Overview of Dynamic Bond Based Hydrogels for Reversible Adhesion Processes
Abstract
1. Introduction
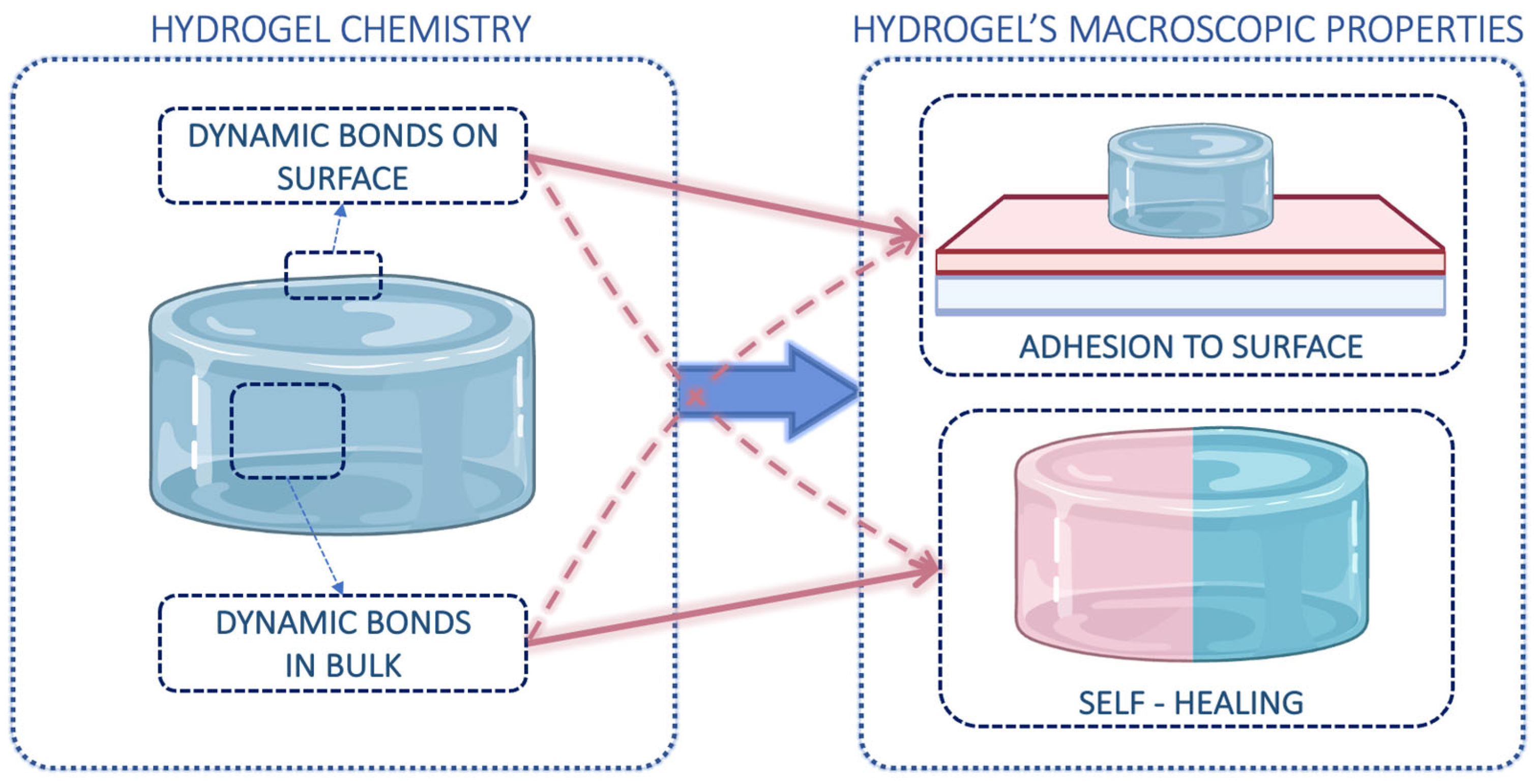
2. Dynamic Hydrogels: General Features
3. Dynamic Bonds: A Chemical Recap
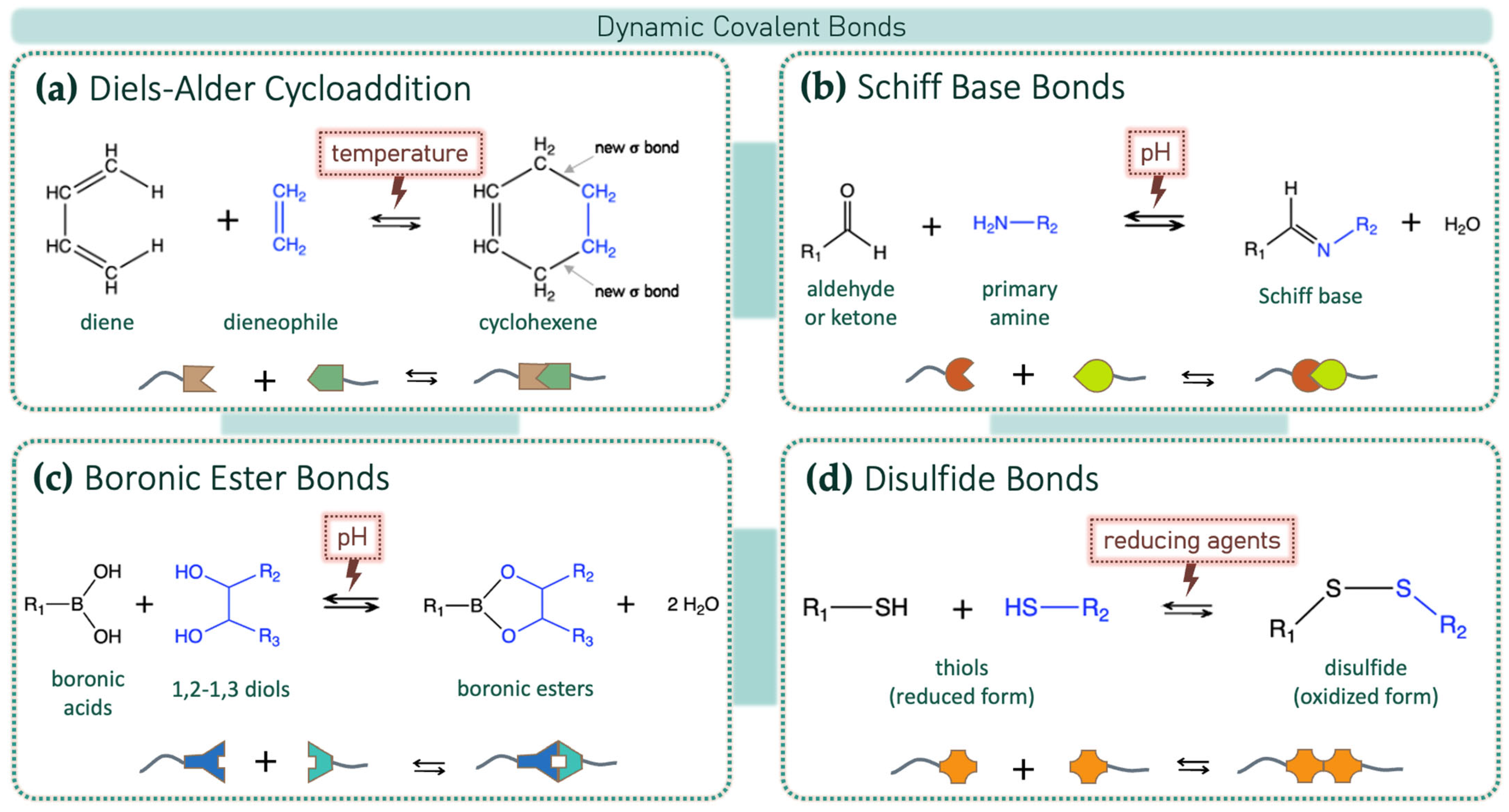
3.1. Dynamic Covalent Bonds
3.1.1. Diels–Alder Cycloaddition
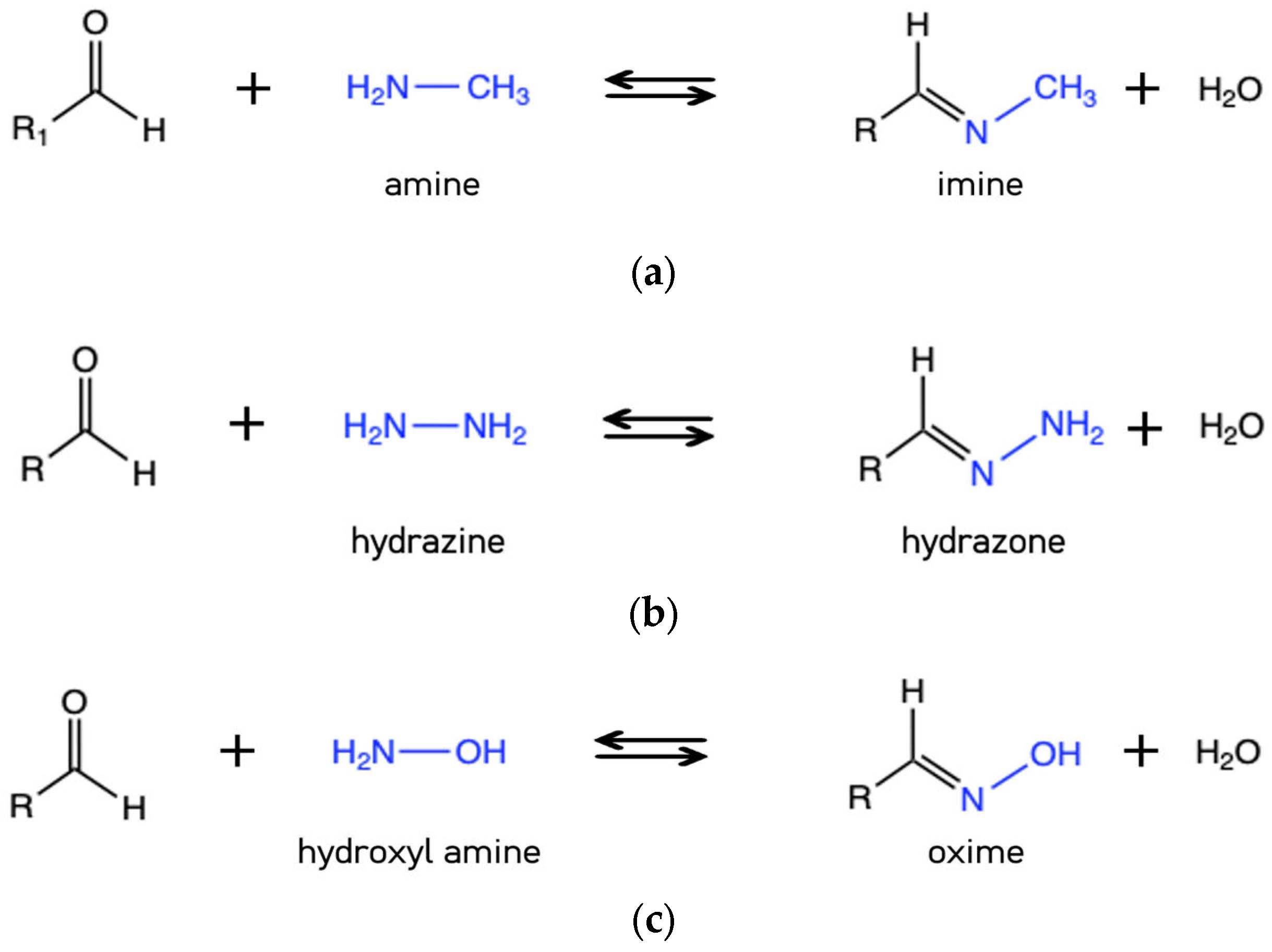
3.1.2. Schiff Bases and R-C=N Dynamic Bonds
3.1.3. Boronic Ester Bonds
3.1.4. Disulfide Bonds
3.2. Dynamic Non-Covalent Interactions
3.2.1. H-Bonding
3.2.2. Host–Guest Interactions
3.2.3. Metal–Ligand Coordination
3.2.4. π–π Interactions
4. Dynamic Hydrogels: Engineering the Network
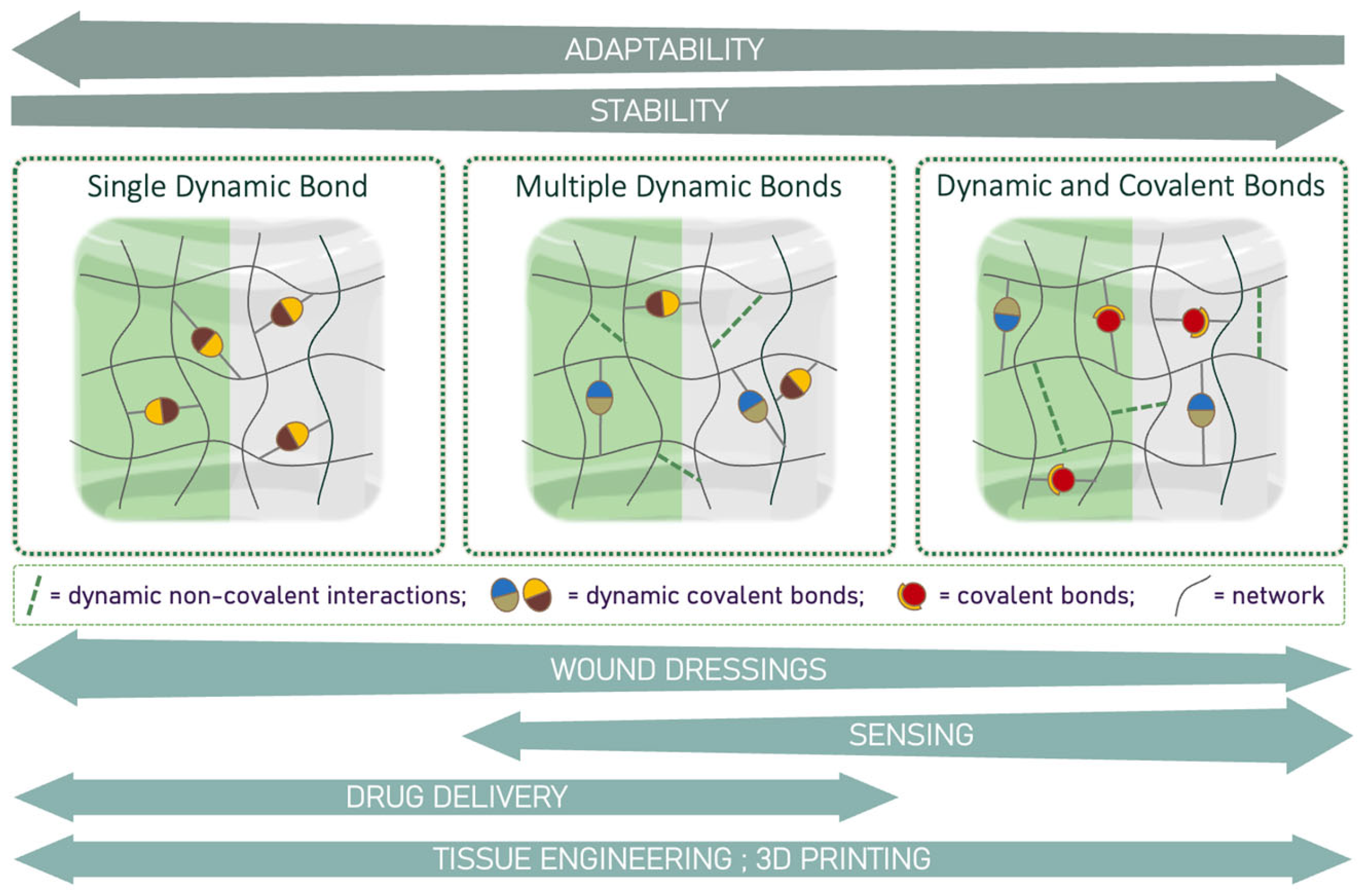
4.1. Hydrogels Based on a Single Dynamic Bond
4.2. Hydrogels Based on Multiple Dynamic Bonds
4.3. Hydrogels Based on Multi-Crosslinked Networks: Dynamic and Covalent Bonds
| Nature of Dynamic Bond | Main Polymer | Components Involved in the Main Linkage | Other Covalent and/or Dynamic Bonds * | Refs. |
|---|---|---|---|---|
| Schiff base bond | chitosan | HTCC-MA + OHA | UV irradiation HTCC-MA | [78] |
| CMCS + modified PCD | Ester bond PVA + borax Host–guest linkage modified PCD | [75] | ||
| gelatin | Gel + OKG | Micheal addition Gel + DA Catechol-Quinone Coupling DA + OKG | [79] | |
| hydrazone bond | hyaluronic acid | HA–furan–adipic dihydrazide + HA–furan–CHO | Diels–Alder Click Reaction furan + imide | [76] |
| sodium alginate | OSA + adipic acid dihydrazide + PEGDA | Schiff base Bond UV irradiation PEGDA | [80] | |
| boronic ester bond | chitosan | CMCS-DA + Alg-PBA | UV irradiation methacrylated CMCS-DA Schiff base Bond CMCS + inter DA | [81] |
| tannic acid | TA-Al3+ + GG-PAM-PBA | Polymerization Methylene bis AM Metal Coordination TA + Al3+ | [82] | |
| H-bond | tannic acid | TA + PEGDA | Chelation Fe + TA Ionic interactions Alg-NHS + Fe UV irradiation PEGDA | [83] |
| TA + PEG | Covalent Crosslinking Gel + TA | [84] | ||
| hyaluronic acid | gallol–HA + gallol–Gel | Covalent Crosslinking Inter-gallol moieties | [85] | |
| metal–ligand coordination | iron (Fe) | PAA + Fe ions in silica nanoparticles | Covalent Crosslinking PAA + silica nanoparticles H-bonds PAA + Gel Electrostatic interaction PAA + glycerol | [86] |
| GelMAC + Fe3+ | UV irradiation GelMAC Covalent Crosslinking GelMAC + PEGDA | [87] | ||
| gallium (Ga) | PAA + Ga | Covalent Crosslinking PAA + Alg | [77] | |
| zirconium (Zr) | PAA + Zr4+ | UV irradiation PAA-MA Enamine bonds PAA-MA + PEI | [88] |
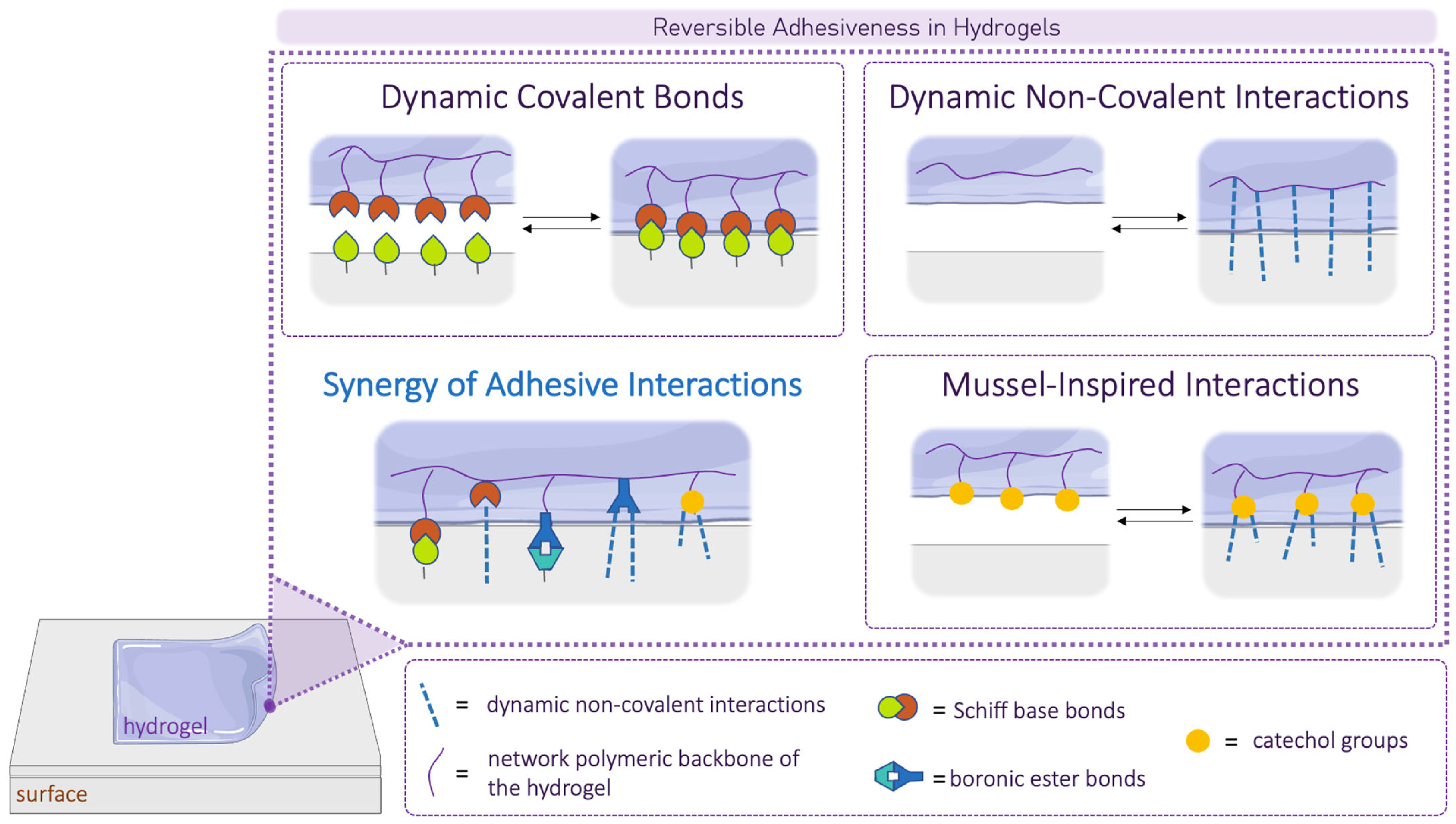
5. Adhesion Mechanisms
5.1. Dynamic Covalent Bonds Acting as Adhesive Bonds
5.2. Dynamic Non-Covalent Interactions Acting as Adhesive Bonds
5.3. Mussel-Inspired Adhesion
5.4. Synergy of Adhesive Interactions
6. Applications
7. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Notation and Abbreviations
| A@B | chemical B modified with chemical A |
| 2-FPBA | 2-formyl-phenyl boronic acid |
| 3-aminoPBA | 3-amino-phenyl boronic acid |
| AA | acrylic acid |
| AM | acrylamide |
| AG | agarose |
| AG-NH2 | agarose–ethylenediamine conjugate |
| AGA | aldehyde-contained glycyrrhizic acid |
| Alg | sodium alginate |
| Amy | amylopectin |
| BA | boric acid |
| CMC | carboxymethyl cellulose |
| CMCS | carboxymethyl chitosan |
| CNCs | cellulose nanocrystals |
| CNTs | carbon nanotubes |
| Col | collagen |
| Cys | cysteine |
| DA | dopamine |
| DF-PEG | dialdehyde-functionalized polyethylene glycol |
| Dopa | 3,4-dihydroxyphenylalanine |
| Gel | gelatin |
| GelMAC | gelatin methacrylol catechol |
| GG | galactomannan |
| GM | gentamicin |
| HA | hyaluronic acid |
| HC | hemicellulose |
| HTCC | N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride |
| HuA | humic acid |
| LA | lipoic acid |
| MA | methacrylated |
| mfps | mussel foot proteins |
| nDMA | nitro-dopamine methacrylamide |
| NS | nanosheets |
| OHA | oxidized hyaluronic acid |
| OKG | oxidized konjac glucomannan |
| OSA | oxidized sodium alginate |
| PA | protocatechualdehyde |
| PAA | polyacrylic acid |
| PAM | polyacrylamide |
| PBA | phenyl boronic acid |
| PCD | poly(β-cyclodextrin) |
| PDA | polydopamine |
| PEG | polyethylene glycol |
| PEGDA | polyethylene glycol diacrylate |
| PEI | polyethylene imine |
| PNIPAM | poly(N-isopropyl acrylamide) |
| PPy | polypyrrole |
| PVA | polyvinyl alcohol |
| PVP | polyvinyl pyrrolidone |
| SL | sodium lipoate |
| TA | tannic acid |
| ThA | thioctic acid |
| XG | xanthan gum |
References
- Revete, A.; Aparicio, A.; Cisterna, B.A.; Revete, J.; Luis, L.; Ibarra, E.; González, E.A.S.; Molino, J.; Reginensi, D. Advancements in the Use of Hydrogels for Regenerative Medicine: Properties and Biomedical Applications. Int. J. Biomater. 2022, 2022, 3606765. [Google Scholar] [CrossRef] [PubMed]
- Ursini, O.; Grieco, M.; Sappino, C.; Capodilupo, A.L.; Giannitelli, S.M.; Mauri, E.; Bucciarelli, A.; Coricciati, C.; de Turris, V.; Gigli, G.; et al. Modulation of Methacrylated Hyaluronic Acid Hydrogels Enables Their Use as 3D Cultured Model. Gels 2023, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef] [PubMed]
- Muir, V.G.; Burdick, J.A. Chemically Modified Biopolymers for the Formation of Biomedical Hydrogels. Chem. Rev. 2021, 121, 10908–10949. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Liu, F.; Abdiryim, T.; Liu, X. Self-Healing Hydrogels: From Synthesis to Multiple Applications. ACS Mater. Lett. 2023, 5, 1787–1830. [Google Scholar] [CrossRef]
- Rosellini, E.; Cascone, M.G.; Rosellini, E.; Cascone, M.G. Microfluidic Fabrication of Natural Polymer-Based Scaffolds for Tissue Engineering Applications: A Review. Biomimetics 2023, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, L.; Wang, J.; Meng, Q.; Zhong, S.; Gao, Y.; Cui, X. Recent advances in polysaccharide-based self-healing hydrogels for biomedical applications. Carbohydr. Polym. 2022, 283, 119161. [Google Scholar] [CrossRef]
- Quan, L.; Xin, Y.; Wu, X.; Ao, Q. Mechanism of Self-Healing Hydrogels and Application in Tissue Engineering. Polymers 2022, 14, 2184. [Google Scholar] [CrossRef]
- Imato, K.; Nishihara, M.; Kanehara, T.; Amamoto, Y.; Takahara, A.; Otsuka, H. Self-Healing of Chemical Gels Cross-Linked by Diarylbibenzofuranone-Based Trigger-Free Dynamic Covalent Bonds at Room Temperature. Angew. Chem. Int. Ed. 2012, 51, 1138–1142. [Google Scholar] [CrossRef]
- Ye, J.; Fu, S.; Zhou, S.; Li, M.; Li, K.; Sun, W.; Zhai, Y. Advances in hydrogels based on dynamic covalent bonding and prospects for its biomedical application. Eur. Polym. J. 2020, 139, 110024. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, Y.; Hasanin, M.S.; Yu, T. Editorial: Adhesive hydrogels: Design, fabrication, and bio-applications. Front. Bioeng. Biotechnol. 2023, 11, 1290228. [Google Scholar] [CrossRef] [PubMed]
- Bovone, G.; Dudaryeva, O.Y.; Marco-Dufort, B.; Tibbitt, M.W. Engineering Hydrogel Adhesion for Biomedical Applications via Chemical Design of the Junction. ACS Biomater. Sci. Eng. 2021, 7, 4048–4076. [Google Scholar] [CrossRef] [PubMed]
- Rowan, S.J.; Cantrill, S.J.; Cousins, G.R.L.; Sanders, J.K.M.; Stoddart, J.F. Dynamic Covalent Chemistry. Angew. Chem. Int. Ed. 2002, 41, 898–952. [Google Scholar] [CrossRef]
- Wanasinghe, S.V.; Dodo, O.J.; Konkolewicz, D. Dynamic Bonds: Adaptable Timescales for Responsive Materials. Angew. Chem. Int. Ed. 2022, 61, e202206938. [Google Scholar] [CrossRef]
- Shahi, S.; Roghani-Mamaqani, H.; Hoogenboom, R.; Talebi, S.; Mardani, H. Stimuli-Responsive Covalent Adaptable Hydrogels Based on Homolytic Bond Dissociation and Chain Transfer Reactions. Chem. Mater. 2022, 34, 468–498. [Google Scholar] [CrossRef]
- Han, Y.; Cao, Y.; Lei, H. Dynamic Covalent Hydrogels: Strong yet Dynamic. Gels 2022, 8, 577. [Google Scholar] [CrossRef]
- Picchioni, F.; Muljana, H. Hydrogels Based on Dynamic Covalent and Non Covalent Bonds: A Chemistry Perspective. Gels 2018, 4, 21. [Google Scholar] [CrossRef]
- Uman, S.; Dhand, A.; Burdick, J.A. Recent advances in shear-thinning and self-healing hydrogels for biomedical applications. J. Appl. Polym. Sci. 2020, 137, 48668. [Google Scholar] [CrossRef]
- Yang, J.; Bai, R.; Chen, B.; Suo, Z. Hydrogel Adhesion: A Supramolecular Synergy of Chemistry, Topology, and Mechanics. Adv. Funct. Mater. 2020, 30, 1901693. [Google Scholar] [CrossRef]
- Karvinen, J.; Kellomäki, M. Characterization of self-healing hydrogels for biomedical applications. Eur. Polym. J. 2022, 181, 111641. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Sun, Z.; Zhu, X.; Zhao, Q.; Zhang, T.; Cholewinski, A.; Yang, F.K.; Zhao, B.; Pinnaratip, R.; et al. Catechol-functionalized hydrogels: Biomimetic design, adhesion mechanism, and biomedical applications. Chem. Soc. Rev. 2020, 49, 433–464. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A thermally re-mendable cross-linked polymeric material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Chakma, P.; Konkolewicz, D. Dynamic Covalent Bonds in Polymeric Materials. Angew. Chem. Int. Ed. 2019, 58, 9682–9695. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.; Roghani-Mamaqani, H.; Talebi, S.; Mardani, H. Chemical stimuli-induced reversible bond cleavage in covalently crosslinked hydrogels. Coord. Chem. Rev. 2022, 455, 214368. [Google Scholar] [CrossRef]
- Kalia, J.; Raines, R.T. Hydrolytic Stability of Hydrazones and Oximes. Angew. Chem. Int. Ed. 2008, 47, 7523–7526. [Google Scholar] [CrossRef]
- Lorand, J.P.; Edwards, J.O. Polyol Complexes and Structure of the Benzeneboronate Ion. J. Org. Chem. 1959, 24, 769–774. [Google Scholar] [CrossRef]
- Marco-Dufort, B.; Tibbitt, M.W. Design of moldable hydrogels for biomedical applications using dynamic covalent boronic esters. Mater. Today Chem. 2019, 12, 16–33. [Google Scholar] [CrossRef]
- Brooks, W.L.A.; Sumerlin, B.S. Synthesis and Applications of Boronic Acid-Containing Polymers: From Materials to Medicine. Chem. Rev. 2015, 116, 1375–1397. [Google Scholar] [CrossRef]
- Sinawang, G.; Osaki, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Biofunctional hydrogels based on host–guest interactions. Polym. J. 2020, 52, 839–859. [Google Scholar] [CrossRef]
- Haas, K.L.; Franz, K.J. Application of Metal Coordination Chemistry to Explore and Manipulate Cell Biology. Chem. Rev. 2009, 109, 4921–4960. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Wu, S.; Sun, Y.; Yang, H.; Lin, B. Physically and chemically dual-crosslinked hydrogels with superior mechanical properties and self-healing behavior. New J. Chem. 2020, 44, 9903–9911. [Google Scholar] [CrossRef]
- Tong, Z.; Jin, L.; Oliveira, J.M.; Reis, R.L.; Zhong, Q.; Mao, Z.; Gao, C. Adaptable hydrogel with reversible linkages for regenerative medicine: Dynamic mechanical microenvironment for cells. Bioact. Mater. 2021, 6, 1375–1387. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, Q.; Fang, Z.; Gu, L.; Bian, L. Structurally Dynamic Hydrogels for Biomedical Applications: Pursuing a Fine Balance between Macroscopic Stability and Microscopic Dynamics. Chem. Rev. 2021, 121, 11149–11193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, R.; Li, Q.; Dai, F.; Lan, G.; Shang, S.; Lu, F. A self-adapting hydrogel based on chitosan/oxidized konjac glucomannan/AgNPs for repairing irregular wounds. Biomater. Sci. 2020, 8, 1910–1922. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Wang, Y.; Hao, J. Rapid-Forming and Self-Healing Agarose-Based Hydrogels for Tissue Adhesives and Potential Wound Dressings. Biomacromolecules 2018, 19, 980–988. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, H.; Li, M.; Li, Z.; Zhang, H.; Guo, B.; Zhang, J. Antibacterial Conductive UV-Blocking Adhesion Hydrogel Dressing with Mild On-Demand Removability Accelerated Drug-Resistant Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 41726–41741. [Google Scholar] [CrossRef] [PubMed]
- Sigen, A.; Xu, Q.; Johnson, M.; Creagh-Flynn, J.; Venet, M.; Zhou, D.; Lara-Sáez, I.; Tai, H.; Wang, W. An injectable multi-responsive hydrogel as self-healable and on-demand dissolution tissue adhesive. Appl. Mater. Today 2021, 22, 100967. [Google Scholar] [CrossRef]
- Muir, V.G.; Qazi, T.H.; Weintraub, S.; Maldonado, B.O.T.; Arratia, P.E.; Burdick, J.A. Sticking Together: Injectable Granular Hydrogels with Increased Functionality via Dynamic Covalent Inter-Particle Crosslinking. Small 2022, 18, e2201115. [Google Scholar] [CrossRef]
- Zhao, P.; Wei, K.; Feng, Q.; Chen, H.; Wong, D.S.H.; Chen, X.; Wu, C.-C.; Bian, L. Mussel-mimetic hydrogels with defined cross-linkers achieved via controlled catechol dimerization exhibiting tough adhesion for wet biological tissues. Chem. Commun. 2017, 53, 12000–12003. [Google Scholar] [CrossRef]
- Chen, M.; Wu, Y.; Chen, B.; Tucker, A.M.; Jagota, A.; Yang, S. Fast, strong, and reversible adhesives with dynamic covalent bonds for potential use in wound dressing. Proc. Natl. Acad. Sci. USA 2022, 119, e2203074119. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Gong, C.; Li, B.; Wu, G. A pH, glucose, and dopamine triple-responsive, self-healable adhesive hydrogel formed by phenylborate–catechol complexation. Polym. Chem. 2017, 8, 2997–3005. [Google Scholar] [CrossRef]
- Du, J.; Wang, F.; Li, J.; Yang, Y.; Guo, D.; Zhang, Y.; Yang, A.; He, X.; Cheng, Y. Green polymer hydrogels from a natural monomer with inherent antioxidative capability for efficient wound healing and spinal cord injury treatment. Biomater. Sci. 2023, 11, 3683–3694. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xiao, Q.; Qi, G.; Chen, F.; Tu, B.; Zhang, S.; Li, Y.; Chen, Y.; Yu, H.; Duan, P. A Hydrogen Bonds-Crosslinked Hydrogels With Self-Healing and Adhesive Properties for Hemostatic. Front. Bioeng. Biotechnol. 2022, 10, 855013. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Jiang, Y.; Liu, Y.; Ren, Y.; Xu, Z.; Li, Z.; Zhao, Y.; Wu, X.; Ren, J. Marine-inspired molecular mimicry generates a drug-free, but immunogenic hydrogel adhesive protecting surgical anastomosis. Bioact. Mater. 2021, 6, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Wang, M.; Meng, L.; Chang, H.; Wang, B.; Xu, F.; Yang, J.; Wan, P. Mussel-Inspired Cellulose Nanocomposite Tough Hydrogels with Synergistic Self-Healing, Adhesive, and Strain-Sensitive Properties. Chem. Mater. 2018, 30, 3110–3121. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wang, Q.; Han, Y.; Chen, H.; Tan, Y. Doubly Dynamic Hydrogel Formed by Combining Boronate Ester and Acylhydrazone Bonds. Polymers 2020, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Su, Q.; Guo, R.; Zhang, J.; Dong, A.; Lin, C.; Zhang, J. A Multitasking Hydrogel Based on Double Dynamic Network with Quadruple-Stimuli Sensitiveness, Autonomic Self-Healing Property, and Biomimetic Adhesion Ability. Macromol. Chem. Phys. 2017, 218, 1700166. [Google Scholar] [CrossRef]
- Su, K.; Deng, D.; Wu, X.; Song, Y.; Sun, Y.; Wang, X.; Zhang, Z.; Li, J.; Yan, Z.; Shang, X.; et al. On-demand detachable adhesive hydrogel based on dual dynamic covalent cross-linked with NIR/pH dual-responsive properties for diabetic wound healing. Chem. Eng. J. 2024, 479, 147646. [Google Scholar] [CrossRef]
- Yi, X.; Cheng, F.; Wei, X.; Li, H.; Qian, J.; He, J. Bioinspired adhesive and self-healing bacterial cellulose hydrogels formed by a multiple dynamic crosslinking strategy for sealing hemostasis. Cellulose 2022, 30, 397–411. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.; Du, R.; Yang, Y.; Liu, Y.; Wan, Z.; Yang, X. Injectable Self-Healing Adhesive Natural Glycyrrhizic Acid Bioactive Hydrogel for Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 17562–17576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Luo, B.; An, Z.; Zheng, P.; Liu, Y.; Zhao, H.; Zhang, Z.; Gao, T.; Cao, Y.; Zhang, Y.; et al. MMP-Responsive Nanoparticle-Loaded, Injectable, Adhesive, Self-Healing Hydrogel Wound Dressing Based on Dynamic Covalent Bonds. Biomacromolecules 2023, 24, 5769–5779. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.; Narayanan, K.B.; Uthappa, U.T.; Park, P.H.; Choi, I.; Han, S.S. Tissue Adhesive, Self-Healing, Biocompatible, Hemostasis, and Antibacterial Properties of Fungal-Derived Carboxymethyl Chitosan-Polydopamine Hydrogels. Pharmaceutics 2022, 14, 1028. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Jiang, Y.; Ren, Z.; Tang, K. Protocatechualdehyde-ferric iron tricomplex embedded gelatin hydrogel with adhesive, antioxidant and photothermal antibacterial capacities for infected wound healing promotion. Int. J. Biol. Macromol. 2023, 242, 125029. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Li, Z.; Pan, G.; Guo, B. Antibacterial conductive self-healable supramolecular hydrogel dressing for infected motional wound healing. Sci. China Chem. 2022, 65, 2238–2251. [Google Scholar] [CrossRef]
- Heidarian, P.; Kouzani, A.Z.; Kaynak, A.; Paulino, M.; Nasri-Nasrabadi, B.; Varley, R. Double dynamic cellulose nanocomposite hydrogels with environmentally adaptive self-healing and pH-tuning properties. Cellulose 2019, 27, 1407–1422. [Google Scholar] [CrossRef]
- Yang, B.; Song, J.; Jiang, Y.; Li, M.; Wei, J.; Qin, J.; Peng, W.; Lasaosa, F.L.; He, Y.; Mao, H.; et al. Injectable Adhesive Self-Healing Multicross-Linked Double-Network Hydrogel Facilitates Full-Thickness Skin Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 57782–57797. [Google Scholar] [CrossRef]
- Chen, T.; Chen, Y.; Rehman, H.U.; Chen, Z.; Yang, Z.; Wang, M.; Li, H.; Liu, H. Ultratough, Self-Healing, and Tissue-Adhesive Hydrogel for Wound Dressing. ACS Appl. Mater. Interfaces 2018, 10, 33523–33531. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Qi, C.; Gao, Y. Injectable Self-Healable Nanocomposite Hydrogels with Mussel-Inspired Adhesive Properties for 3D Printing Ink. ACS Appl. Nano Mater. 2019, 2, 5000–5008. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Y.; Zu, B.; Lei, D.; Guo, Y.; Wang, M.; Dou, X. Reversible adhesive hydrogel with enhanced sampling efficiency boosted by hydrogen bond and van der Waals force for visualized detection. Chem. Eng. J. 2023, 455, 140493. [Google Scholar] [CrossRef]
- Zheng, H.; Lin, N.; He, Y.; Zuo, B. Self-Healing, Self-Adhesive Silk Fibroin Conductive Hydrogel as a Flexible Strain Sensor. ACS Appl. Mater. Interfaces 2021, 13, 40013–40031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, L.; Zhao, M.; Ma, Y.; Zheng, T.; Shi, L. Stretchable, self-healing and adhesive sodium alginate-based composite hydrogels as wearable strain sensors for expansion–contraction motion monitoring. Soft Matter 2022, 18, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Gao, Z.; Li, X.; Gao, G. An amylopectin-enabled skin-mounted hydrogel wearable sensor. J. Mater. Chem. B 2021, 9, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Su, J.; Han, J.; Xiao, T.; Sun, X.; Cui, G.; Duan, X.; Shi, P. An Intrinsic Self-Healable, Anti-Freezable and Ionically Conductive Hydrogel for Soft Ionotronics Induced by Imidazolyl Cross-Linker Molecules Anchored with Dynamic Disulfide Bonds. Macromol. Rapid Commun. 2024, 45, e2300613. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.J.; Liu, S.N.; Gu, Z.Z.; Xu, H. A Skin-Inspired Multifunctional Conductive Hydrogel with High Stretchable, Adhesive, Healable, and Decomposable Properties for Highly Sensitive Dual-Sensing of Temperature and Strain. Small Methods 2023, 7, e2300749. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, L.; Chen, Y. Dual dynamically crosslinked thermosensitive hydrogel with self-fixing as a postoperative anti-adhesion barrier. Acta Biomater. 2020, 110, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Fu, C.; Alam, N.; Ni, Y.; Chen, L.; Huang, L.; Hu, H.-C. Tannic acid modified hemicellulose nanoparticle reinforced ionic hydrogels with multi-functions for human motion strain sensor applications. Ind. Crops Prod. 2022, 176, 114412. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, S.; Xia, X.; Tan, M.; Lv, Y.; Cheng, Y.; Tao, Y.; Lu, J.; Du, J.; Wang, H. High-performance carboxymethyl cellulose-based hydrogel film for food packaging and preservation system. Int. J. Biol. Macromol. 2022, 223, 1126–1137. [Google Scholar] [CrossRef]
- Gao, Y.; Zhan, X.; Huo, S.; Fu, L.; Tang, Z.; Qi, K.; Lv, C.; Liu, C.; Zhu, Y.; Ding, S.; et al. A gentamicin-thioctic acid multifunctional hydrogel for accelerating infected wound healing. J. Mater. Chem. B 2022, 10, 2171–2182. [Google Scholar] [CrossRef]
- Yao, S.; Zhao, Y.; Xu, Y.; Jin, B.; Wang, M.; Yu, C.; Guo, Z.; Jiang, S.; Tang, R.; Fang, X.; et al. Injectable Dual-Dynamic-Bond Cross-Linked Hydrogel for Highly Efficient Infected Diabetic Wound Healing. Adv. Healthc. Mater. 2022, 11, e2200516. [Google Scholar] [CrossRef]
- Zeng, Q.; Wan, S.; Yang, S.; Zhao, X.; He, F.; Zhang, Y.; Cao, X.; Wen, Q.; Feng, Y.; Yu, G.; et al. Super stretchability, strong adhesion, flexible sensor based on Fe3+ dynamic coordination sodium alginate/polyacrylamide dual-network hydrogel. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129733. [Google Scholar] [CrossRef]
- Deng, X.; Huang, B.; Wang, Q.; Wu, W.; Coates, P.; Sefat, F.; Lu, C.; Zhang, W.; Zhang, X. A Mussel-Inspired Antibacterial Hydrogel with High Cell Affinity, Toughness, Self-Healing, and Recycling Properties for Wound Healing. ACS Sustain. Chem. Eng. 2021, 9, 3070–3082. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, Y.; Jin, S.; Li, J.; Wu, P.; Luo, Z. Flexible Lignin-based hydrogels with Self-healing and adhesive ability driven by noncovalent interactions. Chem. Eng. J. 2022, 429, 132252. [Google Scholar] [CrossRef]
- Huang, X.; Li, J.; Luo, J.; Gao, Q.; Mao, A.; Li, J. Research progress on double-network hydrogels. Mater. Today Commun. 2021, 29, 102757. [Google Scholar] [CrossRef]
- Ren, Z.; Ke, T.; Ling, Q.; Zhao, L.; Gu, H. Rapid self-healing and self-adhesive chitosan-based hydrogels by host-guest interaction and dynamic covalent bond as flexible sensor. Carbohydr. Polym. 2021, 273, 118533. [Google Scholar] [CrossRef]
- Yu, F.; Cao, X.; Du, J.; Wang, G.; Chen, X. Multifunctional Hydrogel with Good Structure Integrity, Self-Healing, and Tissue-Adhesive Property Formed by Combining Diels–Alder Click Reaction and Acylhydrazone Bond. ACS Appl. Mater. Interfaces 2015, 7, 24023–24031. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.; Xiao, J.; Chen, S.-W.; Tu, Q.; Yuan, M.-S.; Wang, J. Liquid Metal-Doped Conductive Hydrogel for Construction of Multifunctional Sensors. Anal. Chem. 2023, 95, 3811–3820. [Google Scholar] [CrossRef]
- Qiu, H.; Deng, J.; Wei, R.; Wu, X.; Chen, S.; Yang, Y.; Gong, C.; Cui, L.; Si, Z.; Zhu, Y.; et al. A lubricant and adhesive hydrogel cross-linked from hyaluronic acid and chitosan for articular cartilage regeneration. Int. J. Biol. Macromol. 2023, 243, 125249. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, G.; Wang, H.; Li, Q.; Tang, K. Multi-Crosslinked Hydrogels with Instant Self-Healing and Tissue Adhesive Properties for Biomedical Applications. Macromol. Biosci. 2022, 22, e2100443. [Google Scholar] [CrossRef]
- Aldana, A.A.; Morgan, F.L.C.; Houben, S.; Pitet, L.M.; Moroni, L.; Baker, M.B. Biomimetic double network hydrogels: Combining dynamic and static crosslinks to enable biofabrication and control cell-matrix interactions. J. Polym. Sci. 2021, 59, 2832–2843. [Google Scholar] [CrossRef]
- Zou, C.Y.; Lei, X.X.; Hu, J.J.; Jiang, Y.L.; Li, Q.J.; Song, Y.T.; Zhang, Q.Y.; Li-Ling, J.; Xie, H.Q. Multi-crosslinking hydrogels with robust bio-adhesion and pro-coagulant activity for first-aid hemostasis and infected wound healing. Bioact. Mater. 2022, 16, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, X.; Song, R.; Lv, H.; Zong, S.; Zhao, Q.; Wu, J.; Wen, X.; Jiang, J.; Duan, J. Application of adhesive controllable galactomannan hydrogel initiated by aluminum ions at room temperature in flexible sensors. React. Funct. Polym. 2023, 193, 105738. [Google Scholar] [CrossRef]
- Zheng, Y.; Baidya, A.; Annabi, N. Molecular design of an ultra-strong tissue adhesive hydrogel with tunable multifunctionality. Bioact. Mater. 2023, 29, 214–229. [Google Scholar] [CrossRef]
- Li, H.; Shi, Y.; Zhang, W.; Yu, M.; Chen, X.; Kong, M. Ternary Complex Coacervate of PEG/TA/Gelatin as Reinforced Bioadhesive for Skin Wound Repair. ACS Appl. Mater. Interfaces 2022, 14, 18097–18109. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Galarraga, J.H.; Kwon, M.Y.; Lee, H.; Burdick, J.A. Gallol-derived ECM-mimetic adhesive bioinks exhibiting temporal shear-thinning and stabilization behavior. Acta Biomater. 2019, 95, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Dai, S.; Dong, X.; Li, M.; Xu, Y.; Jiang, Y.; Yuan, N.; Ding, J. A wide-temperature-range sensor based on wide-strain-range self-healing and adhesive organogels. New J. Chem. 2022, 46, 4334–4342. [Google Scholar] [CrossRef]
- Ghovvati, M.; Baghdasarian, S.; Baidya, A.; Dhal, J.; Annabi, N. Engineering a highly elastic bioadhesive for sealing soft and dynamic tissues. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1511–1522. [Google Scholar] [CrossRef]
- Rong, L.; Zhao, W.; Fan, Y.; Zhou, Z.; Zhan, M.; He, X.; Yuan, W.; Qian, C. Environmentally Stable, Stretchable, Adhesive, and Conductive Organohydrogels with Multiple Dynamic Interactions as High-Performance Strain and Temperature Sensors. ACS Appl. Mater. Interfaces 2022, 14, 55075–55087. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Gosecka, M.; Bodaghi, M.; Crespy, D.; Youssef, G.; Dodda, J.M.; Wong, S.H.D.; Imran, A.B.; Gosecki, M.; Jobdeedamrong, A.; et al. Engineering multifunctional dynamic hydrogel for biomedical and tissue regenerative applications. Chem. Eng. J. 2024, 487, 150403. [Google Scholar] [CrossRef]
- Fuchs, S.; Shariati, K.; Ma, M. Specialty Tough Hydrogels and Their Biomedical Applications. Adv. Healthc. Mater. 2020, 9, e1901396. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, X.; Ma, X.; Wang, W.; Yan, F.; Zhao, X.; Chu, X.; Xu, W.; Sun, C. A review of the properties and applications of bioadhesive hydrogels. Polym. Chem. 2021, 12, 3721–3739. [Google Scholar] [CrossRef]
- Nam, S.; Mooney, D. Polymeric Tissue Adhesives. Chem. Rev. 2021, 7, 4048–4076. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Liu, Z.; You, M.; Yin, H.; Yu, H.; Shi, X.; Yang, J.; Qin, G.; Zhu, L.; Chen, Q. Dynamic Disulfide Bond Regulated Tough Adhesion and On-Demand Debonding of the Albumin-Based Double Network Hydrogel to Diverse Substrates. ACS Appl. Polym. Mater. 2024, 6, 330–340. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Q.; Qu, D.-H. Emerging Hydrogen-Bond Design for High-Performance Dynamic Polymeric Materials. ACS Mater. Lett. 2023, 5, 480–490. [Google Scholar] [CrossRef]
- Yuk, H.; Varela, C.E.; Nabzdyk, C.S.; Mao, X.; Padera, R.F.; Roche, E.T.; Zhao, X. Dry double-sided tape for adhesion of wet tissues and devices. Nature 2019, 575, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, L.; He, X.; Xia, Z.; Zhao, Z.; Xi, Z.; Yu, J.; Wang, J. Dynamic Crosslinked Injectable Mussel-Inspired Hydrogels with Adhesive, Self-Healing, and Biodegradation Properties. Polymers 2023, 15, 1876. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, M.; Zhang, Y.; Pei, R. Recent Progress of Highly Adhesive Hydrogels as Wound Dressings. Biomacromolecules 2020, 21, 3966–3983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dai, Y.; Xia, F.; Zhang, X. Interfacial Molecular Lock: A Universal Strategy for Hydrogel Adhesion. ACS Appl. Polym. Mater. 2022, 5, 1037–1045. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Fan, X.; Fang, Y.; Zhou, W.; Yan, L.; Xu, Y.; Zhu, H.; Liu, H. Mussel foot protein inspired tough tissue-selective underwater adhesive hydrogel. Mater. Horiz. 2021, 8, 997–1007. [Google Scholar] [CrossRef]
- Jafari, H.; Ghaffari-Bohlouli, P.; Niknezhad, S.V.; Abedi, A.; Izadifar, Z.; Mohammadinejad, R.; Varma, R.S.; Shavandi, A. Tannic acid: A versatile polyphenol for design of biomedical hydrogels. J. Mater. Chem. B 2022, 10, 5873–5912. [Google Scholar] [CrossRef]
- Hofman, A.H.; Hees, I.A.v.; Yang, J.; Kamperman, M. Bioinspired Underwater Adhesives by Using the Supramolecular Toolbox. Adv. Mater. 2018, 30, e1704640. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, X.; Luo, Y.; Zhu, R.; Zheng, M.; Yang, J.; Bai, S. Silk Fibroin-Based Tough Hydrogels with Strong Underwater Adhesion for Fast Hemostasis and Wound Sealing. Biomacromolecules 2022, 24, 319–331. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Yu, H.; Wang, L.; Sheng, Y.; Huang, Z.; Yang, J.; Ni, Z.; Shen, D. Instant and Tough Adhesives for Rapid Gastric Perforation and Traumatic Pneumothorax Sealing. Adv. Healthc. Mater. 2022, 11, e2201798. [Google Scholar] [CrossRef]
- Fu, Z.; Xiao, S.; Wang, P.; Zhao, J.; Ling, Z.; An, Z.; Shao, J.; Fu, W. Injectable, stretchable, toughened, bioadhesive composite hydrogel for bladder injury repair. RSC Adv. 2023, 13, 10903–10913. [Google Scholar] [CrossRef]
- Chen, K.; Wu, Z.; Liu, Y.; Yuan, Y.; Liu, C. Injectable Double-Crosslinked Adhesive Hydrogels with High Mechanical Resilience and Effective Energy Dissipation for Joint Wound Treatment. Adv. Funct. Mater. 2022, 32, 2109687. [Google Scholar] [CrossRef]
- Xu, A.; Zhang, N.; Su, S.; Shi, H.; Lu, D.; Li, X.; Zhang, X.; Feng, X.; Wen, Z.; Ma, G.; et al. A highly stretchable, adhesive, and antibacterial hydrogel with chitosan and tobramycin as dynamic cross-linkers for treating the infected diabetic wound. Carbohydr. Polym. 2024, 324, 121543. [Google Scholar] [CrossRef]
- Kim, Y.; Hu, Y.; Jeong, J.-P.; Jung, S. Injectable, self-healable and adhesive hydrogels using oxidized Succinoglycan/chitosan for pH-responsive drug delivery. Carbohydr. Polym. 2022, 284, 119195. [Google Scholar] [CrossRef]
- Chen, J.; Wang, D.; Wang, L.-H.; Liu, W.; Chiu, A.; Shariati, K.; Liu, Q.; Wang, X.; Zhong, Z.; Webb, J.; et al. An Adhesive Hydrogel with “Load-Sharing” Effect as Tissue Bandages for Drug and Cell Delivery. Adv. Mater. 2020, 32, e2001628. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Zhang, S.; Li, J.; Quan, P.; Song, Y.; Liu, J.; Fang, L. Multiple dynamic interaction-enabled eutectogel with strong tissue adhesion, mechanical strength and temperature tolerance for transdermal drug delivery: Double monodentate coordination and π-π interaction. Chem. Eng. J. 2023, 476, 146583. [Google Scholar] [CrossRef]
- Park, K.; Kang, K.; Kim, J.; Kim, S.D.; Jin, S.; Shin, M.; Son, D. Balanced Coexistence of Reversible and Irreversible Covalent Bonds in a Conductive Triple Polymeric Network Enables Stretchable Hydrogels with High Toughness and Adhesiveness. ACS Appl. Mater. Interfaces 2022, 14, 56395–56406. [Google Scholar] [CrossRef] [PubMed]
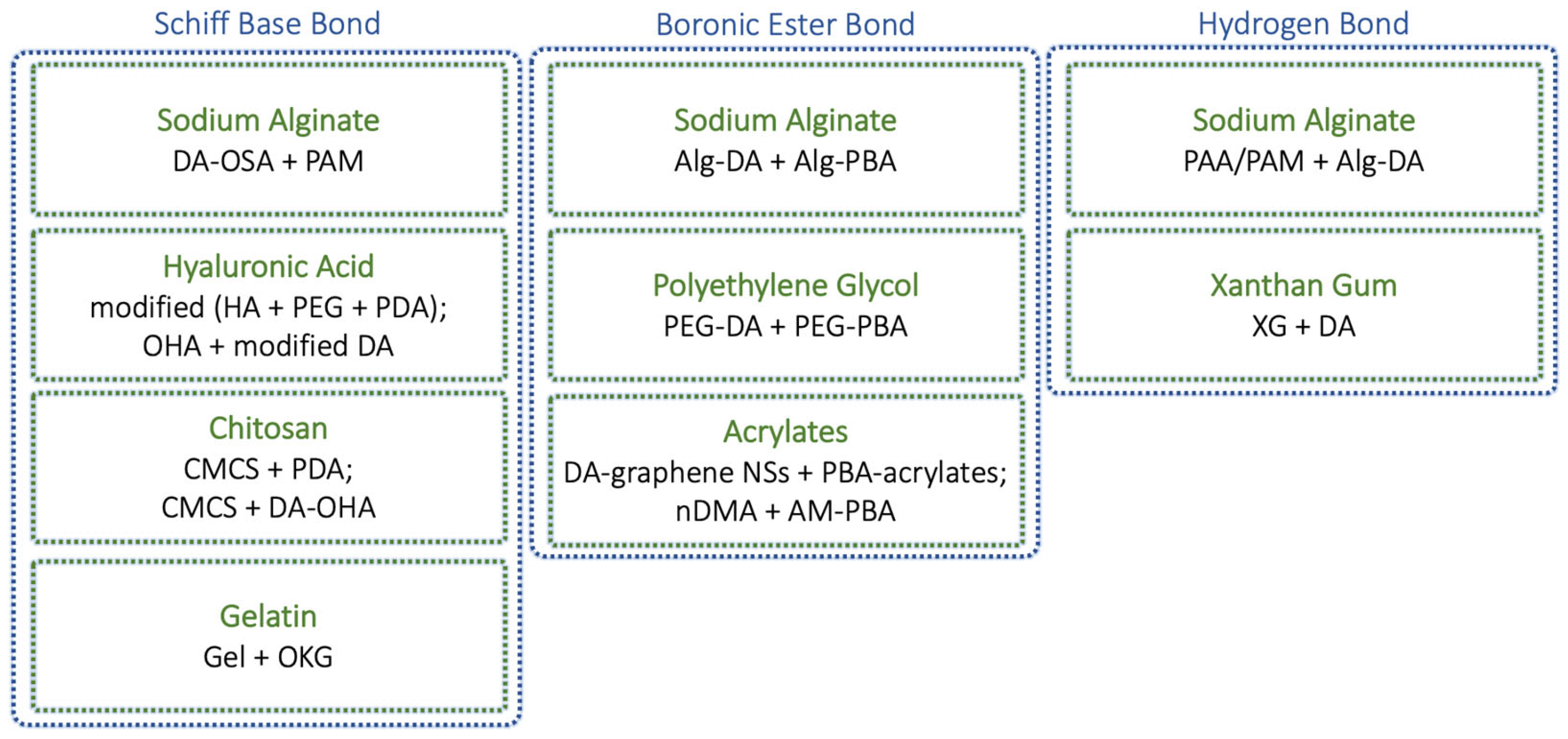
| Nature of Dynamic Bond | Main Polymer | Components Involved in the Main Linkage * | Refs. |
|---|---|---|---|
| Schiff base bond | chitosan | chitosan + OKG | [35] |
| polyethylene glycol | DF-PEG + AG-NH2 | [36] | |
| hyaluronic acid | modified (HA + PEG + PDA) | [37] | |
| hydrazone bond | hyaluronic acid | modified HA + disulfide crosslinker | [38] |
| modified HA | [39] | ||
| boronic ester bond | acrylates | nDMA + AM-PBA | [40] |
| polyvinyl alcohol | PVA + BA | [41] | |
| polyethylene glycol | PEGDA + PEG-PBA | [42] | |
| disulfide bond | lipoic acid | α-LA + SL | [43] |
| H-bond | polyvinyl pyrrolidone | HuA + PVP | [44] |
| xanthan gum | XG + DA | [45] | |
| metal–ligand coordination | aluminium | PAA + Al3+; TA@CNCs | [46] |
| Nature of Dynamic Bond | Main Polymer | Components Involved in the Main Linkage * | Other Dynamic Bonds * | Refs. |
|---|---|---|---|---|
| Schiff base bond | chitosan | CMCS + 2-FPBA | Boronic Ester bond 2-FPBA + modified gallate | [49] |
| CMCS + AGA | H-bond intra AGA | [51] | ||
| CMCS + OHA | Disulfide (-S-Ag-S-) modified Col + Ag | [52] | ||
| gelatin | CMCS + PDA | H-bond CMCS + PDA | [53] | |
| Gel + PA | Metal Coordination PA + Fe | [54] | ||
| Gel + monoaldehyde β-cyclodextrin | Host–guest interaction Chitosan + monoaldehyde β-cyclodextrin | [55] | ||
| Gel + modified cellulose | Metal Coordination TA + Fe | [56] | ||
| OHA + DA@OHA; OHA + micelle | H-bond; π-π interactions DA catechol groups | [57] | ||
| sodium alginate | DA grafted OSA + PAM | H-bond Inter DA grafted OSA | [58] | |
| boronic ester bond | acrylates | DA@graphene NSs + acrylate-PBA | H-bond Inter DA@graphene NSs | [59] |
| polyvinyl alcohol | PVA/TA@cellulose + borax | H-bond Inter PVA; Cys/TA@cellulose + PVA Schiff base Bond Cys + TA for TA modified cellulose | [50] | |
| PVA + borax | H-bond PAA + PVA/borax | [60] | ||
| PVA + borax | H-bond TA + PVA; TA + silk fibroin | [61] | ||
| poly(N-isopropyl acrylamide) | catechol-functionalized PNIPAM + PBA | Disulfide Bond inter sulfide-containing PBA | [48] | |
| sodium alginate | Alg-DA + Alg-PBA | H-bond Inter Alg; CNTs + Alg | [62] | |
| amylopectin | Amy + borax | H-bond Inter Amy; Amy + PAM; Amy + PAA | [63] | |
| disulfide bond | imidazole-type ionic liquid monomer | ionic liquid monomer with disulfide and alkene groups | Hydrophobic interactions Micellization of octadecyl-MA | [64] |
| poly(N-isopropyl acrylamide) | RS-Ag + crosslinker with disulfide groups | H-bond PNIPAM + RS-Ag Metal Coordination RS-Ag | [65] | |
| oxime bond | hyaluronic acid | OHA + modified Pluronic F127 | Hydrophobic interactions Inter-modified Pluronic F127 | [66] |
| H-bond | tannic acid | TA@HC nanoparticles + PAA | Metal Coordination TA@HC nanoparticles + Al3+ | [67] |
| TA + PVA | Coordination bond TA + CMC Amide bond PEI + CMC | [68] | ||
| thioctic acid | poly(ThA) + GM | Disulfide Bond Inter poly(ThA) | [69] | |
| sodium alginate | Histidine + Alg | Metal Coordination Histidine + Zn2+ | [70] | |
| PAM + Alg | Metal Coordination Alg+ Fe3+ | [71] | ||
| PAA/PAM + Alg-DA | Metal Coordination PAA/PAM + Al3+ Electrostatic interaction PAA/PAM + Alg-DA | [72] | ||
| Lignosulfonate + PVP | Hydrophobic interactions Lignosulfonate + PVP | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Condò, I.; Giannitelli, S.M.; Lo Presti, D.; Cortese, B.; Ursini, O. Overview of Dynamic Bond Based Hydrogels for Reversible Adhesion Processes. Gels 2024, 10, 442. https://doi.org/10.3390/gels10070442
Condò I, Giannitelli SM, Lo Presti D, Cortese B, Ursini O. Overview of Dynamic Bond Based Hydrogels for Reversible Adhesion Processes. Gels. 2024; 10(7):442. https://doi.org/10.3390/gels10070442
Chicago/Turabian StyleCondò, Ilaria, Sara Maria Giannitelli, Daniela Lo Presti, Barbara Cortese, and Ornella Ursini. 2024. "Overview of Dynamic Bond Based Hydrogels for Reversible Adhesion Processes" Gels 10, no. 7: 442. https://doi.org/10.3390/gels10070442
APA StyleCondò, I., Giannitelli, S. M., Lo Presti, D., Cortese, B., & Ursini, O. (2024). Overview of Dynamic Bond Based Hydrogels for Reversible Adhesion Processes. Gels, 10(7), 442. https://doi.org/10.3390/gels10070442












