Biomimetic Hydrogel Strategies for Cancer Therapy
Abstract
1. Introduction
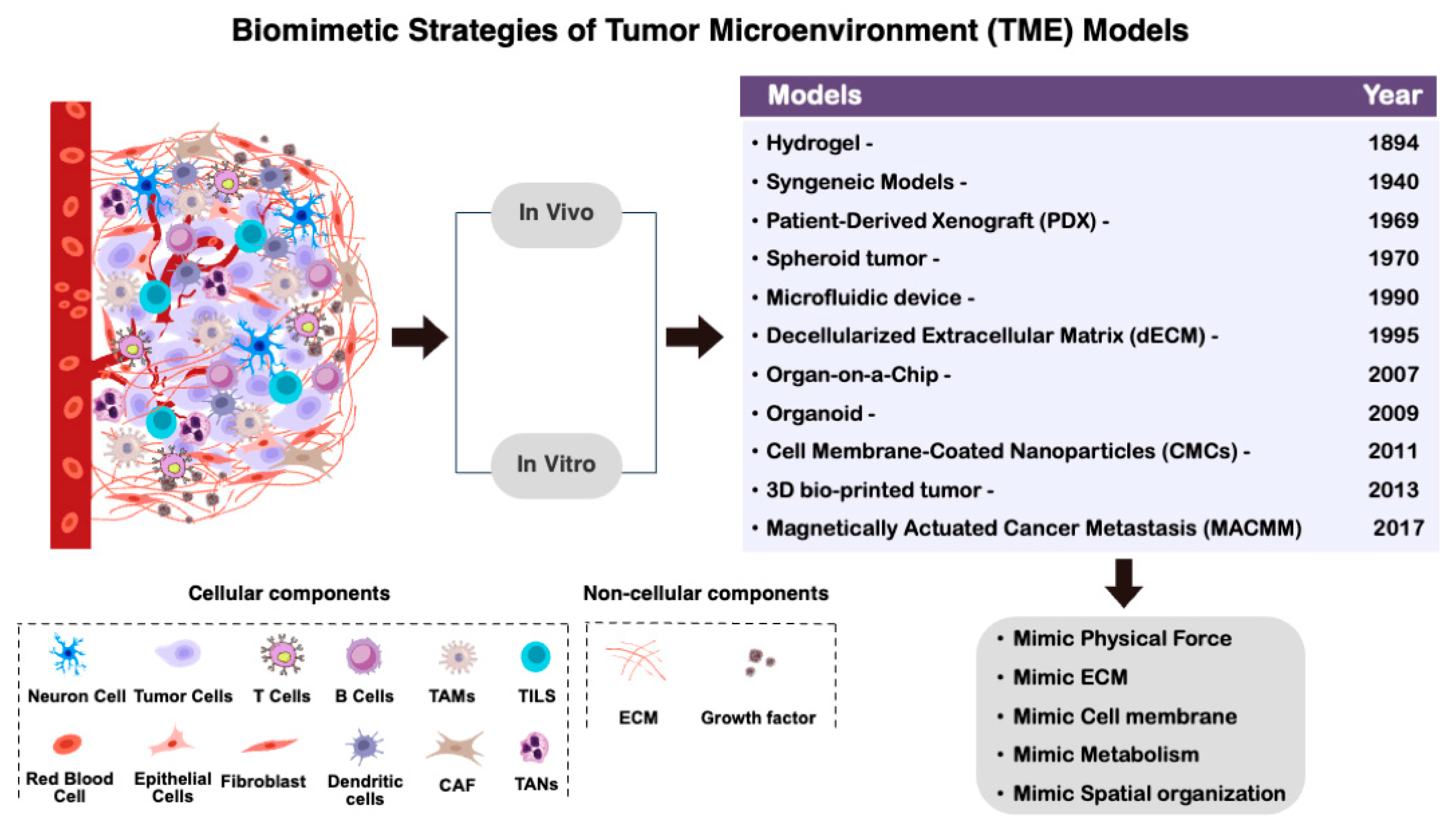
2. Biomimetic Cancer Systems
2.1. Biomimetic Design for Cancer Therapy—Biomimetics and Biomimicry
2.2. Biomimetic Drug Delivery Systems
2.3. Cell Membrane Mimetics
2.4. Biomimetic Hydrogel and Biomimetic Polymer Nanocomposites
2.5. ECM-Based Hydrogels
2.6. Biomimetic ECM Drug Delivery Systems
3. Biological Hydrogels in Cancer Biomimetic Systems
3.1. Hydrogel Tissue Mimetics
3.2. Hydrogels and 3D Tumor Models
3.3. Clay-Based Hydrogels
4. Engineering Biomimetic Models for Mechanobiology of Tumor Progression
4.1. Modeling Cancer Metastasis
4.2. Mechanobiology in Cancer Metastasis
4.3. Cancer Cell–ECM Interaction
5. Future Directions and Trends
5.1. Biomimetic Hallmarks of Cancer
5.1.1. Hallmarks of Cancer and Metabolism
5.1.2. Hallmarks of Cancer and Wound Healing
5.1.3. Hallmark of Cancer and Acidosis of the Tumor Microenvironment
5.2. The Gut Microbiome and Halalmarks of Cancer
5.3. What Is in a Name? The Etymological Origin of Cancer
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schiliro, C.; Firestein, B.L. Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting Enhanced Growth and Proliferation. Cells 2021, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Haileselassie, W.; Mulugeta, T.; Tigeneh, W.; Kaba, M.; Labisso, W.L. The Situation of Cancer Treatment in Ethiopia: Challenges and Opportunities. J. Cancer Prev. 2019, 24, 33–42. [Google Scholar] [CrossRef]
- Pelt, J.; Busatto, S.; Ferrari, M.; Thompson, E.A.; Mody, K.; Wolfram, J. Chloroquine and nanoparticle drug delivery: A promising combination. Pharmacol. Ther. 2018, 191, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhai, B.Z.; Wu, Y.J.; Wang, Y. Recent progress in the development of nanomaterials targeting multiple cancer metabolic pathways: A review of mechanistic approaches for cancer treatment. Drug Deliv. 2023, 30, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aranda, M.; Redondo, M. Immunotherapy: A Challenge of Breast Cancer Treatment. Cancers 2019, 11, 1822. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, D.; Huang, L.; Wang, S.; Jin, Y. Targeting Reactive Oxygen Species Capacity of Tumor Cells with Repurposed Drug as an Anticancer Therapy. Oxid. Med. Cell Longev. 2021, 2021, 8532940. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Alqahtani, H. Deep Learning-Based Cancer Detection-Recent Developments, Trend and Challenges. Comput. Model. Eng. Sci. 2022, 130, 1271–1307. [Google Scholar] [CrossRef]
- Sobral, D.; Martins, M.; Kaplan, S.; Golkaram, M.; Salmans, M.; Khan, N.; Vijayaraghavan, R.; Casimiro, S.; Fernandes, A.; Borralho, P.; et al. Genetic and microenvironmental intra-tumor heterogeneity impacts colorectal cancer evolution and metastatic development. Commun. Biol. 2022, 5, 937. [Google Scholar] [CrossRef]
- Idikio, H.A. Human cancer classification: A systems biology-based model integrating morphology, cancer stem cells, proteomics, and genomics. J. Cancer 2011, 2, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Collisson, E.A.; Cho, R.J.; Gray, J.W. What are we learning from the cancer genome? Nat. Rev. Clin. Oncol. 2012, 9, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Zhang, X.; Aubel, D.; Bai, Y.; Li, X.; Wei, Y.; Fussenegger, M.; Deng, X. Role of YAP/TAZ in Cell Lineage Fate Determination and Related Signaling Pathways. Front. Cell Dev. Biol. 2020, 8, 735. [Google Scholar] [CrossRef] [PubMed]
- Tennakoon, J.B.; Shi, Y.; Han, J.J.; Tsouko, E.; White, M.A.; Burns, A.R.; Zhang, A.; Xia, X.; Ilkayeva, O.R.; Xin, L.; et al. Androgens regulate prostate cancer cell growth via an AMPK-PGC-1alpha-mediated metabolic switch. Oncogene 2014, 33, 5251–5261. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Zhou, W.; Yao, W.; Yang, F.; Zhang, S.; Singh, R.; Chen, J.; Chen, J.J.; Zhang, Y.; Wei, F.; et al. Downregulation of YAP-dependent Nupr1 promotes tumor-repopulating cell growth in soft matrices. Oncogenesis 2016, 5, e220. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Reaume, M.; Hennenfent, M.; Lee, B.P.; Rajachar, R. Biomimetic hydrogels with spatial- and temporal-controlled chemical cues for tissue engineering. Biomater. Sci. 2020, 8, 3248–3269. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xu, K.; Zhu, P.; Wang, C.; Chi, Q. Smart hydrogels with high tunability of stiffness as a biomimetic cell carrier. Cell Biol. Int. 2019, 43, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, H.; Zhao, X. Designer Self-Assembling Peptide Hydrogels to Engineer 3D Cell Microenvironments for Cell Constructs Formation and Precise Oncology Remodeling in Ovarian Cancer. Adv. Sci. 2020, 7, 1903718. [Google Scholar] [CrossRef] [PubMed]
- Camci-Unal, G.; Cuttica, D.; Annabi, N.; Demarchi, D.; Khademhosseini, A. Synthesis and characterization of hybrid hyaluronic acid-gelatin hydrogels. Biomacromolecules 2013, 14, 1085–1092. [Google Scholar] [CrossRef]
- Guan, X.; Avci-Adali, M.; Alarcin, E.; Cheng, H.; Kashaf, S.S.; Li, Y.; Chawla, A.; Jang, H.L.; Khademhosseini, A. Development of hydrogels for regenerative engineering. Biotechnol. J. 2017, 12, 1600394. [Google Scholar] [CrossRef]
- Batool, N.; Sarfraz, R.M.; Mahmood, A.; Rehman, U.; Zaman, M.; Akbar, S.; Almasri, D.M.; Gad, H.A. Development and Evaluation of Cellulose Derivative and Pectin Based Swellable pH Responsive Hydrogel Network for Controlled Delivery of Cytarabine. Gels 2023, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, S.; Mu, L.; Si, T.; Gai, M.; Sun, M.; Frueh, J.; He, Q. Magnetically-propelled hydrogel particle motors produced by ultrasound assisted hydrodynamic electrospray ionization jetting. Colloids Surf. B Biointerfaces 2019, 175, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Redmond, J.; McCarthy, H.; Buchanan, P.; Levingstone, T.J.; Dunne, N.J. Advances in biofabrication techniques for collagen-based 3D in vitro culture models for breast cancer research. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111944. [Google Scholar] [CrossRef]
- Alshehri, A.M.; Wilson, O.C., Jr.; Dahal, B.; Philip, J.; Luo, X.; Raub, C.B. Magnetic nanoparticle-loaded alginate beads for local micro-actuation of in vitro tissue constructs. Colloids Surf. B Biointerfaces 2017, 159, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Cui, Y.; Hao, W.; Chen, M.; Liu, Q.; Wang, Y.; Yang, M.; Li, Z.; Gong, W.; Song, S.; et al. Carrier-free highly drug-loaded biomimetic nanosuspensions encapsulated by cancer cell membrane based on homology and active targeting for the treatment of glioma. Bioact. Mater. 2021, 6, 4402–4414. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhao, P.; Jiang, Y.; Tang, Y.; Jin, H.; Pan, Z.; He, H.; Yang, V.C.; Huang, Y. Blood-Brain-Barrier-Penetrating Albumin Nanoparticles for Biomimetic Drug Delivery via Albumin-Binding Protein Pathways for Antiglioma Therapy. ACS Nano 2016, 10, 9999–10012. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Halperin-Sternfeld, M.; Adler-Abramovich, L. Bio Mimicking of Extracellular Matrix. In Biological and Bio-Inspired Nanomaterials: Properties and Assembly Mechanisms; Perrett, S., Buell, A.K., Knowles, T.P.J., Eds.; Springer: Singapore, 2019; pp. 371–399. [Google Scholar]
- Northcutt, L.A.; Suarez-Arnedo, A.; Rafat, M. Emerging Biomimetic Materials for Studying Tumor and Immune Cell Behavior. Ann. Biomed. Eng. 2020, 48, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Liu, L.; Pan, H.; Cai, L. Biomimetic Active Materials Guided Immunogenic Cell Death for Enhanced Cancer Immunotherapy. Small Methods 2023, 7, e2201412. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Cheng, P.; Pu, K. Recent Advances in Cell Membrane-Camouflaged Nanoparticles for Cancer Phototherapy. Small 2019, 15, e1804105. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zheng, D.; Lin, X.; Wei, Z.; Zhang, D.; Li, Z.; Zhang, Y.; Wu, M.; Liu, X. Cancer cell membrane-coated magnetic nanoparticles for MR/NIR fluorescence dual-modal imaging and photodynamic therapy. Biomater. Sci. 2018, 6, 1834–1845. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Wilson, O.C. Biobased Materials for Medical Applications. In Biomedical Materials; Springer: Cham, Switzerland, 2021; pp. 139–193. [Google Scholar]
- Hwang, J.; Jeong, Y.; Park, J.M.; Lee, K.H.; Hong, J.W.; Choi, J. Biomimetics: Forecasting the future of science, engineering, and medicine. Int. J. Nanomed. 2015, 10, 5701–5713. [Google Scholar]
- Schuessler, T.K.; Chan, X.Y.; Chen, H.J.; Ji, K.; Park, K.M.; Roshan-Ghias, A.; Sethi, P.; Thakur, A.; Tian, X.; Villasante, A.; et al. Biomimetic tissue-engineered systems for advancing cancer research: NCI Strategic Workshop report. Cancer Res. 2014, 74, 5359–5363. [Google Scholar] [CrossRef]
- Pn, N.; Mehla, S.; Begum, A.; Chaturvedi, H.K.; Ojha, R.; Hartinger, C.; Plebanski, M.; Bhargava, S.K. Smart Nanozymes for Cancer Therapy: The Next Frontier in Oncology. Adv. Healthc. Mater. 2023, 12, e2300768. [Google Scholar]
- Guido, C.; Maiorano, G.; Cortese, B.; D’Amone, S.; Palama, I.E. Biomimetic Nanocarriers for Cancer Target Therapy. Bioengineering 2020, 7, 111. [Google Scholar] [CrossRef]
- Cash, K.J.; Clark, H.A. Nanosensors and nanomaterials for monitoring glucose in diabetes. Trends Mol. Med. 2010, 16, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, E.B.; Elvira, K.S. Biomimetic artificial cells to model the effect of membrane asymmetry on chemoresistance. Chem. Commun. 2021, 57, 6534–6537. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Wangpraseurt, D. Engineered photoresponsive biohybrids for tumor therapy. Smart Med. 2023, 2, e20220041. [Google Scholar] [CrossRef]
- Zeugolis, D.I.; Pandit, A. Biofunctional Biomaterials—The Next Frontier. Bioconjug Chem. 2015, 26, 1157. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, Z.; Shi, X.; Shen, M. Engineered cancer cell membranes: An emerging agent for efficient cancer theranostics. Exploration 2022, 2, 20210171. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Hu, X.; Peng, R.; Huang, H.; Liu, Q.; Tan, W. Biomimetic Carriers Based on Giant Membrane Vesicles for Targeted Drug Delivery and Photodynamic/Photothermal Synergistic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 43811–43819. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, J.M.; Gan, Y.C.; Qiu, X.Z.; Gao, Z.C.; Wang, H.; Chen, S.X.; Xiong, Y.; Liu, G.H.; Lin, S.E.; et al. Biomimetic natural biomaterials for tissue engineering and regenerative medicine: New biosynthesis methods, recent advances, and emerging applications. Mil. Med. Res. 2023, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Tang, J.; Shi, H.; Ye, X.; He, X.; Xu, F.; Yan, L.; Qiao, Z.; Wang, K. Nature-Inspired Smart DNA Nanodoctor for Activatable In Vivo Cancer Imaging and In Situ Drug Release Based on Recognition-Triggered Assembly of Split Aptamer. Anal. Chem. 2016, 88, 11699–11706. [Google Scholar] [CrossRef] [PubMed]
- Beh, C.Y.; Prajnamitra, R.P.; Chen, L.L.; Hsieh, P.C. Advances in Biomimetic Nanoparticles for Targeted Cancer Therapy and Diagnosis. Molecules 2021, 26, 5052. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cuzzucoli, F.; Escobar, A.; Lu, S.; Liang, L.; Wang, S. Tumor-on-a-chip platforms for assessing nanoparticle-based cancer therapy. Nanotechnology 2018, 29, 332001. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Sun, Y.; Liu, H. Cell membrane biomimetic nanomedicines for cancer phototherapy. Interdiscip. Med. 2023, 1, e20220012. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Y.; Dai, W.; Lu, J.; Cui, J.; Wu, H.; Yuan, L.; Zhang, H.; Wang, X.; Wang, J.; et al. The use of a tumor metastasis targeting peptide to deliver doxorubicin-containing liposomes to highly metastatic cancer. Biomaterials 2012, 33, 8451–8460. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Versluis, A.J.; Rensen, P.C.; Rump, E.T.; Van Berkel, T.J.; Bijsterbosch, M.K. Low-density lipoprotein receptor-mediated delivery of a lipophilic daunorubicin derivative to B16 tumours in mice using apolipoprotein E-enriched liposomes. Br. J. Cancer 1998, 78, 1607–1614. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shigehiro, T.; Kasai, T.; Murakami, M.; Sekhar, S.C.; Tominaga, Y.; Okada, M.; Kudoh, T.; Mizutani, A.; Murakami, H.; Sa-lomon, D.S.; et al. Efficient drug delivery of Paclitaxel glycoside: A novel solubility gradient encapsulation into liposomes coupled with immunoliposomes preparation. PLoS ONE 2014, 9, e107976. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Kim, D.W.; Chung, J.Y.; Shin, S.G.; Kim, S.C.; Heo, D.S.; Kim, N.K.; Bang, Y.J. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin. Cancer Res. 2004, 10, 3708–3716. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xu, Z.; Ma, M.; Xu, T. Dendrimers as drug carriers: Applications in different routes of drug administration. J. Pharm. Sci. 2008, 97, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, L.S.; Lau, C.H.; Han Chang, T.J.; Tam, D.Y.; Leung, H.M.; Tin, C.; Lo, P.K. Synthetic α-l-Threose Nucleic Acids Targeting BcL-2 Show Gene Silencing and in Vivo Antitumor Activity for Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 38510–38518. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Huang, N.; Li, M.; Zheng, M.; Wang, Z.; Zhang, X.; Gao, H.; Lao, Y.; Zhang, J.; Ding, B. Dendritic Cell-Derived Exosomes Driven Drug Co-Delivery Biomimetic Nanosystem for Effective Combination of Malignant Melanoma Immunotherapy and Gene Therapy. Drug Des. Dev. Ther. 2023, 17, 2087–2106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.L.; Wang, Y.J.; Sun, J.; Zhou, J.; Xing, C.; Huang, G.; Li, J.; Yang, H. Artificial chimeric exosomes for anti-phagocytosis and targeted cancer therapy. Chem. Sci. 2019, 10, 1555–1561. [Google Scholar] [CrossRef]
- Zhou, X.; Zhuang, Y.; Liu, X.; Gu, Y.; Wang, J.; Shi, Y.; Zhang, L.; Li, R.; Zhao, Y.; Chen, H.; et al. Study on tumour cell-derived hybrid exosomes as dasatinib nanocarriers for pancreatic cancer therapy. Artif. Cells Nanomed. Biotechnol. 2023, 51, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Cho, H.Y.; Kim, K.J.; Choi, J.W. In situ monitoring of doxorubicin release from biohybrid nanoparticles modified with antibody and cell-penetrating peptides in breast cancer cells using surface-enhanced Raman spectroscopy. Biosens. Bioelectron. 2015, 71, 300–305. [Google Scholar] [CrossRef]
- Davis, M.E.; Chen, Z.G.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef]
- Cao, J.; Qi, J.; Lin, X.; Xiong, Y.; He, F.; Deng, W.; Liu, G. Biomimetic Black Phosphorus Nanosheet-Based Drug Delivery System for Targeted Photothermal-Chemo Cancer Therapy. Front. Bioeng. Biotechnol. 2021, 9, 707208. [Google Scholar] [CrossRef]
- Li, Y.; Ke, J.; Jia, H.; Ren, J.; Wang, L.; Zhang, Z.; Wang, C. Cancer cell membrane coated PLGA nanoparticles as biomimetic drug delivery system for improved cancer therapy. Colloids Surf. B Biointerfaces 2023, 222, 113131. [Google Scholar] [CrossRef]
- Pei, M.; Liu, K.; Qu, X.; Wang, K.; Chen, Q.; Zhang, Y.; Wang, X.; Wang, Z.; Li, X.; Chen, F.; et al. Enzyme-catalyzed synthesis of selenium-doped manganese phosphate for synergistic therapy of drug-resistant colorectal cancer. J. Nanobiotechnol. 2023, 21, 72. [Google Scholar] [CrossRef]
- Turtoi, M.; Anghelache, M.; Bucatariu, S.M.; Deleanu, M.; Voicu, G.; Safciuc, F.; Manduteanu, I.; Fundueanu, G.; Simionescu, M.; Calin, M. A novel platform for drug testing: Biomimetic three-dimensional hyaluronic acid-based scaffold seeded with human hepatocarcinoma cells. Int. J. Biol. Macromol. 2021, 185, 604–619. [Google Scholar] [CrossRef]
- Dunne, L.W.; Huang, Z.; Meng, W.; Fan, X.; Zhang, N.; Zhang, Q.; An, Z. Human decellularized adipose tissue scaffold as a model for breast cancer cell growth and drug treatments. Biomaterials 2014, 35, 4940–4949. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.; Pan, Q. Capecitabine for treating head and neck cancer. Expert Opin. Investig. Drugs 2016, 25, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liu, X.; Cheng, Y.; Zhong, S.; Shi, X.; Wang, S.; Liu, M.; Ding, J.; Zhou, W. Core-Shell Nanosystems for Self-Activated Drug-Gene Combinations against Triple-Negative Breast Cancer. ACS Appl. Mater. Interfaces 2020, 12, 53654–53664. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Luk, B.T.; Hu, C.M.; Zhang, L. Engineered nanoparticles mimicking cell membranes for toxin neutralization. Adv. Drug Deliv. Rev. 2015, 90, 69–80. [Google Scholar] [CrossRef]
- Li, J.; Zhen, X.; Lyu, Y.; Jiang, Y.; Huang, J.; Pu, K. Cell Membrane Coated Semiconducting Polymer Nanoparticles for Enhanced Multimodal Cancer Phototheranostics. ACS Nano 2018, 12, 8520–8530. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Q.; Zhu, Y.; Pei, M.; Wang, K.; Qu, X.; Zhang, Y.; Gao, J.; Qin, H. Targeting inorganic nanoparticles to tumors using biological membrane-coated technology. MedComm 2022, 3, e192. [Google Scholar] [CrossRef]
- Kroll, A.V.; Jiang, Y.; Zhou, J.; Holay, M.; Fang, R.H.; Zhang, L. Biomimetic Nanoparticle Vaccines for Cancer Therapy. Adv. Biosyst. 2019, 3, e1800219. [Google Scholar] [CrossRef] [PubMed]
- Narkhede, A.A.; Shevde, L.A.; Rao, S.S. Biomimetic strategies to recapitulate organ specific microenvironments for studying breast cancer metastasis. Int. J. Cancer 2017, 141, 1091–1109. [Google Scholar] [CrossRef]
- Gujrati, V.; Kim, S.; Kim, S.H.; Min, J.J.; Choy, H.E.; Kim, S.C.; Jon, S. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano 2014, 8, 1525–1537. [Google Scholar] [CrossRef] [PubMed]
- Luk, B.T.; Zhang, L. Cell membrane-camouflaged nanoparticles for drug delivery. J. Control Release 2015, 220 Pt B, 600–607. [Google Scholar] [CrossRef]
- Han, X.; Wang, C.; Liu, Z. Red Blood Cells as Smart Delivery Systems. Bioconjug Chem. 2018, 29, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Fan, Q.; Xu, J.; Bai, J.; Han, X.; Dong, Z.; Zhou, X.; Liu, Z.; Gu, Z.; Wang, C. Calming Cytokine Storm in Pneumonia by Targeted Delivery of TPCA-1 Using Platelet-Derived Extracellular Vesicles. Matter 2020, 3, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Xia, C.; Wu, Y.; Zhou, N.; Chen, Z.; Li, W. Research Progress on Cell Membrane-Coated Biomimetic Delivery Systems. Front. Bioeng. Biotechnol. 2021, 9, 772522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Z.; Zhang, Y.; Cao, F.; Dong, K.; Ren, J.; Qu, X. Erythrocyte Membrane Cloaked Metal-Organic Framework Nanoparticle as Biomimetic Nanoreactor for Starvation-Activated Colon Cancer Therapy. ACS Nano 2018, 12, 10201–10211. [Google Scholar] [CrossRef]
- Athar, S.H.M.D.; Khan, G.J.; Ansari, M.A.; Raza, S.; Shaikh, S.N. Overview on virosomes as a novel carrier for drug delivery. J. Drug Deliv. Ther. 2019, 8, 429–434. [Google Scholar]
- Chen, H.; Zhou, M.; Zeng, Y.; Miao, T.; Luo, H.; Tong, Y.; Zhao, M.; Mu, R.; Gu, J.; Yang, S.; et al. Biomimetic Lipopolysaccharide-Free Bacterial Outer Membrane-Functionalized Nanoparticles for Brain-Targeted Drug Delivery. Adv. Sci. 2022, 9, e2105854. [Google Scholar] [CrossRef]
- Vijayan, V.; Uthaman, S.; Park, I.K. Cell Membrane-Camouflaged Nanoparticles: A Promising Biomimetic Strategy for Cancer Theragnostics. Polymers 2018, 10, 983. [Google Scholar] [CrossRef] [PubMed]
- Burkett, B.J.; Bartlett, D.J.; McGarrah, P.W.; Lewis, A.R.; Johnson, D.R.; Berberoglu, K.; Pandey, M.K.; Packard, A.T.; Halfdanarson, T.R.; Hruska, C.B.; et al. A Review of Theranostics: Perspectives on Emerging Approaches and Clinical Advancements. Radiol. Imaging Cancer 2023, 5, e220157. [Google Scholar] [CrossRef] [PubMed]
- Lankoff, A.; Czerwinska, M.; Kruszewski, M. Nanoparticle-Based Radioconjugates for Targeted Imaging and Therapy of Prostate Cancer. Molecules 2023, 28, 4122. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.; Hinchliffe, T.; Roy, V.; Shah, N.; Jones, C.N.; Obaid, G. Biomimetic Nanotechnology: A Natural Path Forward for Tumor-Selective and Tumor-Specific NIR Activable Photonanomedicines. Pharmaceutics 2021, 13, 786. [Google Scholar] [CrossRef] [PubMed]
- Raza, F.; Zafar, H.; Zhang, S.; Kamal, Z.; Su, J.; Yuan, W.E.; Mingfeng, Q. Recent Advances in Cell Membrane-Derived Biomimetic Nanotechnology for Cancer Immunotherapy. Adv. Healthc. Mater. 2021, 10, e2002081. [Google Scholar] [CrossRef] [PubMed]
- Sushnitha, M.; Evangelopoulos, M.; Tasciotti, E.; Taraballi, F. Cell Membrane-Based Biomimetic Nanoparticles and the Immune System: Immunomodulatory Interactions to Therapeutic Applications. Front. Bioeng. Biotechnol. 2020, 8, 627. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tan, Y.; Dong, Z.; Lu, J.; Han, X.; Jin, Q.; Zhu, W.; Shen, J.; Cheng, L.; Liu, Z.; et al. Injectable Anti-inflammatory Nanofiber Hydrogel to Achieve Systemic Immunotherapy Post Local Administration. Nano Lett. 2020, 20, 6763–6773. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Lim, J.; An, J.; Yoon, J.; Choi, J.W. Nanomaterial-based biohybrid hydrogel in bioelectronics. Nano Converg. 2023, 10, 8. [Google Scholar] [CrossRef]
- Blanco-Fernandez, B.; Gaspar, V.M.; Engel, E.; Mano, J.F. Proteinaceous Hydrogels for Bioengineering Advanced 3D Tumor Models. Adv. Sci. 2021, 8, 2003129. [Google Scholar] [CrossRef]
- Zhang, L.; He, G.; Yu, Y.; Zhang, Y.; Li, X.; Wang, S. Design of Biocompatible Chitosan/Polyaniline/Laponite Hydrogel with Photothermal Conversion Capability. Biomolecules 2022, 12, 1089. [Google Scholar] [CrossRef]
- Devi, V.K.A.; Shyam, R.; Palaniappan, A.; Jaiswal, A.K.; Oh, T.H.; Nathanael, A.J. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers 2021, 13, 3782. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, R.; Johnson, M.; A, S.; He, Z.; Milne, C.; Wang, X.; Lara-Saez, I.; Xu, Q.; Wang, W. An Injectable Chitosan-Based Self-Healable Hydrogel System as an Antibacterial Wound Dressing. Materials 2021, 14, 5956. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O. Eligard: Leuprolide acetate in a novel sustained-release delivery system. Urology 2003, 61, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Hamstra, D.A.; Mariados, N.; Sylvester, J.; Shah, D.; Karsh, L.; Hudes, R.; Beyer, D.; Kurtzman, S.; Bogart, J.; Hsi, R.A.; et al. Continued Benefit to Rectal Separation for Prostate Radiation Therapy: Final Results of a Phase III Trial. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Krukiewicz, K.; Zak, J.K. Biomaterial-based regional chemotherapy: Local anticancer drug delivery to enhance chemotherapy and minimize its side-effects. Mater. Sci. Eng. C 2016, 62, 927–942. [Google Scholar] [CrossRef]
- Prince, E.; Cruickshank, J.; Ba-Alawi, W.; Hodgson, K.; Haight, J.; Tobin, C.; Wakeman, A.; Avoulov, A.; Topolskaia, V.; El-liott, M.J.; et al. Biomimetic hydrogel supports initiation and growth of patient-derived breast tumor organoids. Nat. Commun. 2022, 13, 1466. [Google Scholar] [CrossRef] [PubMed]
- Bilalis, P.; Skoulas, D.; Karatzas, A.; Marakis, J.; Stamogiannos, A.; Tsimblouli, C.; Sereti, E.; Stratikos, E.; Dimas, K.; Vlas-sopoulos, D.; et al. Self-Healing pH- and Enzyme Stimuli-Responsive Hydrogels for Targeted Delivery of Gemcitabine to Treat Pancreatic Cancer. Biomacromolecules 2018, 19, 3840–3852. [Google Scholar] [CrossRef]
- Sobczak, M. Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems—State of Knowledge and Future Prospects. Int. J. Mol. Sci. 2022, 23, 4421. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Tan, C.; Qin, G.; Yao, S.K. Promising Clinical Applications of Hydrogels Associated with Precise Cancer Treatment: A Review. Technol. Cancer Res. Treat. 2023, 22, 15330338221150322. [Google Scholar] [CrossRef]
- Jorgensen, A.M.; Chou, Z.; Gillispie, G.; Lee, S.J.; Yoo, J.J.; Soker, S.; Atala, A. Decellularized Skin Extracellular Matrix (dsECM) Improves the Physical and Biological Properties of Fibrinogen Hydrogel for Skin Bioprinting Applications. Nanomaterials 2020, 10, 1484. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Z. A pH-Responsive Hydrogel Based on a Tumor-Targeting Mesoporous Silica Nanocomposite for Sustained Cancer Labeling and Therapy. Macromol. Rapid Commun. 2016, 37, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Pautu, V.; Lepeltier, E.; Mellinger, A.; Riou, J.; Debuigne, A.; Jerome, C.; Clere, N.; Passirani, C. pH-Responsive Lipid Nanocapsules: A Promising Strategy for Improved Resistant Melanoma Cell Internalization. Cancers 2021, 13, 2028. [Google Scholar] [CrossRef] [PubMed]
- Alsharabasy, A.M.; Pandit, A.; Farras, P. Recent Advances in the Design and Sensing Applications of Hemin/Coordination Polymer-Based Nanocomposites. Adv. Mater. 2021, 33, e2003883. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Korc, M.; Lin, C.C. Biomimetic and enzyme-responsive dynamic hydrogels for studying cell-matrix interactions in pancreatic ductal adenocarcinoma. Biomaterials 2018, 160, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Nguyen, L.T.; Ngiam, M.; Wang, C.; Cheng, Z.; Chan, C.K.; Ramakrishna, S. Biomimetic nanocomposites to control osteogenic differentiation of human mesenchymal stem cells. Adv. Healthc. Mater. 2014, 3, 737–751. [Google Scholar] [CrossRef]
- Liverani, C.; Mercatali, L.; Cristofolini, L.; Giordano, E.; Minardi, S.; Porta, G.D.; De Vita, A.; Miserocchi, G.; Spadazzi, C.; Tasciotti, E.; et al. Investigating the Mechanobiology of Cancer Cell-ECM Interaction through Collagen-Based 3D Scaffolds. Cell Mol. Bioeng. 2017, 10, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Rothe, R.; Voigt, D.; Sayed, A.; Huang, C.; Hauser, S.; Lee, P.W.; Cui, M.; Saenz, J.P.; Boccaccini, A.R.; et al. A self-assembled dynamic extracellular matrix-like hydrogel system with multi-scale structures for cell bioengineering applications. Acta Biomater. 2023, 162, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Xia, X.; Hu, F.; Yu, J. Extracellular matrix remodeling in the tumor immunity. Front. Immunol. 2023, 14, 1340634. [Google Scholar] [CrossRef]
- Meredith, J.E., Jr.; Fazeli, B.; Schwartz, M.A. The extracellular matrix as a cell survival factor. Mol. Biol. Cell 1993, 4, 953–961. [Google Scholar] [CrossRef]
- Ngai, D.; Lino, M.; Bendeck, M.P. Cell-Matrix Interactions and Matricrine Signaling in the Pathogenesis of Vascular Calcification. Front. Cardiovasc. Med. 2018, 5, 174. [Google Scholar] [CrossRef] [PubMed]
- Barney, L.E.; Dandley, E.C.; Jansen, L.E.; Reich, N.G.; Mercurio, A.M.; Peyton, S.R. A cell-ECM screening method to predict breast cancer metastasis. Integr. Biol. 2015, 7, 198–212. [Google Scholar] [CrossRef]
- Dzobo, K.; Dandara, C. The Extracellular Matrix: Its Composition, Function, Remodeling, and Role in Tumorigenesis. Biomimetics 2023, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, Y.; Liu, Q.; Gao, T.; Feng, J.Q.; Dechow, P.; D’Souza, R.N.; Qin, C.; Liu, X. Biomimetic engineering of nanofibrous gelatin scaffolds with noncollagenous proteins for enhanced bone regeneration. Tissue Eng. Part A 2013, 19, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Liu, D.; Cao, Z.; Zheng, N.; Mao, C.; Liu, S.; He, L.; Liu, S. Research Status and Prospect of Non-Viral Vectors Based on siRNA: A Review. Int. J. Mol. Sci. 2023, 24, 3375. [Google Scholar] [CrossRef] [PubMed]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, C.; Bai, H.; Zhang, J.; Wang, Z.; Li, Z.; Zhao, X.; Wang, J.; Liu, H. Functionalization of biomimetic mineralized collagen for bone tissue engineering. Mater. Today Bio 2023, 20, 100660. [Google Scholar] [CrossRef]
- Liang, K.; Zhao, C.; Song, C.; Zhao, L.; Qiu, P.; Wang, S.; Zhu, J.; Gong, Z.; Liu, Z.; Tang, R.; et al. In Situ Biomimetic Mineralization of Bone-Like Hydroxyapatite in Hydrogel for the Acceleration of Bone Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 292–308. [Google Scholar] [CrossRef]
- Negut, I.; Bita, B.; Groza, A. Polymeric Coatings and Antimicrobial Peptides as Efficient Systems for Treating Implantable Medical Devices Associated-Infections. Polymers 2022, 14, 1611. [Google Scholar] [CrossRef]
- Bao, X.; Huo, S.; Wang, Z.; Yang, S.; Dou, L.; Liu, Y.; Huang, J.; Cai, C.; Fang, B.; Xu, G. Multifunctional biomimetic hydrogel dressing provides anti-infection treatment and improves immunotherapy by reprogramming the infection-related wound microenvironment. J. Nanobiotechnol. 2024, 22, 80. [Google Scholar] [CrossRef]
- Ghimire, S.; Sarkar, P.; Rigby, K.; Maan, A.; Mukherjee, S.; Crawford, K.E.; Mukhopadhyay, K. Polymeric Materials for Hemostatic Wound Healing. Pharmaceutics 2021, 13, 2127. [Google Scholar] [CrossRef]
- Rausch, M.K.; Parekh, S.H.; Dortdivanlioglu, B.; Rosales, A.M. Synthetic hydrogels as blood clot mimicking wound healing materials. Prog. Biomed. Eng. 2021, 3, 042006. [Google Scholar] [CrossRef]
- Wang, M.; Deng, Z.; Guo, Y.; Xu, P. Engineering functional natural polymer-based nanocomposite hydrogels for wound healing. Nanoscale Adv. 2022, 5, 27–45. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Najda, A.; Kaur, P.; Shah, M.; Singh, H.; Kaur, P.; Cavalu, S.; Jaroszuk-Sierocinska, M.; Rahman, M.H. Potentiality of Nanoenzymes for Cancer Treatment and Other Diseases: Current Status and Future Challenges. Materials 2021, 14, 5965. [Google Scholar] [CrossRef]
- Niland, S.; Eble, J.A. Hold on or Cut? Integrin- and MMP-Mediated Cell-Matrix Interactions in the Tumor Microenvironment. Int. J. Mol. Sci. 2020, 22, 238. [Google Scholar] [CrossRef]
- Ray, A.; Slama, Z.M.; Morford, R.K.; Madden, S.A.; Provenzano, P.P. Enhanced Directional Migration of Cancer Stem Cells in 3D Aligned Collagen Matrices. Biophys. J. 2017, 112, 1023–1036. [Google Scholar] [CrossRef]
- Yueh, T.C.; Hung, Y.C.; Lee, H.T.; Yang, M.D.; Wang, Z.H.; Yang, Y.C.; Ke, T.W.; Pei, J.S.; Tsai, C.W.; Bau, D.T.; et al. Role of Matrix Metallopeptidase-2 Genotypes in Taiwanese Patients with Colorectal Cancer. Anticancer. Res. 2022, 42, 5335–5342. [Google Scholar] [CrossRef]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef]
- Pape, J.; Emberton, M.; Cheema, U. 3D Cancer Models: The Need for a Complex Stroma, Compartmentalization and Stiffness. Front. Bioeng. Biotechnol. 2021, 9, 660502. [Google Scholar] [CrossRef]
- Ricketts, K.P.; Cheema, U.; Nyga, A.; Castoldi, A.; Guazzoni, C.; Magdeldin, T.; Emberton, M.; Gibson, A.P.; Royle, G.J.; Loiz-idou, M. A 3D in vitro cancer model as a platform for nanoparticle uptake and imaging investigations. Small 2014, 10, 3954–3961. [Google Scholar] [CrossRef]
- Loessner, D.; Stok, K.S.; Lutolf, M.P.; Hutmacher, D.W.; Clements, J.A.; Rizzi, S.C. Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials 2010, 31, 8494–8506. [Google Scholar] [CrossRef]
- Guo, J.; Kamel-Reid, S.; Rutherford, M.; Hart, J.; Melamed, S.; Pollett, A. Personalized medicine: CCO’s vision, accomplishments and future plans. Healthc. Q. 2015, 17, 41–43. [Google Scholar] [CrossRef][Green Version]
- Crotti, S.; Piccoli, M.; Rizzolio, F.; Giordano, A.; Nitti, D.; Agostini, M. Extracellular Matrix and Colorectal Cancer: How Surrounding Microenvironment Affects Cancer Cell Behavior? J. Cell. Physiol. 2017, 232, 967–975. [Google Scholar] [CrossRef]
- Wu, M.; Frieboes, H.B.; McDougall, S.R.; Chaplain, M.A.; Cristini, V.; Lowengrub, J. The effect of interstitial pressure on tumor growth: Coupling with the blood and lymphatic vascular systems. J. Theor. Biol. 2013, 320, 131–151. [Google Scholar] [CrossRef]
- Belgodere, J.A.; King, C.T.; Bursavich, J.B.; Burow, M.E.; Martin, E.C.; Jung, J.P. Engineering Breast Cancer Microenvironments and 3D Bioprinting. Front. Bioeng. Biotechnol. 2018, 6, 66. [Google Scholar] [CrossRef]
- Malandrino, A.; Trepat, X.; Kamm, R.D.; Mak, M. Dynamic Filopodial Forces Induce Accumulation, Damage, and Plastic Remodeling of 3D Extracellular Matrices. PLoS Comput. Biol. 2019, 15, e1006684. [Google Scholar] [CrossRef]
- Moon, S.; Ok, Y.; Hwang, S.; Lim, Y.S.; Kim, H.Y.; Na, Y.J.; Yoon, S. A Marine Collagen-Based Biomimetic Hydrogel Recapitulates Cancer Stem Cell Niche and Enhances Progression and Chemoresistance in Human Ovarian Cancer. Mar. Drugs 2020, 18, 498. [Google Scholar] [CrossRef]
- Huynh, R.N.; Yousof, M.; Ly, K.L.; Gombedza, F.C.; Luo, X.; Bandyopadhyay, B.C.; Raub, C.B. Microstructural densification and alignment by aspiration-ejection influence cancer cell interactions with three-dimensional collagen networks. Biotechnol. Bioeng. 2020, 117, 1826–1838. [Google Scholar] [CrossRef]
- Castro-Abril, H.; Heras, J.; Del Barrio, J.; Paz, L.; Alcaine, C.; Aliacar, M.P.; Garzon-Alvarado, D.; Doblare, M.; Ochoa, I. The Role of Mechanical Properties and Structure of Type I Collagen Hydrogels on Colorectal Cancer Cell Migration. Macromol. Biosci. 2023, 23, e2300108. [Google Scholar] [CrossRef]
- Hutson, C.B.; Nichol, J.W.; Aubin, H.; Bae, H.; Yamanlar, S.; Al-Haque, S.; Koshy, S.T.; Khademhosseini, A. Synthesis and characterization of tunable poly(ethylene glycol): Gelatin methacrylate composite hydrogels. Tissue Eng. Part A 2011, 17, 1713–1723. [Google Scholar] [CrossRef]
- Cui, L.; Yao, Y.; Yim, E.K.F. The effects of surface topography modification on hydrogel properties. APL Bioeng. 2021, 5, 031509. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, H.; Tang, D.; Li, Y.; Li, X.; Xu, F. Bioactuators based on stimulus-responsive hydrogels and their emerging biomedical applications. NPG Asia Mater. 2019, 11, 64. [Google Scholar] [CrossRef]
- Stowers, R.S.; Allen, S.C.; Suggs, L.J. Dynamic phototuning of 3D hydrogel stiffness. Proc. Natl. Acad. Sci. USA 2015, 112, 1953–1958. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, R.; Ouyang, L.; Ding, H.; Zhang, T.; Zhang, K.; Cheng, S.; Sun, W. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 2014, 6, 035001. [Google Scholar] [CrossRef]
- Nomicisio, C.; Ruggeri, M.; Bianchi, E.; Vigani, B.; Valentino, C.; Aguzzi, C.; Viseras, C.; Rossi, S.; Sandri, G. Natural and Synthetic Clay Minerals in the Pharmaceutical and Biomedical Fields. Pharmaceutics 2023, 15, 1368. [Google Scholar] [CrossRef]
- Wilson, O.; Olorunyolemi, T.; Jaworski, A.; Borum, L.; Young, D.; Siriwat, A.; Dickens, E.; Oriahki, C.; Lerner, M. Surface and interfacial properties of polymer-intercalated layered double hydroxide nanocomposites. Appl. Clay Sci. 1999, 15, 265–279. [Google Scholar] [CrossRef]
- Tipa, C.; Cidade, M.T.; Borges, J.P.; Costa, L.C.; Silva, J.C.; Soares, P.I.P. Clay-Based Nanocomposite Hydrogels for Biomedical Applications: A Review. Nanomaterials 2022, 12, 498. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.I.; Kanczler, J.M.; Yang, X.B.; Attard, G.S.; Oreffo, R.O. Clay gels for the delivery of regenerative microenvironments. Adv. Mater. 2011, 23, 3304–3308. [Google Scholar] [CrossRef]
- Saadh, M.J.; Abdulsahib, W.K.; Mustafa, A.N.; Zabibah, R.S.; Adhab, Z.H.; Rakhimov, N.; Alsaikhan, F. Recent advances in natural nanoclay for diagnosis and therapy of cancer: A review. Colloids Surf. B Biointerfaces 2024, 235, 113768. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Morales-Guadarrama, G.; Garcia-Becerra, R.; Mendez-Perez, E.A.; Garcia-Quiroz, J.; Avila, E.; Diaz, L. Vasculogenic Mimicry in Breast Cancer: Clinical Relevance and Drivers. Cells 2021, 10, 1758. [Google Scholar] [CrossRef]
- Langley, R.R.; Fidler, I.J. The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 2011, 128, 2527–2535. [Google Scholar] [CrossRef]
- Kraning-Rush, C.M.; Califano, J.P.; Reinhart-King, C.A. Cellular traction stresses increase with increasing metastatic potential. PLoS ONE 2012, 7, e32572. [Google Scholar] [CrossRef]
- Zhao, X.B.; Chen, Y.P.; Tan, M.; Zhao, L.; Zhai, Y.Y.; Sun, Y.L.; Gong, Y.; Feng, X.Q.; Du, J.; Fan, Y.B. Extracellular Matrix Stiffness Regulates DNA Methylation by PKCalpha-Dependent Nuclear Transport of DNMT3L. Adv. Healthc. Mater. 2021, 10, e2100821. [Google Scholar] [CrossRef]
- Yamashita, H.; Ichikawa, T.; Matsuyama, D.; Kimura, Y.; Ueda, K.; Craig, S.W.; Harada, I.; Kioka, N. The role of the interaction of the vinculin proline-rich linker region with vinexin alpha in sensing the stiffness of the extracellular matrix. J. Cell Sci. 2014, 127 Pt 9, 1875–1886. [Google Scholar]
- Herath, S.C.; Yue, D.; Hui, S.; Kim, M.C.; Wang, D.A.; Wang, Q.; Van Vliet, K.J.; Asada, H.; Chen, P.C. Quantification of magnetically induced changes in ECM local apparent stiffness. Biophys. J. 2014, 106, 332–341. [Google Scholar] [CrossRef]
- Omachi, T.; Ichikawa, T.; Kimura, Y.; Ueda, K.; Kioka, N. Vinculin association with actin cytoskeleton is necessary for stiffness-dependent regulation of vinculin behavior. PLoS ONE 2017, 12, e0175324. [Google Scholar] [CrossRef] [PubMed]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Siegel, P.M.; Shu, W.; Drobnjak, M.; Kakonen, S.M.; Cordon-Cardo, C.; Guise, T.A.; Massague, J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003, 3, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Riching, K.M.; Cox, B.L.; Salick, M.R.; Pehlke, C.; Riching, A.S.; Ponik, S.M.; Bass, B.R.; Crone, W.C.; Jiang, Y.; Weaver, A.M.; et al. 3D collagen alignment limits protrusions to enhance breast cancer cell persistence. Biophys. J. 2014, 107, 2546–2558. [Google Scholar] [CrossRef] [PubMed]
- Carey, S.P.; Goldblatt, Z.E.; Martin, K.E.; Romero, B.; Williams, R.M.; Reinhart-King, C.A. Local extracellular matrix alignment directs cellular protrusion dynamics and migration through Rac1 and FAK. Integr. Biol. 2016, 8, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Tata, U.; Lin, V.; Chiao, J.-C. The Migration of Cancer Cells in Gradually Varying Chemical Gradients and Mechanical Constraints. Micromachines 2014, 5, 13–26. [Google Scholar] [CrossRef]
- Verbruggen, S.W.; Thompson, C.L.; Duffy, M.P.; Lunetto, S.; Nolan, J.; Pearce, O.M.T.; Jacobs, C.R.; Knight, M.M. Mechanical Stimulation Modulates Osteocyte Regulation of Cancer Cell Phenotype. Cancers 2021, 13, 2906. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Boudou, T.; Wang, W.G.; Chen, C.S.; Reich, D.H. Decoupling cell and matrix mechanics in engineered microtissues using magnetically actuated microcantilevers. Adv. Mater. 2013, 25, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Liu, F.; Tonkova, E.A.; Lee, S.Y.; Tschumperlin, D.J.; Brenner, M.B. Soft matrix is a natural stimulator for cellular invasiveness. Mol. Biol. Cell 2014, 25, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Zhao, S.; Bunaciu, R.P.; Yen, A.; Wu, M. A 3D in situ cell counter reveals that breast tumor cell (MDA-MB-231) proliferation rate is reduced by the collagen matrix density. Biotechnol. Prog. 2015, 31, 990–996. [Google Scholar] [CrossRef]
- Mason, B.N.; Califano, J.P.; Reinhart-King, C.A. Matrix Stiffness: A Regulator of Cellular Behavior and Tissue Formation. In Engineering Biomaterials for Regenerative Medicine; Bhatia, S.K., Ed.; Springer: New York, NY, USA, 2012; pp. 19–37. [Google Scholar]
- Park, J.; Kim, D.H.; Kim, H.N.; Wang, C.J.; Kwak, M.K.; Hur, E.; Suh, K.Y.; An, S.S.; Levchenko, A. Directed migration of cancer cells guided by the graded texture of the underlying matrix. Nat. Mater. 2016, 15, 792–801. [Google Scholar] [CrossRef]
- Tilghman, R.W.; Cowan, C.R.; Mih, J.D.; Koryakina, Y.; Gioeli, D.; Slack-Davis, J.K.; Blackman, B.R.; Tschumperlin, D.J.; Parsons, J.T. Matrix Rigidity Regulates Cancer Cell Growth and Cellular Phenotype. PLoS ONE 2010, 5, e12905. [Google Scholar] [CrossRef]
- Cavo, M.; Fato, M.; Penuela, L.; Beltrame, F.; Raiteri, R.; Scaglione, S. Microenvironment complexity and matrix stiffness regulate breast cancer cell activity in a 3D in vitro model. Sci. Rep. 2016, 6, 35367. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Feng, J.; Jones, C.A.R.; Mao, X.; Sander, L.M.; Levine, H.; Sun, B. Stress-induced plasticity of dynamic collagen networks. Nat. Commun. 2017, 8, 842. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wyckoff, J.B.; Frohlich, V.C.; Oleynikov, Y.; Hüttelmaier, S.; Zavadil, J.; Cermak, L.; Bottinger, E.P.; Singer, R.H.; White, J.G.; et al. Single Cell Behavior in Metastatic Primary Mammary Tumors Correlated with Gene Expression Patterns Revealed by Molecular Profiling. Cancer Res. 2002, 62, 6278–6288. [Google Scholar] [PubMed]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Holle, A.W.; Young, J.L.; Van Vliet, K.J.; Kamm, R.D.; Discher, D.; Janmey, P.; Spatz, J.P.; Saif, T. Cell-Extracellular Matrix Mechanobiology: Forceful Tools and Emerging Needs for Basic and Translational Research. Nano Lett. 2018, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tzvetkova-Chevolleau, T.; Stephanou, A.; Fuard, D.; Ohayon, J.; Schiavone, P.; Tracqui, P. The motility of normal and cancer cells in response to the combined influence of the substrate rigidity and anisotropic microstructure. Biomaterials 2008, 29, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.T.; Tsai, I.Y.; Russell, T.P.; Hanks, S.K.; Wang, Y.L. Cellular responses to substrate topography: Role of myosin II and focal adhesion kinase. Biophys. J. 2006, 90, 3774–3782. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.T.; Raghunathan, V.K.; Nealey, P.F.; Russell, P.; Murphy, C.J. Topographic modulation of the orientation and shape of cell nuclei and their influence on the measured elastic modulus of epithelial cells. Biophys. J. 2011, 101, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Staunton, J.R.; Tanner, K. Independent Control of Topography for 3D Patterning of the ECM Microenvironment. Adv. Mater. 2016, 28, 132–137. [Google Scholar] [CrossRef]
- Rizwan, A.; Bulte, C.; Kalaichelvan, A.; Cheng, M.; Krishnamachary, B.; Bhujwalla, Z.M.; Jiang, L.; Glunde, K. Metastatic breast cancer cells in lymph nodes increase nodal collagen density. Sci. Rep. 2015, 5, 10002. [Google Scholar] [CrossRef]
- Petrie, R.J.; Doyle, A.D.; Yamada, K.M. Random versus directionally persistent cell migration. Nat. Rev. Mol. Cell Biol. 2009, 10, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, K.; Inoue, T.; Hamada, Y.; Matsumoto, T. A novel patterned magnetic micropillar array substrate for analysis of cellular mechanical responses. J. Biomech. 2017, 65, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Tse, J.M.; Cheng, G.; Tyrrell, J.A.; Wilcox-Adelman, S.A.; Boucher, Y.; Jain, R.K.; Munn, L.L. Mechanical compression drives cancer cells toward invasive phenotype. Proc. Natl. Acad. Sci. USA 2012, 109, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.Y.; Morison, J.; Dawson, M.A. Epigenetics in the hematologic malignancies. Haematologica 2014, 99, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- Boggs, A.E.; Vitolo, M.I.; Whipple, R.A.; Charpentier, M.S.; Goloubeva, O.G.; Ioffe, O.B.; Tuttle, K.C.; Slovic, J.; Lu, Y.; Mills, G.B.; et al. alpha-Tubulin acetylation elevated in metastatic and basal-like breast cancer cells promotes microtentacle formation, adhesion, and invasive migration. Cancer Res. 2015, 75, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, R.; Gautreau, A. Quantitative and unbiased analysis of directional persistence in cell migration. Nat. Protoc. 2014, 9, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Cortes, A.; Abarca, E.; Silva, L.; Velastegui, E.; Leon-Sosa, A.; Karolys, G.; Cabrera, F.; Caicedo, A. Identification of key proteins in the signaling crossroads between wound healing and cancer hallmark phenotypes. Sci. Rep. 2021, 11, 17245. [Google Scholar] [CrossRef] [PubMed]
- MacCarthy-Morrogh, L.; Martin, P. The hallmarks of cancer are also the hallmarks of wound healing. Sci. Signal 2020, 13, eaay8690. [Google Scholar] [CrossRef]
- Lee, S.; Shanti, A. Effect of Exogenous pH on Cell Growth of Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 9910. [Google Scholar] [CrossRef]
- Bogdanov, A.; Bogdanov, A.; Chubenko, V.; Volkov, N.; Moiseenko, F.; Moiseyenko, V. Tumor acidity: From hallmark of cancer to target of treatment. Front. Oncol. 2022, 12, 979154. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Posener, K.; Negelein, E. Über den Stoffwechsel der Carcinomzelle. Die Naturwissenschaften 1924, 12, 1131–1137. [Google Scholar] [CrossRef]
- Greathouse, K.L.; Wyatt, M.; Johnson, A.J.; Toy, E.P.; Khan, J.M.; Dunn, K.; Clegg, D.J.; Reddy, S. Diet-microbiome interactions in cancer treatment: Opportunities and challenges for precision nutrition in cancer. Neoplasia 2022, 29, 100800. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Thomson, C.A.; Sullivan, K.R.; Howe, C.L.; Kushi, L.H.; Caan, B.J.; Neuhouser, M.L.; Bandera, E.V.; Wang, Y.; Robien, K.; et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA A Cancer J. Clin. 2022, 72, 230–262. [Google Scholar] [CrossRef] [PubMed]
- Regenstein, J.M.; Chaudry, M.M.; Regenstein, C.E. The Kosher and Halal Food Laws. Compr. Rev. Food Sci. Food Saf. 2003, 2, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Crowder, S.L.; Jim, H.S.L.; Hogue, S.; Carson, T.L.; Byrd, D.A. Gut microbiome and cancer implications: Potential opportunities for fermented foods. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188897. [Google Scholar] [CrossRef] [PubMed]
- Al-Qadami, G.H.; Secombe, K.R.; Subramaniam, C.B.; Wardill, H.R.; Bowen, J.M. Gut Microbiota-Derived Short-Chain Fatty Acids: Impact on Cancer Treatment Response and Toxicities. Microorganisms 2022, 10, 2048. [Google Scholar] [CrossRef]
- Nobs, S.P.; Elinav, E. A microbial iron fist to fight tumors. Nat. Immunol. 2024, 25, 720–721. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, A.; Kim, I.; Cha, D.G.; Kim, S.; Park, E.S.; Noh, J.G.; Lee, J.; Ku, J.H.; Choi, Y.H.; et al. A dietary commensal microbe enhances antitumor immunity by activating tumor macrophages to sequester iron. Nat. Immunol. 2024, 25, 790–801. [Google Scholar] [CrossRef]
- Iwanaga, S. Biochemical principle of Limulus test for detecting bacterial endotoxins. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2007, 83, 110–119. [Google Scholar] [CrossRef]
- Vernen, F.; Harvey, P.J.; Dias, S.A.; Veiga, A.S.; Huang, Y.H.; Craik, D.J.; Lawrence, N.; Troeira Henriques, S. Characterization of Tachyplesin Peptides and Their Cyclized Analogues to Improve Antimicrobial and Anticancer Properties. Int. J. Mol. Sci. 2019, 20, 4184. [Google Scholar] [CrossRef] [PubMed]
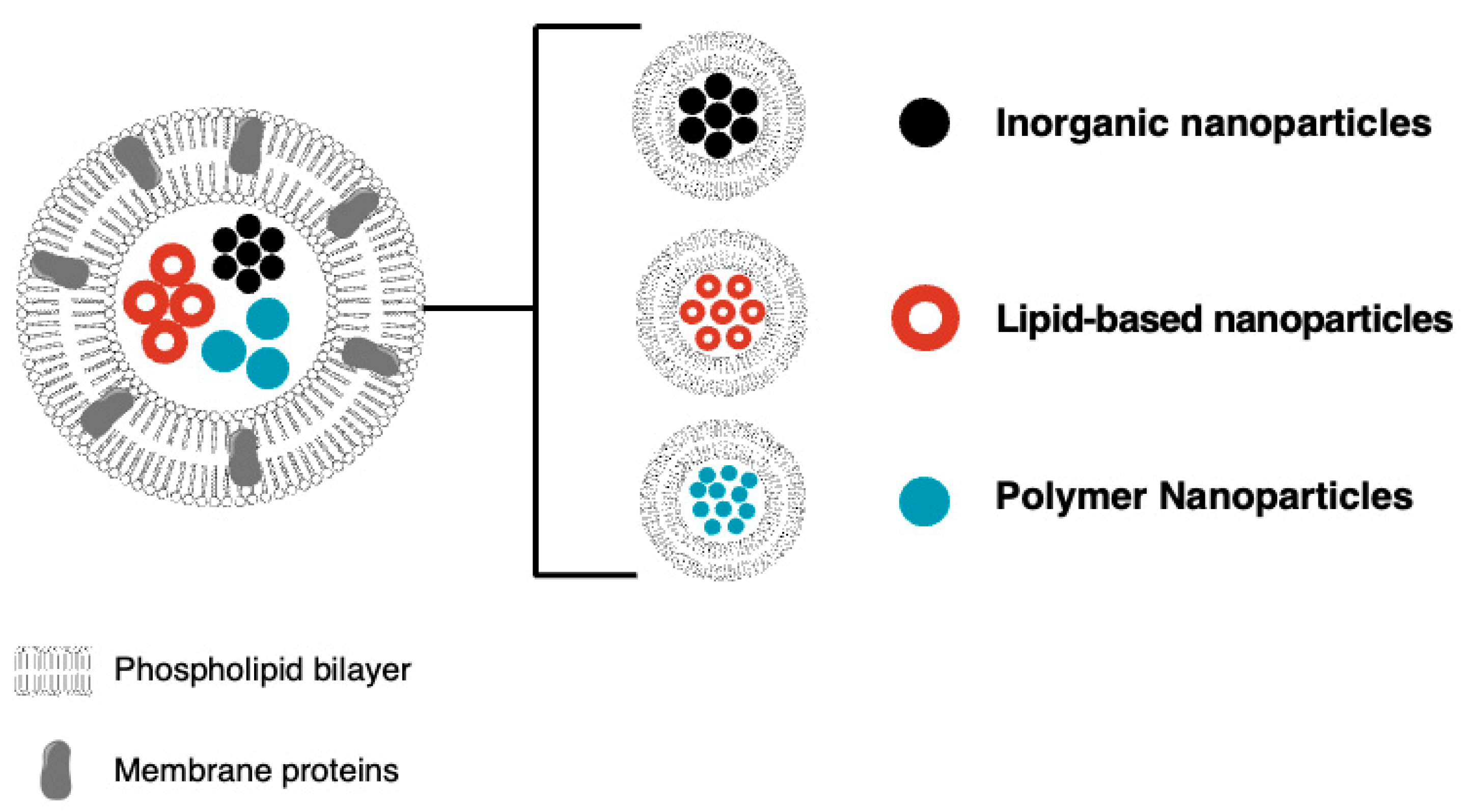

| Biomimetic Drug Delivery System | Drug Name | Ref. |
|---|---|---|
| Liposomes | Doxorubicin (Doxil) | [51,52] |
| Daunorubicin (DaunoXome) | [53] | |
| Polymeric Micelles | Paclitaxel (Genexol-PM) | [54,55] |
| Dendrimers | Cisplatin, Methotrexate | [56] |
| Viral-like Particles (VLPs) | Nucleic acids for gene therapy | [57] |
| Exosomes | Chemotherapeutics, Proteins | [58,59,60] |
| Cell-Penetrating Peptides (CPPs) | Various drugs and biomolecules | [61] |
| Biomimetic Nanoparticles | Imaging agents, Chemotherapeutics | [62] |
| Biomimetic Membranes | Antibiotics, Bioactive molecules | [63,64] |
| Biomimetic Enzyme Mimetics | Prodrug activators | [65] |
| Biomimetic Scaffolds | Growth factors, Antibiotics | [66,67] |
| Biomimetic Prodrugs | Capecitabine | [68] |
| Biomimetic Surface Coatings | Drug-eluting stents (e.g., sirolimus, paclitaxel) | [25,69] |
| Biomimetic Application | Key Functionality | Inspiration from Nature | Key Properties of Polymer Nanocomposites | Example | Ref. |
|---|---|---|---|---|---|
| Drug Delivery | Controlled Release, Targeting | Drug-carrying viruses, Release based on specific stimuli | Biocompatibility, Degradation, Targeting ligands | Stimuli-responsive polymer nanocomposites for targeted drug delivery to cancer cells (e.g., pH-sensitive polymers releasing drugs in the acidic tumor microenvironment) | [76] |
| Tissue Engineering | Cell Adhesion, Proliferation, Differentiation | Extracellular matrix, Scaffolding for tissue growth | Biocompatibility, Mechanical properties (Mimicking target tissue), Controlled porosity | Biomimetic scaffolds mimicking natural bone structure for bone regeneration (e.g., scaffolds with interconnected pores mimicking bone) | [109] |
| Biosensors | Biorecognition, Signal Transduction | Biological receptors, Enzyme–substrate interactions | Biocompatibility, Selectivity, Sensitivity | Nanocomposite-based sensors for glucose detection mimicking taste receptors (e.g., incorporating enzymes that react with glucose) | [40] |
| Gene Delivery | Efficient Gene Delivery, Controlled Release | Viral vectors for gene transfer | Biocompatibility, Low immunogenicity, Controlled release of genetic material | Polymer nanocomposites for siRNA delivery to silence genes involved in diseases (e.g., using cationic polymers to complex with negatively charged siRNA) | [110] |
| Implants | Improved Biocompatibility, Enhanced Osseointegration | Natural bone structure, Load-bearing capacity | Biocompatibility, Mechanical strength (Mimicking bone), Osseoconductive properties | Biomimetic hydroxyapatite-based implants for improved bone bonding (e.g., mimicking the mineral component of bone) | [111,112,113,114] |
| Antibacterial Coatings |
Bacterial Adhesion Inhibition, Biofilm Prevention | Antimicrobial peptides on insect wings |
Biocompatibility, Antimicrobial activity, Surface modification for long-lasting effect | Polymer nanocomposite coatings incorporating natural antimicrobial peptides to prevent bacterial infections on medical devices | [115] |
| Wound Healing Dressings |
Enhanced Healing, Reduced Inflammation |
Skin barrier function, Moist wound environment |
Biocompatibility, Controlled drug release, Biodegradability | Biomimetic wound dressings mimicking the skin’s barrier function while promoting healing and reducing inflammation | [116] |
| Hemostatic Materials |
Blood Clotting Acceleration, Reduced Bleeding |
Platelet aggregation, Blood clotting cascade |
Biocompatibility, Hemostatic activity, Shape conformity | Polymer nanocomposites mimicking the structure and function of platelets to accelerate blood clotting at wound sites | [117,118,119] |
| Biomimetic Enzymes |
Targeted Enzyme Therapy, Enhanced Biocatalysis |
Natural enzymes with high efficiency and specificity | Biocompatibility, Enzyme immobilization, Controlled activity | Polymer nanocomposite-based artificial enzymes mimicking natural enzymes for targeted treatment of diseases | [38,120] |
| Biomimetic Hydrogels |
Tissue Regeneration, Drug Delivery |
Extracellular matrix, Natural hydrogels |
Biocompatibility, Biodegradability, Tunable mechanical properties | Biomimetic hydrogels mimicking the properties of the extracellular matrix for tissue engineering and controlled drug delivery | [16,27,46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshehri, A.M.; Wilson, O.C., Jr. Biomimetic Hydrogel Strategies for Cancer Therapy. Gels 2024, 10, 437. https://doi.org/10.3390/gels10070437
Alshehri AM, Wilson OC Jr. Biomimetic Hydrogel Strategies for Cancer Therapy. Gels. 2024; 10(7):437. https://doi.org/10.3390/gels10070437
Chicago/Turabian StyleAlshehri, Awatef M., and Otto C. Wilson, Jr. 2024. "Biomimetic Hydrogel Strategies for Cancer Therapy" Gels 10, no. 7: 437. https://doi.org/10.3390/gels10070437
APA StyleAlshehri, A. M., & Wilson, O. C., Jr. (2024). Biomimetic Hydrogel Strategies for Cancer Therapy. Gels, 10(7), 437. https://doi.org/10.3390/gels10070437







