Influence of a Solid Surface on PNIPAM Microgel Films
Abstract
:1. Introduction
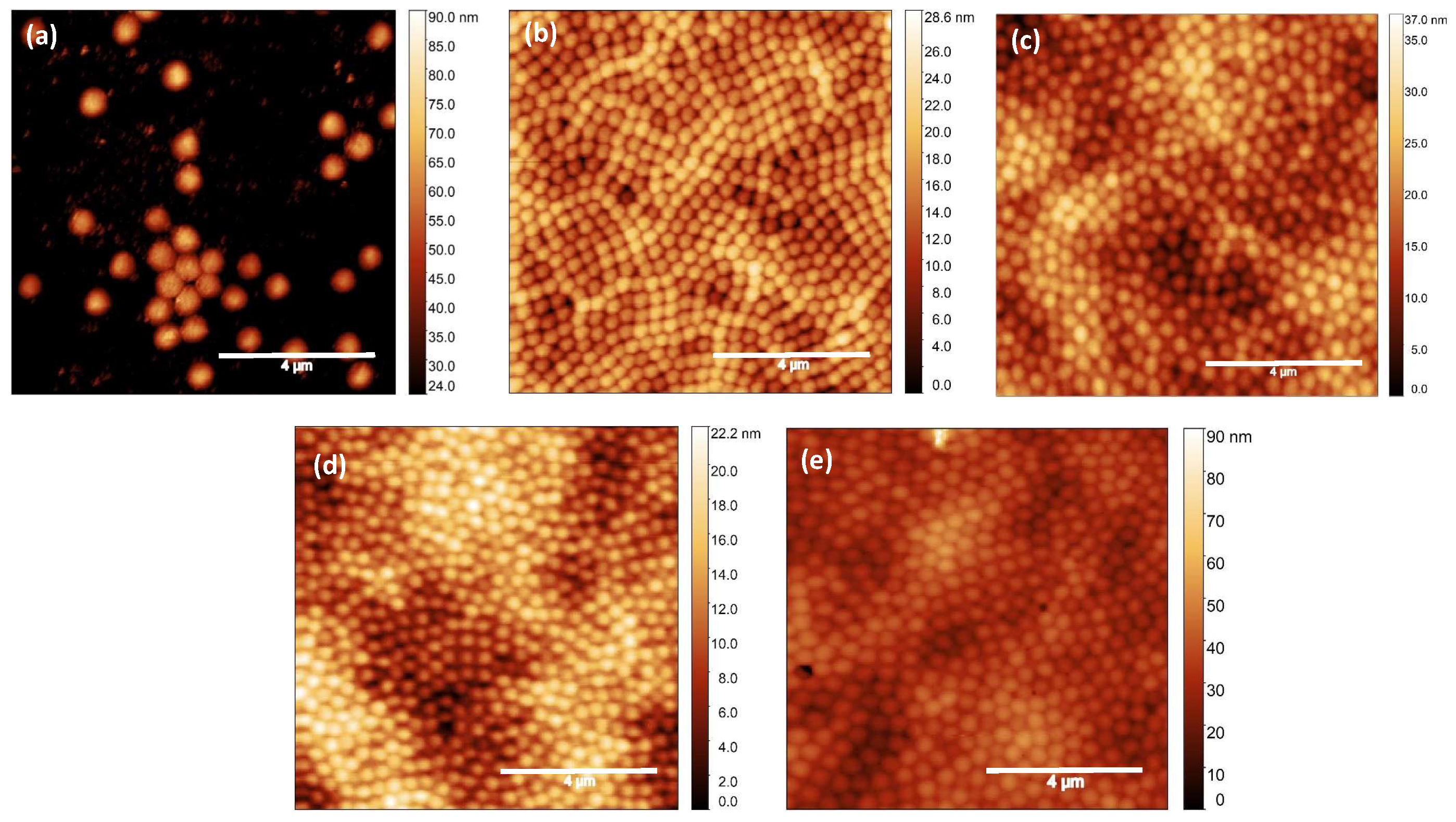
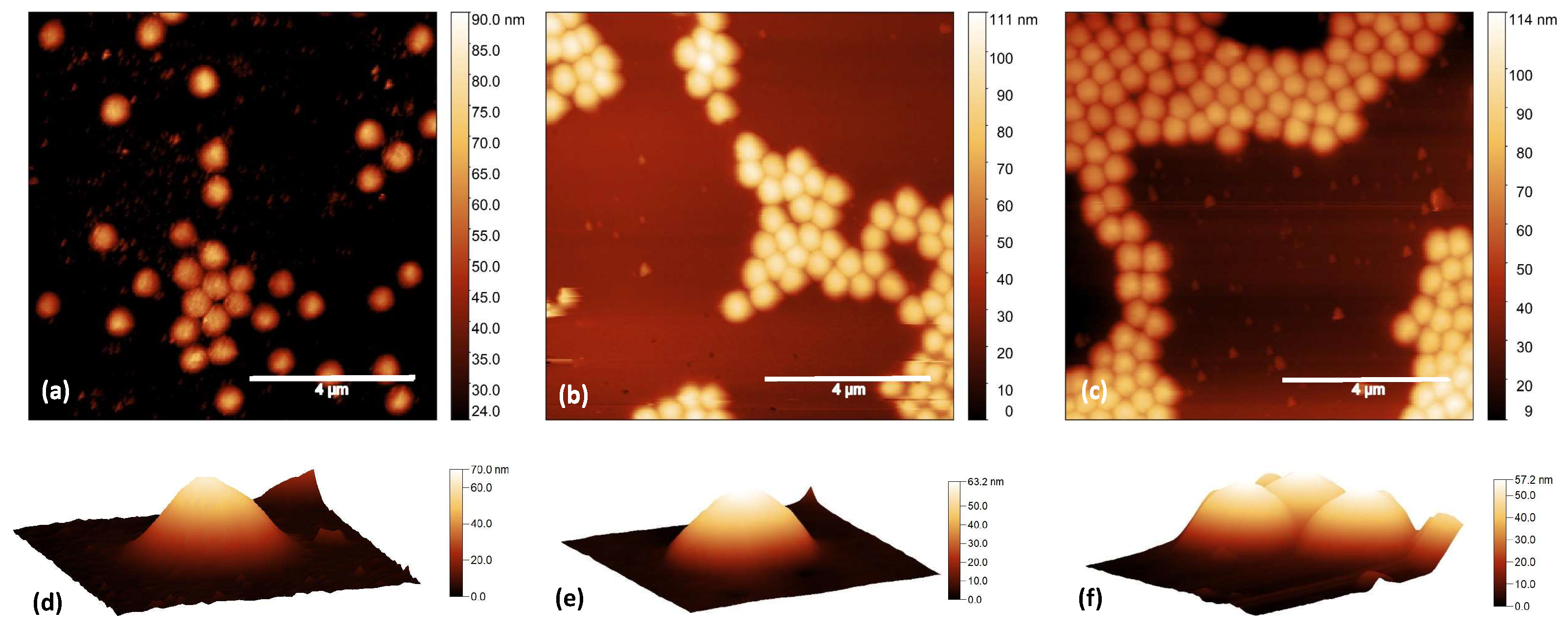
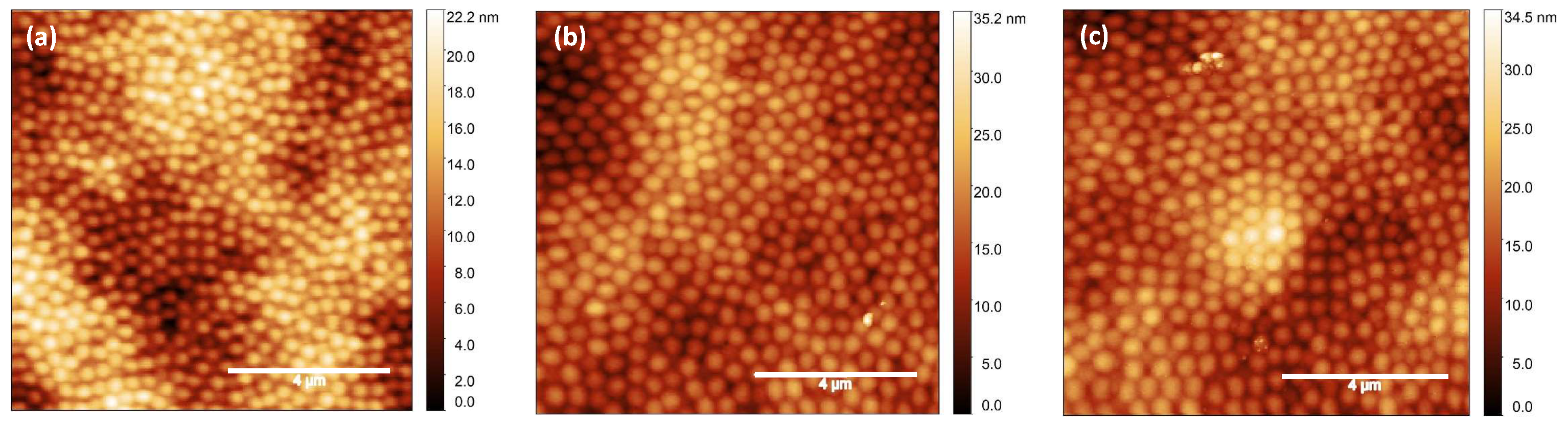
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Microgel Synthesis
4.3. Surface Modification
4.4. Thin-Film Deposition
4.5. Characterization
4.5.1. Dynamic Light Scattering
4.5.2. Atomic Force Microscopy
4.5.3. Contact Angle Measurements
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheffold, F. Pathways and challenges towards a complete characterization of microgels. Nat. Commun. 2020, 11, 4315. [Google Scholar] [CrossRef]
- Bergman, M.J.; Gnan, N.; Obiols-Rabasa, M.; Meijer, J.M.; Rovigatti, L.; Zaccarelli, E.; Schurtenberger, P. A new look at effective interactions between microgel particles. Nat. Commun. 2018, 9, 5039. [Google Scholar] [CrossRef]
- Karg, M.; Pich, A.; Hellweg, T.; Hoare, T.; Lyon, L.A.; Crassous, J.J.; Suzuki, D.; Gumerov, R.A.; Schneider, S.; Potemkin, I.I.; et al. Nanogels and Microgels: From Model Colloids to Applications, Recent Developments, and Future Trends. Langmuir 2019, 35, 6231–6255. [Google Scholar] [CrossRef]
- Vinogradov, S.V. Colloidal microgels in drug delivery applications. Curr. Pharm. Des. 2006, 12, 4703–4712. [Google Scholar] [CrossRef]
- Nasimova, I.R.; Vyshivannaya, O.V.; Gallyamov, M.O.; Kozhunova, E.Y. Thermo- and pH-Sensitive Microgels Based on Interpenetrating Networks as Components for Creating Polymeric Materials. Polym. Sci. Ser. A 2019, 61, 773–779. [Google Scholar] [CrossRef]
- Lyon, L.A.; Hendrickson, G.R.; Meng, Z.; St. John Iyer, A.N. Exploiting the Optical Properties of Microgels and Hydrogels as Microlenses and Photonic Crystals in Sensing Applications. In Microgel Suspensions; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; Chapter 14; pp. 355–374. [Google Scholar] [CrossRef]
- Di Napoli, B.; Franco, S.; Severini, L.; Tumiati, M.; Buratti, E.; Titubante, M.; Nigro, V.; Gnan, N.; Micheli, L.; Ruzicka, B.; et al. Gellan Gum Microgels as Effective Agents for a Rapid Cleaning of Paper. ACS Appl. Polym. Mater. 2020, 2, 2791–2801. [Google Scholar] [CrossRef]
- Agrawal, G.; Agrawal, R. Functional Microgels: Recent Advances in Their Biomedical Applications. Small 2018, 14, 1801724. [Google Scholar] [CrossRef]
- Oberdisse, J.; Hellweg, T. Recent advances in stimuli-responsive core-shell microgel particles: Synthesis, characterisation, and applications. Colloid Polym. Sci. 2020, 298, 921–935. [Google Scholar] [CrossRef]
- Xing, Z.; Wang, C.; Yan, J.; Zhang, L.; Li, L.; Zha, L. pH/temperature dual stimuli-responsive microcapsules with interpenetrating polymer network structure. Colloid Polym. Sci. 2010, 288, 1723–1729. [Google Scholar] [CrossRef]
- Liu, X.; Guo, H.; Zha, L. Study of pH/temperature dual stimuli-responsive nanogels with interpenetrating polymer network structure. Polym. Int. 2012, 61, 1144–1150. [Google Scholar] [CrossRef]
- Nigro, V.; Angelini, R.; Bertoldo, M.; Bruni, F.; Ricci, M.; Ruzicka, B. Local structure of temperature and pH-sensitive colloidal microgels. J. Chem. Phys. 2015, 143, 114904. [Google Scholar] [CrossRef] [PubMed]
- Cors, M.; Wiehemeier, L.; Oberdisse, J.; Hellweg, T. Deuteration-Induced Volume Phase Transition Temperature Shift of PNIPMAM Microgels. Polymers 2019, 11, 620. [Google Scholar] [CrossRef]
- Nigro, V.; Angelini, R.; Bertoldo, M.; Ruzicka, B. Swelling of responsive-microgels: Experiments versus models. Colloids Surf. A 2017, 532, 389–396. [Google Scholar] [CrossRef]
- Zhu, P.W.; Napper, D.H. Light scattering studies of poly(N-isopropylacrylamide) microgel particle in mixed water-acetic acid solvents. Macromol. Chem. Phys. 1999, 200, 1950–1955. [Google Scholar] [CrossRef]
- Franco, S.; Buratti, E.; Ruzicka, B.; Nigro, V.; Zoratto, N.; Matricardi, P.; Zaccarelli, E.; Angelini, R. Volume fraction determination of microgel composed of interpenetrating polymer networks of PNIPAM and polyacrylic acid. J. Phys. Condens. Matter 2021, 33, 174004. [Google Scholar] [CrossRef] [PubMed]
- Nigro, V.; Angelini, R.; Rosi, B.; Bertoldo, M.; Buratti, E.; Casciardi, S.; Sennato, S.; Ruzicka, B. Study of network composition in interpenetrating polymer networks of poly(N isopropylacrylamide) microgels: The role of poly(acrylic acid). J. Colloid Interface Sci. 2019, 545, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Brijitta, J.; Schurtenberger, P. Responsive hydrogel colloids: Structure, interactions, phase behavior, and equilibrium and nonequilibrium transitions of microgel dispersions. Curr. Opin. Colloid Interface Sci. 2019, 40, 87–103. [Google Scholar] [CrossRef]
- Debord, J.D.; Lyon, L.A. Synthesis and Characterization of pH-Responsive Copolymer Microgels with Tunable Volume Phase Transition Temperatures. Langmuir 2003, 19, 7662–7664. [Google Scholar] [CrossRef]
- Tavagnacco, L.; Zaccarelli, E.; Chiessi, E. On the molecular origin of the cooperative coil-to-globule transition of poly(N-isopropylacrylamide) in water. Phys. Chem. Chem. Phys. 2018, 20, 9997–10010. [Google Scholar] [CrossRef]
- Zahid, S.; Alzahrani, A.K.; Kizilbash, N.; Ambreen, J.; Ajmal, M.; Farooqi, Z.H.; Siddiq, M. Preparation of stimuli responsive microgel with silver nanoparticles for biosensing and catalytic reduction of water pollutants. RSC Adv. 2022, 12, 33215–33228. [Google Scholar] [CrossRef]
- Zhu, L.; Meng, Y.; Zhou, J.; Hu, Y.; Gao, S.; Li, S.; Liu, J.; Wang, S.; Xia, Y. Tough and Transparent Photonic Hydrogel Nanocomposites for Display, Sensing, and Actuation Applications. ACS Appl. Nano Mater. 2023, 6, 6984–6991. [Google Scholar] [CrossRef]
- Meena, L.K.; Rather, H.; Kedaria, D.; Vasita, R. Polymeric microgels for bone tissue engineering applications—A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 381–397. [Google Scholar] [CrossRef]
- Dirksen, M.; Kinder, T.A.; Brändel, T.; Hellweg, T. Temperature Controlled Loading and Release of the Anti-Inflammatory Drug Cannabidiol by Smart Microgels. Molecules 2021, 26, 3181. [Google Scholar] [CrossRef]
- Saunders, B.R.; Laajam, N.; Daly, E.; Teow, S.; Hu, X.; Stepto, R. Microgels: From responsive polymer colloids to biomaterials. Adv. Colloid Interface Sci. 2009, 147–148, 251–262. [Google Scholar] [CrossRef]
- Islam, M.R.; Nguyen, R.; Lyon, L.A. Emergence of Non-Hexagonal Crystal Packing of Deswollen and Deformed Ultra-Soft Microgels under Osmotic Pressure Control. Macromol. Rapid Commun. 2021, 42, 2100372. [Google Scholar] [CrossRef]
- Li, F.; Luo, Y.; Feng, X.; Guo, Y.; Zhou, Y.; He, D.; Xie, Z.; Zhang, H.; Liu, Y. Two-dimensional colloidal crystal of soft microgel spheres: Development, preparation and applications. Colloids Surf. B Biointerfaces 2022, 212, 112358. [Google Scholar] [CrossRef] [PubMed]
- Rey, M.; Hou, X.; Tang, J.S.J.; Vogel, N. Interfacial arrangement and phase transitions of PNiPAm microgels with different crosslinking densities. Soft Matter 2017, 13, 8717–8727. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Hellweg, T.; von Klitzing, R. Packing Density Control in P(NIPAM-co-AAc) Microgel Monolayers: Effect of Surface Charge, pH, and Preparation Technique. Langmuir 2008, 24, 12595–12602. [Google Scholar] [CrossRef]
- Hiltl, S.; Schürings, M.P.; Balaceanu, A.; Mayorga, V.; Liedel, C.; Pich, A.; Böker, A. Guided self-assembly of microgels: From particle arrays to anisotropic nanostructures. Soft Matter 2011, 7, 8231–8238. [Google Scholar] [CrossRef]
- Uhlig, K.; Wegener, T.; He, J.; Zeiser, M.; Bookhold, J.; Dewald, I.; Godino, N.; Jaeger, M.; Hellweg, T.; Fery, A.; et al. Patterned Thermoresponsive Microgel Coatings for Noninvasive Processing of Adherent Cells. Biomacromolecules 2016, 17, 1110–1116. [Google Scholar] [CrossRef]
- South, A.B.; Whitmire, R.E.; García, A.J.; Lyon, L.A. Centrifugal Deposition of Microgels for the Rapid Assembly of Nonfouling Thin Films. ACS Appl. Mater. Interfaces 2009, 1, 2747–2754. [Google Scholar] [CrossRef] [PubMed]
- Keskin, D.; Mergel, O.; van der Mei, H.C.; Busscher, H.J.; van Rijn, P. Inhibiting Bacterial Adhesion by Mechanically Modulated Microgel Coatings. Biomacromolecules 2019, 20, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Serpe, M.J.; Jones, C.D.; Lyon, L.A. Layer-by-Layer Deposition of Thermoresponsive Microgel Thin Films. Langmuir 2003, 19, 8759–8764. [Google Scholar] [CrossRef]
- Nigro, V.; Buratti, E.; Limosani, F.; Angelini, R.; Dinelli, F.; Franco, S.; Nichelatti, E.; Piccinini, M.; Vincenti, M.A.; Montereali, R.M.; et al. Spin-coating deposition of thermoresponsive microgel thin films. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131918. [Google Scholar] [CrossRef]
- Shu, T.; Hu, L.; Hunter, H.; Balasuriya, N.; Fang, C.; Zhang, Q.; Serpe, M.J. Multi-responsive micro/nanogels for optical sensing. Adv. Phys. X 2022, 7, 2043185. [Google Scholar] [CrossRef]
- Kim, J.; Nayak, S.; Lyon, L.A. Bioresponsive Hydrogel Microlenses. J. Am. Chem. Soc. 2005, 127, 9588–9592. [Google Scholar] [CrossRef]
- Marcisz, K.; Karbarz, M.; Stojek, Z. Electrochemical chemo- and biosensors based on microgels immobilized on electrode surface. Electrochem. Sci. Adv. 2022, 2, e2100162. [Google Scholar] [CrossRef]
- Caldwell, A.S.; Aguado, B.A.; Anseth, K.S. Designing Microgels for Cell Culture and Controlled Assembly of Tissue Microenvironments. Adv. Funct. Mater. 2020, 30, 1907670. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Tang, Y.; He, X.; Pan, F.; Li, Z.; Xu, H.; Lu, J.R. Patterned Thermoresponsive Microgel Surfaces to Control Cell Detachment. Biomacromolecules 2016, 17, 572–579. [Google Scholar] [CrossRef]
- Sanzari, I.; Buratti, E.; Huang, R.; Tusan, C.G.; Dinelli, F.; Evans, N.D.; Prodromakis, T.; Bertoldo, M. Poly(N-isopropylacrylamide) based thin microgel films for use in cell culture applications. Sci. Rep. 2020, 10, 6126. [Google Scholar] [CrossRef]
- Schmidt, S.; Zeiser, M.; Hellweg, T.; Duschl, C.; Fery, A.; Möhwald, H. Adhesion and Mechanical Properties of PNIPAM Microgel Films and Their Potential Use as Switchable Cell Culture Substrates. Adv. Funct. Mater. 2010, 20, 3235–3243. [Google Scholar] [CrossRef]
- Flechner, M.; Schaller, J.; Stahl, M.; Achberger, K.; Gerike, S.; Hannappel, Y.; Fu, J.; Jaeger, M.; Hellweg, T.; Duschl, C.; et al. Adhesion, proliferation, and detachment of various cell types on thermoresponsive microgel coatings. Biotechnol. Bioeng. 2022, 119, 1728–1739. [Google Scholar] [CrossRef]
- Xia, Y.; Tang, D.; Wu, H.; Wang, S. Cell attachment/detachment behavior on poly(N-isopropylacrylamide)-based microgel films: The effect of microgel structure and swelling ratio. J. Mater. Sci. 2018, 53, 8795–8806. [Google Scholar] [CrossRef]
- Buratti, E.; Sanzari, I.; Dinelli, F.; Prodromakis, T.; Bertoldo, M. Formation and Stability of Smooth Thin Films with Soft Microgels Made of Poly(N-Isopropylacrylamide) and Poly(Acrylic Acid). Polymers 2020, 12, 2638. [Google Scholar] [CrossRef]
- Hoppe Alvarez, L.; Rudov, A.A.; Gumerov, R.A.; Lenssen, P.; Simon, U.; Potemkin, I.I.; Wöll, D. Controlling microgel deformation via deposition method and surface functionalization of solid supports. Phys. Chem. Chem. Phys. 2021, 23, 4927–4934. [Google Scholar] [CrossRef] [PubMed]
- Cutright, C.C.; Harris, J.L.; Ramesh, S.; Khan, S.A.; Genzer, J.; Menegatti, S. Surface-Bound Microgels for Separation, Sensing, and Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2104164. [Google Scholar] [CrossRef]
- Kyrey, T.; Witte, J.; Pipich, V.; Feoktystov, A.; Koutsioubas, A.; Vezhlev, E.; Frielinghaus, H.; von Klitzing, R.; Wellert, S.; Holderer, O. Influence of the cross-linker content on adsorbed functionalised microgel coatings. Polymer 2019, 169, 29–35. [Google Scholar] [CrossRef]
- Cutright, C.; Brotherton, Z.; Alexander, L.; Harris, J.; Shi, K.; Khan, S.; Genzer, J.; Menegatti, S. Packing density, homogeneity, and regularity: Quantitative correlations between topology and thermoresponsive morphology of PNIPAM-co-PAA microgel coatings. Appl. Surf. Sci. 2020, 508, 145129. [Google Scholar] [CrossRef]
- Wang, Y.P.; Yuan, K.; Li, Q.L.; Wang, L.P.; Gu, S.J.; Pei, X.W. Preparation and characterization of poly(N-isopropylacrylamide) films on a modified glass surface via surface initiated redox polymerization. Mater. Lett. 2005, 59, 1736–1740. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kamra, T.; Uddin, K.M.A.; Snezhkova, O.; Jayawardena, H.S.N.; Yan, M.; Montelius, L.; Schnadt, J.; Ye, L. Controlled short-linkage assembly of functional nano-objects. Appl. Surf. Sci. 2014, 300, 22–28. [Google Scholar] [CrossRef]
- Hoppe Alvarez, L.; Eisold, S.; Gumerov, R.A.; Strauch, M.; Rudov, A.A.; Lenssen, P.; Merhof, D.; Potemkin, I.I.; Simon, U.; Wöll, D. Deformation of Microgels at Solid–Liquid Interfaces Visualized in Three-Dimension. Nano Lett. 2019, 19, 8862–8867. [Google Scholar] [CrossRef] [PubMed]
- Alghunaim, A.; Brink, E.T.; min Zhang Newby, B. Surface immobilization of thermo-responsive poly(N-isopropylacrylamide) by simple entrapment in a 3-aminopropyltriethoxysilane network. Polymer 2016, 101, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Kohlrausch, R. Theorie des elektrischen rckstandes in der leidener flasche. Ann. Phys. 1854, 2, 179–214. [Google Scholar] [CrossRef]
- Williams, G.; Watts, D.C. Non-Symmetrical Dielectric Relaxation Behavior Arising from a Simple Empirical Decay Function. J. Chem. Soc. Faraday Trans. 1970, 66, 80–85. [Google Scholar] [CrossRef]
- Gwyddion 2.65. Available online: http://gwyddion.net/ (accessed on 16 July 2024).
- Dino-Lite Digital Microscope. Available online: https://www.dino-lite.eu/en/ (accessed on 16 July 2024).
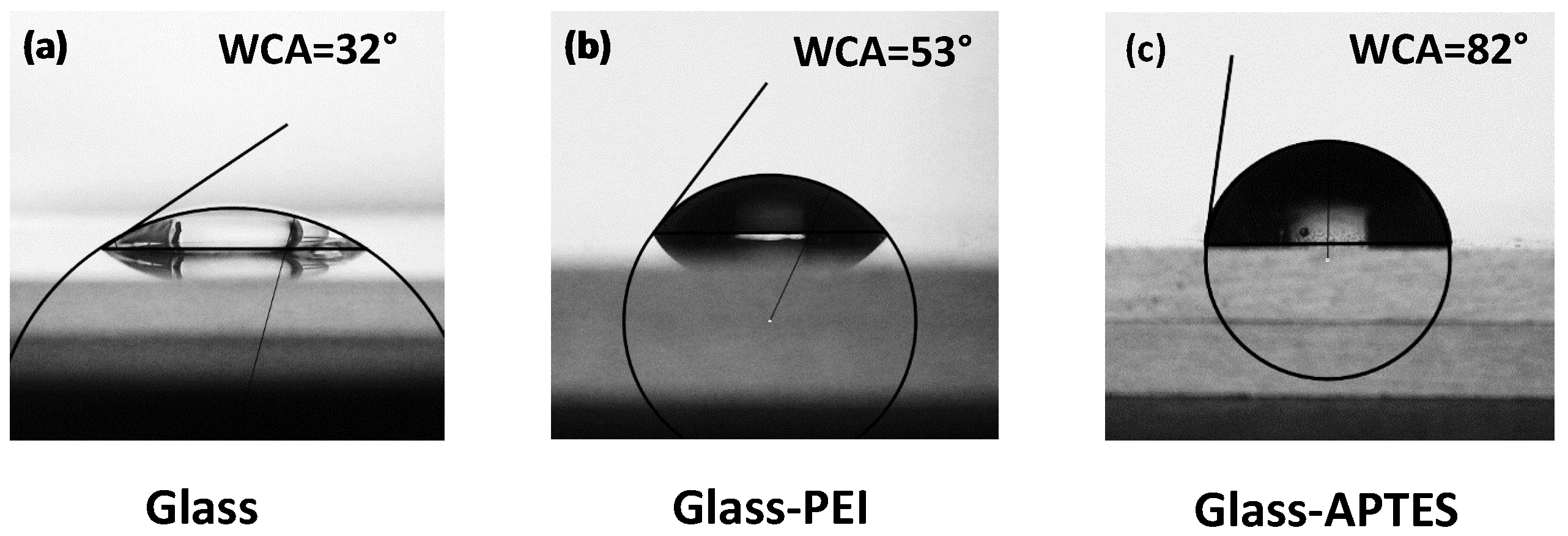
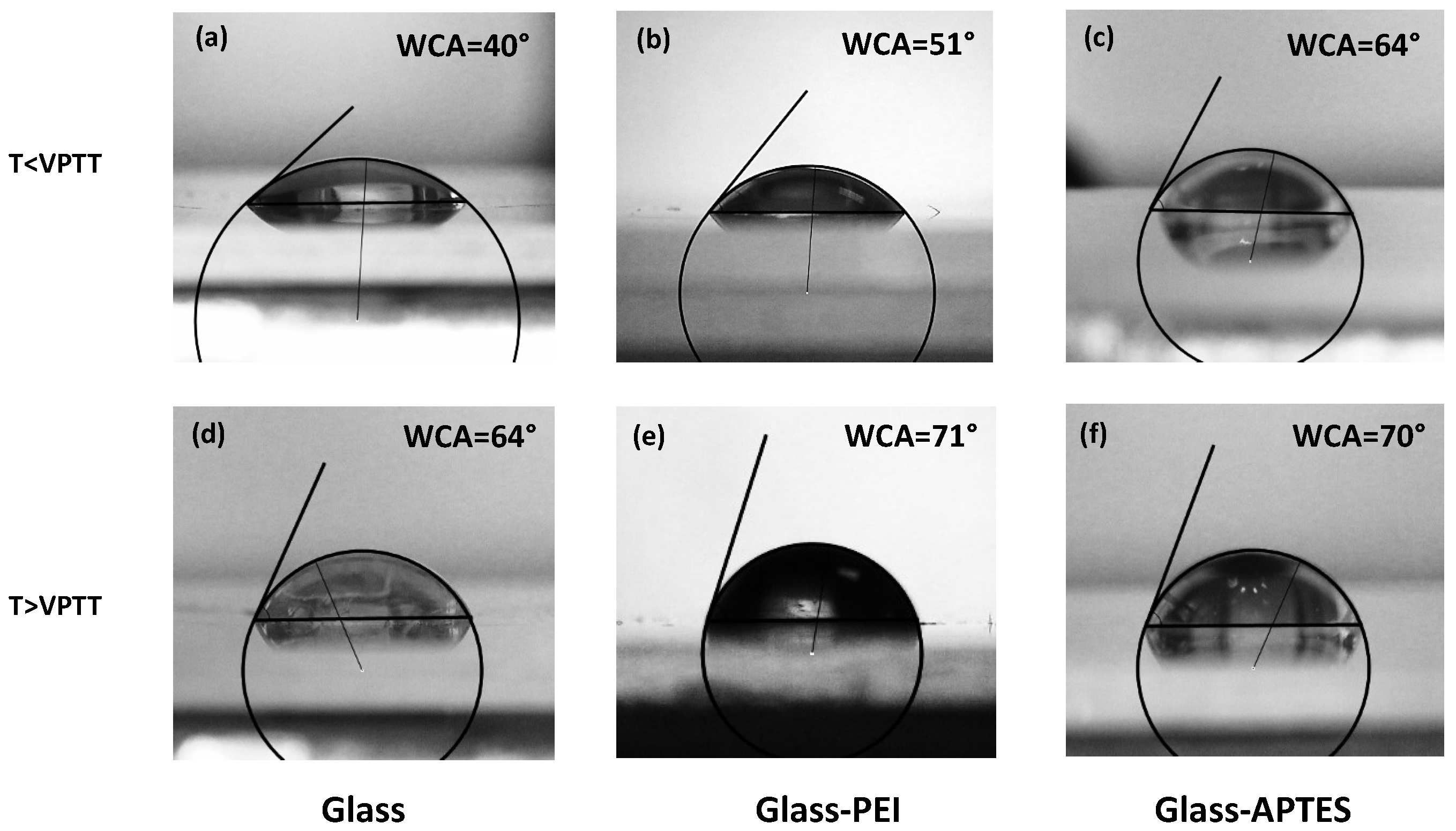

| Substrate | WCA | Particle Height (nm) | RMS Roughness (nm) | Size (nm) |
|---|---|---|---|---|
| Glass | 32° | 70 ± 1 | 9.2 ± 0.2 | 571 ± 18 |
| Glass–PEI | 53° | 63 ± 3 | 19 ± 2 | 633 ± 15 |
| Glass–APTES | 82° | 57 ± 3 | 20 ± 2 | 662 ± 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nigro, V.; Angelini, R.; Buratti, E.; Colantonio, C.; D’Amato, R.; Dinelli, F.; Franco, S.; Limosani, F.; Montereali, R.M.; Nichelatti, E.; et al. Influence of a Solid Surface on PNIPAM Microgel Films. Gels 2024, 10, 473. https://doi.org/10.3390/gels10070473
Nigro V, Angelini R, Buratti E, Colantonio C, D’Amato R, Dinelli F, Franco S, Limosani F, Montereali RM, Nichelatti E, et al. Influence of a Solid Surface on PNIPAM Microgel Films. Gels. 2024; 10(7):473. https://doi.org/10.3390/gels10070473
Chicago/Turabian StyleNigro, Valentina, Roberta Angelini, Elena Buratti, Claudia Colantonio, Rosaria D’Amato, Franco Dinelli, Silvia Franco, Francesca Limosani, Rosa Maria Montereali, Enrico Nichelatti, and et al. 2024. "Influence of a Solid Surface on PNIPAM Microgel Films" Gels 10, no. 7: 473. https://doi.org/10.3390/gels10070473





