Degradable Gel for Temporary Plugging in High Temperature Reservoir and Its Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of Orthogonal Experiment Results
2.2. FTIR Analysis
2.3. 13C NMR Analysis
2.4. TG Analysis
2.5. Viscoelastic Properties of Degradable Gel
2.6. Compressive Performance of Degradable Gel
2.7. Plugging Performance and Core Damage Performance of Degradable Gel
2.8. Degradation Properties
2.8.1. The Degraded Residual Liquid Viscosity
2.8.2. SEM Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of the Crosslinker
4.3. Preparation of Degradable Gel
4.4. Characterization and Performance Evaluation
4.4.1. Characterization Tests
- (1)
- Fourier Transform Infrared Spectroscopy (FTIR)
- (2)
- 13C NMR measurement
- (3)
- Thermal Analysis
- (4)
- Scanning electron microscope
4.4.2. Performance Evaluation of Degradable Gel
- (1)
- Measurement of gelation time and degradation time
- (2)
- Viscoelastic properties test
- (3)
- Compressive strength test
- (4)
- Plugging performance test and core damage performance test
- (5)
- Measurement of degraded residual liquid viscosity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, Z.; Liu, Y.-T.; Jia, H.; Xia, B.; Ou, H.-M.; Pu, Q.-B.; Hussein, I.A. Progress and Prospects of In Situ Polymer Gels for Sealing Operation in Wellbore and Near-Well Zone. Energy Fuels 2024, 38, 3539–3563. [Google Scholar] [CrossRef]
- Xu, C.; Yang, X.; Liu, C.; Kang, Y.; Bai, Y.; You, Z. Dynamic Fracture Width Prediction for Lost Circulation Control and Formation Damage Prevention in Ultra-Deep Fractured Tight Reservoir. Fuel 2022, 307, 121770. [Google Scholar] [CrossRef]
- Sun, J.; Bai, Y.; Cheng, R.; Lyu, K.; Liu, F.; Feng, J.; Lei, S.; Zhang, J.; Hao, H. Research Progress and Prospect of Plugging Technologies for Fractured Formation with Severe Lost Circulation. Pet. Explor. Dev. 2021, 48, 732–743. [Google Scholar] [CrossRef]
- Jia, H.; Kang, Z.; Zhu, J.; Ren, L.; Cai, M.; Wang, T.; Xu, Y.; Li, Z. High Density Bromide-Based Nanocomposite Gel for Temporary Plugging in Fractured Reservoirs with Multi-Pressure Systems. J. Pet. Sci. Eng. 2021, 205, 108778. [Google Scholar] [CrossRef]
- Jia, H.; Niu, C.; Liang, W.; He, W.; Sun, J. High-Density Solid-Free Flexible Microgel Fluid Loss Pill in High-Temperature and High-Pressure Reservoirs: Curing Mechanism and Working Performance. SPE J. 2023, 28, 917–933. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, H.; She, J.; Jiang, G.; Peng, C.; You, Z. Experimental Study on Fracture Plugging Effect of Irregular-Shaped Lost Circulation Materials. Energy 2023, 276, 127544. [Google Scholar] [CrossRef]
- Liu, J.; Fu, H.; Luo, Z.; Chen, W.; Liu, F.; Zhao, M. Preparation and Performance of PH-Temperature Responsive Low-Damage Gel Temporary Plugging Agent. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 130990. [Google Scholar] [CrossRef]
- Cheng, L.; Qin, Y.; Su, Y.; Pan, Y.; Wang, Y.; Liao, R.; Li, Z. Development of a High-Strength and Adhesive Polyacrylamide Gel for Well Plugging. ACS Omega 2022, 7, 6151–6159. [Google Scholar] [CrossRef]
- Savari, S.; Whitfill, D.L.; Walker, J. Acid-Soluble Lost Circulation Material for Use in Large, Naturally Fractured Formations and Reservoirs. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 6–9 March 2017. [Google Scholar]
- Yang, J.; Bai, Y.; Sun, J.; Lv, K.; Lang, Y. Recent Advances of Thermosetting Resin and Its Application Prospect in Oil and Gas Drilling and Production Engineering. Geoenergy Sci. Eng. 2023, 230, 212222. [Google Scholar] [CrossRef]
- Huang, X.; Sun, J.; Lv, K.; Liu, J.; Shen, H.; Zhang, F. Application of Core-Shell Structural Acrylic Resin/Nano-SiO2 Composite in Water Based Drilling Fluid to Plug Shale Pores. J. Nat. Gas Sci. Eng. 2018, 55, 418–425. [Google Scholar] [CrossRef]
- Xu, C.; Zhu, L.; Xu, F.; Kang, Y.; Jing, H.; You, Z. Experimental Study on the Mechanical Controlling Factors of Fracture Plugging Strength for Lost Circulation Control in Shale Gas Reservoir. Geoenergy Sci. Eng. 2023, 231, 212285. [Google Scholar] [CrossRef]
- Xu, C.; Liu, L.; Yang, Y.; Kang, Y.; You, Z. An Innovative Fracture Plugging Evaluation Method for Drill-in Fluid Loss Control and Formation Damage Prevention in Deep Fractured Tight Reservoirs. Fuel 2024, 358, 130123. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Liao, R.; Zhang, G.; Zhang, M.; Li, X. Prediction of Hydrogel Degradation Time Based on Central Composite Design. ACS Omega 2024, 9, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, F.; Liu, J.; Liu, C.; Su, G.; Yang, H.; Yu, X. Synthesis and Properties of PAM/PLA Composite Degradable Particle Temporary Plugging Agent. J. Appl. Polym. Sci. 2022, 139, e53216. [Google Scholar] [CrossRef]
- Okromelidze, G.V.; Garshina, O.V.; Nekrasova, I.L.; Iljyasov, S.E. Method of Well- Killing Operation by Using Visco-Elastic Gels with Controllable Destruction Terms. In Proceedings of the SPE Russian Oil and Gas Exploration & Production Technical Conference and Exhibition, Moscow, Russia, 14–16 October 2014. [Google Scholar]
- Wang, Y.; Zhao, M.; Dong, X.; Li, Q.; Yang, Z.; Ding, M. Potential of the Base-Activated Persulfate for Polymer-Plugging Removal in Low Temperature Reservoirs. J. Pet. Sci. Eng. 2020, 189, 107000. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Tao, Y.; Ma, H.; Wu, J. Research and Application of the HTHS Anti-H2S Gel Valve for Underbalanced Drilling. J. Pet. Sci. Eng. 2018, 164, 174–181. [Google Scholar] [CrossRef]
- Kang, Z.; Jia, H.; Li, Z.-G.; Xia, B.; Wang, Y.; Jiang, Y.; Peng, H.-L. Comprehensive Evaluation of Chemical Breakers for Multistage Network Ultra-High Strength Gel. Pet. Sci. 2023, 20, 2864–2878. [Google Scholar] [CrossRef]
- Wang, K.; Liu, G.; Guo, Y.; Yang, H.; Chen, Z.; Su, G.; Wang, Y.; Wei, B.; Yu, X. Preparation and Properties of Degradable Hydrogels as a Temporary Plugging Agent Used for Acidizing Treatments to Enhance Oil Recovery. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 128218. [Google Scholar] [CrossRef]
- Ji, R.; Yu, X.; Yang, H.; Wang, X.; Su, G. Preparation and Degradable Mechanism of Self-Breaking Gel Valve for Underbalanced Drilling. Geoenergy Sci. Eng. 2024, 235, 212705. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Liao, R.; Zhang, G.; Zhang, M.; Li, X. Study of Adhesive Self-Degrading Gel for Wellbore Sealing. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129567. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, X.; Li, L.; Zhang, J.; Shi, X.; Hu, G. Research Progress of High-Temperature Resistant Functional Gel Materials and Their Application in Oil and Gas Drilling. Gels 2022, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zou, H.; Wang, Y.; Zhu, M.; Su, G.; Huang, Z.; Yu, X.; Yang, H. Degradation Behavior and Mechanism of P(AM/AA/AMPS)@PLA Core-Shell Self-Degrading Temporary Plugging Agent. J. Mol. Liq. 2024, 393, 123656. [Google Scholar] [CrossRef]
- Song, T.; Tian, X.; Bai, B.; Eriyagama, Y.; Ahdaya, M.; Alotibi, A.; Schuman, T. Laboratory Evaluation of High-Temperature Resistant Lysine-Based Polymer Gel Systems for Leakage Control. Geoenergy Sci. Eng. 2024, 234, 212685. [Google Scholar] [CrossRef]
- Zou, C.; Dai, C.; Liu, Y.; You, Q.; Ding, F.; Huang, Y.; Sun, N. A Novel Self-Degradable Gel (SDG) as Liquid Temporary Plugging Agent for High-Temperature Reservoirs. J. Mol. Liq. 2023, 386, 122463. [Google Scholar] [CrossRef]
- Du, J.; Liu, J.; Zhao, L.; Liu, P.; Chen, X.; Wang, Q.; Yu, M. Water-Soluble Polymers for High-Temperature Resistant Hydraulic Fracturing: A Review. J. Nat. Gas Sci. Eng. 2022, 104, 104673. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, L.; Cao, Z.; Hao, T.; Liu, Y.; Wu, W. High-temperature Performance and Cross-linking Mechanism of Different Types of Gel Systems in Saline Environment. J. Appl. Polym. Sci. 2022, 139, 51452. [Google Scholar] [CrossRef]
- Shamlooh, M.; Elaf, R.; Hussein, I.A.; Saad, M.; Bai, B. Chitosan/Polyacrylamide Green Gels for Water Control in High-Temperature Reservoirs. Energy Fuels 2022, 36, 3816–3824. [Google Scholar] [CrossRef]
- Yu, B.; Zhao, S.; Long, Y.; Bai, B.; Schuman, T. Comprehensive Evaluation of a High-Temperature Resistant Re-Crosslinkable Preformed Particle Gel for Water Management. Fuel 2022, 309, 122086. [Google Scholar] [CrossRef]
- Ding, H.; Tang, H.; Wang, F.; Zhang, H.; Guo, Y. The Molecular Structure Control of Butyl Methacrylate/Acrylamide (Co)Polymers. Mater. Des. 2015, 88, 820–826. [Google Scholar] [CrossRef]
- Fazeli, M.; Escrochi, M.; Hosseini, Z.S.; Vaferi, B. Experimental Analyzing the Effect of N-Heptane Concentration and Angular Frequency on the Viscoelastic Behavior of Crude Oil Containing Asphaltene. Sci. Rep. 2022, 12, 3965. [Google Scholar] [CrossRef]
- Sydansk, R.D. A Newly Developed Chromium(Lll) Gel Technology. SPE Reserv. Eng. 1990, 5, 346–352. [Google Scholar] [CrossRef]
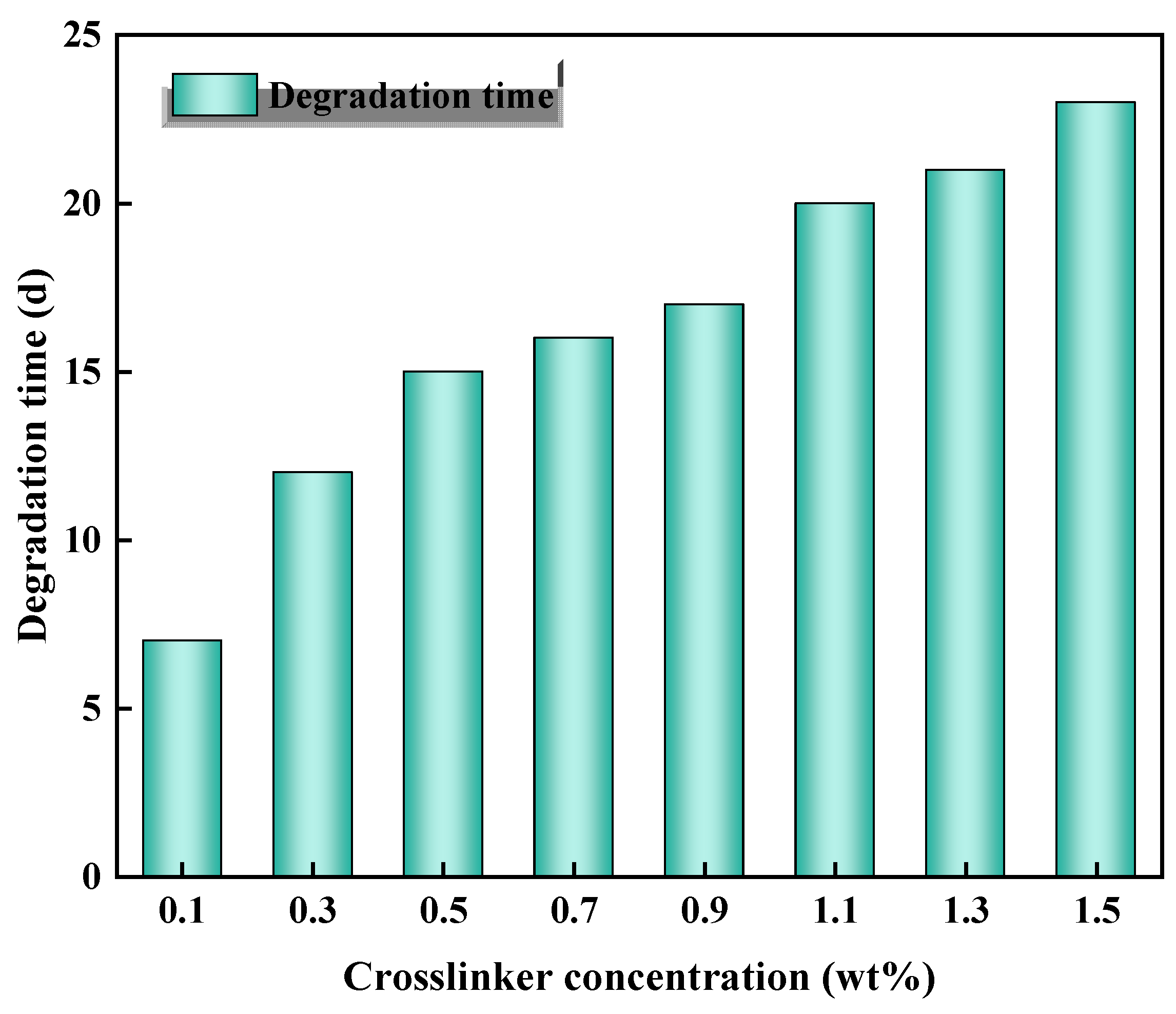

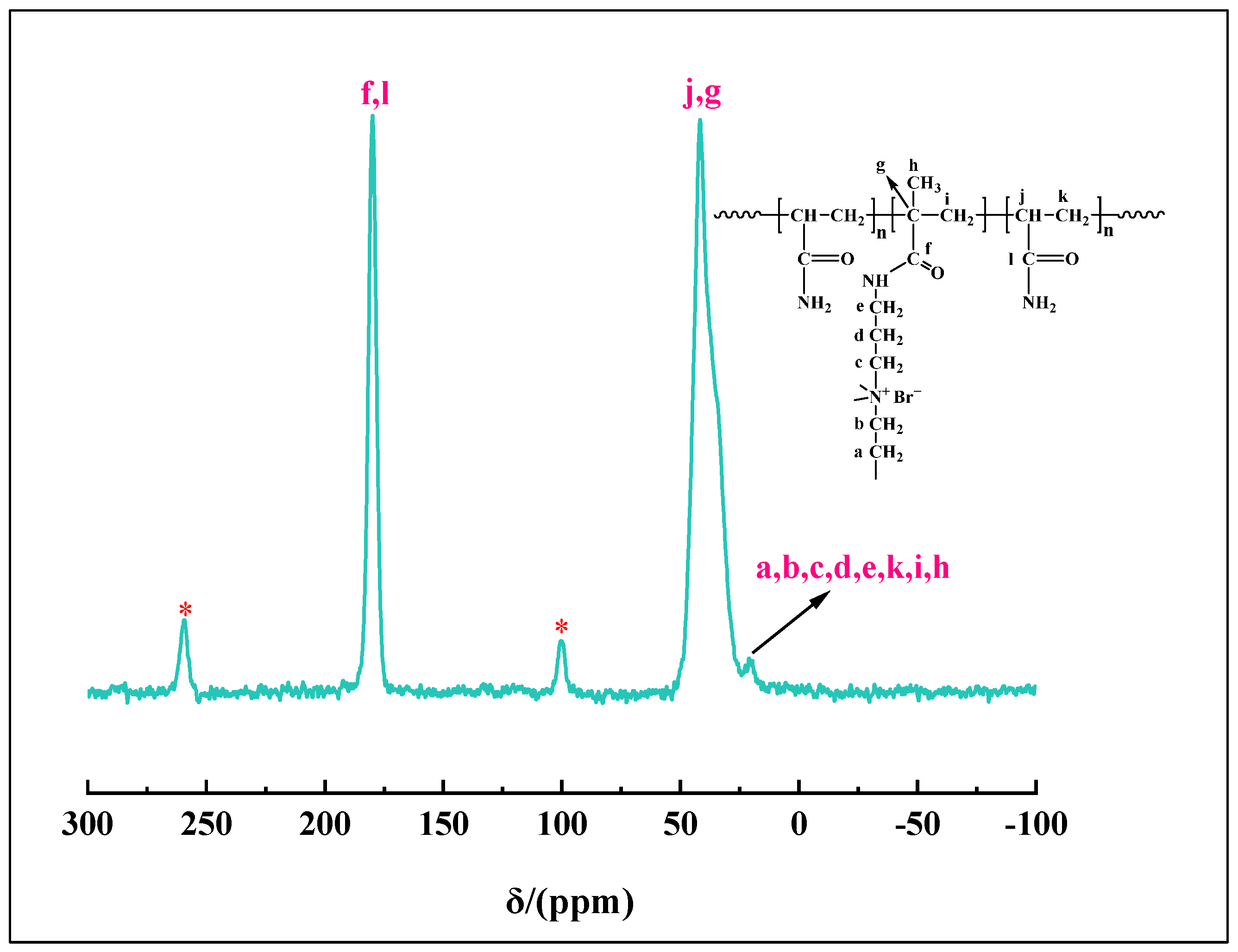
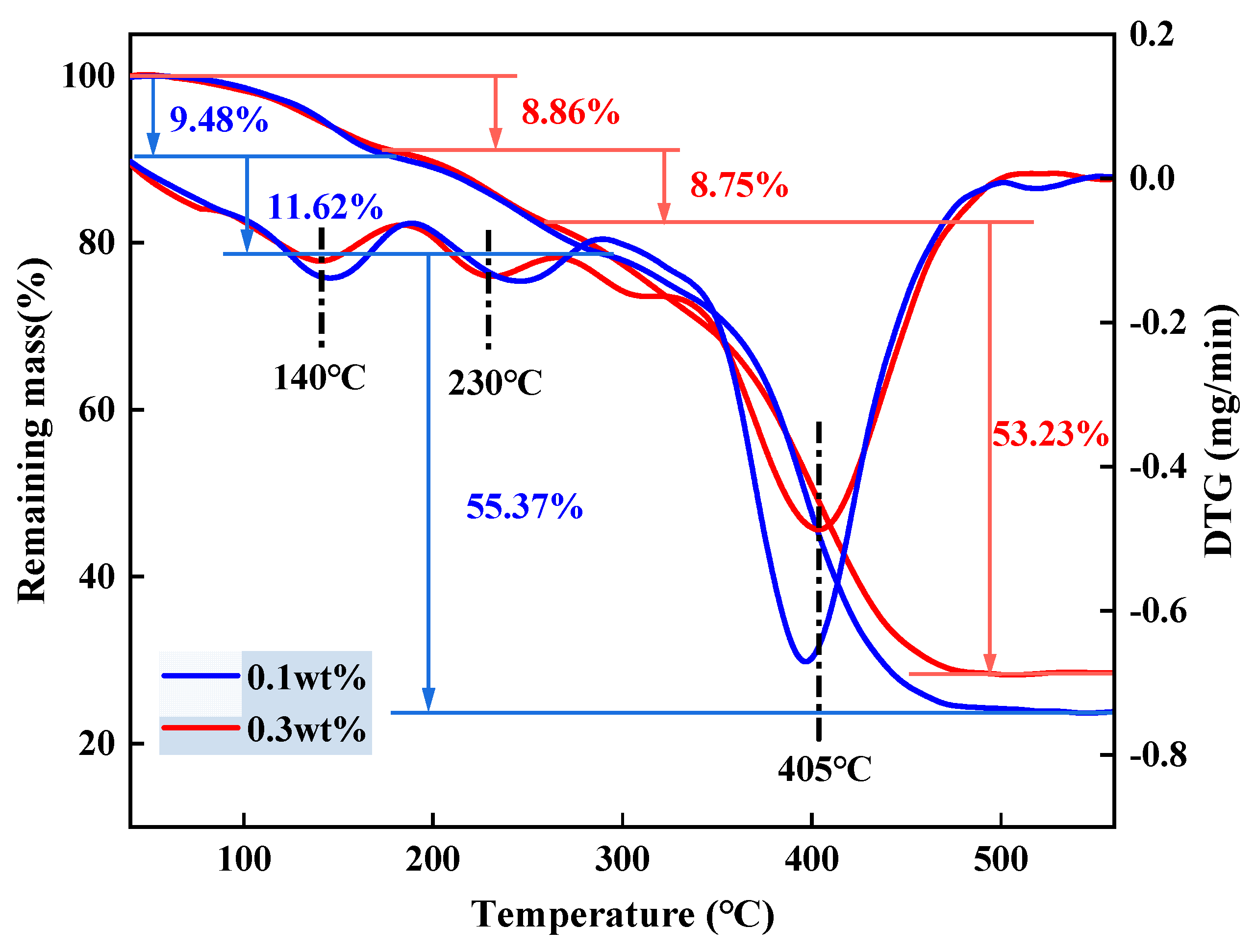




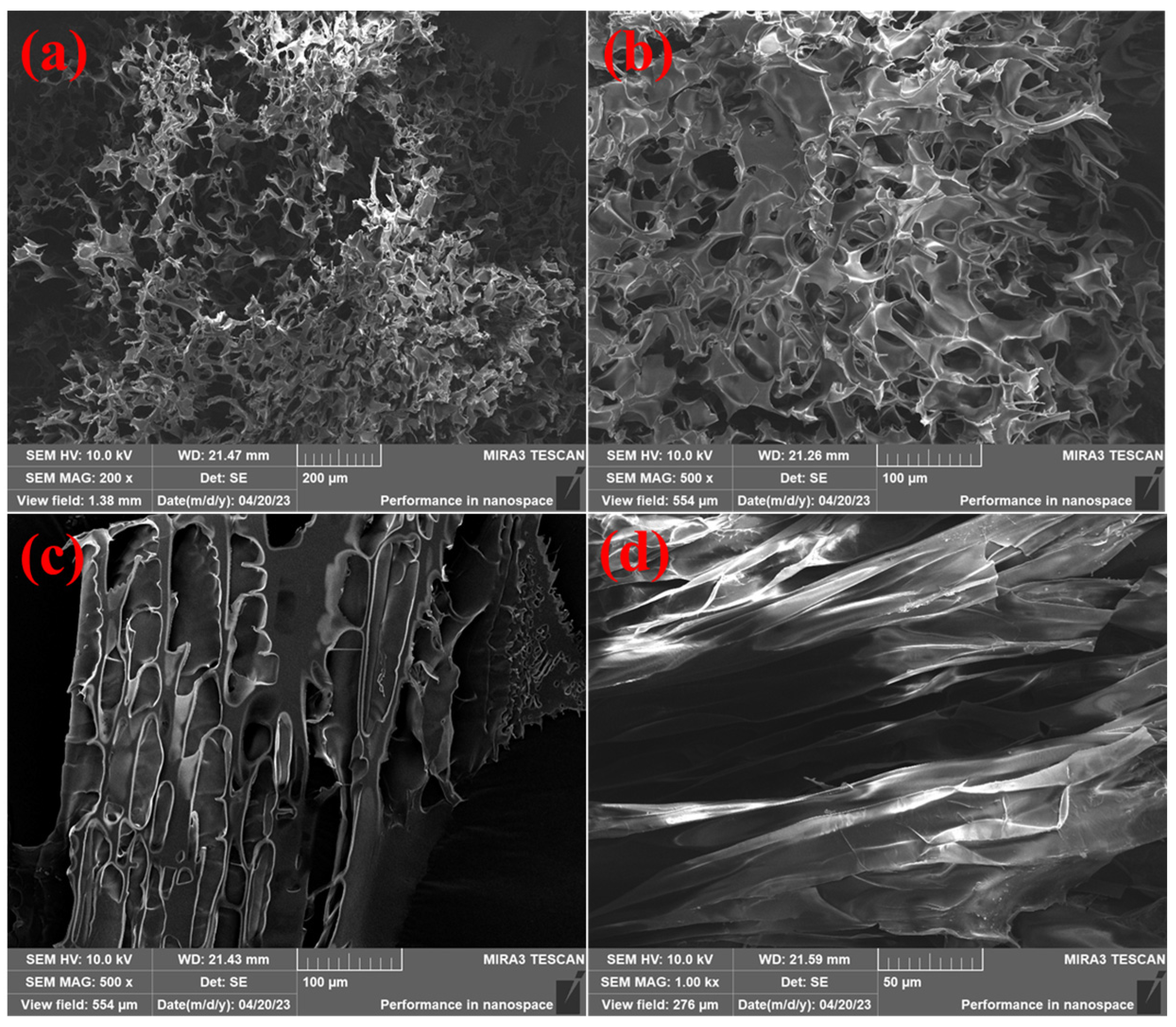


| Number | Monomer Concentration (wt%) | Initiator Concentration (wt%) | Crosslinker Concentration (wt%) | Temperature (°C) | Degradation Time (d) |
|---|---|---|---|---|---|
| 1 | 8 | 0.01 | 0.5 | 90 | 15 |
| 2 | 8 | 0.02 | 1 | 110 | 10 |
| 3 | 8 | 0.03 | 1.5 | 130 | 9 |
| 4 | 10 | 0.01 | 1.5 | 110 | 14 |
| 5 | 10 | 0.02 | 0.5 | 130 | 5 |
| 6 | 10 | 0.03 | 1 | 90 | 19 |
| 7 | 12 | 0.01 | 1 | 130 | 7 |
| 8 | 12 | 0.02 | 1.5 | 90 | 25 |
| 9 | 12 | 0.03 | 0.5 | 110 | 8 |
| 11.333 | 12.000 | 9.333 | 19.667 | - | |
| 12.667 | 13.333 | 12.000 | 10.667 | - | |
| 13.333 | 12.000 | 16.000 | 7.000 | - | |
| 2.000 | 1.333 | 6.667 | 12.667 | - |
| Sand-Filled Tube Number | Initial Permeability (mD) | Plugging Pressure (MPa) | Post-Degradation Permeability (mD) | Core Damage Rate (%) |
|---|---|---|---|---|
| A | 2794.1 | 17.8 | 2685.4 | 3.89 |
| B | 1559.2 | 20.1 | 1489.5 | 4.47 |
| C | 1185.6 | 22.7 | 1137.9 | 4.02 |
| D | 746.3 | 23.4 | 710.9 | 4.74 |
| E | 538.2 | 25.2 | 511.7 | 4.92 |
| Factor | Level | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Monomer concentration (wt%) | 8 | 10 | 12 |
| Crosslinker concentration (wt%) | 0.5 | 1 | 1.5 |
| Initiator concentration (wt%) | 0.01 | 0.02 | 0.03 |
| Temperature (°C) | 90 | 110 | 130 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Liu, J.; Ji, R.; Yu, X.; Yang, H.; Su, G. Degradable Gel for Temporary Plugging in High Temperature Reservoir and Its Properties. Gels 2024, 10, 445. https://doi.org/10.3390/gels10070445
Yang F, Liu J, Ji R, Yu X, Yang H, Su G. Degradable Gel for Temporary Plugging in High Temperature Reservoir and Its Properties. Gels. 2024; 10(7):445. https://doi.org/10.3390/gels10070445
Chicago/Turabian StyleYang, Fan, Jinhua Liu, Renjing Ji, Xiaorong Yu, Huan Yang, and Gaoshen Su. 2024. "Degradable Gel for Temporary Plugging in High Temperature Reservoir and Its Properties" Gels 10, no. 7: 445. https://doi.org/10.3390/gels10070445





