Innovative Approach to Accelerate Wound Healing: Synthesis and Validation of Enzymatically Cross-Linked COL–rGO Biocomposite Hydrogels
Abstract
1. Introduction
2. Results and Discussion
2.1. Morphological Characterization of COL–rGO Hydrogels
2.2. Chemical Characterization of COL–rGO Hydrogels
2.3. Thermal Stability of COL–rGO Hydrogels
2.4. Swelling Capacity, Penetration Mechanism, and Wettability
2.5. Conductivity and Surface Charge
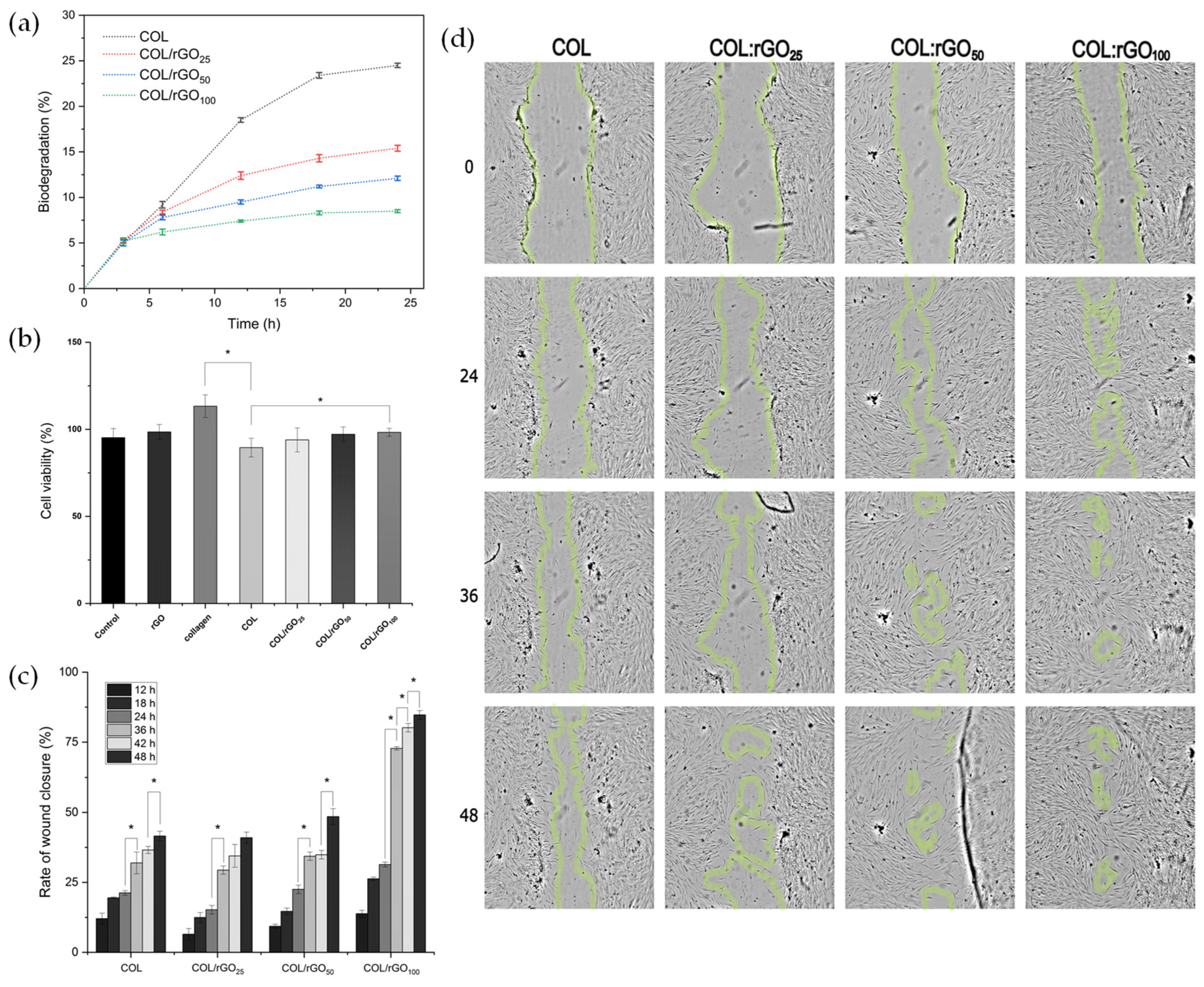
2.6. Biodegradability and Cytotoxicity Assays
2.7. In Vitro Wound Healing Assay (Scratch Test)
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Graphene Oxide (GO) and Reduced Graphene Oxide (rGO)
4.3. Hydrogels synthesis
4.4. Biocomposite Hydrogel’s Characterization
4.5. In Vitro Biodegradation of COL–rGO Hydrogel
4.6. Cytotoxicity Assay
4.7. In Vitro Wound Healing Assay (Scratch Test)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, B.; Dong, R.; Liang, Y.; Li, M. Haemostatic Materials for Wound Healing Applications. Nat. Rev. Chem. 2021, 5, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Guo, B. Smart Wound Dressings for Wound Healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, X.; Liu, X.; Wen, G.; Yu, Y. Recent Advances in Decellularized Biomaterials for Wound Healing. Mater. Today Bio 2023, 19, 100589. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Kumar, D.; Priyadarshani, A.; Jain, G.K.; Mittal, G.; Kesharwani, P.; Aggarwal, G. Recent Progress in Polymeric Biomaterials and Their Potential Applications in Skin Regeneration and Wound Care Management. J. Drug Deliv. Sci. Technol. 2023, 82, 104319. [Google Scholar] [CrossRef]
- Saberianpour, S.; Melotto, G.; Forss, R.; Redhead, L.; Elsom, J.; Terrazzini, N.; Sandeman, S.; Sarker, D.; Bucca, G.; Hesketh, A.; et al. Development of Theranostic Wound Dressings: Harnessing the Knowledge of Biospecific Interactions at the Biomaterial Interface to Promote Healing and Identify Biomarkers. Expert. Rev. Med. Devices 2023, 20, 163–165. [Google Scholar] [CrossRef]
- Sathyaraj, W.V.; Prabakaran, L.; Bhoopathy, J.; Dharmalingam, S.; Karthikeyan, R.; Atchudan, R. Therapeutic Efficacy of Polymeric Biomaterials in Treating Diabetic Wounds—An Upcoming Wound Healing Technology. Polymers 2023, 15, 1205. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Escribano, V.; Torrecillas-Baena, B.; Dorado, G.; Gálvez-Moreno, M.Á.; Camacho-Cardenosa, M.; Casado-Díaz, A. Combination of Biomaterials and Extracellular Vesicles from Mesenchymal Stem-Cells: New Therapeutic Strategies for Skin-Wound Healing. Appl. Sci. 2023, 13, 2702. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, H.; Guo, B. Conductive Biomaterials as Bioactive Wound Dressing for Wound Healing and Skin Tissue Engineering. Nanomicro Lett. 2021, 14, 1. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A Mini Review on Hydrogels Classification and Recent Developments in Miscellaneous Applications. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of Synthesis of Hydrogels … A Review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Ligorio, C.; Zhou, M.; Wychowaniec, J.K.; Zhu, X.; Bartlam, C.; Miller, A.F.; Vijayaraghavan, A.; Hoyland, J.A.; Saiani, A. Graphene Oxide Containing Self-Assembling Peptide Hybrid Hydrogels as a Potential 3D Injectable Cell Delivery Platform for Intervertebral Disc Repair Applications. Acta Biomater. 2019, 92, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Z.; Xu, C.; Li, Y.; Gao, J.; Wang, W.; Liu, Y. High Strength Graphene Oxide/Polyvinyl Alcohol Composite Hydrogels. J. Mater. Chem. 2011, 21, 10399–10406. [Google Scholar] [CrossRef]
- Hu, Y.; Zhuo, H.; Zhang, Y.; Lai, H.; Yi, J.; Chen, Z.; Peng, X.; Wang, X.; Liu, C.; Sun, R.; et al. Graphene Oxide Encapsulating Liquid Metal to Toughen Hydrogel. Adv. Funct. Mater. 2021, 31, 2106761. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Nadolna, K.; Owczarek, A. Chapter 6—The Physical and Chemical Properties of Hydrogels Based on Natural Polymers. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 151–172. ISBN 978-0-12-816421-1. [Google Scholar]
- Chen, J.; Li, Y.; Huang, L.; Li, C.; Shi, G. High-Yield Preparation of Graphene Oxide from Small Graphite Flakes via an Improved Hummers Method with a Simple Purification Process. Carbon 2015, 81, 826–834. [Google Scholar] [CrossRef]
- Hu, X.; Qi, R.; Zhu, J.; Lu, J.; Luo, Y.; Jin, J.; Jiang, P. Preparation and Properties of Dopamine Reduced Graphene Oxide and Its Composites of Epoxy. J. Appl. Polym. Sci. 2014, 131, 39754. [Google Scholar] [CrossRef]
- Xu, L.Q.; Yang, W.J.; Neoh, K.-G.; Kang, E.-T.; Fu, G.D. Dopamine-Induced Reduction and Functionalization of Graphene Oxide Nanosheets. Macromolecules 2010, 43, 8336–8339. [Google Scholar] [CrossRef]
- Agarwal, V.; Zetterlund, P.B. Strategies for Reduction of Graphene Oxide—A Comprehensive Review. Chem. Eng. J. 2021, 405, 127018. [Google Scholar] [CrossRef]
- Shin, S.R.; Zihlmann, C.; Akbari, M.; Assawes, P.; Cheung, L.; Zhang, K.; Manoharan, V.; Zhang, Y.S.; Yüksekkaya, M.; Wan, K.; et al. Reduced Graphene Oxide-GelMA Hybrid Hydrogels as Scaffolds for Cardiac Tissue Engineering. Small 2016, 12, 3677–3689. [Google Scholar] [CrossRef]
- Bahrami, S.; Baheiraei, N.; Shahrezaee, M. Biomimetic Reduced Graphene Oxide Coated Collagen Scaffold for in Situ Bone Regeneration. Sci. Rep. 2021, 11, 16783. [Google Scholar] [CrossRef]
- Chu, J.; Shi, P.; Yan, W.; Fu, J.; Yang, Z.; He, C.; Deng, X.; Liu, H. PEGylated Graphene Oxide-Mediated Quercetin-Modified Collagen Hybrid Scaffold for Enhancement of MSCs Differentiation Potential and Diabetic Wound Healing. Nanoscale 2018, 10, 9547–9560. [Google Scholar] [CrossRef]
- Guo, W.; Wang, S.; Yu, X.; Qiu, J.; Li, J.; Tang, W.; Li, Z.; Mou, X.; Liu, H.; Wang, Z. Construction of a 3D RGO–Collagen Hybrid Scaffold for Enhancement of the Neural Differentiation of Mesenchymal Stem Cells. Nanoscale 2016, 8, 1897–1904. [Google Scholar] [CrossRef]
- Kang, S.; Park, J.B.; Lee, T.-J.; Ryu, S.; Bhang, S.H.; La, W.-G.; Noh, M.-K.; Hong, B.H.; Kim, B.-S. Covalent Conjugation of Mechanically Stiff Graphene Oxide Flakes to Three-Dimensional Collagen Scaffolds for Osteogenic Differentiation of Human Mesenchymal Stem Cells. Carbon 2015, 83, 162–172. [Google Scholar] [CrossRef]
- Liu, S.; Mou, S.; Zhou, C.; Guo, L.; Zhong, A.; Yang, J.; Yuan, Q.; Wang, J.; Sun, J.; Wang, Z. Off-the-Shelf Biomimetic Graphene Oxide–Collagen Hybrid Scaffolds Wrapped with Osteoinductive Extracellular Matrix for the Repair of Cranial Defects in Rats. ACS Appl. Mater. Interfaces 2018, 10, 42948–42958. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Lee, Y.; Le Thi, P.; Park, K.D. Engineered Horseradish Peroxidase-Catalyzed Hydrogels with High Tissue Adhesiveness for Biomedical Applications. J. Ind. Eng. Chem. 2019, 78, 34–52. [Google Scholar] [CrossRef]
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis Route of Reduced Graphene Oxide via Thermal Reduction of Chemically Exfoliated Graphene Oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- Kaminska, I.; Das, M.R.; Coffinier, Y.; Niedziolka-Jonsson, J.; Sobczak, J.; Woisel, P.; Lyskawa, J.; Opallo, M.; Boukherroub, R.; Szunerits, S. Reduction and Functionalization of Graphene Oxide Sheets Using Biomimetic Dopamine Derivatives in One Step. ACS Appl. Mater. Interfaces 2012, 4, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-L.; Yan, X.-P.; Meng, K.; Wang, S.-F. Graphene Oxide Based Photoinduced Charge Transfer Label-Free Near-Infrared Fluorescent Biosensor for Dopamine. Anal. Chem. 2011, 83, 8787–8793. [Google Scholar] [CrossRef]
- Kim, J.-K.; Lee, J.-S.; Jung, H.-J.; Cho, J.-H.; Heo, J.-I.; Chang, Y.-H. Preparation and Properties of Collagen/Modified Hyaluronic Acid Hydrogel for Biomedical Application. J. Nanosci. Nanotechnol. 2007, 7, 3852–3856. [Google Scholar] [CrossRef]
- Zhu, S.; Gu, Z.; Xiong, S.; An, Y.; Liu, Y.; Yin, T.; You, J.; Hu, Y. Fabrication of a Novel Bio-Inspired Collagen–Polydopamine Hydrogel and Insights into the Formation Mechanism for Biomedical Applications. RSC Adv. 2016, 6, 66180–66190. [Google Scholar] [CrossRef]
- Sadeghi, M.; Hosseinzadeh, H. Synthesis and Super-Swelling Behavior of a Novel Low Salt-Sensitive Protein-Based Superabsorbent Hydrogel: Collagen-g-Poly(AMPS). Turk. J. Chem. 2010, 34, 739–752. [Google Scholar] [CrossRef]
- Šupová, M.; Suchý, T.; Chlup, H.; Štípek, J.; Žitný, R.; Landfeld, A.; Skočilas, J.; Žaloudková, M.; Rýglová, Š.; Braun, M.; et al. The Comprehensive Evaluation of Two Collagen Gels Used for Sausage Casing Extrusion Purposes: The Role of the Structural and Mechanical Properties. J. Food Eng. 2023, 343, 111387. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97. [Google Scholar] [CrossRef]
- Chakrapani, V.Y.; Gnanamani, A.; Giridev, V.R.; Madhusoothanan, M.; Sekaran, G. Electrospinning of Type I Collagen and PCL Nanofibers Using Acetic Acid. J. Appl. Polym. Sci. 2012, 125, 3221–3227. [Google Scholar] [CrossRef]
- De Guzzi Plepis, A.M.; Goissis, G.; Das-Gupta, D.K. Dielectric and Pyroelectric Characterization of Anionic and Native Collagen. Polym. Eng. Sci. 1996, 36, 2932–2938. [Google Scholar] [CrossRef]
- Andonegi, M.; Las Heras, K.; Santos-Vizcaíno, E.; Igartua, M.; Hernandez, R.M.; de la Caba, K.; Guerrero, P. Structure-Properties Relationship of Chitosan/Collagen Films with Potential for Biomedical Applications. Carbohydr. Polym. 2020, 237, 116159. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Liu, A.; Wang, W. Improved Thermal-Stability and Mechanical Properties of Type I Collagen by Crosslinking with Casein, Keratin and Soy Protein Isolate Using Transglutaminase. Int. J. Biol. Macromol. 2017, 98, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Liu, F.; Yu, Z.; Chang, B.; Goff, H.D.; Zhong, F. Effect of Aging Treatment on the Physicochemical Properties of Collagen Films. Food Hydrocoll. 2019, 87, 436–447. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Hidayah, N.M.S.; Liu, W.-W.; Lai, C.-W.; Noriman, N.Z.; Khe, C.-S.; Hashim, U.; Lee, H.C. Comparison on Graphite, Graphene Oxide and Reduced Graphene Oxide: Synthesis and Characterization. AIP Conf. Proc. 2017, 1892, 150002. [Google Scholar] [CrossRef]
- Lerf, A.; Buchsteiner, A.; Pieper, J.; Schöttl, S.; Dekany, I.; Szabo, T.; Boehm, H.P. Hydration Behavior and Dynamics of Water Molecules in Graphite Oxide. J. Phys. Chem. Solids 2006, 67, 1106–1110. [Google Scholar] [CrossRef]
- Hua, Y.; Ma, C.; Wei, T.; Zhang, L.; Shen, J. Collagen/Chitosan Complexes: Preparation, Antioxidant Activity, Tyrosinase Inhibition Activity, and Melanin Synthesis. Int. J. Mol. Sci. 2020, 21, 313. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Ridzuan, P.M.; Bahari, H. Current Insights into Collagen Type I. Polymers 2021, 13, 2642. [Google Scholar] [CrossRef] [PubMed]
- Julia, S.; Sari, Y.W.; Nurlely; Soejoko, D.S. Formation and Characterization of Collagen and Hydroxyapatite Composite. J. Phys. Conf. Ser. 2019, 1248, 12082. [Google Scholar] [CrossRef]
- Michalicha, A.; Canal, C.; Espona-Noguera, A.; Piet, M.; Budzyńska, B.; Przywara, S.; Belcarz, A. Collagen-Sealed Polyester Vascular Prostheses Functionalized by Polycatecholamine Coatings. Int. J. Mol. Sci. 2022, 23, 9369. [Google Scholar] [CrossRef] [PubMed]
- Girão, A.F.; Gonçalves, G.; Bhangra, K.S.; Phillips, J.B.; Knowles, J.; Irurueta, G.; Singh, M.K.; Bdkin, I.; Completo, A.; Marques, P.A.A.P. Electrostatic Self-Assembled Graphene Oxide-Collagen Scaffolds towards a Three-Dimensional Microenvironment for Biomimetic Applications. RSC Adv. 2016, 6, 49039–49051. [Google Scholar] [CrossRef]
- Mitra, T.; Manna, P.J.; Raja, S.T.K.; Gnanamani, A.; Kundu, P.P. Curcumin Loaded Nano Graphene Oxide Reinforced Fish Scale Collagen—A 3D Scaffold Biomaterial for Wound Healing Applications. RSC Adv. 2015, 5, 98653–98665. [Google Scholar] [CrossRef]
- Calderon, L.; Collin, E.; Murphy, M.; O’Halloran, D.; Pandit, A. Pandit Type Ii Collagen-Hyaluronan Hydrogel—A Step towards a Scaffold for Intervertebral Disc Tissue Engineering. Eur. Cell Mater. 2010, 20, 134–148. [Google Scholar] [CrossRef]
- Frayssinet, A.; Petta, D.; Illoul, C.; Haye, B.; Markitantova, A.; Eglin, D.; Mosser, G.; D’Este, M.; Hélary, C. Extracellular Matrix-Mimetic Composite Hydrogels of Cross-Linked Hyaluronan and Fibrillar Collagen with Tunable Properties and Ultrastructure. Carbohydr. Polym. 2020, 236, 116042. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, B.; Xu, G.; Hao, W. Swelling Behaviours and Mechanical Properties of Silk Fibroin–Polyurethane Composite Hydrogels. Compos. Sci. Technol. 2013, 84, 15–22. [Google Scholar] [CrossRef]
- Mora, C.; Buljan, A.; Figueroa, T. Reaction Conditions and Aqueous Washing Process: Implications for Graphene Oxide Production and Its Applications. ACS Appl. Nano Mater. 2022, 5, 4648–4662. [Google Scholar] [CrossRef]
- Sionkowska, A. The Influence of UV Light on Collagen/Poly(Ethylene Glycol) Blends. Polym. Degrad. Stab. 2006, 91, 305–312. [Google Scholar] [CrossRef]
- Jafarigol, E.; Salehi, M.B.; Mortaheb, H.R. Preparation and Assessment of Electro-Conductive Poly(Acrylamide-Co-Acrylic Acid) Carboxymethyl Cellulose/Reduced Graphene Oxide Hydrogel with High Viscoelasticity. Chem. Eng. Res. Des. 2020, 162, 74–84. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, B.; Wu, H.; Liang, Y.; Ma, P.X. Injectable Antibacterial Conductive Nanocomposite Cryogels with Rapid Shape Recovery for Noncompressible Hemorrhage and Wound Healing. Nat. Commun. 2018, 9, 2784. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Y.; Zhang, J.; Hu, X.; Yang, Z.; Guo, Y.; Wang, Y. In-Situ Doping of a Conductive Hydrogel with Low Protein Absorption and Bacterial Adhesion for Electrical Stimulation of Chronic Wounds. Acta Biomater. 2019, 89, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable Conductive Injectable Hydrogels as Novel Antibacterial, Anti-Oxidant Wound Dressings for Wound Healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- He, J.; Shi, M.; Liang, Y.; Guo, B. Conductive Adhesive Self-Healing Nanocomposite Hydrogel Wound Dressing for Photothermal Therapy of Infected Full-Thickness Skin Wounds. Chem. Eng. J. 2020, 394, 124888. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Song, S.; Yang, K.; Liu, H.; Yang, Z.; Wang, J.; Yang, B.; Lin, Q. Skin-Inspired Antibacterial Conductive Hydrogels for Epidermal Sensors and Diabetic Foot Wound Dressings. Adv. Funct. Mater. 2019, 29, 1901474. [Google Scholar] [CrossRef]
- Norahan, M.H.; Pourmokhtari, M.; Saeb, M.R.; Bakhshi, B.; Soufi Zomorrod, M.; Baheiraei, N. Electroactive Cardiac Patch Containing Reduced Graphene Oxide with Potential Antibacterial Properties. Mater. Sci. Eng. C 2019, 104, 109921. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Lofland, S.; Hu, X. Thermal Conductivity of Protein-Based Materials: A Review. Polymers 2019, 11, 456. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Mi, Y.; Zhao, S.; Qi, S.; Sun, M.; Peng, B.; Xu, Q.; Niu, Y.; Zhou, Y. Transparent Stretchable Hydrogel Sensors: Materials, Design and Applications. J. Mater. Chem. C Mater. 2022, 10, 13351–13371. [Google Scholar] [CrossRef]
- Dayana Priyadharshini, S.; Manikandan, S.; Kiruthiga, R.; Rednam, U.; Babu, P.S.; Subbaiya, R.; Karmegam, N.; Kim, W.; Govarthanan, M. Graphene Oxide-Based Nanomaterials for the Treatment of Pollutants in the Aquatic Environment: Recent Trends and Perspectives—A Review. Environ. Pollut. 2022, 306, 119377. [Google Scholar] [CrossRef]
- Li, Y.; Asadi, A.; Monroe, M.R.; Douglas, E.P. PH Effects on Collagen Fibrillogenesis in Vitro: Electrostatic Interactions and Phosphate Binding. Mater. Sci. Eng. C 2009, 29, 1643–1649. [Google Scholar] [CrossRef]
- Shi, L.; Tian, H.; Wang, Y.; Hao, G.; Chen, J.; Weng, W. Effect of PH on Properties of Golden Pompano Skin Collagen-based Fibril Gels by Self-assembly in Vitro. J. Sci. Food Agric. 2020, 100, 4801–4807. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Zhou, J.; Wang, M.; Su, D.; Ma, Q.; Lv, G.; Chen, J. In Situ Formed Collagen-Hyaluronic Acid Hydrogel as Biomimetic Dressing for Promoting Spontaneous Wound Healing. Mater. Sci. Eng. C 2019, 101, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Ennaas, N.; Hammami, R.; Gomaa, A.; Bédard, F.; Biron, É.; Subirade, M.; Beaulieu, L.; Fliss, I. Collagencin, an Antibacterial Peptide from Fish Collagen: Activity, Structure and Interaction Dynamics with Membrane. Biochem. Biophys. Res. Commun. 2016, 473, 642–647. [Google Scholar] [CrossRef]
- Pal, P.; Dadhich, P.; Srivas, P.K.; Das, B.; Maulik, D.; Dhara, S. Bilayered Nanofibrous 3D Hierarchy as Skin Rudiment by Emulsion Electrospinning for Burn Wound Management. Biomater. Sci. 2017, 5, 1786–1799. [Google Scholar] [CrossRef]
- Torres, F.G.; Commeaux, S.; Troncoso, O.P. Starch-Based Biomaterials for Wound-Dressing Applications. Starch Stärke 2013, 65, 543–551. [Google Scholar] [CrossRef]
- Li, B.; Xiong, F.; Yao, B.; Du, Q.; Cao, J.; Qu, J.; Feng, W.; Yuan, H. Preparation and Characterization of Antibacterial Dopamine-Functionalized Reduced Graphene Oxide/PLLA Composite Nanofibers. RSC Adv. 2020, 10, 18614–18623. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, C.; Mou, S.; Li, J.; Zhou, M.; Zeng, Y.; Luo, C.; Sun, J.; Wang, Z.; Xu, W. Biocompatible Graphene Oxide–Collagen Composite Aerogel for Enhanced Stiffness and in Situ Bone Regeneration. Mater. Sci. Eng. C 2019, 105, 110137. [Google Scholar] [CrossRef]
- Zhou, M.; Lozano, N.; Wychowaniec, J.K.; Hodgkinson, T.; Richardson, S.M.; Kostarelos, K.; Hoyland, J.A. Graphene Oxide: A Growth Factor Delivery Carrier to Enhance Chondrogenic Differentiation of Human Mesenchymal Stem Cells in 3D Hydrogels. Acta Biomater. 2019, 96, 271–280. [Google Scholar] [CrossRef]
- Neacsu, I.A.; Leau, S.-A.; Marin, S.; Holban, A.M.; Vasile, B.-S.; Nicoara, A.-I.; Ene, V.L.; Bleotu, C.; Albu Kaya, M.G.; Ficai, A. Collagen-Carboxymethylcellulose Biocomposite Wound-Dressings with Antimicrobial Activity. Materials 2021, 14, 1153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tan, B.; Wu, Y.; Zhang, M.; Liao, J. A Review on Hydrogels with Photothermal Effect in Wound Healing and Bone Tissue Engineering. Polymers 2021, 13, 2100. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Dai, J.; Zhang, J.; Li, Z. Accelerated Skin Wound Healing by Electrical Stimulation. Adv. Heal. Mater. 2021, 10, 2100557. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.N.M.; Doulgkeroglou, M.N.; Zeugolis, D.I. Electric Field Stimulation for Tissue Engineering Applications. BMC Biomed. Eng. 2021, 3, 1. [Google Scholar] [CrossRef]
- Cinar, K.; Comlekci, S.; Senol, N. Effects of a Specially Pulsed Electric Field on an Animal Model of Wound Healing. Lasers Med. Sci. 2009, 24, 735–740. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Rigogliuso, S.; Salamone, M.; Barbarino, E.; Barbarino, M.; Nicosia, A.; Ghersi, G. Production of Injectable Marine Collagen-Based Hydrogel for the Maintenance of Differentiated Chondrocytes in Tissue Engineering Applications. Int. J. Mol. Sci. 2020, 21, 5798. [Google Scholar] [CrossRef]

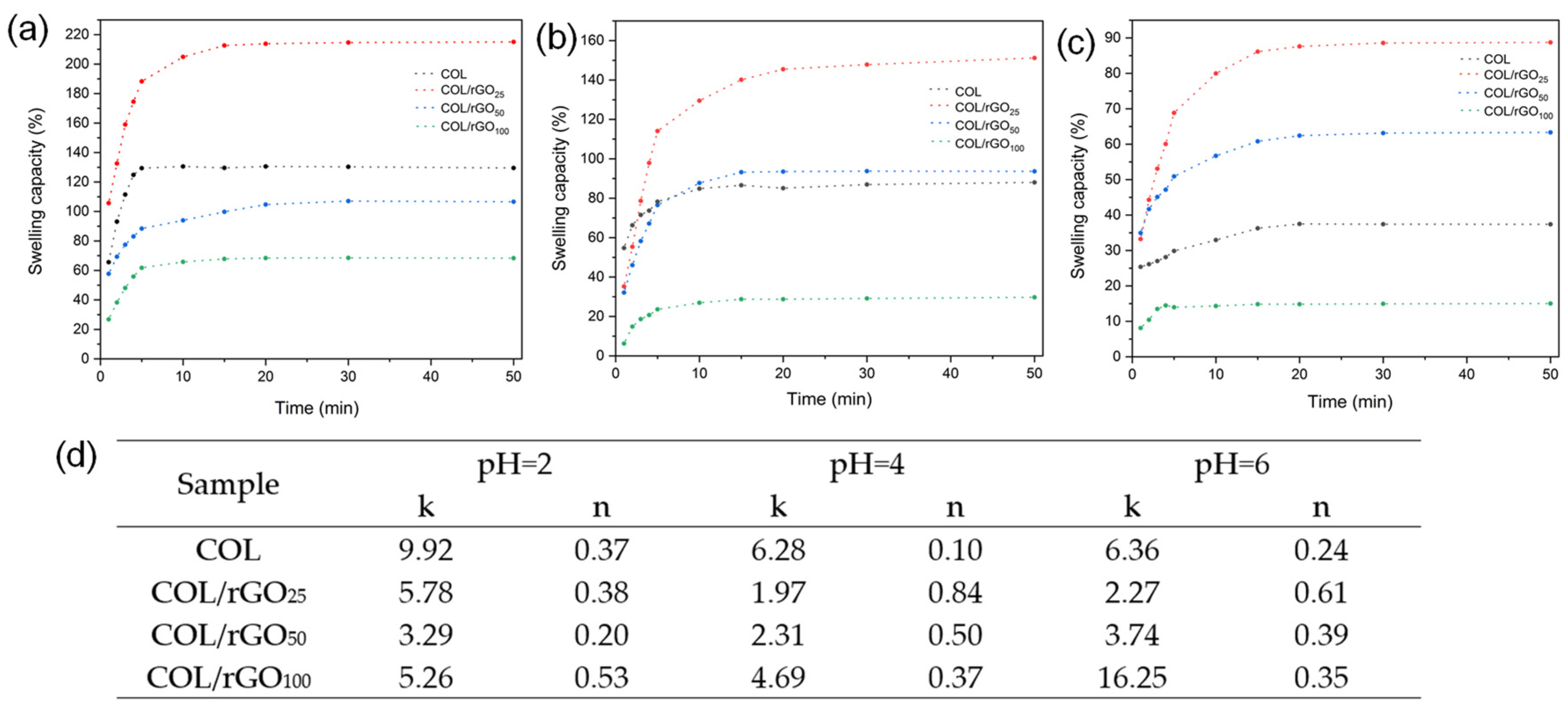
| Sample | Th 1 (°C) | Ti 2 (°C) | Tmax 3 (°C) | Tb 4 (°C) | M600 5 (%) |
|---|---|---|---|---|---|
| COL hydrogel | 83 | 225 | 286 | 337 | 7.6 |
| COL/rGO25 | 92 | 211 | 280 | 335 | 13.6 |
| COL/rGO50 | 100 | 193 | 275 | 331 | 18.7 |
| COL/rGO100 | 115 | 184 | 270 | 317 | 24.2 |
| Sample | Contact Angle (°) | Conductivity (mS/m) | Surface Charge (mV) |
|---|---|---|---|
| GO | 61.8 ± 1.4 | 121.4 ± 12.3 | −32.8 ± 2.3 |
| rGO | 84.5 ± 2.0 | 232.6 ± 10.6 | −70.2 ± 5.4 |
| COL hydrogel | 50.6 ± 1.3 a | 20.27 ± 0.25 a | 4.5 ± 0.8 a |
| COL/rGO25 | 54.1 ± 1.6 a | 24.61 ± 0.03 b | −46.8 ± 1.1 b |
| COL/rGO50 | 73.6 ± 2.6 b | 29.72 ± 0.61 c | −65.2 ± 2.2 c |
| COL/rGO100 | 76.4 ± 2.8 b | 39.57 ± 0.19 d | −79.0 ± 3.1 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, L.; Espinoza, V.; Tapia, M.; Aedo, V.; Ruiz, I.; Meléndrez, M.; Aguayo, C.; Atanase, L.I.; Fernández, K. Innovative Approach to Accelerate Wound Healing: Synthesis and Validation of Enzymatically Cross-Linked COL–rGO Biocomposite Hydrogels. Gels 2024, 10, 448. https://doi.org/10.3390/gels10070448
González L, Espinoza V, Tapia M, Aedo V, Ruiz I, Meléndrez M, Aguayo C, Atanase LI, Fernández K. Innovative Approach to Accelerate Wound Healing: Synthesis and Validation of Enzymatically Cross-Linked COL–rGO Biocomposite Hydrogels. Gels. 2024; 10(7):448. https://doi.org/10.3390/gels10070448
Chicago/Turabian StyleGonzález, Luisbel, Víctor Espinoza, Mauricio Tapia, Valentina Aedo, Isleidy Ruiz, Manuel Meléndrez, Claudio Aguayo, Leonard I. Atanase, and Katherina Fernández. 2024. "Innovative Approach to Accelerate Wound Healing: Synthesis and Validation of Enzymatically Cross-Linked COL–rGO Biocomposite Hydrogels" Gels 10, no. 7: 448. https://doi.org/10.3390/gels10070448
APA StyleGonzález, L., Espinoza, V., Tapia, M., Aedo, V., Ruiz, I., Meléndrez, M., Aguayo, C., Atanase, L. I., & Fernández, K. (2024). Innovative Approach to Accelerate Wound Healing: Synthesis and Validation of Enzymatically Cross-Linked COL–rGO Biocomposite Hydrogels. Gels, 10(7), 448. https://doi.org/10.3390/gels10070448









