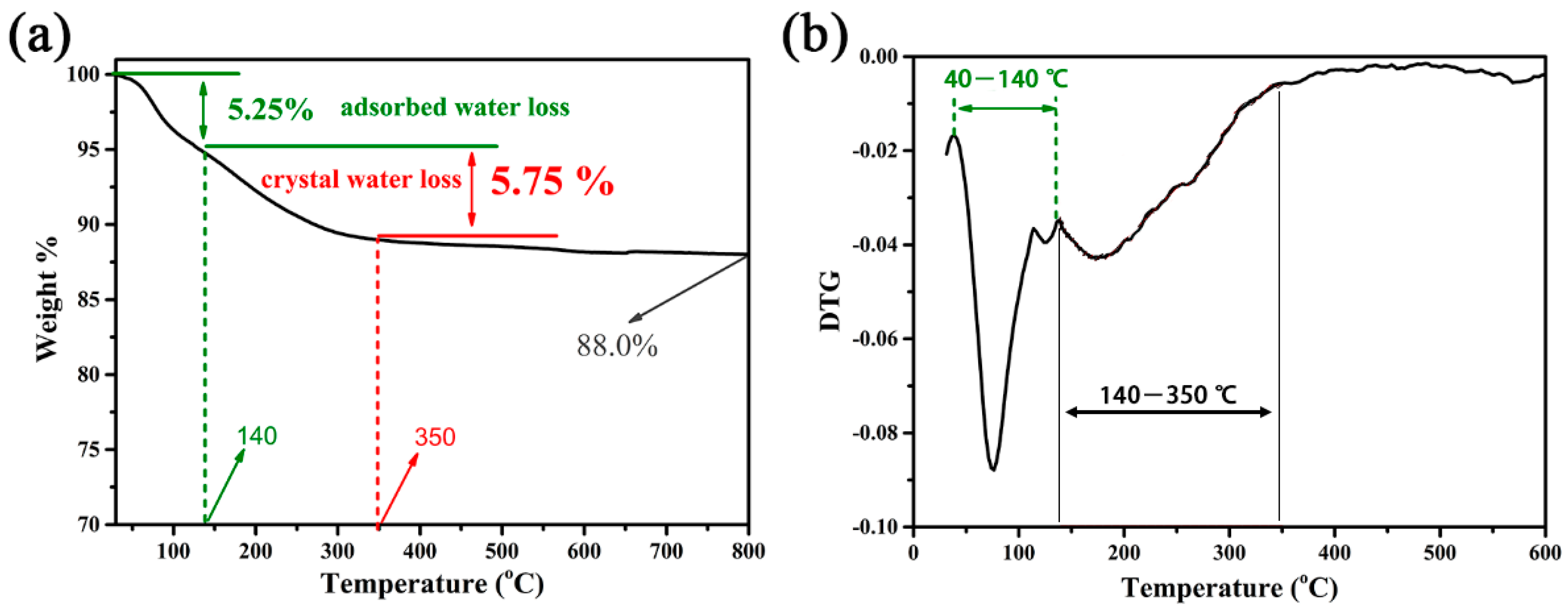
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Procedures of Zeolite Synthesis
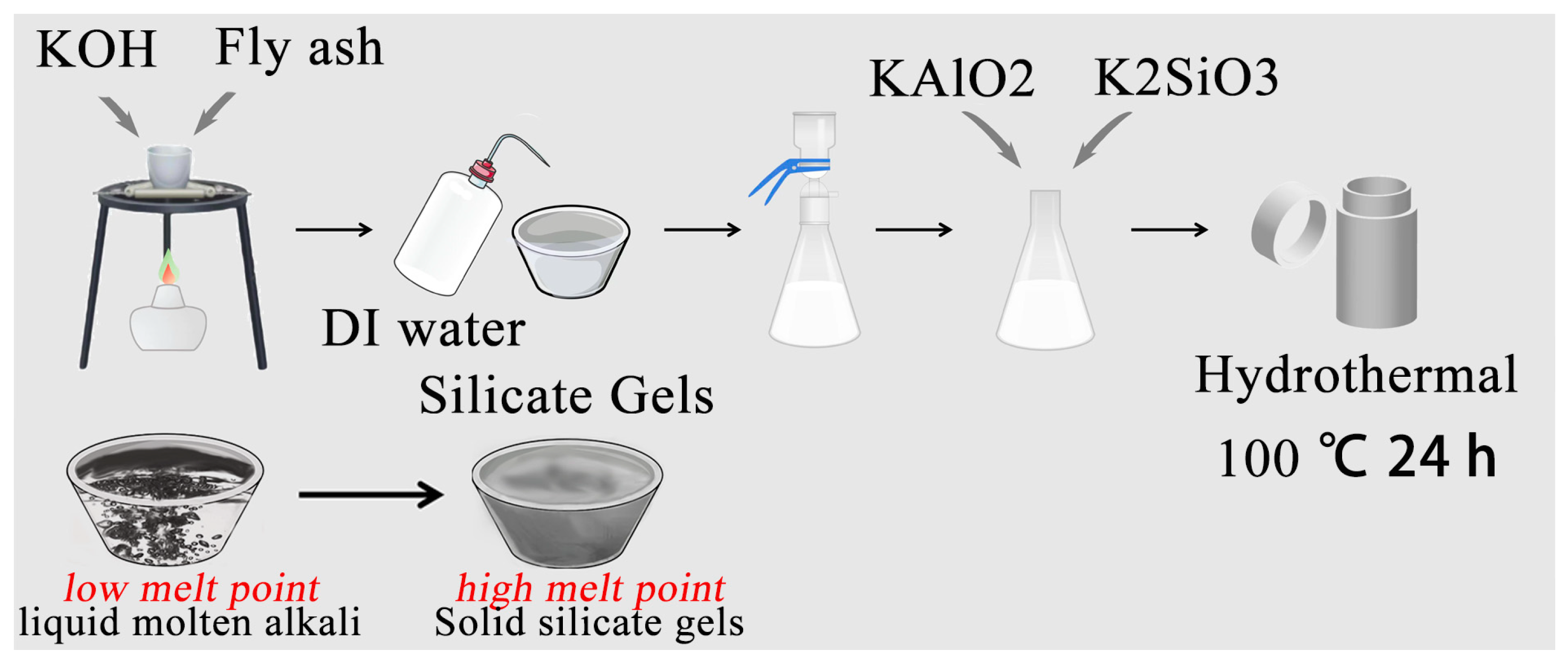
4.2.1. The Formation of Silicate Gels
4.2.2. Hydrothermal Synthesis of Zeolite-F
4.2.3. Experiment of Ammonium Adsorption
4.3. Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, R.; Zhu, H.; Zhou, J.; Luo, H.; Xue, K.; Yu, L.; Zhang, Y. Highly Water-Stable and Efficient Hydrogen-Producing Heterostructure Synthesized from Mn0.5Cd0.5S and a Zeolitic Imidazolate Framework ZIF-8 via Ligand and Cation Exchange. ACS Appl. Mater. Interfaces 2023, 15, 36477–36488. [Google Scholar] [CrossRef]
- He, J.; Deng, J.; Lan, T.; Liu, X.; Shen, Y.; Han, L.; Wang, J.; Zhang, D. Strong metal oxide-zeolite interactions during selective catalytic reduction of nitrogen oxides. J. Hazard. Mater. 2023, 465, 133164. [Google Scholar] [CrossRef]
- Mokrzycki, J.; Franus, W.; Panek, R.; Sobczyk, M.; Rusiniak, P.; Szerement, J.; Jarosz, R.; Marcińska-Mazur, L.; Bajda, T.; Mierzwa-Hersztek, M. Zeolite Composite Materials from Fly Ash: An Assessment of Physicochemical and Adsorption Properties. Materials 2023, 16, 2142. [Google Scholar] [CrossRef]
- Tesana, S.; Kennedy, J.V.; Yip, A.C.K.; Golovko, V.B. In Situ Incorporation of Atomically Precise Au Nanoclusters within Zeolites for Ambient Temperature CO Oxidation. Nanomaterials 2023, 13, 3120. [Google Scholar] [CrossRef]
- Gu, J.; Liu, L.; Zhu, R.; Song, Q.; Yu, H.; Jiang, P.; Miao, C.; Du, Y.; Fu, R.; Wang, Y.; et al. Recycling Coal Fly Ash for Super-Thermal-Insulating Aerogel Fiber Preparation with Simultaneous Al2O3 Extraction. Molecules 2023, 28, 7978. [Google Scholar] [CrossRef]
- Shishkin, A.; Abramovskis, V.; Zalite, I.; Singh, A.K.; Mezinskis, G.; Popov, V.; Ozolins, J. Physical, Thermal, and Chemical Properties of Fly Ash Cenospheres Obtained from Different Sources. Materials 2023, 16, 2035. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Cecilia, J.A.; Ballesteros-Plata, D.; Barroso-Martín, I.; Núñez, P.; Infantes-Molina, A.; Rodríguez-Castellón, E. Microwave-Assisted Synthesis of Zeolite A from Metakaolinite for CO2 Adsorption. Int. J. Mol. Sci. 2023, 24, 14040. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, X.; Qiu, Q.; Zhao, Y.; Long, L. Synthesis of high-quality NaP1 zeolite from municipal solid waste incineration fly ash by microwave-assisted hydrothermal method and its adsorption capacity. Sci. Total Environ. 2023, 855, 158741. [Google Scholar] [CrossRef]
- Wang, B.; Wu, J.; Yuan, Z.-Y.; Li, N.; Xiang, S. Synthesis of MCM-22 zeolite by an ultrasonic-assisted aging procedure. Ultrason. Sonochem. 2008, 15, 334–338. [Google Scholar] [CrossRef]
- Panitchakarn, P.; Laosiripojana, N.; Viriya-Umpikul, N.; Pavasant, P. Synthesis of high-purity Na-A and Na-X zeolite from coal fly ash. J. Air Waste Manag. Assoc. 2014, 64, 586–596. [Google Scholar] [CrossRef]
- Koshlak, H. Synthesis of Zeolites from Coal Fly Ash Using Alkaline Fusion and Its Applications in Removing Heavy Metals. Materials 2023, 16, 4837. [Google Scholar] [CrossRef]
- Küçük, M.E.; Makarava, I.; Kinnarinen, T.; Häkkinen, A. Simultaneous adsorption of Cu(II), Zn(II), Cd(II) and Pb(II) from synthetic wastewater using NaP and LTA zeolites prepared from biomass fly ash. Heliyon 2023, 9, e20253. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, S.; Ding, B.; Jia, H.; Chen, P.; Du, T.; Wang, Y. Optimization of preparation of NaA zeolite from fly ash for CO2 capture. Environ. Sci. Pollut. Res. 2023, 30, 102803–102817. [Google Scholar] [CrossRef]
- Che, Q.; Yang, M.; Wang, X.; Yang, Q.; Chen, Y.; Chen, X.; Chen, W.; Hu, J.; Zeng, K.; Yang, H.; et al. Preparation of mesoporous ZSM-5 catalysts using green templates and their performance in biomass catalytic pyrolysis. Bioresour. Technol. 2019, 289, 121729. [Google Scholar] [CrossRef]
- Tarach, K.A.; Pyra, K.; Góra-Marek, K. Opening up ZSM-5 Hierarchical Zeolite’s Porosity through Sequential Treatments for Improved Low-Density Polyethylene Cracking. Molecules 2020, 25, 2878. [Google Scholar] [CrossRef]
- Visa, M.; Enesca, A. Opportunities for Recycling PV Glass and Coal Fly Ash into Zeolite Materials Used for Removal of Heavy Metals (Cd, Cu, Pb) from Wastewater. Materials 2022, 16, 239. [Google Scholar] [CrossRef]
- Haghjoo, S.; Lengauer, C.L.; Kazemian, H.; Roushani, M. Facile and innovative application of surfactant-modified-zeolite from Austrian fly ash for glyphosate removal from water solution. J. Environ. Manag. 2023, 346, 118976. [Google Scholar] [CrossRef]
- Joseph, I.V.; Tosheva, L.; Miller, G.; Doyle, A.M. FAU-Type Zeolite Synthesis from Clays and Its Use for the Simultaneous Adsorption of Five Divalent Metals from Aqueous Solutions. Materials 2021, 14, 3738. [Google Scholar] [CrossRef]
- Panek, R.; Medykowska, M.; Wiśniewska, M.; Szewczuk-Karpisz, K.; Jędruchniewicz, K.; Franus, M. Simultaneous Removal of Pb2+ and Zn2+ Heavy Metals Using Fly Ash Na-X Zeolite and Its Carbon Na-X(C) Composite. Materials 2021, 14, 2832. [Google Scholar] [CrossRef]
- Osacký, M.; Binčík, T.; Hudcová, B.; Vítková, M.; Pálková, H.; Hudec, P.; Bačík, P.; Czímerová, A. Low-cost zeolite-based sorbents prepared from industrial perlite by-product material for Zn2+ and Ni2+ removal from aqueous solutions: Synthesis, properties and sorption efficiency. Heliyon 2022, 8, e12029. [Google Scholar] [CrossRef]
- Fan, Y.; Huang, R.; Liu, Q.; Cao, Q.; Guo, R. Synthesis of zeolite A from fly ash and its application in the slow release of urea. Waste Manag. 2023, 158, 47–55. [Google Scholar] [CrossRef]
- Wang, G.; Chen, C.; Li, J.; Yang, F.; Wang, L.; Lin, X.; Wu, H.; Zhang, J. A clean method for gallium recovery and the coproduction of silica-potassium compound fertilizer and zeolite F from brown corundum fly ash. J. Hazard. Mater. 2024, 461, 132625. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, J.; Miao, J.; Yue, L.; Cheng, M.; Li, Y.; Jing, Z. Self-regulated immobilization behavior of multiple heavy metals via zeolitization towards a novel hydrothermal technology for soil remediation. Environ. Res. 2023, 216 Pt 3, 114726. [Google Scholar] [CrossRef]
- Fan, W.; Morozumi, K.; Kimura, R.; Yokoi, T.; Okubo, T. Synthesis of nanometer-sized sodalite without adding organic additives. Langmuir 2008, 24, 6952–6958. [Google Scholar] [CrossRef]
- Liu, H. Conversion of Harmful Fly Ash Residue to Zeolites: Innovative Processes Focusing on Maximum Activation, Extraction, and Utilization of Aluminosilicate. ACS Omega 2022, 7, 20347–20356. [Google Scholar] [CrossRef]
- Hardin, J.L.; Oyler, N.A.; Steinle, E.D.; Meints, G.A. Spectroscopic analysis of interactions between alkylated silanes and alumina nanoporous membranes. J. Colloid Interface Sci. 2010, 342, 614–619. [Google Scholar] [CrossRef]
- Ellerbrock, R.; Stein, M.; Schaller, J. Comparing amorphous silica, short-range-ordered silicates and silicic acid species by FTIR. Sci. Rep. 2022, 12, 11708. [Google Scholar] [CrossRef]
- Fu, H.; Li, Y.; Yu, Z.; Shen, J.; Li, J.; Zhang, M.; Ding, T.; Xu, L.; Lee, S.S. Ammonium removal using a calcined natural zeolite modified with sodium nitrate. J. Hazard. Mater. 2020, 393, 122481. [Google Scholar] [CrossRef]
- Zhou, C.; An, Y.; Zhang, W.; Yang, D.; Tang, J.; Ye, J.; Zhou, Z. Inhibitory effects of Ca2+ on ammonium exchange by zeolite in the long-term exchange and NaClO-NaCl regeneration process. Chemosphere 2021, 263, 128216. [Google Scholar] [CrossRef]
- He, X.; Chen, W.; Sun, F.; Jiang, Z.; Li, B.; Li, X.-Y.; Lin, L. Enhanced NH4+ Removal and Recovery from Wastewater Using Na-Zeolite-based Flow-Electrode Capacitive Deionization: Insight from Ion Transport Flux. Environ. Sci. Technol. 2023, 57, 8828–8838. [Google Scholar] [CrossRef]




| Materials | Melt Point/°C | Solubility/ | Source |
|---|---|---|---|
| NaOH | 318 (low) | strong corrosive | Aladdin |
| KOH | 361 (low) | strong corrosive | Aladdin |
| Na2SiO3 | 1089 (high) | water soluble | Aladdin |
| NaAlO2 | 1650 (high) | water soluble | Aladdin |
| K2SiO3 | 976 (high) | water soluble | Aladdin |
| Fly ash | / | water insoluble | SiO2 48%, Al2O3 33% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, J.; Yang, Y.; Zhang, L.; Li, M.; Wang, Y.; Chen, Y.; Ling, R.; Yan, J.; Chen, Y.; Hu, J.; et al. Molten Alkali-Assisted Formation of Silicate Gels and Its Application for Preparing Zeolites. Gels 2024, 10, 392. https://doi.org/10.3390/gels10060392
Ye J, Yang Y, Zhang L, Li M, Wang Y, Chen Y, Ling R, Yan J, Chen Y, Hu J, et al. Molten Alkali-Assisted Formation of Silicate Gels and Its Application for Preparing Zeolites. Gels. 2024; 10(6):392. https://doi.org/10.3390/gels10060392
Chicago/Turabian StyleYe, Juan, Yanchun Yang, Li Zhang, Man Li, Yiling Wang, Yuxuan Chen, Ruhui Ling, Jiefeng Yan, Yan Chen, Jinxing Hu, and et al. 2024. "Molten Alkali-Assisted Formation of Silicate Gels and Its Application for Preparing Zeolites" Gels 10, no. 6: 392. https://doi.org/10.3390/gels10060392





