Streamlining Skin Regeneration: A Ready-To-Use Silk Bilayer Wound Dressing
Abstract
:1. Introduction
2. Results and Discussion
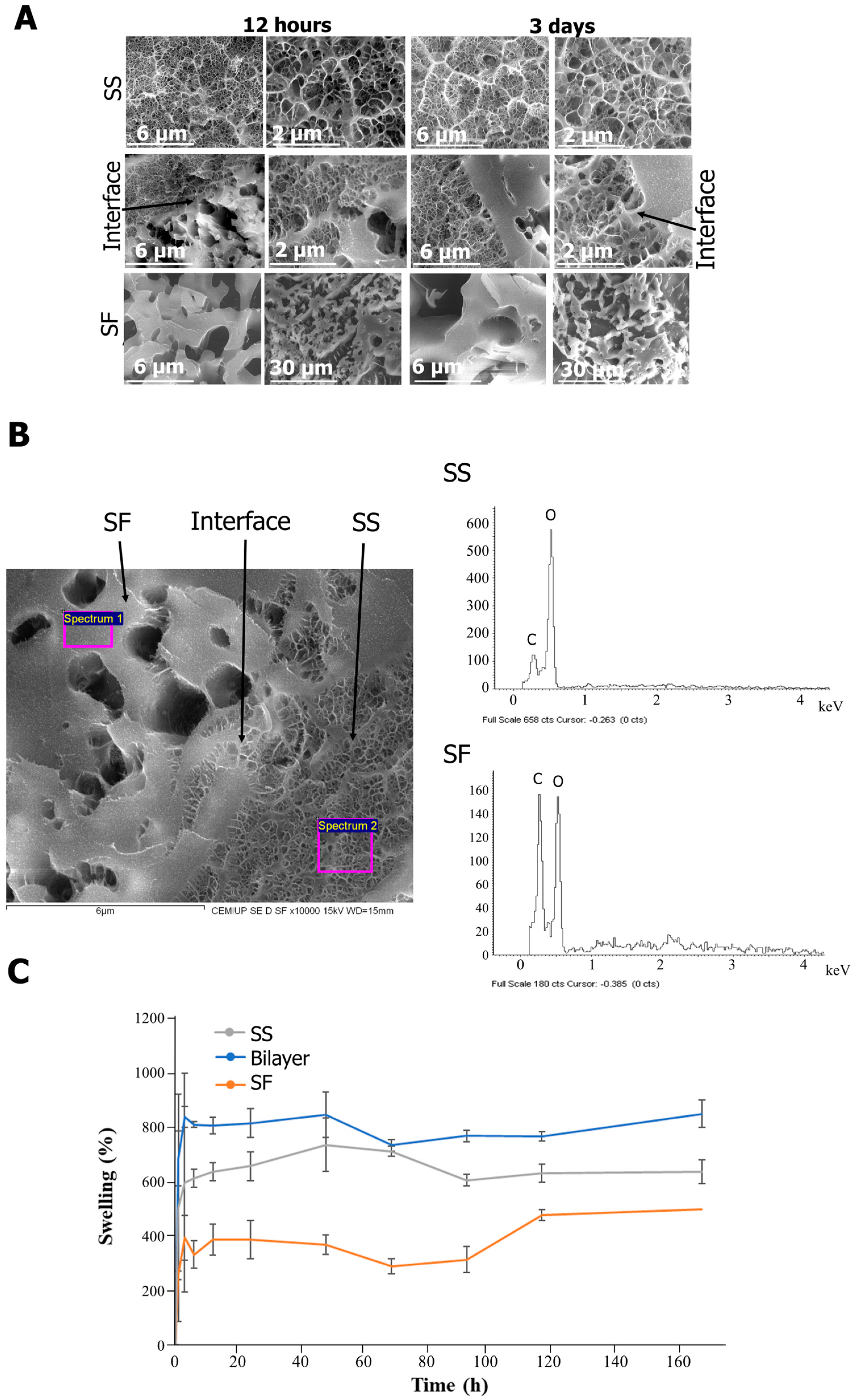
2.1. Structure and Composition
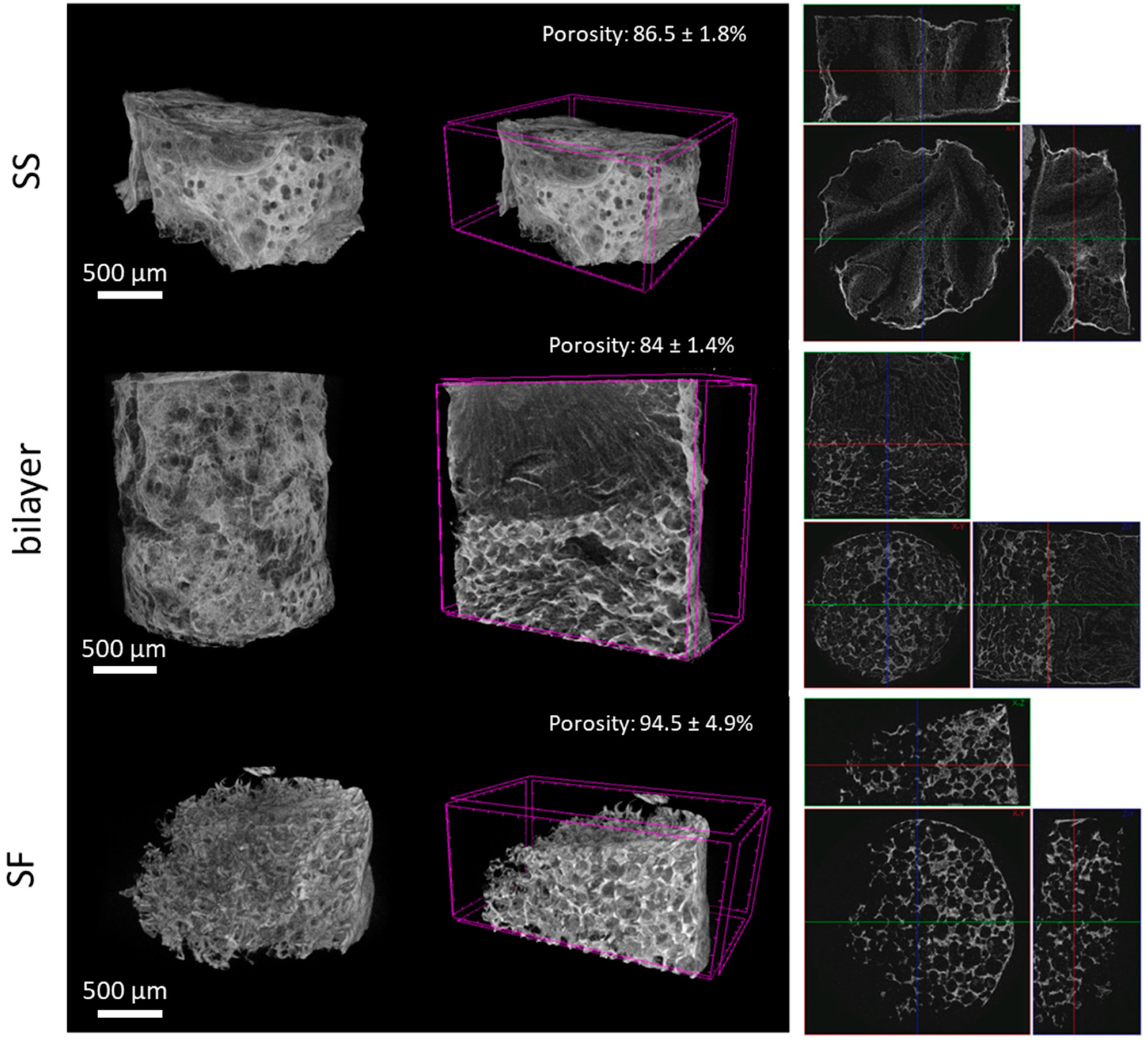
2.2. Rehydration Capacity and Porosity
2.3. Mechanical Properties
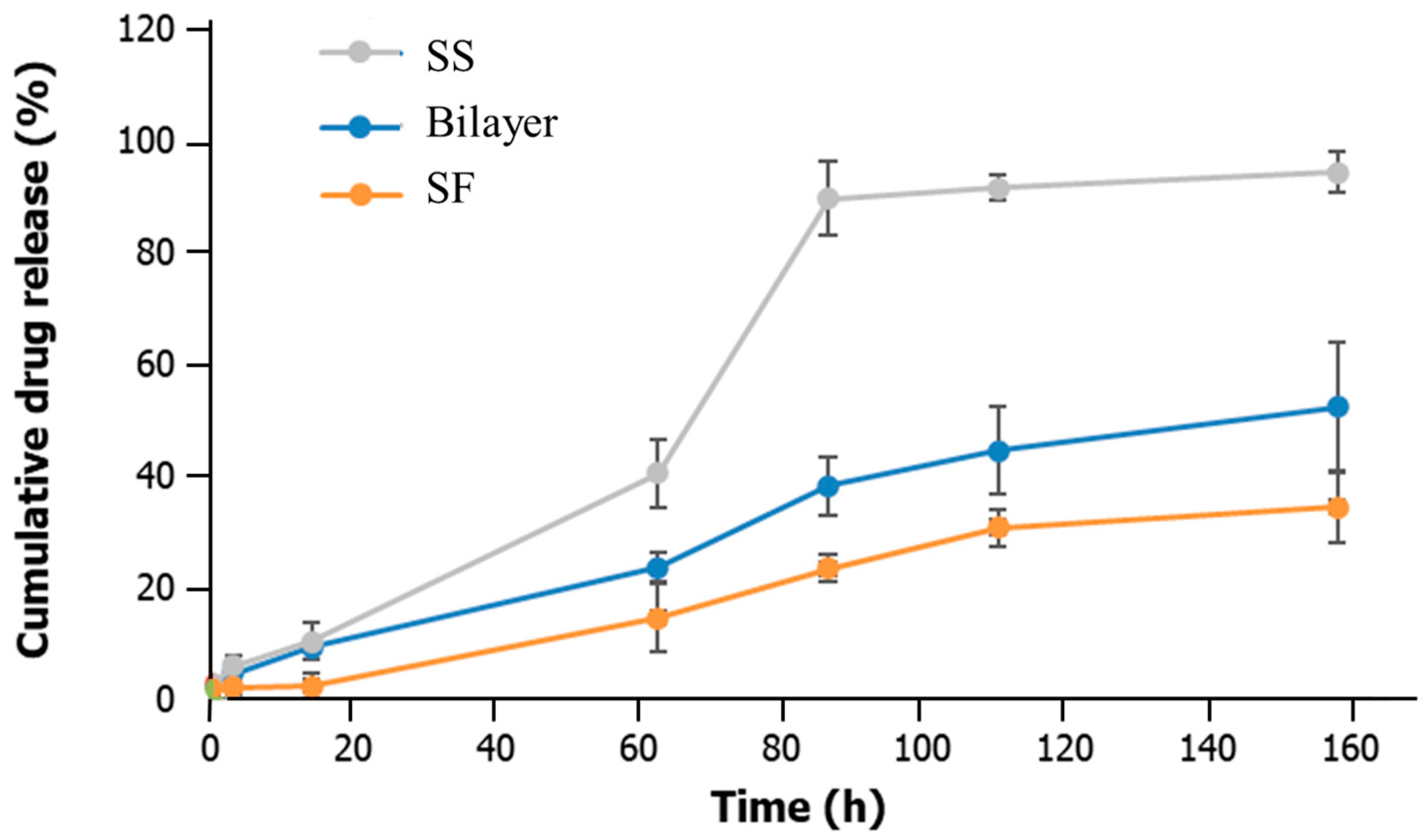
2.4. Drug Delivery Capacity
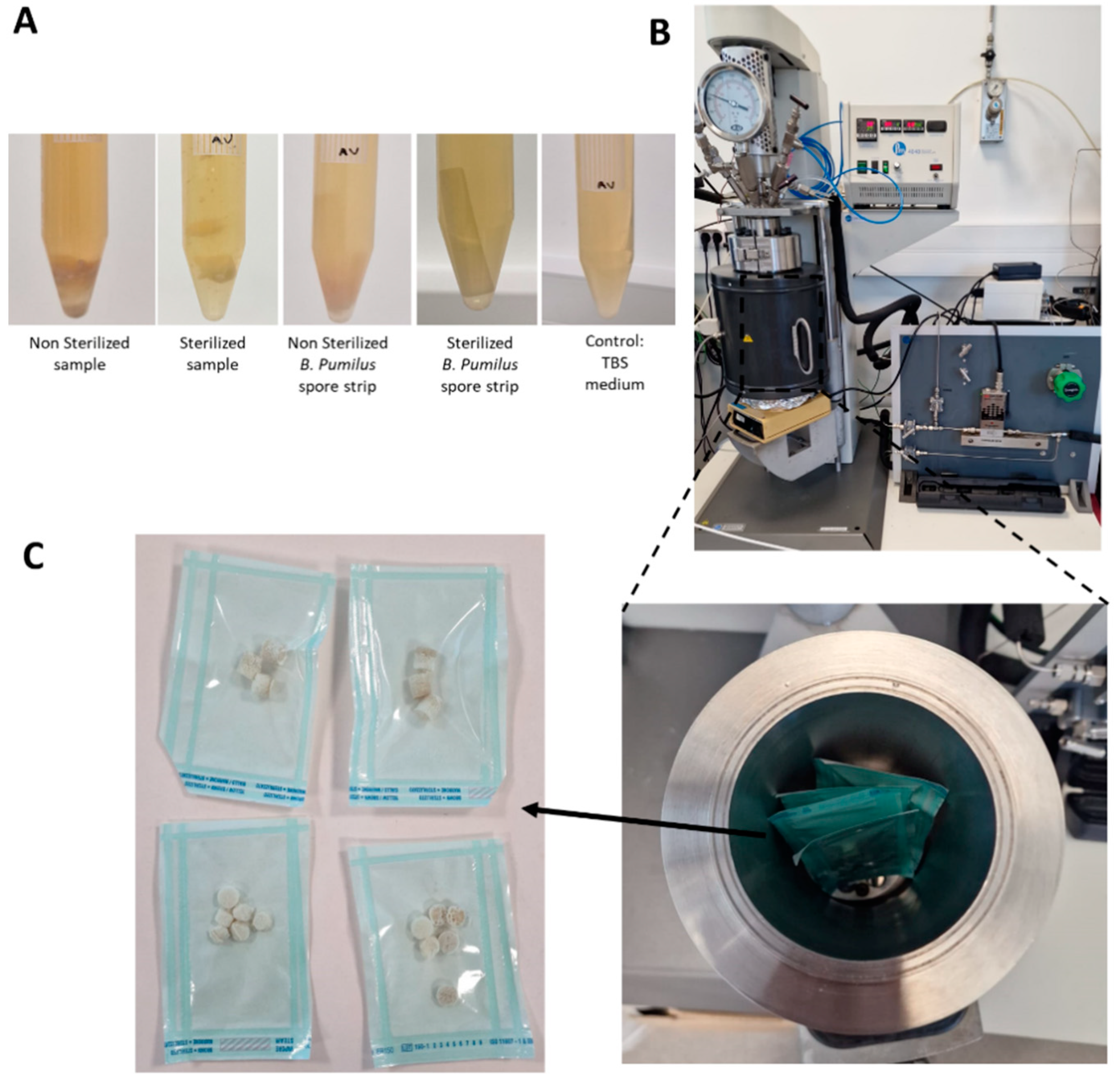
2.5. Sterility Tests
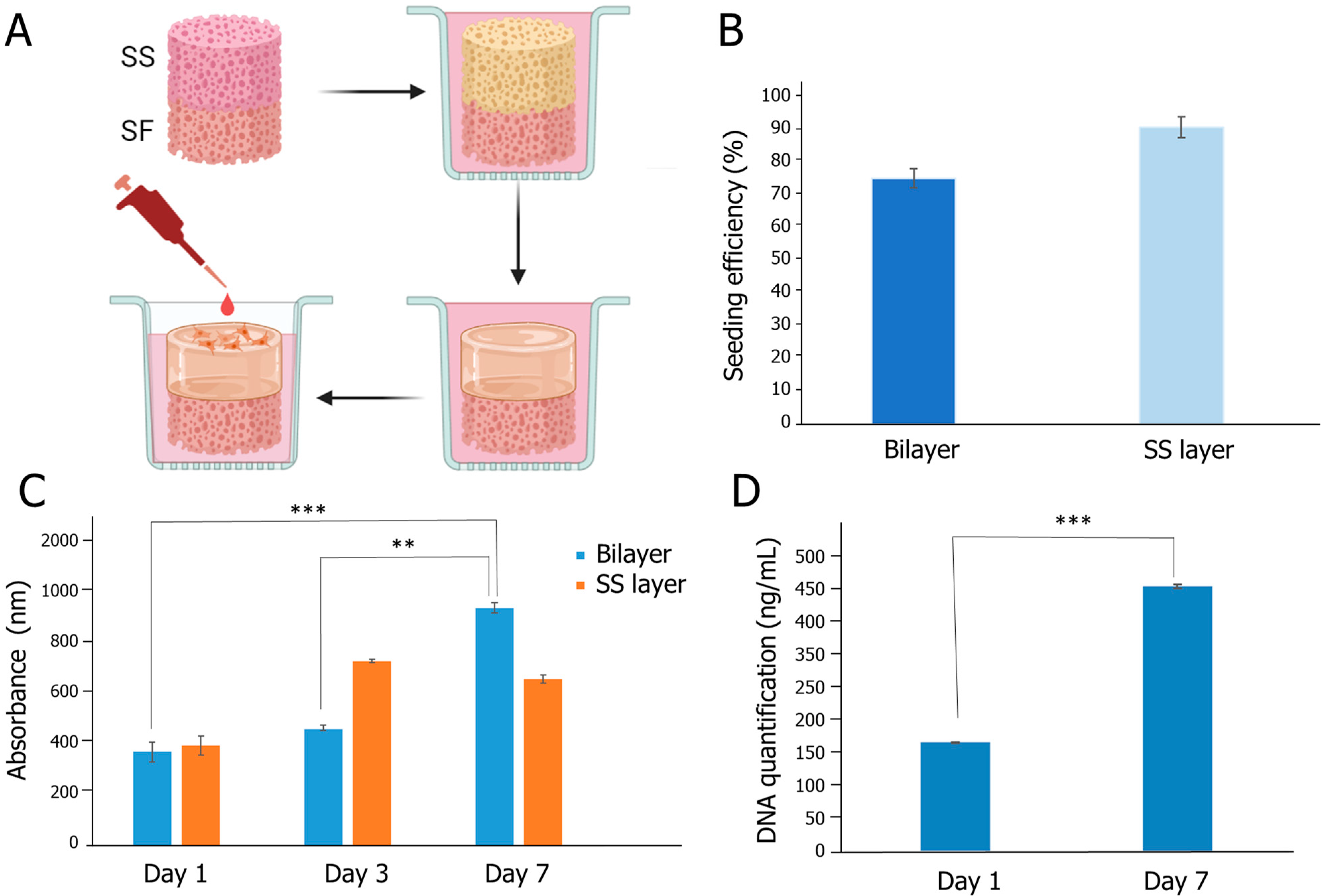
2.6. Biological Evaluation
3. Conclusions
4. Materials and Methods
4.1. Scaffold Production
4.1.1. Sericin Extraction and Processing
4.1.2. Fibroin Extraction and Processing
4.1.3. SS/SF Bilayer Assembly
4.1.4. Supercritical CO2 (sCO2) Sterilization
4.2. Physicochemical Characterization
4.2.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
4.2.2. Scanning Electron Microscopy (SEM), Cryo-Scanning Electron Microscopy (Cryo-SEM), and Energy Dispersive Spectroscopy (EDS)
4.2.3. Microcomputed Tomography (Micro-CT)
4.2.4. Mechanical Properties
4.2.5. Drug Delivery Capacity
4.2.6. Swelling Properties
4.2.7. Sterility Assessment
4.3. In Vitro Biological Assessment
4.3.1. Cell Culture
4.3.2. Micro Seeding
4.3.3. Cell Metabolic Activity
4.3.4. Fluorescence Staining
4.3.5. Cell Proliferation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins-Green, M. Cutaneous chronic wounds: A worldwide silent epidemic. Open Access Gov. 2023, 38, 51–53. [Google Scholar] [CrossRef]
- Schlottmann, F.; Bucan, V.; Vogt, P.M.; Krezdorn, N. A short history of skin grafting in burns: From the gold standard of autologous skin grafting to the possibilities of allogeneic skin grafting with immunomodulatory approaches. Medicina 2021, 57, 225. [Google Scholar] [CrossRef] [PubMed]
- Gefen, A.; Alves, P.; Beeckman, D.; Lázaro-Martínez, J.L.; Lev-Tov, H.; Najafi, B.; Swanson, T.; Woo, K. Mechanical and contact characteristics of foam materials within wound dressings: Theoretical and practical considerations in treatment. Int. Wound J. 2023, 20, 1960–1978. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, M.S.; Cybulska, A.M.; Skonieczna-Żydecka, K.; Augustyniuk, K.; Grochans, E.; Karakiewicz, B. Effectiveness of Hydrocolloid Dressings for Treating Pressure Ulcers in Adult Patients: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 7881. [Google Scholar] [CrossRef]
- Kalantari, K.; Mostafavi, E.; Afifi, A.M.; Izadiyan, Z.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Wound dressings functionalized with silver nanoparticles: Promises and pitfalls. Nanoscale 2020, 12, 2268–2291. [Google Scholar] [CrossRef]
- Rajendran, S.; Anand, S. Insight into the development of non-adherent, absorbent dressings. J. Wound Care 2002, 11, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef]
- Luneva, O.; Olekhnovich, R.; Uspenskaya, M. Bilayer Hydrogels for Wound Dressing and Tissue Engineering. Polymers 2022, 14, 3135. [Google Scholar] [CrossRef]
- Yan, L.P.; Oliveira, J.M.; Oliveira, A.L.; Reis, R.L. Silk fibroin/cano-CaP bilayered scaffolds for osteochondral Tissue Engineering. Key Eng. Mater. 2013, 587, 245–248. [Google Scholar] [CrossRef]
- Andrews, K.L.; Derby, K.M.; Jacobson, T.M.; Sievers, B.A.; Kiemele, L.J. Prevention and Management of Chronic Wounds. In Braddom’s Physical Medicine and Rehabilitation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 469–484.e4. [Google Scholar] [CrossRef]
- Wietlisbach, C.M. Wound Care. In Cooper’s Fundamentals of Hand Therapy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 154–166. [Google Scholar] [CrossRef]
- Peng, S.; Liang, Y.; Xiao, W.; Liu, Y.; Yu, M.; Liu, L. Anaphylaxis induced by intra-articular injection of chitosan: A case report and literature review. Clin. Case Rep. 2022, 10, e6596. [Google Scholar] [CrossRef]
- Fu, S.; Thacker, A.; Sperger, D.M.; Boni, R.L.; Velankar, S.; Munson, E.J.; Block, L.H. Rheological Evaluation of Inter-grade and Inter-batch Variability of Sodium Alginate. AAPS PharmSciTech 2010, 11, 1662–1674. [Google Scholar] [CrossRef]
- Ng, V.W.; Chan, J.M.; Sardon, H.; Ono, R.J.; García, J.M.; Yang, Y.Y.; Hedrick, J.L. Antimicrobial hydrogels: A new weapon in the arsenal against multidrug-resistant infections. Adv. Drug Deliv. Rev. 2014, 78, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Willard, J.J.; Drexler, J.W.; Das, A.; Roy, S.; Shilo, S.; Shoseyov, O.; Powell, H.M. Plant-Derived Human Collagen Scaffolds for Skin Tissue Engineering. Tissue Eng. Part A 2013, 19, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, V.; Krithica, N.; Madhan, B.; Sehgal, P.K. Preparation and properties of tannic acid cross-linked collagen scaffold and its application in wound healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101B, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Helary, C.; Abed, A.; Mosser, G.; Louedec, L.; Letourneur, D.; Coradin, T.; Giraud-Guille, M.M.; Meddahi-Pellé, A. Evaluation of dense collagen matrices as medicated wound dressing for the treatment of cutaneous chronic wounds. Biomater. Sci. 2015, 3, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Eskandarinia, A.; Kefayat, A.; Agheb, M.; Rafienia, M.; Baghbadorani, M.A.; Navid, S.; Ebrahimpour, K.; Khodabakhshi, D.; Ghahremani, F. A Novel Bilayer Wound Dressing Composed of a Dense Polyurethane/Propolis Membrane and a Biodegradable Polycaprolactone/Gelatin Nanofibrous Scaffold. Sci. Rep. 2020, 10, 3063. [Google Scholar] [CrossRef] [PubMed]
- Hasatsri, S.; Aungsapat, A.; Aramwit, P. Randomized Clinical Trial of the Innovative Bilayered Wound Dressing Made of Silk and Gelatin: Safety and Efficacy Tests Using a Split-Thickness Skin Graft Model. Evidence-Based Complement. Altern. Med. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Nakipoglu, M.; Özkabadayı, Y.; Karahan, S.; Tezcaner, A. Bilayer wound dressing composed of asymmetric polycaprolactone membrane and chitosan-carrageenan hydrogel incorporating storax balsam. Int. J. Biol. Macromol. 2024, 254, 128020. [Google Scholar] [CrossRef]
- Xu, H.-L.; ZhuGe, D.-L. Silk fibroin nanomaterials. In Biopolymeric Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 171–202. [Google Scholar] [CrossRef]
- Vidya, M.; Rajagopal, S. Silk Fibroin: A Promising Tool for Wound Healing and Skin Regeneration. Int. J. Polym. Sci. 2021, 2021, 9069924. [Google Scholar] [CrossRef]
- Capar, G.; Pilevneli, T.; Yetis, U.; Dilek, F.B. Life cycle assessment of sericin recovery from silk degumming wastewaters. Sustain. Chem. Pharm. 2022, 30, 100889. [Google Scholar] [CrossRef]
- Aramwit, P.; Kanokpanont, S.; Nakpheng, T.; Srichana, T. The Effect of Sericin from Various Extraction Methods on Cell Viability and Collagen Production. Int. J. Mol. Sci. 2010, 11, 2200–2211. [Google Scholar] [CrossRef]
- Suzuki, S.; Sakiragaoglu, O.; Chirila, T.V. Study of the Antioxidative Effects of Bombyx mori Silk Sericin in Cultures of Murine Retinal Photoreceptor Cells. Molecules 2022, 27, 4635. [Google Scholar] [CrossRef]
- Aramwit, P.; Damrongsakkul, S.; Kanokpanont, S.; Srichana, T. Properties and antityrosinase activity of sericin from various extraction methods. Biotechnol. Appl. Biochem. 2010, 55, 91–98. [Google Scholar] [CrossRef]
- Rahimpour, S.; Jabbari, H.; Yousofi, H.; Fathi, A.; Mahmoodi, S.; Jafarian, M.J.; Shomali, N.; Shotorbani, S.S. Regulatory effect of sericin protein in inflammatory pathways; A comprehensive review. Pathol. Res. Pract. 2023, 243, 154369. [Google Scholar] [CrossRef]
- Sano, M.; Tamada, Y.; Niwa, K.; Morita, T.; Yoshino, G. Sulfated sericin is a novel anticoagulant influencing the blood coagulation cascade. J. Biomater. Sci. Polym. Ed. 2009, 20, 773–783. [Google Scholar] [CrossRef]
- Kaewkorn, W.; Limpeanchob, N.; Tiyaboonchai, W.; Pongcharoen, S.; Sutheerawattananonda, M. Effects of silk sericin on the proliferation and apoptosis of colon cancer cells. Biol. Res. 2012, 45, 45–50. [Google Scholar] [CrossRef]
- Javali, U.C.; Padaki, N.V.; Das, B.; Malali, K.B. Developments in the use of silk by-products and silk waste. In Advances in Silk Science and Technology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 261–270. [Google Scholar] [CrossRef]
- Siavashani, A.Z.; Mohammadi, J.; Rottmar, M.; Senturk, B.; Nourmohammadi, J.; Sadeghi, B.; Huber, L.; Maniura-Weber, K. Silk fibroin/sericin 3D sponges: The effect of sericin on structural and biological properties of fibroin. Int. J. Biol. Macromol. 2020, 153, 317–326. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Bernardes, B.G.; Borges, S.; Rodrigues, I.; Fernandes, R.; Gomes-Guerreiro, S.; Pinto, M.T.; Pintado, M.; Soares, R.; Costa, R.; et al. Exploring Silk Sericin for Diabetic Wounds: An In Situ-Forming Hydrogel to Protect against Oxidative Stress and Improve Tissue Healing and Regeneration. Biomolecules 2022, 12, 801. [Google Scholar] [CrossRef]
- Tao, G.; Cai, R.; Wang, Y.; Liu, L.; Zuo, H.; Zhao, P.; Umar, A.; Mao, C.; Xia, Q.; He, H. Bioinspired design of AgNPs embedded silk sericin-based sponges for efficiently combating bacteria and promoting wound healing. Mater. Des. 2019, 180, 107940. [Google Scholar] [CrossRef]
- White, A.; Burns, D.; Christensen, T.W. Effective terminal sterilization using supercritical carbon dioxide. J. Biotechnol. 2006, 123, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, N.; Soares, G.C.; Santos-Rosales, V.; Concheiro, A.; Alvarez-Lorenzo, C.; García-González, C.A.; Oliveira, A.L. A new era for sterilization based on supercritical CO2 technology. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 399–428. [Google Scholar] [CrossRef] [PubMed]
- Santos-Rosales, V.; Magariños, B.; Starbird, R.; Suárez-González, J.; Fariña, J.B.; Alvarez-Lorenzo, C.; García-González, C.A. Supercritical CO2 technology for one-pot foaming and sterilization of polymeric scaffolds for bone regeneration. Int. J. Pharm. 2021, 605, 120801. [Google Scholar] [CrossRef]
- Ribeiro, V.P.; Costa, J.B.; Carneiro, S.M.; Pina, S.; Veloso, A.C.A.; Reis, R.L.; Oliveira, J.M. Bioinspired Silk Fibroin-Based Composite Grafts as Bone Tunnel Fillers for Anterior Cruciate Ligament Reconstruction. Pharmaceutics 2022, 14, 697. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.P.; Pina, S.; Costa, J.B.; Cengiz, I.F.; García-Fernández, L.; Fernández-Gutiérrez, M.d.M.; Paiva, O.C.; Oliveira, A.L.; San-Román, J.; Oliveira, J.M.; et al. Enzymatically Cross-Linked Silk Fibroin-Based Hierarchical Scaffolds for Osteochondral Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 3781–3799. [Google Scholar] [CrossRef] [PubMed]
- Haris, P.I. Infrared Spectroscopy of Protein Structure. In Encyclopedia of Biophysics; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1095–1106. [Google Scholar] [CrossRef]
- Chiang, K.-Y.; Matsumura, F.; Yu, C.-C.; Qi, D.; Nagata, Y.; Bonn, M.; Meister, K. True Origin of Amide I Shifts Observed in Protein Spectra Obtained with Sum Frequency Generation Spectroscopy. J. Phys. Chem. Lett. 2023, 14, 4949–4954. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Purwar, R. Influence of metal oxide nanoparticles on morphological, structural, rheological and conductive properties of mulberry silk fibroin nanocomposite solutions. Polym. Test. 2021, 93, 106916. [Google Scholar] [CrossRef]
- Lan, Z.; Kar, R.; Chwatko, M.; Shoga, E.; Cosgriff-Hernandez, E. High porosity PEG-based hydrogel foams with self-tuning moisture balance as chronic wound dressings. J. Biomed. Mater. Res. Part A 2023, 111, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.P.; Reagan, M.R.; Bohara, R.A. Silk fibroin and silk-based biomaterial derivatives for ideal wound dressings. Int. J. Biol. Macromol. 2020, 164, 4613–4627. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, Y.; Wang, L.; Liu, D.; Qin, C.; Shi, Y. A review of preparation methods of porous skin tissue engineering scaffolds. Mater. Today Commun. 2022, 32, 104109. [Google Scholar] [CrossRef]
- Xu, R.; Xia, H.; He, W.; Li, Z.; Zhao, J.; Liu, B.; Wang, Y.; Lei, Q.; Kong, Y.; Bai, Y.; et al. Controlled water vapor transmission rate promotes wound-healing via wound re-epithelialization and contraction enhancement. Sci. Rep. 2016, 6, 24596. [Google Scholar] [CrossRef]
- Yannas, I.V.; Lee, E.; Orgill, D.P.; Skrabut, E.M.; Murphy, G.F. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. USA 1989, 86, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Doillon, C.J.; Whyne, C.F.; Brandwein, S.; Silver, F.H. Collagen-based wound dressings: Control of the pore structure and morphology. J. Biomed. Mater. Res. 1986, 20, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Henderson, P.W.; Choi, N.W.; Bonassar, L.J.; Spector, J.A.; Stroock, A.D. Microstructured templates for directed growth and vascularization of soft tissue in vivo. Biomaterials 2011, 32, 5391–5401. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Hong, K.D.; Shin, H.W.; Kim, S.H.; Kim, S.H.; Lee, M.S.; Jang, W.Y.; Khang, G.; Lee, H.B. Preparation of porcine small intestinal submucosa sponge and their application as a wound dressing in full-thickness skin defect of rat. Int. J. Biol. Macromol. 2005, 36, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Kunz, R.I.; Brancalhão, R.M.C.; Ribeiro, L.d.F.C.; Natali, M.R.M. Silkworm sericin: Properties and biomedical applications. BioMed Res. Int. 2016, 2016, 8175701. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Aramwit, P. Sustainable Uses of Byproducts from Silk Processing, 1st ed.; Wiley: Hoboken, NJ, USA, 2021; ISBN 978-3-527-34786-5. [Google Scholar]
- Tosun, N.G.; Ozer, A.; Bektas, T.; Oksuz, K.E.; Tayhan, S.E.; Ozdemir, T. Silk sericin-hydroxyapatite nanoribbons toward structurally stable osteogenic scaffolds. J. Aust. Ceram. Soc. 2023, 59, 1291–1301. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Ju, H.W.; Moon, B.M.; Park, H.J.; Kim, J.H.; Lee, O.J.; Park, C.H. A novel approach to fabricate silk nanofibers containing hydroxyapatite nanoparticles using a three-way stopcock connector. Nanoscale Res. Lett. 2013, 8, 303. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Borges, S.; Costa-Pinto, A.R.; Costa, R.; Amorim, M.; Dias, J.R.; Ramos, Ó.; Alves, P.; Granja, P.L.; Soares, R.; et al. In Situ Forming Silk Sericin-Based Hydrogel: A Novel Wound Healing Biomaterial. ACS Biomater. Sci. Eng. 2021, 7, 1573–1586. [Google Scholar] [CrossRef]
- Minsart, M.; Van Vlierberghe, S.; Dubruel, P.; Mignon, A. Commercial wound dressings for the treatment of exuding wounds: An in-depth physico-chemical comparative study. Burn. Trauma 2022, 10, tkac024. [Google Scholar] [CrossRef]
- Mutlu, G.; Calamak, S.; Ulubayram, K.; Guven, E. Curcumin-loaded electrospun PHBV nanofibers as potential wound-dressing material. J. Drug Deliv. Sci. Technol. 2018, 43, 185–193. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Jones, V.; Grey, J.E.; Harding, K.G. Wound dressings. Br. Med. J. 2006, 332, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Gefen, A.; Alves, P.; Beeckman, D.; Cullen, B.; Lázaro-Martínez, J.L.; Lev-Tov, H.A.; Najafi, B.; Santamaria, N.; Sharpe, A.; Swanson, T.; et al. How Should Clinical Wound Care and Management Translate to Effective Engineering Standard Testing Requirements from Foam Dressings? Mapping the Existing Gaps and Needs. Adv. Wound Care 2024, 13, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Beer, F.P. Mechanics of Materials; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Sadighi, M.; Salami, S. An investigation on low-velocity impact response of elastomeric & crushable foams. Open Eng. 2012, 2, 627–637. [Google Scholar] [CrossRef]
- Ohura, N.; Ichioka, S.; Nakatsuka, T.; Shibata, M. Evaluating dressing materials for the prevention of shear force in the treatment of pressure ulcers. J. Wound Care 2005, 14, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Eskandarinia, A.; Morowvat, M.H.; Niknezhad, S.V.; Baghbadorani, M.A.; Michálek, M.; Chen, S.; Nemati, M.M.; Negahdaripour, M.; Heidari, R.; Azadi, A.; et al. A photocrosslinkable and hemostatic bilayer wound dressing based on gelatin methacrylate hydrogel and polyvinyl alcohol foam for skin regeneration. Int. J. Biol. Macromol. 2024, 266, 131231. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, H.; Zhou, Y.; Yu, Z.; Yuan, H.; Feng, B.; van Rijn, P.; Zhang, Y. Alkali-Mediated Miscibility of Gelatin/Polycaprolactone for Electrospinning Homogeneous Composite Nanofibers for Tissue Scaffolding. Macromol. Biosci. 2017, 17, 1700268. [Google Scholar] [CrossRef]
- Sadeghi, A.; Fatemi, M.J.; Zandi, M.; Bagheri, T.; Ghadimi, T.; Tamimi, M.; Pezeshki-Modaress, M. Multilayered 3-D nanofibrous scaffold with chondroitin sulfate sustained release as dermal substitute. Int. J. Biol. Macromol. 2022, 206, 718–729. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, J.; Sun, B.; Jin, Q.; Li, H.; Xia, C.; Wang, H.; Mo, X.; Wu, J. Harnessing electrospun nanofibers to recapitulate hierarchical fibrous structures of meniscus. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-P.; Oliveira, J.M.; Oliveira, A.L.; Reis, R.L. Core-shell silk hydrogels with spatially tuned conformations as drug-delivery system. J. Tissue Eng. Regen. Med. 2017, 11, 3168–3177. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Santschi, M.; Ferguson, S.J. A Biomimetic Macroporous Hybrid Scaffold with Sustained Drug Delivery for Enhanced Bone Regeneration. Biomacromolecules 2021, 22, 2460–2471. [Google Scholar] [CrossRef]
- Pignatelli, C.; Perotto, G.; Nardini, M.; Cancedda, R.; Mastrogiacomo, M.; Athanassiou, A. Electrospun silk fibroin fibers for storage and controlled release of human platelet lysate. Acta Biomater. 2018, 73, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Wongpanit, P.; Ueda, H.; Tabata, Y.; Rujiravanit, R. In Vitro and In Vivo Release of Basic Fibroblast Growth Factor Using a Silk Fibroin Scaffold as Delivery Carrier. J. Biomater. Sci. Polym. Ed. 2010, 21, 1403–1419. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Peng, X.; Zheng, Y.; Cheng, Z.; Sun, S.; Ding, Q.; Liu, W.; Ding, C. A poloxamer/hyaluronic acid/chitosan-based thermosensitive hydrogel that releases dihydromyricetin to promote wound healing. Int. J. Biol. Macromol. 2022, 216, 475–486. [Google Scholar] [CrossRef]
- Zhang, Y.; Tangfeng, W.; Shen, C.; Xu, G.; Chen, H.; Yan, H.; Xiong, M.; Zhang, G. A Robust Sericin Hydrogel Formed by a Native Sericin from Silkworm Bodies. Fibers Polym. 2022, 23, 1826–1833. [Google Scholar] [CrossRef]
- Yan, C.; Liang, J.; Fang, H.; Meng, X.; Chen, J.; Zhong, Z.; Liu, Q.; Hu, H.; Zhang, X. Fabrication and Evaluation of Silk Sericin-Derived Hydrogel for the Release of the Model Drug Berberine. Gels 2021, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qi, C.; Tao, K.; Zhang, J.; Zhang, J.; Xu, L.; Jiang, X.; Zhang, Y.; Huang, L.; Li, Q.; et al. Sericin/Dextran Injectable Hydrogel as an Optically Trackable Drug Delivery System for Malignant Melanoma Treatment. ACS Appl. Mater. Interfaces 2016, 8, 6411–6422. [Google Scholar] [CrossRef]
- Shitole, M.; Dugam, S.; Tade, R.; Nangare, S. Pharmaceutical applications of silk sericin. Ann. Pharm. Fr. 2020, 78, 469–486. [Google Scholar] [CrossRef]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Zheng, Y.; Cui, D.; Haick, H. Multifunctional Dressing for Wound Diagnosis and Rehabilitation. Adv. Health Mater. 2021, 10, 2101292. [Google Scholar] [CrossRef]
- Siritientong, T.; Srichana, T.; Aramwit, P. The effect of sterilization methods on the physical properties of silk sericin scaffolds. AAPS PharmSciTech 2011, 12, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Silk sericin-based materials for biomedical applications. Biomaterials 2022, 287, 121638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; He, X.; Fang, A.; Jiang, R.; Wu, T.; Chen, H.; Cao, X.; Liang, P.; Xia, D.; et al. A sterile self-assembled sericin hydrogel via a simple two-step process. Polym. Test. 2019, 80, 106016. [Google Scholar] [CrossRef]
- Foster, O.; Shaidani, S.; Theodossiou, S.K.; Falcucci, T.; Hiscox, D.; Smiley, B.M.; Romano, C.; Kaplan, D.L. Sudan Black B Pretreatment to Suppress Autofluorescence in Silk Fibroin Scaffolds. ACS Biomater. Sci. Eng. 2023, 9, 3193–3205. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Luo, G.; Xia, H.; He, W.; Zhao, J.; Liu, B.; Tan, J.; Zhou, J.; Liu, D.; Wang, Y.; et al. Novel bilayer wound dressing composed of silicone rubber with particular micropores enhanced wound re-epithelialization and contraction. Biomaterials 2015, 40, 1–11. [Google Scholar] [CrossRef]
- Karizmeh, M.S.; Poursamar, S.A.; Kefayat, A.; Farahbakhsh, Z.; Rafienia, M. An in vitro and in vivo study of PCL/chitosan electrospun mat on polyurethane/propolis foam as a bilayer wound dressing. Mater. Sci. Eng. C 2022, 135, 112667. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-Y.; Um, I.C.; Kim, M.-K.; Kwon, K.-J.; Kim, S.-G.; Park, Y.-W. Effectiveness of Woven Silk Dressing Materials on Full-skin Thickness Burn Wounds in Rat Model. Maxillofac. Plast. Reconstr. Surg. 2014, 36, 280–284. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Chen, J.; Wang, L.; Gui, X.; Ran, J.; Xu, G.; Zhao, H.; Zeng, M.; Ji, J.; et al. Silk Fibroin Biomaterial Shows Safe and Effective Wound Healing in Animal Models and a Randomized Controlled Clinical Trial. Adv. Health Mater. 2017, 6, 1700121. [Google Scholar] [CrossRef]
- Guo, P.; Du, P.; Zhao, P.; Chen, X.; Liu, C.; Du, Y.; Li, J.; Tang, X.; Yang, F.; Lv, G. Regulating the mechanics of silk fibroin scaffolds promotes wound vascularization. Biochem. Biophys. Res. Commun. 2021, 574, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, T.; Yuan, X.-F.; Bayat, A. Electrospun silk fibroin fiber diameter influences in vitro dermal fibroblast behavior and promotes healing of ex vivo wound models. J. Tissue Eng. 2014, 5, 204173141455166. [Google Scholar] [CrossRef]
- Lu, J.; Fan, X.; Hu, J.; Li, J.; Rong, J.; Wang, W.; Chen, Y.; Liu, W.; Chen, J.; Chen, Y. Construction and function of robust and moist bilayer chitosan-based hydrogel wound dressing. Mater. Des. 2023, 226, 111604. [Google Scholar] [CrossRef]
- Kanokpanont, S.; Damrongsakkul, S.; Ratanavaraporn, J.; Aramwit, P. An innovative bi-layered wound dressing made of silk and gelatin for accelerated wound healing. Int. J. Pharm. 2012, 436, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Boucard, N.; Viton, C.; Agay, D.; Mari, E.; Roger, T.; Chancerelle, Y.; Domard, A. The use of physical hydrogels of chitosan for skin regeneration following third-degree burns. Biomaterials 2007, 28, 3478–3488. [Google Scholar] [CrossRef]
- Liu, X.; Meng, H. Consideration for the scale-up manufacture of nanotherapeutics—A critical step for technology transfer. VIEW 2021, 2, 20200190. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, Y.; Cheng, Y.; Zhang, Z.; Shi, X.; Qin, H. Effects of Lyophilization on the Release Profiles of 3D Printed Delivery Systems Fabricated with Carboxymethyl Cellulose Hydrogel. Polymers 2021, 13, 749. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.P.; Morais, A.d.S.; Maia, F.R.; Canadas, R.F.; Costa, J.B.; Oliveira, A.L.; Oliveira, J.M.; Reis, R.L. Combinatory approach for developing silk fibroin scaffolds for cartilage regeneration. Acta Biomater. 2018, 72, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.B.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Fast Setting Silk Fibroin Bioink for Bioprinting of Patient-Specific Memory-Shape Implants. Adv. Health Mater. 2017, 6. [Google Scholar] [CrossRef]
- Soare, G.C.; Silva, M.; da Silva, S.B.; Learmonth, D.A.; Vallejo, M.; Sousa, R.A.; Oliveira, A.L. New Insights on Biopolymer Sterilization Using Supercritical CO2 Technology. In Proceedings of the 16th European Meeting on Supercritical Fluids, Lisbon, Portugal, 25–28 April 2017; Available online: https://www.researchgate.net/publication/327463494_New_Insights_on_Biopolymer_Sterilization_Using_Supercritical_CO2_Technology (accessed on 1 February 2024).
- Veiga, A.; Magalhães, R.; Duarte, M.M.; Dias, J.R.; Alves, N.M.; Costa-Pinto, A.R.; Castro, F.; Rocha, F.; Oliveira, A.L. Continuous Production of Highly Tuned Silk/Calcium-Based Composites: Exploring New Pathways for Skin Regeneration. Molecules 2022, 27, 2249. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veiga, A.; Silva, I.V.; Dias, J.R.; Alves, N.M.; Oliveira, A.L.; Ribeiro, V.P. Streamlining Skin Regeneration: A Ready-To-Use Silk Bilayer Wound Dressing. Gels 2024, 10, 439. https://doi.org/10.3390/gels10070439
Veiga A, Silva IV, Dias JR, Alves NM, Oliveira AL, Ribeiro VP. Streamlining Skin Regeneration: A Ready-To-Use Silk Bilayer Wound Dressing. Gels. 2024; 10(7):439. https://doi.org/10.3390/gels10070439
Chicago/Turabian StyleVeiga, Anabela, Inês V. Silva, Juliana R. Dias, Nuno M. Alves, Ana L. Oliveira, and Viviana P. Ribeiro. 2024. "Streamlining Skin Regeneration: A Ready-To-Use Silk Bilayer Wound Dressing" Gels 10, no. 7: 439. https://doi.org/10.3390/gels10070439






