The Diels-Alder Cross-Linked Gelatin/Dextran Nanocomposite Hydrogels with Silver Nanoparticles for Wound Healing Applications: Synthesis, Characterization, and In Vitro Evaluation
Abstract
1. Introduction
2. Results and Discussion
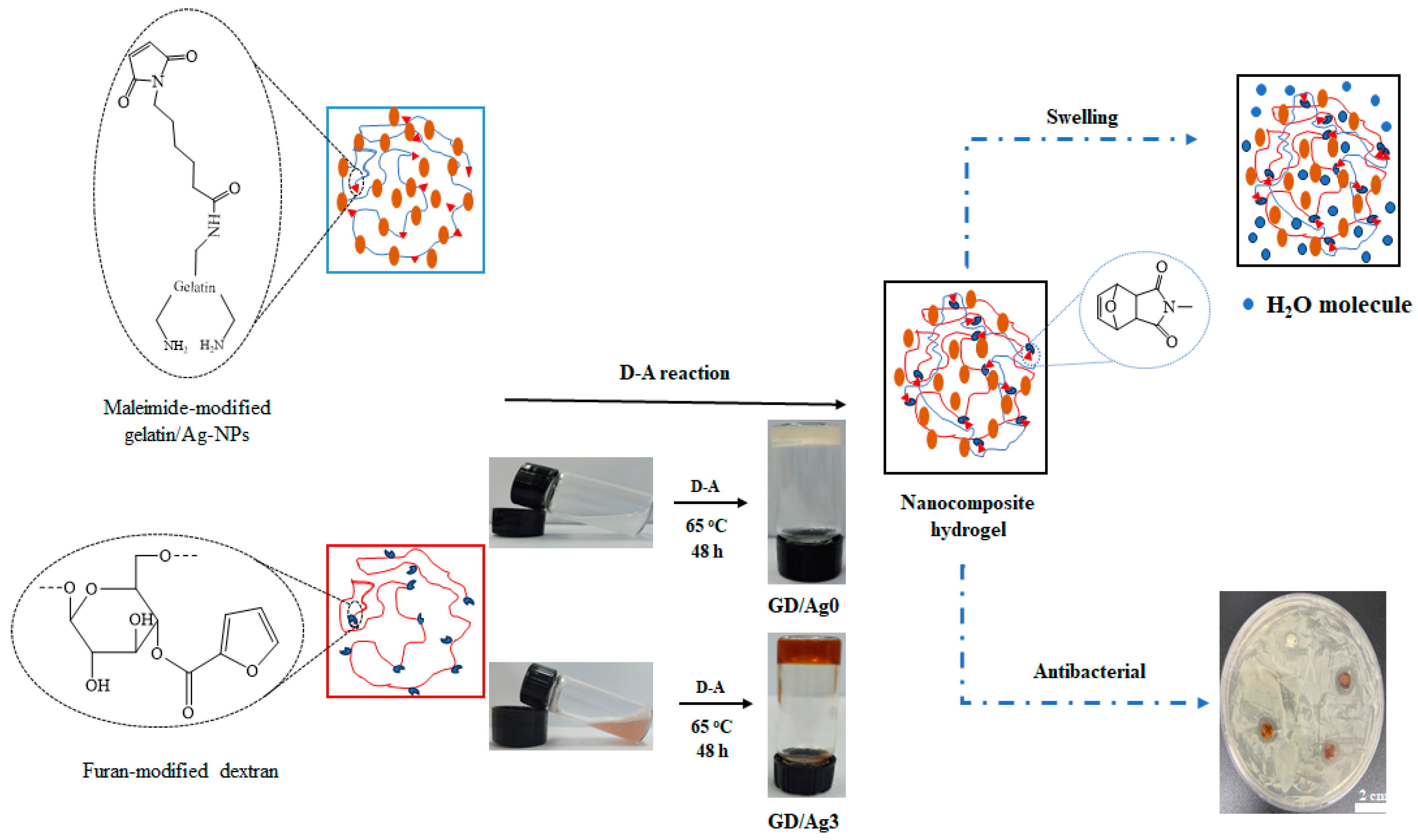
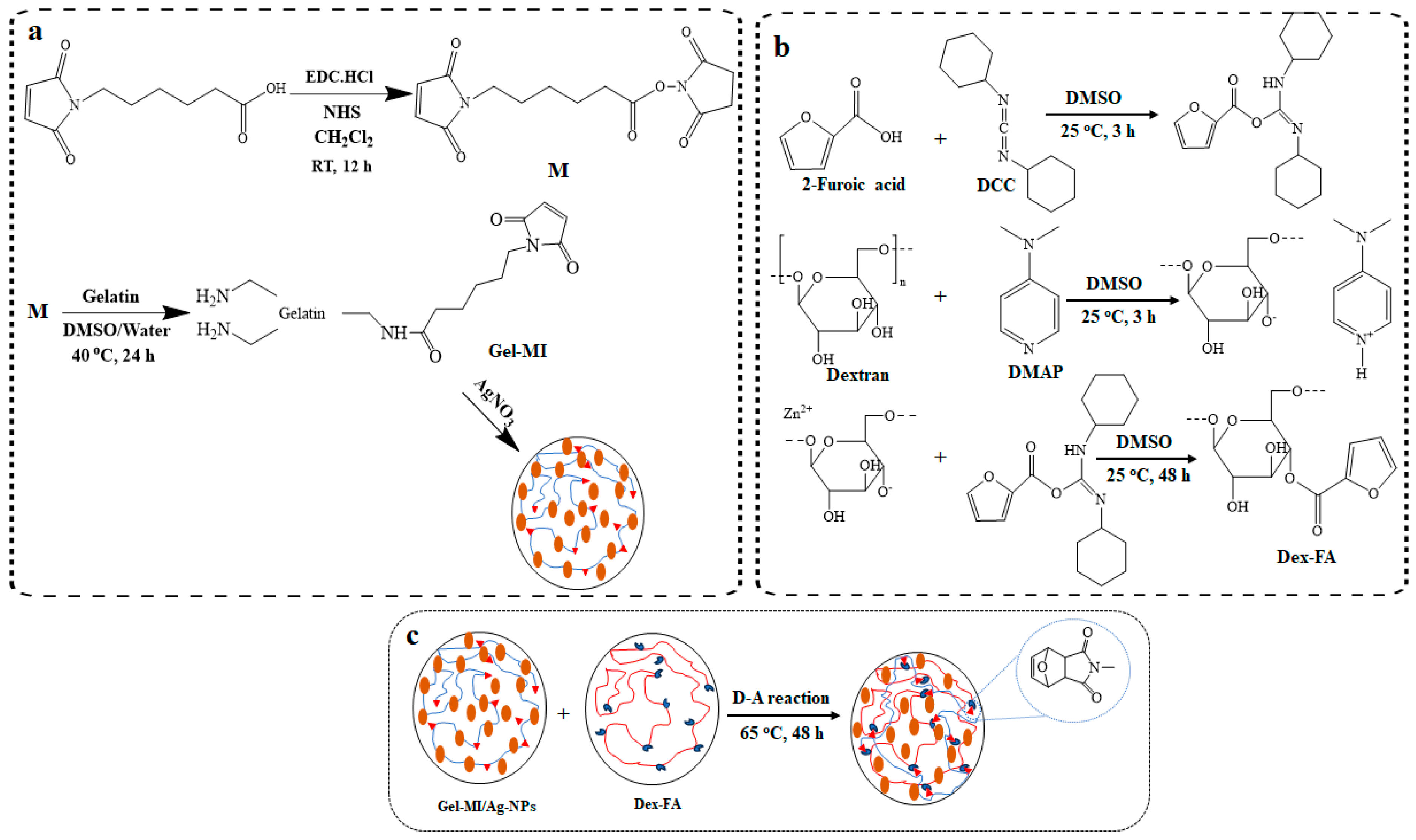
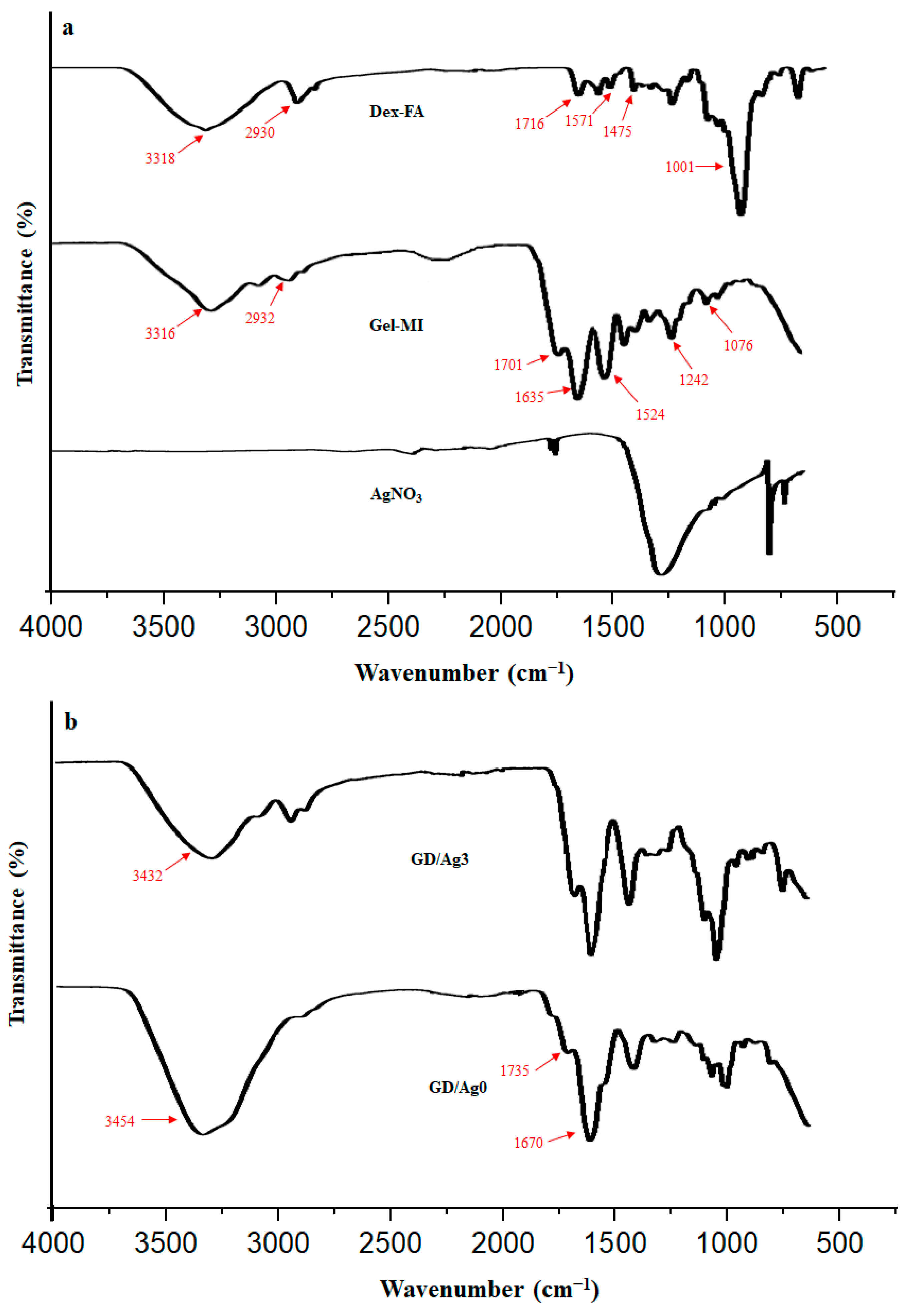
2.1. Preparation of Gel-MI and Dex-FA
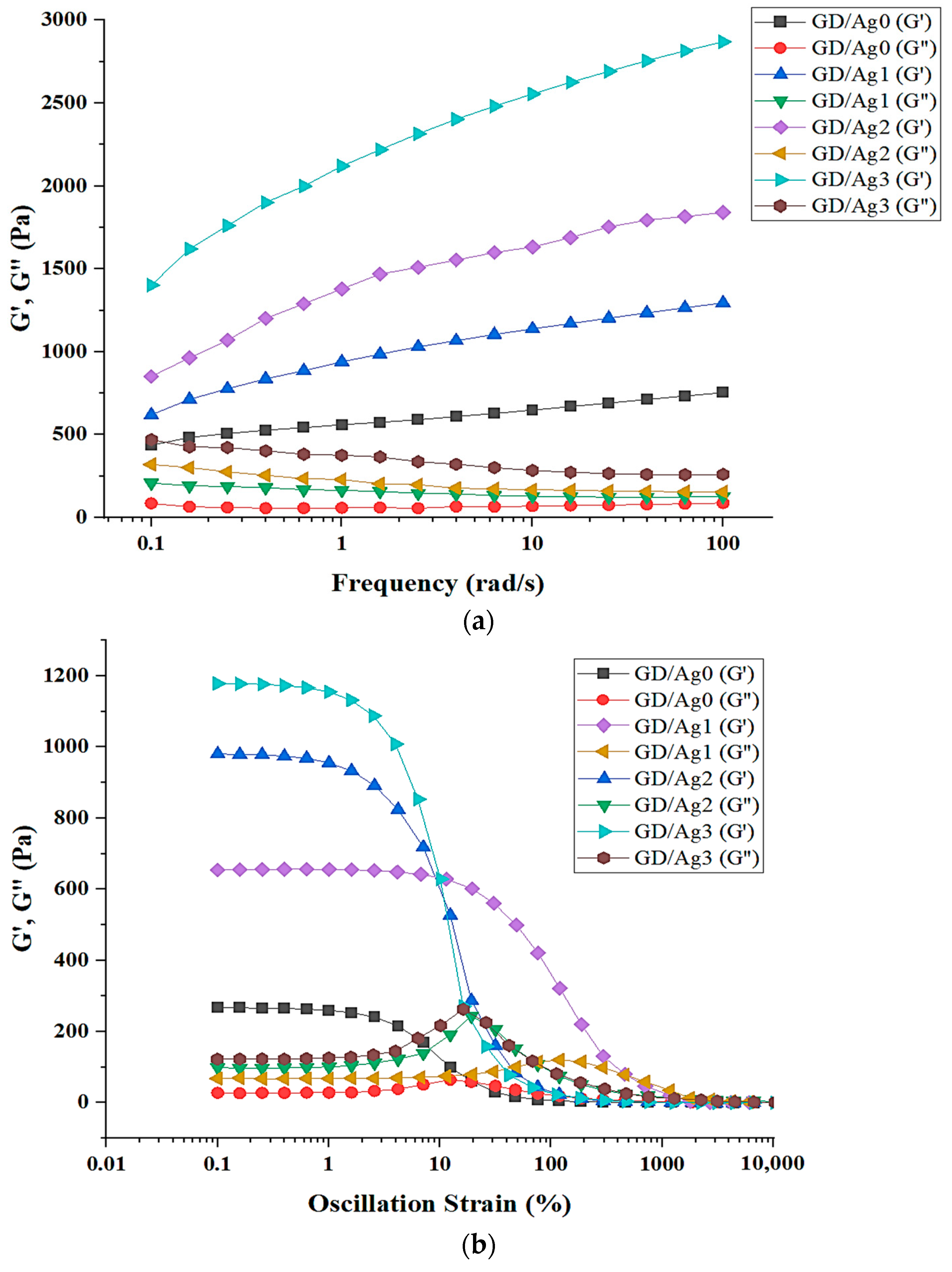
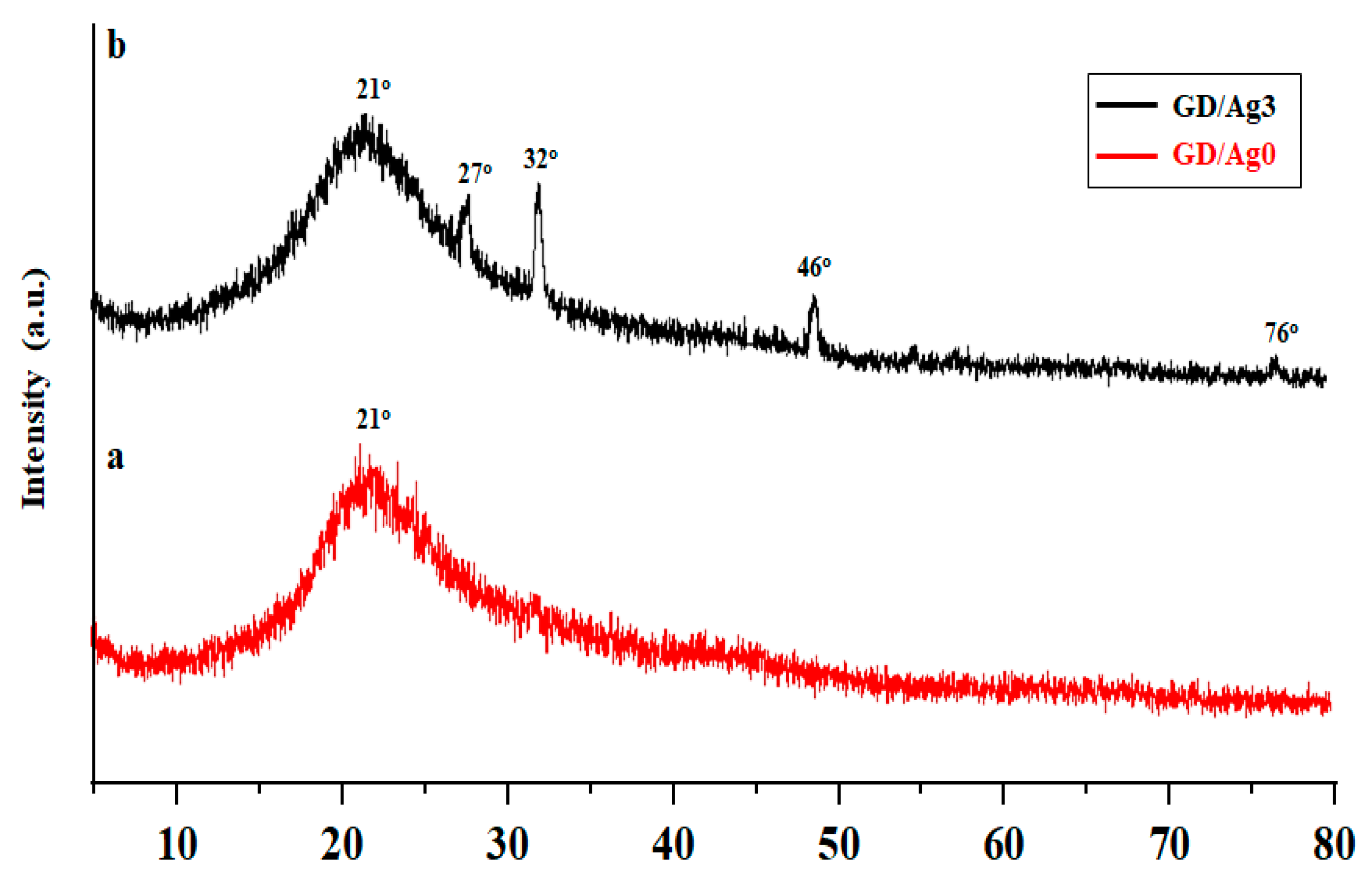
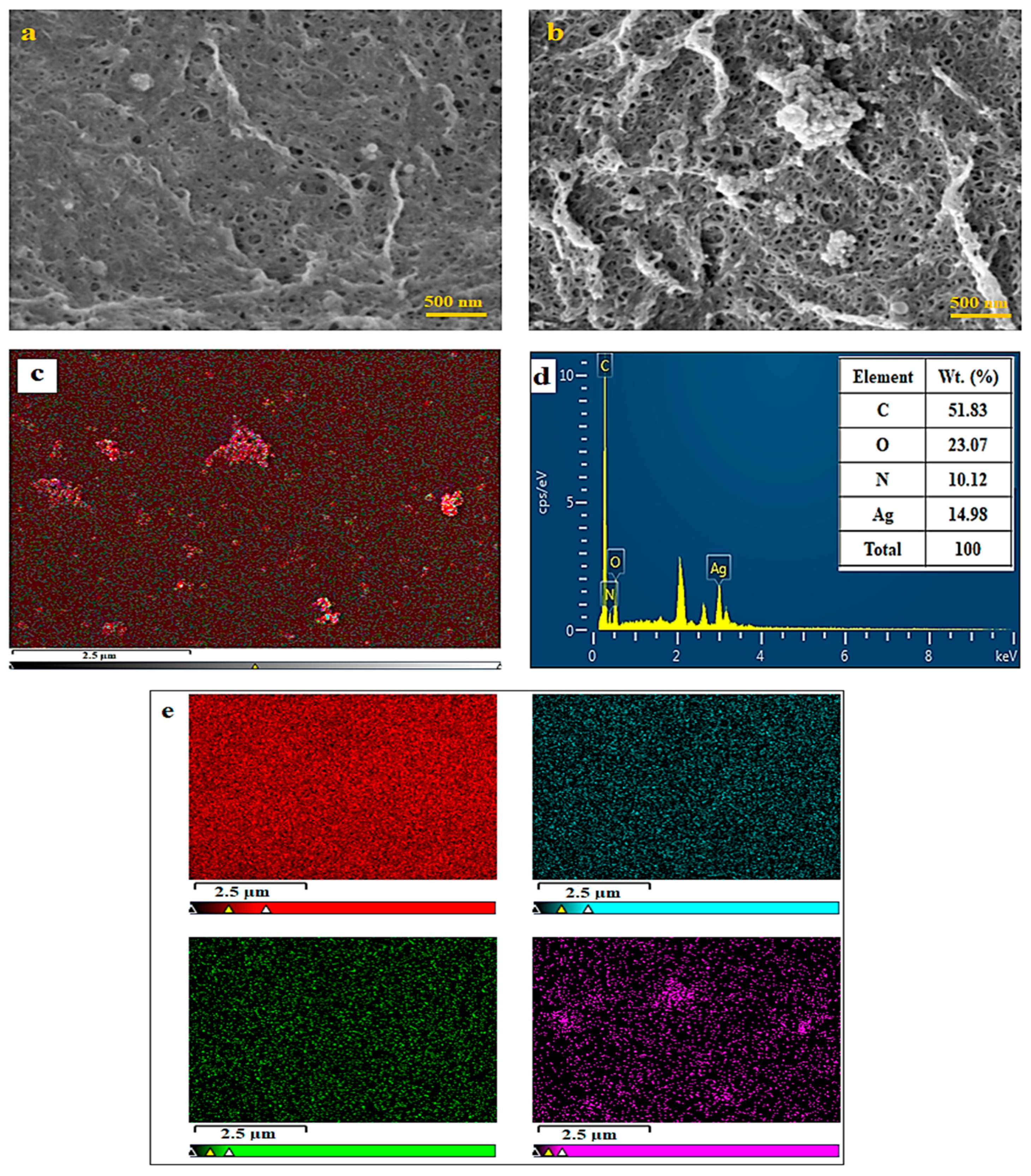
2.2. Hydrogel Formation through D-A Reaction
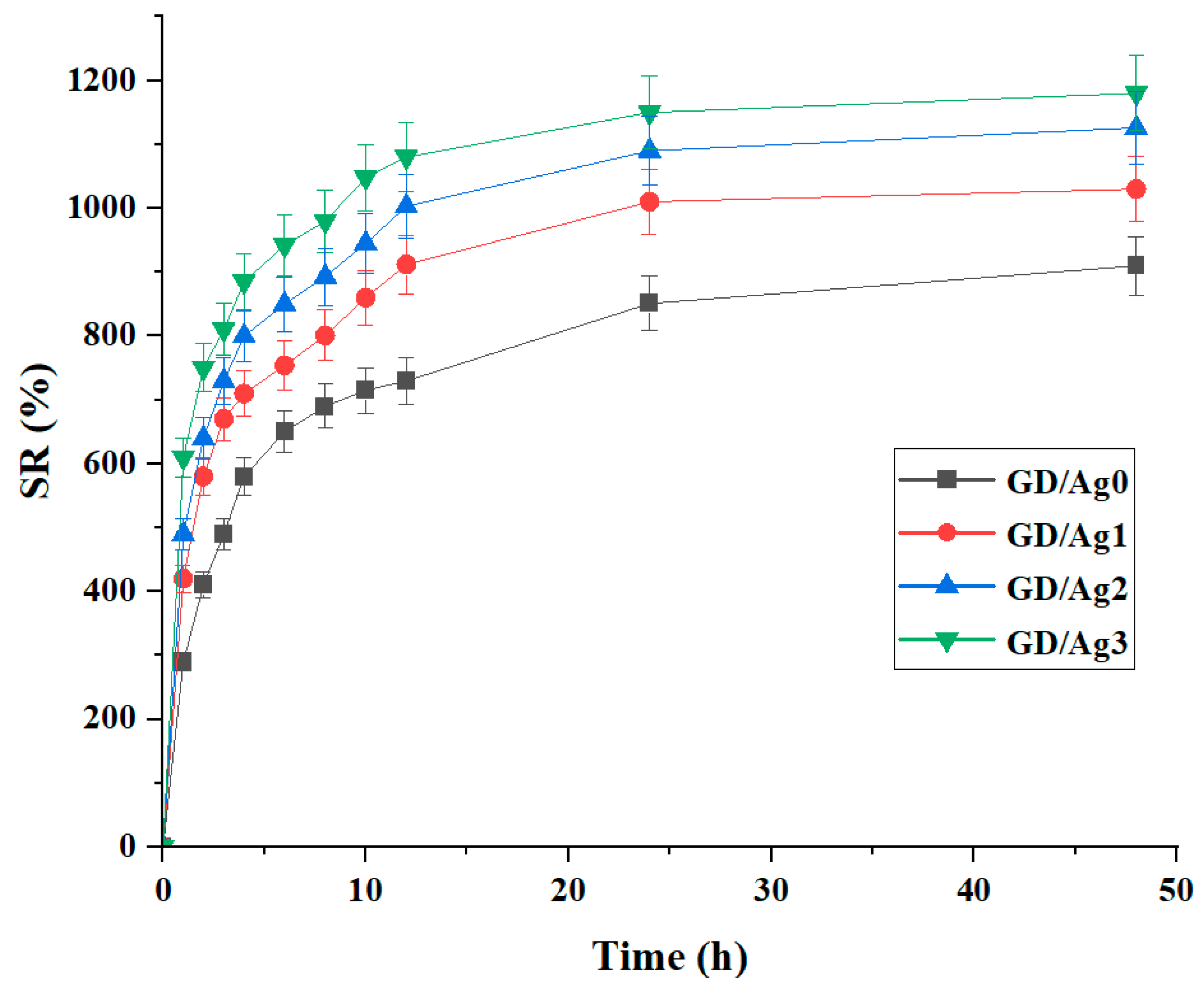
2.3. Swelling Ratio Behavior
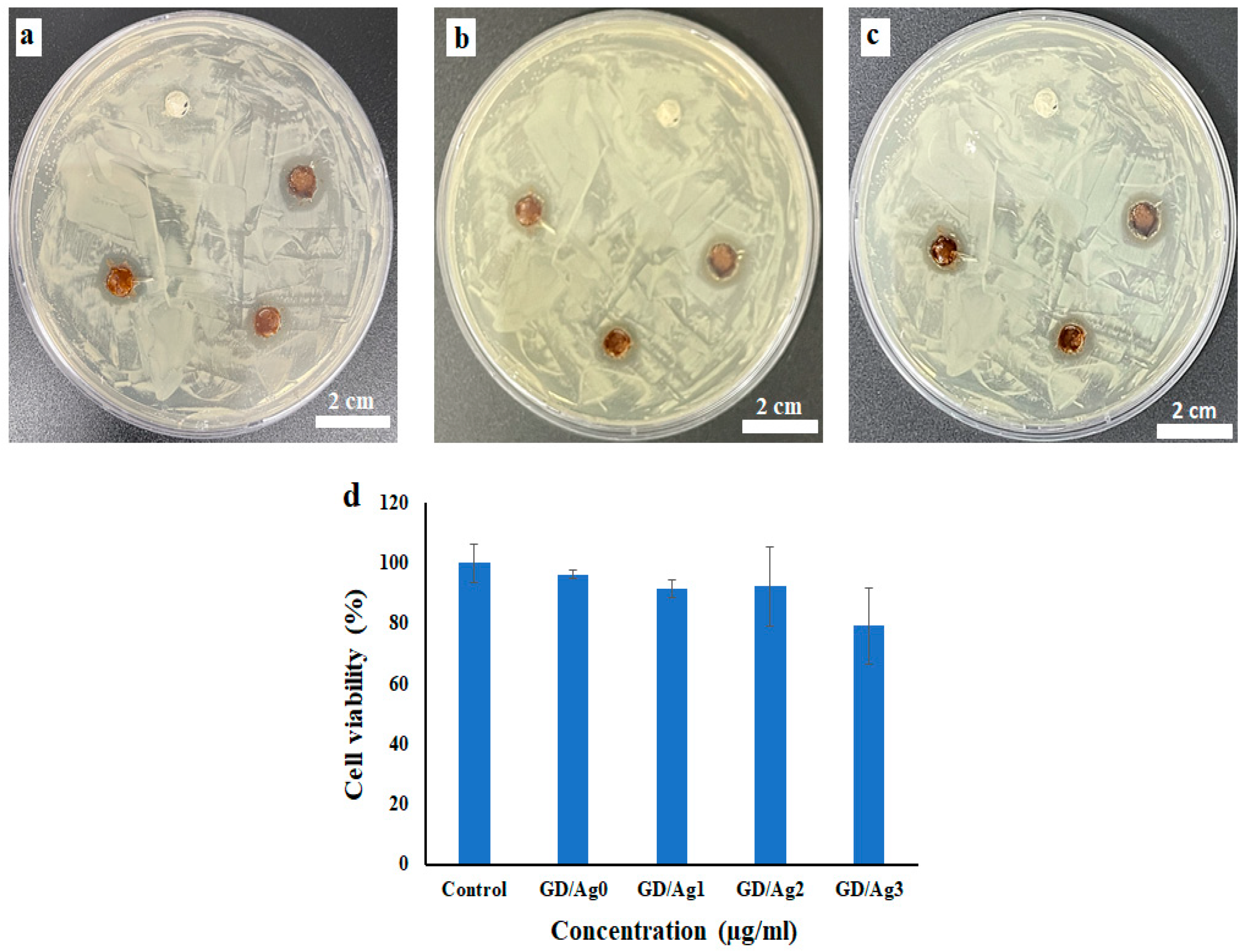
2.4. Antibacterial Activity and In Vitro Cell Viability Assay
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Measurements
4.3. Formulating Hydrogels by Preparing Precursors
4.3.1. Synthesis of 6-Maleimidohexanoic Acid-Modified Gelatin (Gel-MI)
4.3.2. Synthesis of 2-Furoic Acid-Modified Dextran (Dex-FA)
4.3.3. Formation of Nanocomposite Hydrogels Containing Ag-NPs
4.4. Rheological Analysis
4.5. Swelling Determination of Nanocomposite Hydrogels
4.6. In Vitro Cell Viability Assay
4.7. Antibacterial Properties
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic Hydrogels to Promote Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 718377. [Google Scholar] [CrossRef]
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional Hydrogels for Treatment of Chronic Wounds. Gels 2022, 8, 127. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, H.; Pan, X.; Zhang, C.; Zhang, K.; Chen, Z.; Dong, W.; Xie, A.; Qi, X. Dendritic Hydrogels with Robust Inherent Antibacterial Properties for Promoting Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 11144–11155. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Qi, X.; Shi, Y.; Zhang, C.; Cai, E.; Ge, X.; Xiang, Y.; Li, Y.; Zeng, B.; Shen, J. A Hybrid Hydrogel with Intrinsic Immunomodulatory Functionality for Treating Multidrug-Resistant Pseudomonas aeruginosa Infected Diabetic Foot Ulcers. ACS Mater. Lett. 2024, 6, 2533–2547. [Google Scholar] [CrossRef]
- Maleki, A.; He, J.; Bochani, S.; Nosrati, V.; Shahbazi, M.-A.; Guo, B. Multifunctional Photoactive Hydrogels for Wound Healing Acceleration. ACS Nano 2021, 15, 18895–18930. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, M.; Xu, M.; Miao, F.; Merzougui, C.; Zhang, X.; Wei, Y.; Chen, W.; Huang, D. The Fabrication of Antibacterial Hydrogels for Wound Healing. Eur. Polym. J. 2021, 146, 110268. [Google Scholar] [CrossRef]
- Song, S.; Liu, X.; Ding, L.; Liu, Z.; Abubaker, M.A.; Xu, Y.; Zhang, J. A Bacterial Cellulose/Polyvinyl Alcohol/Nitro Graphene Oxide Double Layer Network Hydrogel Efficiency Antibacterial and Promotes Wound Healing. Int. J. Biol. Macromol. 2024, 269, 131957. [Google Scholar] [CrossRef]
- Dong, Y.; Rodrigues, M.; Li, X.; Kwon, S.H.; Kosaric, N.; Khong, S.; Gao, Y.; Wang, W.; Gurtner, G.C. Injectable and Tunable Gelatin Hydrogels Enhance Stem Cell Retention and Improve Cutaneous Wound Healing. Adv. Funct. Mater. 2017, 27, 1606619. [Google Scholar] [CrossRef]
- Rizwan, A.; Gulfam, M.; Jo, S.-H.; Seo, J.-W.; Ali, I.; Thang Vu, T.; Joo, S.-B.; Park, S.-H.; Taek Lim, K. Gelatin-Based NIR and Reduction-Responsive Injectable Hydrogels Cross-Linked through IEDDA Click Chemistry for Drug Delivery Application. Eur. Polym. J. 2023, 191, 112019. [Google Scholar] [CrossRef]
- Zhao, Y.; Jalili, S. Dextran, as a Biological Macromolecule for the Development of Bioactive Wound Dressing Materials: A Review of Recent Progress and Future Perspectives. Int. J. Biol. Macromol. 2022, 207, 666–682. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, Y.; Li, D.; Mo, X.; Pan, J. Hofmeister Effect-Enhanced Gelatin/Oxidized Dextran Hydrogels with Improved Mechanical Properties and Biocompatibility for Wound Healing. Acta Biomater. 2022, 151, 235–253. [Google Scholar] [CrossRef]
- Dzulkharnien, N.S.F.; Rohani, R. A Review on Current Designation of Metallic Nanocomposite Hydrogel in Biomedical Applications. Nanomaterials 2022, 12, 1629. [Google Scholar] [CrossRef]
- Massironi, A.; Franco, A.R.; Babo, P.S.; Puppi, D.; Chiellini, F.; Reis, R.L.; Gomes, M.E. Development and Characterization of Highly Stable Silver NanoParticles as Novel Potential Antimicrobial Agents for Wound Healing Hydrogels. Int. J. Mol. Sci. 2022, 23, 2161. [Google Scholar] [CrossRef]
- Kumar, S.S.D.; Rajendran, N.K.; Houreld, N.N.; Abrahamse, H. Recent Advances on Silver Nanoparticle and Biopolymer-Based Biomaterials for Wound Healing Applications. Int. J. Biol. Macromol. 2018, 115, 165–175. [Google Scholar] [CrossRef]
- Diniz, F.; Maia, R.; de Andrade, L.R.; Andrade, L.; Vinicius Chaud, M.; da Silva, C.; Corrêa, C.; de Albuquerque Junior, R.; Pereira da Costa, L.; Shin, S.; et al. Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing In Vivo. Nanomaterials 2020, 10, 390. [Google Scholar] [CrossRef]
- Sudarsan, S.; Selvi, M.S.; Chitra, G.; Sakthivel, S.; Franklin, D.S.; Guhanathan, S. Nontoxic PH-Sensitive Silver Nanocomposite Hydrogels for Potential Wound Healing Applications. Polym. Plast. Technol. Mater. 2021, 60, 84–104. [Google Scholar] [CrossRef]
- Amiri, N.; Ghaffari, S.; Hassanpour, I.; Chae, T.; Jalili, R.; Kilani, R.T.; Ko, F.; Ghahary, A.; Lange, D. Antibacterial Thermosensitive Silver–Hydrogel Nanocomposite Improves Wound Healing. Gels 2023, 9, 542. [Google Scholar] [CrossRef]
- Wei, H.; Li, W.; Chen, H.; Wen, X.; He, J.; Li, J. Simultaneous Diels-Alder Click Reaction and Starch Hydrogel Microsphere Production via Spray Drying. Carbohydr. Polym. 2020, 241, 116351. [Google Scholar] [CrossRef]
- Amiryaghoubi, N.; Fathi, M.; Safary, A.; Javadzadeh, Y.; Omidi, Y. In Situ Forming Alginate/Gelatin Hydrogel Scaffold through Schiff Base Reaction Embedded with Curcumin-Loaded Chitosan Microspheres for Bone Tissue Regeneration. Int. J. Biol. Macromol. 2024, 256, 128335. [Google Scholar] [CrossRef]
- Khodadadi Yazdi, M.; Sajadi, S.M.; Seidi, F.; Rabiee, N.; Fatahi, Y.; Rabiee, M.; Dominic, C.D.M.; Zarrintaj, P.; Formela, K.; Saeb, M.R.; et al. Clickable Polysaccharides for Biomedical Applications: A Comprehensive Review. Prog. Polym. Sci. 2022, 133, 101590. [Google Scholar] [CrossRef]
- Yadav, S.; Ramesh, K.; Kumar, P.; Jo, S.-H.; Yoo, S.I.; Gal, Y.-S.; Park, S.-H.; Lim, K.T. Near-Infrared Light-Responsive Shell-Crosslinked Micelles of Poly(d,l-Lactide)-b-Poly((Furfuryl Methacrylate)-Co-(N-Acryloylmorpholine)) Prepared by Diels-Alder Reaction for the Triggered Release of Doxorubicin. Materials 2021, 14, 7913. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in Crosslinking Strategies of Biomedical Hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Cadamuro, F.; Russo, L.; Nicotra, F. Biomedical Hydrogels Fabricated Using Diels-Alder Crosslinking. Eur. J. Org. Chem. 2021, 2021, 374–382. [Google Scholar] [CrossRef]
- Salaheldin, H.I.; Almalki, M.H.K.; Osman, G.E.H. Green Synthesis of Silver Nanoparticles Using Bovine Skin Gelatin and Its Antibacterial Effect on Clinical Bacterial Isolates. IET Nanobiotechnol. 2017, 11, 420–425. [Google Scholar] [CrossRef]
- Li, W.-M.; Liu, D.-M.; Chen, S.-Y. Amphiphilically-Modified Gelatin Nanoparticles: Self-Assembly Behavior, Controlled Biodegradability, and Rapid Cellular Uptake for Intracellular Drug Delivery. J. Mater. Chem. 2011, 21, 12381. [Google Scholar] [CrossRef]
- Rodin, V.V.; Izmailova, V.N. NMR Method in the Study of the Interfacial Adsorption Layer of Gelatin. Colloids Surf. A Physicochem. Eng. Asp. 1996, 106, 95–102. [Google Scholar] [CrossRef]
- Debone Piazza, R.; Brandt, J.V.; Carvalho dos Santos, C.; Fernando Costa Marques, R.; Jafelicci Junior, M. Gelatin/Dextran-based Hydrogel Cross-linked by Diels-Alder Click Chemistry: The Swelling and Potassium Diclofenac Releasing. Med. Devices Sens. 2021, 4, e10151. [Google Scholar] [CrossRef]
- Gilchrist, A.E.; Serrano, J.F.; Ngo, M.T.; Hrnjak, Z.; Kim, S.; Harley, B.A.C. Encapsulation of Murine Hematopoietic Stem and Progenitor Cells in a Thiol-Crosslinked Maleimide-Functionalized Gelatin Hydrogel. Acta Biomater. 2021, 131, 138–148. [Google Scholar] [CrossRef]
- Sahatsapan, N.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P.; Tonglairoum, P. 6-Maleimidohexanoic Acid-Grafted Chitosan: A New Generation Mucoadhesive Polymer. Carbohydr. Polym. 2018, 202, 258–264. [Google Scholar] [CrossRef]
- Li, D.; Wang, S.; Meng, Y.; Guo, Z.; Cheng, M.; Li, J. Fabrication of Self-Healing Pectin/Chitosan Hybrid Hydrogel via Diels-Alder Reactions for Drug Delivery with High Swelling Property, PH-Responsiveness, and Cytocompatibility. Carbohydr. Polym. 2021, 268, 118244. [Google Scholar] [CrossRef]
- Ramirez, H.L.; Valdivia, A.; Cao, R.; Fragoso, A.; Torres Labandeira, J.J.; Baños, M.; Villalonga, R. Preparation of β-Cyclodextrin-Dextran Polymers and Their Use as Supramolecular Carrier Systems for Naproxen. Polym. Bull. 2007, 59, 597–605. [Google Scholar] [CrossRef]
- Damodaran, V.B.; Place, L.W.; Kipper, M.J.; Reynolds, M.M. Enzymatically Degradable Nitric Oxide Releasing S-Nitrosated Dextran Thiomers for Biomedical Applications. J. Mater. Chem. 2012, 22, 23038. [Google Scholar] [CrossRef]
- González, K.; García-Astrain, C.; Santamaria-Echart, A.; Ugarte, L.; Avérous, L.; Eceiza, A.; Gabilondo, N. Starch/Graphene Hydrogels via Click Chemistry with Relevant Electrical and Antibacterial Properties. Carbohydr. Polym. 2018, 202, 372–381. [Google Scholar] [CrossRef]
- García-Astrain, C.; Avérous, L. Synthesis and Evaluation of Functional Alginate Hydrogels Based on Click Chemistry for Drug Delivery Applications. Carbohydr. Polym. 2018, 190, 271–280. [Google Scholar] [CrossRef]
- Samanta, H.S.; Ray, S.K. Effect of Polyethylene Glycol and Nano Clay on Swelling, Diffusion, Network Parameters and Drug Release Behavior of Interpenetrating Network Copolymer. J. Appl. Polym. Sci. 2022, 139, 51678. [Google Scholar] [CrossRef]
- Reshma, G.; Reshmi, C.R.; Nair, S.V.; Menon, D. Superabsorbent Sodium Carboxymethyl Cellulose Membranes Based on a New Cross-Linker Combination for Female Sanitary Napkin Applications. Carbohydr. Polym. 2020, 248, 116763. [Google Scholar] [CrossRef]
- Li, J.; Chen, F.; Lin, X.; Ding, T. Hydrogen-Bonding-Assisted Toughening of Hierarchical Carboxymethyl Cellulose Hydrogels for Biomechanical Sensing. Carbohydr. Polym. 2021, 269, 118252. [Google Scholar] [CrossRef]
- Rizwan, A.; Ali, I.; Jo, S.-H.; Vu, T.T.; Gal, Y.-S.; Kim, Y.H.; Park, S.-H.; Lim, K.T. Facile Fabrication of NIR-Responsive Alginate/CMC Hydrogels Derived through IEDDA Click Chemistry for Photothermal–Photodynamic Anti-Tumor Therapy. Gels 2023, 9, 961. [Google Scholar] [CrossRef]
- Han, J.; Lei, T.; Wu, Q. High-Water-Content Mouldable Polyvinyl Alcohol-Borax Hydrogels Reinforced by Well-Dispersed Cellulose Nanoparticles: Dynamic Rheological Properties and Hydrogel Formation Mechanism. Carbohydr. Polym. 2014, 102, 306–316. [Google Scholar] [CrossRef]
- Ali, I.; Gulfam, M.; Jo, S.-H.; Seo, J.-W.; Rizwan, A.; Park, S.-H.; Lim, K.T. Reduction-Responsive and Bioorthogonal Carboxymethyl Cellulose Based Soft Hydrogels Cross-Linked via IEDDA Click Chemistry for Cancer Therapy Application. Int. J. Biol. Macromol. 2022, 219, 109–120. [Google Scholar] [CrossRef]
- Hezari, S.; Olad, A.; Dilmaghani, A. Modified Gelatin/Iron- Based Metal-Organic Framework Nanocomposite Hydrogel as Wound Dressing: Synthesis, Antibacterial Activity, and Camellia Sinensis Release. Int. J. Biol. Macromol. 2022, 218, 488–505. [Google Scholar] [CrossRef]
- Kokila, T.; Ramesh, P.S.; Geetha, D. Biosynthesis of Silver Nanoparticles from Cavendish Banana Peel Extract and Its Antibacterial and Free Radical Scavenging Assay: A Novel Biological Approach. Appl. Nanosci. 2015, 5, 911–920. [Google Scholar] [CrossRef]
- Gholamali, I.; Asnaashariisfahani, M.; Alipour, E. Silver Nanoparticles Incorporated in PH-Sensitive Nanocomposite Hydrogels Based on Carboxymethyl Chitosan-Poly (Vinyl Alcohol) for Use in a Drug Delivery System. Regen. Eng. Transl. Med. 2020, 6, 138–153. [Google Scholar] [CrossRef]
- García-Astrain, C.; Chen, C.; Burón, M.; Palomares, T.; Eceiza, A.; Fruk, L.; Corcuera, M.Á.; Gabilondo, N. Biocompatible Hydrogel Nanocomposite with Covalently Embedded Silver Nanoparticles. Biomacromolecules 2015, 16, 1301–1310. [Google Scholar] [CrossRef]
- Bunyatova, U.; Hammouda, M.B.; Zhang, J.Y. Preparation of Injectable Hydrophilic Dextran/AgNPs Nanocomposite Product: White Light Active Biomolecules as an Antitumor Agent. Int. J. Biol. Macromol. 2023, 245, 125215. [Google Scholar] [CrossRef]
- Lan, G.; Zhu, S.; Chen, D.; Zhang, H.; Zou, L.; Zeng, Y. Highly Adhesive Antibacterial Bioactive Composite Hydrogels With Controllable Flexibility and Swelling as Wound Dressing for Full-Thickness Skin Healing. Front. Bioeng. Biotechnol. 2021, 9, 785302. [Google Scholar] [CrossRef]
- Pal, N.; Banerjee, S.; Roy, P.; Pal, K. Cellulose Nanocrystals-silver Nanoparticles-Reduced Graphene Oxide Based Hybrid PVA Nanocomposites and Its Antimicrobial Properties. Int. J. Biol. Macromol. 2021, 191, 445–456. [Google Scholar] [CrossRef]
- Le Thi, P.; Lee, Y.; Hoang Thi, T.T.; Park, K.M.; Park, K.D. Catechol-Rich Gelatin Hydrogels in Situ Hybridizations with Silver Nanoparticle for Enhanced Antibacterial Activity. Mater. Sci. Eng. C 2018, 92, 52–60. [Google Scholar] [CrossRef]
- Yadollahi, M.; Farhoudian, S.; Namazi, H. One-Pot Synthesis of Antibacterial Chitosan/Silver Bio-Nanocomposite Hydrogel Beads as Drug Delivery Systems. Int. J. Biol. Macromol. 2015, 79, 37–43. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Zhang, J.; Xie, G.; Xiong, T.; Xu, H. Preparation and Characterization of Sodium Alginate/Gelatin/Ag Nanocomposite Antibacterial Film and Its Application in the Preservation of Tangerine. Food Packag. Shelf Life 2022, 33, 100928. [Google Scholar] [CrossRef]
- Baukum, J.; Pranjan, J.; Kaolaor, A.; Chuysinuan, P.; Suwantong, O.; Supaphol, P. The Potential Use of Cross-Linked Alginate/Gelatin Hydrogels Containing Silver Nanoparticles for Wound Dressing Applications. Polym. Bull. 2020, 77, 2679–2695. [Google Scholar] [CrossRef]
- Shao, W.; Liu, H.; Liu, X.; Wang, S.; Wu, J.; Zhang, R.; Min, H.; Huang, M. Development of Silver Sulfadiazine Loaded Bacterial Cellulose/Sodium Alginate Composite Films with Enhanced Antibacterial Property. Carbohydr. Polym. 2015, 132, 351–358. [Google Scholar] [CrossRef]
- Shao, W.; Liu, X.; Min, H.; Dong, G.; Feng, Q.; Zuo, S. Preparation, Characterization, and Antibacterial Activity of Silver Nanoparticle-Decorated Graphene Oxide Nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 6966–6973. [Google Scholar] [CrossRef]
- Hsu, T.-H.; Chiang, S.-J.; Chu, Y.-H. Quartz Crystal Microbalance Analysis of Diels-Alder Reactions of Alkene Gases to Functional Ionic Liquids on Chips. Anal. Chem. 2016, 88, 10837–10841. [Google Scholar] [CrossRef]

| GD/Ag0 | GD/Ag1 | GD/Ag2 | GD/Ag3 | ||
|---|---|---|---|---|---|
| Antibacterial diameter (mm) | 24 h | 0 | 13.28 | 13.07 | 15.29 |
| 36 h | 0 | 12.75 | 12.98 | 14.53 | |
| 48 h | 0 | 11.69 | 12.55 | 14.04 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gholamali, I.; Jo, S.-H.; Han, W.; Lim, J.; Rizwan, A.; Park, S.-H.; Lim, K.T. The Diels-Alder Cross-Linked Gelatin/Dextran Nanocomposite Hydrogels with Silver Nanoparticles for Wound Healing Applications: Synthesis, Characterization, and In Vitro Evaluation. Gels 2024, 10, 408. https://doi.org/10.3390/gels10060408
Gholamali I, Jo S-H, Han W, Lim J, Rizwan A, Park S-H, Lim KT. The Diels-Alder Cross-Linked Gelatin/Dextran Nanocomposite Hydrogels with Silver Nanoparticles for Wound Healing Applications: Synthesis, Characterization, and In Vitro Evaluation. Gels. 2024; 10(6):408. https://doi.org/10.3390/gels10060408
Chicago/Turabian StyleGholamali, Iman, Sung-Han Jo, Won Han, Juhee Lim, Ali Rizwan, Sang-Hyug Park, and Kwon Taek Lim. 2024. "The Diels-Alder Cross-Linked Gelatin/Dextran Nanocomposite Hydrogels with Silver Nanoparticles for Wound Healing Applications: Synthesis, Characterization, and In Vitro Evaluation" Gels 10, no. 6: 408. https://doi.org/10.3390/gels10060408
APA StyleGholamali, I., Jo, S.-H., Han, W., Lim, J., Rizwan, A., Park, S.-H., & Lim, K. T. (2024). The Diels-Alder Cross-Linked Gelatin/Dextran Nanocomposite Hydrogels with Silver Nanoparticles for Wound Healing Applications: Synthesis, Characterization, and In Vitro Evaluation. Gels, 10(6), 408. https://doi.org/10.3390/gels10060408






