Effect of Drying Methods on the Thermal and Mechanical Behavior of Bacterial Cellulose Aerogel
Abstract
:1. Introduction
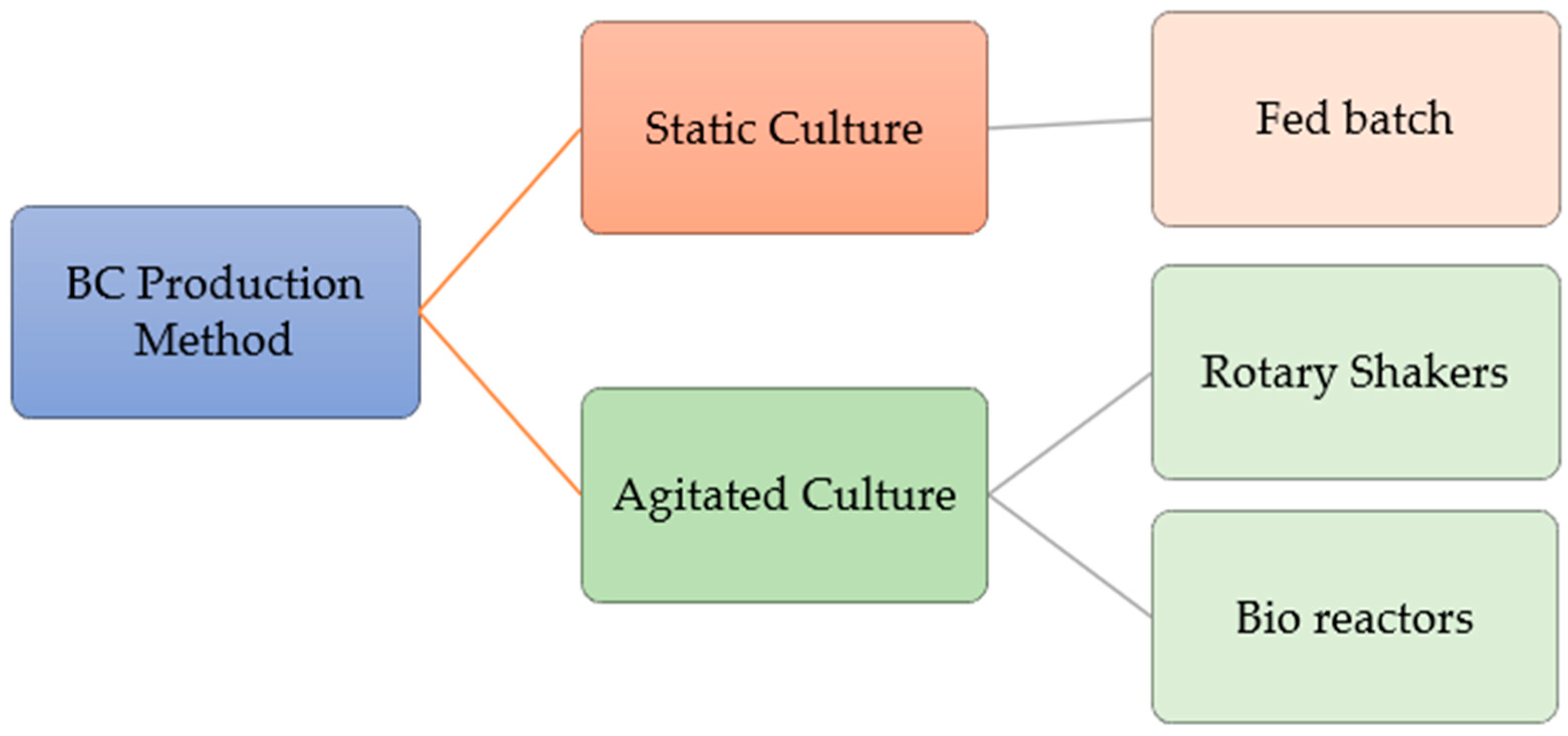
2. Production of Bacterial Cellulose Aerogel
2.1. Static Culture
Fed-Batch

2.2. Agitated/Shaking Culture
Bioreactor Culture
3. Effect of Drying Characteristics of Bacterial Cellulose Aerogels on Their Thermal and Mechanical Behavior
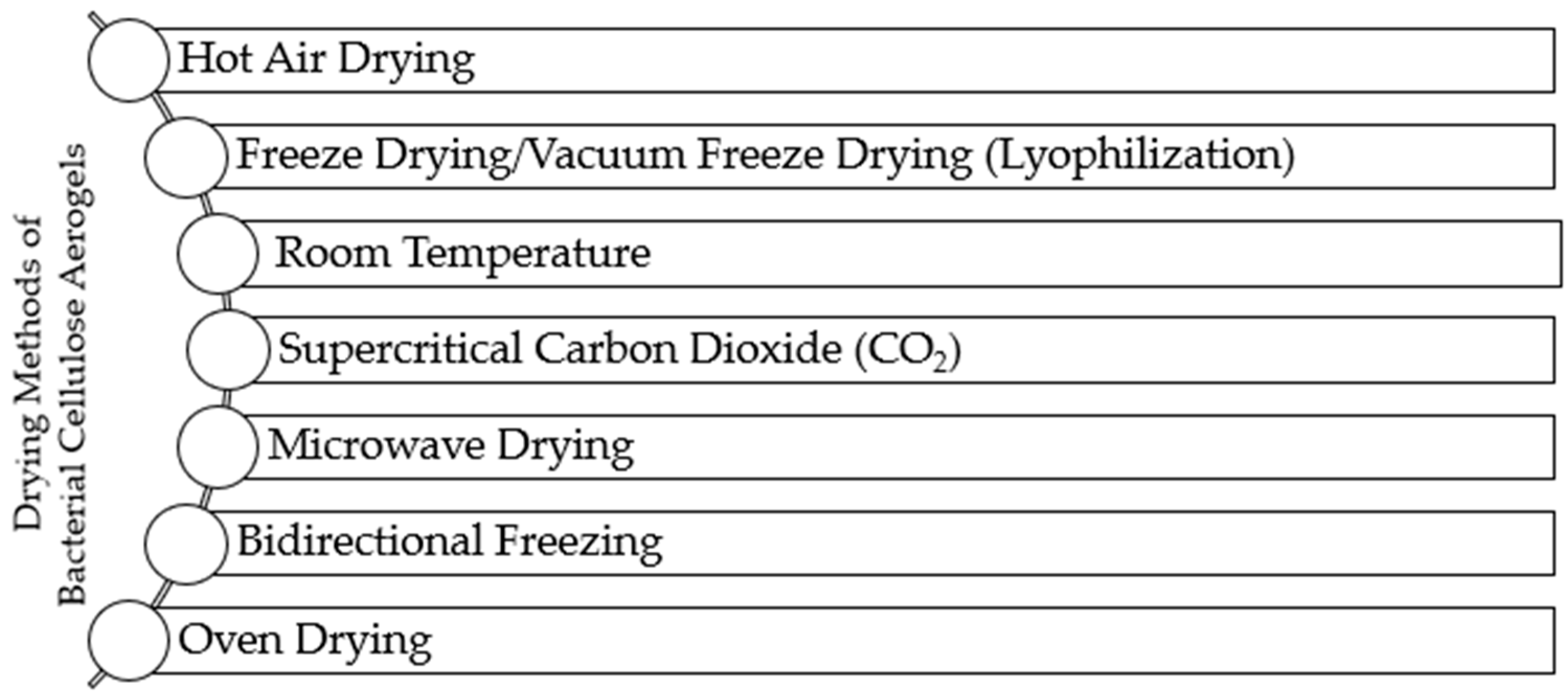
3.1. Most Used Drying Methods
3.1.1. Hot Air Drying
3.1.2. Freeze-Drying
3.1.3. Room Temperature
3.1.4. Supercritical CO2 Drying
3.1.5. Microwave Drying
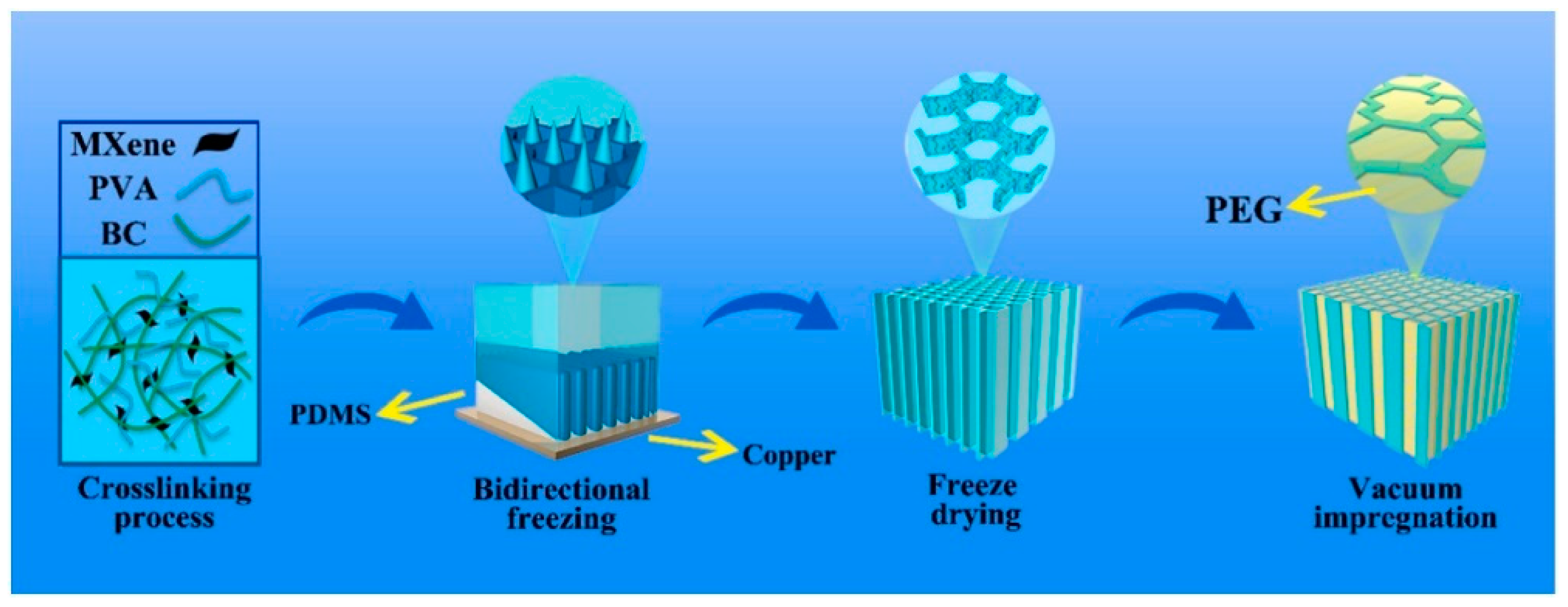
3.1.6. Bidirectional Freezing
3.1.7. Oven Drying
3.2. Effect of Drying Methods of BC Aerogels on Thermal Behavior
3.2.1. Effect of BC Aerogels Composed of Polymeric Additives on Thermal Properties
3.2.2. Effect of BC Aerogels Composed of Inorganic and Different Types of Additives on Thermal Properties
3.3. Effect of Drying Methods of BC Aerogels on Mechanical Behavior
3.3.1. Effect of BC Aerogels Composed of Polymeric Additives on Mechanical Behavior
3.3.2. Effect of BC Aerogels Composed of Inorganic and Different Types of Additives on Mechanical Properties
| BC Aerogels | Effect of Drying Methods on Thermal & Mechanical Behavior of BC Aerogels | Drying Methods | Applications | Ref. |
|---|---|---|---|---|
| Gluconacetobacter xylinus BC membrane | The material’s ability to swell is much more decreased with evaporation drying than freeze-drying. Gas permeability of freeze-dried membranes is higher than evaporation-dried membranes. The Young’s modulus of polymer membranes varies depending on the bacterial strain used. | Freeze-drying, Evaporation drying | Wet wound dressing | [91] |
| Fermented coffee kombucha (CK) BC aerogels by Gluconacetobacter | Oven-dried BCs exhibited the highest tensile stress at break, measuring 24.67 ± 4.40 MPa. All BCs decomposed between 230 and 400 °C regardless of drying conditions. | Oven drying (OD), freeze-drying, vacuum oven drying, and Büchner funnel vacuum drying (BFVD). | Distinctive applications in various industries | [92] |
| Gluconacetobacter sucrofermentans H-110 BC aerogels | With an aerogel density of 22.8 kg/m3, the modulus of elasticity at 80% compression was 0.1 MPa. Lower aerogel density resulted in larger pore sizes (20 to 1000 μm) and reduced modulus of elasticity. | Freeze-drying | Heat- and sound-insulating materials | [80] |
| Comparing of native BC aerogel and TEMPO ((2,2,6,6-tetramethylpiperidin-1-yl)oxyl)) oxidized BC aerogels | Aerogels synthesized from oxidized BC demonstrate increased durability and reduced shrinkage relative to those produced from native BC through freeze-drying. Additionally, TEMPO-mediated oxidation of BC, in conjunction with Mg2+ addition, yields aerogels with substantially improved mechanical strength and a more uniform microporous architecture. | Freeze-drying | Biomedical applications | [81] |
| Natively produced BC pellicles by Gluconacetobacter hansenii | Native cellulose aerogels exhibited a very low thermal conductivity of 13 mW/(K·m). Mechanical and thermogravimetric analysis demonstrated the potential of BC aerogels with added carboxymethyl cellulose for building insulation. These aerogels could enhance thermal insulation and add fireproofing properties in multilayer insulation blankets. | Super critical carbon dioxide drying | Building envelope applications | [71] |
| Bacterial cellulose films | Microwave drying of films is 95% faster than air convection drying. The structure, color, and mechanical properties of BC films dried by microwave and air convection heating were nearly identical. However, microwave-dried films had slightly lower crystallinity and higher swelling. Elongation of samples with air convection drying was higher than microwave oven drying at break. | Microwave oven and air convection heating drying | Food packaging and edible film | [28] |
| Gelatinous Bacterial Cellulose Film by Gluconacetobacter Xylinum | The mechanical properties of BC films prepared by vacuum freeze-drying were inferior to those prepared by other methods; hot air drying showed the best results on mechanical properties. | Hot air drying, vacuum drying and vacuum freeze-drying | - | [32] |
| Producing of Komagataeibacter hansenii 23769 and Herman Schermann BC aerogels | Oven-dried samples’ Young’s modulus, tensile strength, and lower strain are higher than those of the freeze-dried ones. Oven-dried BC had higher crystallinity, LOI (lateral order index), and lower porosity with narrower fiber diameter and distribution than freeze-dried BC, regardless of bacterial strains. | Oven and freeze-drying | Anodic applications | [29] |
| Kombucha bacterial cellulose (KBC) | The microwave drying method showed a lower activation energy (131.70 Wg−1), higher moisture diffusivity (48.27 × 10−11 m2s−1), and greater tensile strength (59.45 MPa). It was more efficient due to faster drying, higher rehydration ratio, and increased tensile strength. In contrast, room temperature drying was better for water affinity. | Microwave drying (180–900 W), hot air oven drying (30–70 °C), and shade drying (25 °C). | Various applications | [93] |
| Dehydration of wet bacterial cellulose (BC) from OPF (oil palm frond) juice | Thermal analysis using TGA and DSC showed that hot-pressed BC had higher thermal resistivity but lower thermal stability than freeze-dried BC. | Hot-pressed and freeze-dried | - | [94] |
| BC aerogel from fruit waste by kombucha fermentation | The alterations resulted in significant improvements in aerogel mechanical properties, with rebound values exceeding 90%. Derived from three raw materials, the aerogels display low density, high porosity, and reduced thermal conductivity, suggesting suitability for insulation applications. | Two directional freeze-drying | Oil–water separation and thermal insulation | [95] |
4. Conclusions & Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ummartyotin, S.; Manuspiya, H. A critical review on cellulose: From fundamental to an approach on sensor technology. Renew. Sustain. Energy Rev. 2015, 41, 402–412. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications-A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Chunshom, N.; Chuysinuan, P.; Thanyacharoen, T.; Techasakul, S.; Ummartyotin, S. Development of gallic acid/cyclodextrin inclusion complex in freeze-dried bacterial cellulose and poly (vinyl alcohol) hydrogel: Controlled-release characteristic and antioxidant properties. Mater. Chem. Phys. 2019, 232, 294–300. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Ciolacu, D.E.; Suflet, D.M. 11-Cellulose-Based Hydrogels for Medical/Pharmaceutical Applications. In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Popa, V., Volf, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 401–439. [Google Scholar] [CrossRef]
- Hu, M.-X.; Niu, H.-M.; Chen, X.-L.; Zhan, H.-B. Natural cellulose microfiltration membranes for oil/water nanoemulsions separation. Colloids Surf. Physicochem. Eng. Asp. 2019, 564, 142–151. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Wu, Y.; He, K.; Li, Y.; Luo, X.; Li, B.; Wang, C.; Liu, S. Flexible cellulose nanofibrils as novel pickering stabilizers: The emulsifying property and packing behavior. Food Hydrocoll. 2019, 88, 180–189. [Google Scholar] [CrossRef]
- Moise, I.V.; Manea, M.M.; Vasilca, S.; Pintilie, C.; Virgolici, M.; Cutrubinis, M.; Stanculescu, I.R.; Meltzer, V. The crosslinking behaviour of cellulose in gamma irradiated paper. Polym. Degrad. Stab. 2019, 160, 53–59. [Google Scholar] [CrossRef]
- Mangiante, G.; Alcouffe, P.; Gaborieau, M.; Zeno, E.; Petit-Conil, M.; Bernard, J.; Charlot, A.; Fleury, E. Biohybrid cellulose fibers: Toward paper materials with wet strength properties. Carbohydr. Polym. 2018, 193, 353–361. [Google Scholar] [CrossRef]
- Andriani, D.; Apriyana, A.Y.; Karina, M. The optimization of bacterial cellulose production and its applications: A review. Cellulose 2020, 27, 6747–6766. [Google Scholar] [CrossRef]
- Yan, M.; Fu, Y.; Pan, Y.; Cheng, X.; Gong, L.; Zhou, Y.; Ahmed, H.; Zhang, H. Highly elastic and fatigue resistant wood/silica composite aerogel operated at extremely low temperature. Compos. Part B Eng. 2022, 230, 109496. [Google Scholar] [CrossRef]
- Hoseini, A.; McCague, C.; Andisheh-Tadbir, M.; Bahrami, M. Aerogel blankets: From mathematical modeling to material characterization and experimental analysis. Int. J. Heat Mass Transf. 2016, 93, 1124–1131. [Google Scholar] [CrossRef]
- Sambucci, M.; Savoni, F.; Valente, M. Aerogel Technology for Thermal Insulation of Cryogenic Tanks—Numerical Analysis for Comparison with Traditional Insulating Materials. Gels 2023, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chowdhury, S.; Balasubramanian, R. New insights into the role of nitrogen-bonding configurations in enhancing the photocatalytic activity of nitrogen-doped graphene aerogels. J. Colloid Interface Sci. 2019, 534, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.N.; Meador, M.A.B.; Scheiman, D.; McCorkle, L. Polyimide Aerogels Using Triisocyanate as Cross-linker. ACS Appl. Mater. Interfaces 2017, 9, 27313–27321. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Starch based aerogels: Production, properties and applications. Trends Food Sci. Technol. 2019, 89, 1–10. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Adnan, A.S.; Yahya, E.B.; Olaiya, N.G.; Safrida, S.; Hossain, M.S.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.K.; Oyekanmi, A.A.; et al. A Review on Plant Cellulose Nanofibre-Based Aerogels for Biomedical Applications. Polymers 2020, 12, 1759. [Google Scholar] [CrossRef] [PubMed]
- Sozcu, S.; Venkataraman, M.; Wiener, J.; Tomkova, B.; Militky, J.; Mahmood, A. Incorporation of Cellulose-Based Aerogels into Textile Structures. Materials 2024, 17, 27. [Google Scholar] [CrossRef]
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New Trends in Bio-Based Aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose Aerogels: Synthesis, Applications, and Prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef]
- Yang, W.-J.; Yuen, A.C.Y.; Li, A.; Lin, B.; Chen, T.B.Y.; Yang, W.; Lu, H.-D.; Yeoh, G.H. Recent progress in bio-based aerogel absorbents for oil/water separation. Cellulose 2019, 26, 6449–6476. [Google Scholar] [CrossRef]
- Jonas, R.; Farah, L.F. Production and application of microbial cellulose. Polym. Degrad. Stab. 1998, 59, 101–106. [Google Scholar] [CrossRef]
- Pa’e, N.; Salehudin, M.H.; Hassan, N.D.; Marsin, A.M.; Muhamad, I.I. Thermal Behavior of Bacterial Cellulose-Based Hydrogels with Other Composites and Related Instrumental Analysis. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 763–787. [Google Scholar] [CrossRef]
- Pecoraro, É.; Manzani, D.; Messaddeq, Y.; Ribeiro, S.J.L. Chapter 17-Bacterial Cellulose from Glucanacetobacter xylinus: Preparation, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 369–383. [Google Scholar] [CrossRef]
- Mehrotra, R.; Sharma, S.; Shree, N.; Kaur, K. Bacterial Cellulose: An Ecological Alternative as a Biotextile. Biosci. Biotechnol. Res. Asia 2023, 20, 449–463. [Google Scholar] [CrossRef]
- Stanisławska, A.; Staroszczyk, H.; Szkodo, M. The effect of dehydration/rehydration of bacterial nanocellulose on its tensile strength and physicochemical properties. Carbohydr. Polym. 2020, 236, 116023. [Google Scholar] [CrossRef] [PubMed]
- Indriyati, I.; Irmawati, Y.; Puspitasari, T. Comparative Study of Bacterial Cellulose Film Dried Using Microwave and Air Convection Heating. J. Eng. Technol. Sci. 2019, 51, 121–132. [Google Scholar] [CrossRef]
- Illa, M.P.; Sharma, C.S.; Khandelwal, M. Tuning the physiochemical properties of bacterial cellulose: Effect of drying conditions. J. Mater. Sci. 2019, 54, 12024–12035. [Google Scholar] [CrossRef]
- Erbas Kiziltas, E.; Kiziltas, A.; Gardner, D.J. Synthesis of bacterial cellulose using hot water extracted wood sugars. Carbohydr. Polym. 2015, 124, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Nan, J.; Tu, X.; He, L.; Wei, B.; Xu, C.; Xu, Y.; Li, S.; Wang, H.; Zhang, J. Improved thermostability and cytocompatibility of bacterial cellulose/collagen composite by collagen fibrillogenesis. Cellulose 2019, 26, 6713–6724. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, L.; Zhao, J.; Zhu, P. Effect of Drying Methods on Structure and Mechanical Properties of Bacterial Cellulose Films. Adv. Mater. Res. 2011, 239–242, 2667–2670. [Google Scholar] [CrossRef]
- Vasconcellos, V.; Farinas, C. The effect of the drying process on the properties of bacterial cellulose films from gluconacetobacter hansenii. Chem. Eng. Trans. 2018, 64, 145–150. [Google Scholar] [CrossRef]
- Choi, S.M.; Rao, K.M.; Zo, S.M.; Shin, E.J.; Han, S.S. Bacterial Cellulose and Its Applications. Polymers 2022, 14, 1080. [Google Scholar] [CrossRef] [PubMed]
- Urbina, L.; Corcuera, M.Á.; Gabilondo, N.; Eceiza, A.; Retegi, A. A review of bacterial cellulose: Sustainable production from agricultural waste and applications in various fields. Cellulose 2021, 28, 8229–8253. [Google Scholar] [CrossRef]
- Betlej, I.; Zakaria, S.; Krajewski, K.; Boruszewski, P. Bacterial Cellulose-Properties and Its Potential Application. Sains Malays. 2021, 50, 493–505. [Google Scholar] [CrossRef]
- Zhong, C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Gao, H.; Liao, B.; Wu, J.; Zhang, W.; Huang, J.; Liu, M.; Huang, J.; Chang, Z.; Jin, M.; et al. Characterization and optimization of production of bacterial cellulose from strain CGMCC 17276 based on whole-genome analysis. Carbohydr. Polym. 2020, 232, 115788. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.d.A.A.; Pedro, A.C.; Ribeiro, V.R.; Bortolini, D.G.; Ozaki, M.S.C.; Maciel, G.M.; Haminiuk, C.W.I. Bacterial cellulose: From production optimization to new applications. Int. J. Biol. Macromol. 2020, 164, 2598–2611. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.S.A.; Vaishnavi, T.; Vidyasri, G.S.; Sathya, K.; Priyanka, P.; Venkatachalam, P.; Karuppiah, S. Production of bacterial cellulose using Gluconacetobacter kombuchae immobilized on Luffa aegyptiaca support. Sci. Rep. 2021, 11, 2912. [Google Scholar] [CrossRef] [PubMed]
- Raiszadeh-Jahromi, Y.; Rezazadeh-Bari, M.; Almasi, H.; Amiri, S. Optimization of bacterial cellulose production by Komagataeibacter xylinus PTCC 1734 in a low-cost medium using optimal combined design. J. Food Sci. Technol. 2020, 57, 2524–2533. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.K.; El-Gendi, H.; Ray, J.B.; Taha, T.H. A low-cost effective media from starch kitchen waste for bacterial cellulose production and its application as simultaneous absorbance for methylene blue dye removal. Biomass Convers. Biorefin. 2023, 13, 12437–12449. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef]
- Shoda, M.; Sugano, Y. Recent advances in bacterial cellulose production. Biotechnol. Bioprocess Eng. 2005, 10, 1–8. [Google Scholar] [CrossRef]
- Gururaj Bhadri, S.H. Statistical Optimization of Medium Components by Response Surface Methodology for Enhanced Production of Bacterial Cellulose by Gluconacetobacter persimmonis. J. Bioprocess. Biotech. 2013, 4, 1000142. [Google Scholar] [CrossRef]
- Keshk, S.M. Bacterial Cellulose Production and its Industrial Applications. J. Bioprocess. Biotech. 2014, 4, 1000150. [Google Scholar] [CrossRef]
- Muthu, S.S.; Rathinamoorthy, R. (Eds.) Sustainability and Fashion. In Bacterial Cel-lulose: Sustainable Material for Textiles; Springer: Singapore, 2021; pp. 1–17. [Google Scholar] [CrossRef]
- Hsieh, J.-T.; Wang, M.-J.; Lai, J.-T.; Liu, H.-S. A novel static cultivation of bacterial cellulose production by intermit-tent feeding strategy. J. Taiwan Inst. Chem. Eng. C 2016, 63, 46–51. [Google Scholar] [CrossRef]
- Raghavendran, V.; Asare, E.; Roy, I. Chapter Three—Bacterial cellulose: Biosynthesis, production, and applications. In Advances in Microbial Physiology; Poole, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 89–138. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of bacterial cellulose in food. Food Hydrocoll. 2014, 35, 539–545. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Kari, Z.A.; Noor, N.H.M.; Ray, R.R. Bacte-rial Cellulose: Production, Characterization, and Application as Antimicrobial Agent. Int. J. Mol. Sci. 2021, 22, 12984. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.; Mayer, R.; Benziman, M. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 1991, 55, 35–58. [Google Scholar] [CrossRef]
- Watanabe, A.; Morita, S.; Ozaki, Y. Temperature-Dependent Changes in Hydrogen Bonds in Cellulose Iα Studied by Infrared Spectroscopy in Combination with Perturbation-Correlation Moving-Window Two-Dimensional Correlation Spectroscopy: Comparison with Cellulose Iβ. Biomacromolecules 2007, 8, 2969–2975. [Google Scholar] [CrossRef]
- Krystynowicz, A.; Czaja, W.; Wiktorowska-Jezierska, A.; Gonçalves-Miśkiewicz, M.; Turkiewicz, M.; Bielecki, S. Factors affecting the yield and properties of bacterial cellulose. J. Ind. Microbiol. Biotechnol. 2002, 29, 189–195. [Google Scholar] [CrossRef]
- Hestrin, S.; Schramm, M. Synthesis of cellulose by Acetobacter xylinum. 2. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose*. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef]
- Antal, T. Comparative study of three drying methods: Freeze, hot air-assisted freeze and infrared-assisted freeze modes. Agron. Res. 2015, 13, 863–878. [Google Scholar]
- Sakthi, S. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Zeng, M.; Laromaine, A.; Roig, A. Bacterial cellulose films: Influence of bacterial strain and drying route on film properties. Cellulose 2014, 21, 4455–4469. [Google Scholar] [CrossRef]
- Tsotsas, E.; Mujumdar, A.S. Modern Drying Technology, Volume 3: Product Quality and Formulation; John Wiley & Sons: Hoboken, NJ, USA, 2011; Available online: https://www.wiley.com/en-us/Modern+Drying+Technology%2C+Volume+3%3A+Product+Quality+and+Formulation-p-9783527643998 (accessed on 13 June 2024).
- Orsat, V.; Changrue, V.; Raghavan, V. Microwave drying of fruits and vegetables. Stewart Postharvest Rev. 2006, 2, 1–7. [Google Scholar] [CrossRef]
- Directional Freezing, Wikipedia. 2024. Available online: https://en.wikipedia.org/w/index.php?title=Directional_freezing&oldid=1202284259 (accessed on 15 June 2024).
- Bai, H.; Chen, Y.; Delattre, B.; Tomsia, A.; Ritchie, R. Bioinspired Large-Scale Aligned Porous Materials Assembled with Dual Temperature Gradients. Sci. Adv. 2015, 1, e1500849. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zou, B.; Bing, N.; Xie, H.; Yu, W. Bidirectional anisotropic bacterial cellulose/polyvinyl alcohol/MXene aerogel phase change composites for photothermal conversion enhancement. Sol. Energy Mater. Sol. Cells 2024, 271, 112818. [Google Scholar] [CrossRef]
- Mettler Toledo. Moisture Content Determination. Available online: https://www.mt.com/in/en/home/applications/Laboratory_weighing/moisture-content-determination.html (accessed on 15 June 2024).
- Sederavičiūtė, F.; Domskienė, J.; Baltina, I. Influence of Drying Temperature on Tensile and Bursting Strength of Bacterial Cellulose Biofilm. Mater. Sci. 2019, 25, 316–321. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, X.; Zhang, Y.; Zhang, Y.; Liu, T. Lightweight, strong, and super-thermal insulating polyimide composite aerogels under high temperature. Compos. Sci. Technol. 2019, 173, 47–52. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Xue, T.; Yang, F.; Fan, W.; Liu, T. Bidirectional anisotropic polyimide/bacterial cellulose aerogels by freeze-drying for super-thermal insulation. Chem. Eng. J. 2020, 385, 123963. [Google Scholar] [CrossRef]
- Albu, M.G.; Vuluga, Z.; Panaitescu, D.M.; Vuluga, D.M.; Căşărică, A.; Ghiurea, M. Morphology and thermal stability of bacterial cellulose/collagen composites. Cent. Eur. J. Chem. 2014, 12, 968–975. [Google Scholar] [CrossRef]
- Liebner, F.; Pircher, N.; Rosenau, T. Chapter 5—Bacterial NanoCellulose Aerogels. In Bacterial Nanocellulose; Gama, M., Dourado, F., Bielecki, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 73–108. [Google Scholar] [CrossRef]
- Demilecamps, A. Synthesis and Characterization of Polysaccharide-Silica Composite Aerogels for Thermal Superin-Sulation. Ph.D. Thesis, Ecole Nationale Supérieure des Mines de Paris, Paris, France, 2015. Available online: https://pastel.archives-ouvertes.fr/tel-01279456 (accessed on 5 February 2022).
- Fleury, B.; Abraham, E.; De La Cruz, J.A.; Chandrasekar, V.S.; Senyuk, B.; Liu, Q.; Cherpak, V.; Park, S.; Ten Hove, J.B.; Smalyukh, I.I. Aerogel from Sustainably Grown Bacterial Cellulose Pellicles as a Thermally Insulative Film for Building Envelopes. ACS Appl. Mater. Interfaces 2020, 12, 34115–34121. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, X.; Wu, X.; Lu, C. Flame Retardant, Heat Insulating Cellulose Aerogels from Waste Cotton Fabrics by in Situ Formation of Magnesium Hydroxide Nanoparticles in Cellulose Gel Nanostructures. ACS Sustain. Chem. Eng. 2015, 3, 1853–1859. [Google Scholar] [CrossRef]
- Viggiano, R.P.; Williams, J.C.; Schiraldi, D.A.; Meador, M.A.B. Effect of Bulky Substituents in the Polymer Backbone on the Properties of Polyimide Aerogels. ACS Appl. Mater. Interfaces 2017, 9, 8287–8296. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, L.; Wang, X.; Song, L.; Hu, Y. Hypophosphorous acid cross-linked layer-by-layer assembly of green poly-electrolytes on polyester-cotton blend fabrics for durable flame-retardant treatment. Carbohydr. Polym. 2018, 201, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bober, P.; Liu, J.; Mikkonen, K.S.; Ihalainen, P.; Pesonen, M.; Plumed-Ferrer, C.; von Wright, A.; Lindfors, T.; Xu, C.; Latonen, R.-M. Biocomposites of nanofibrillated cellulose, polypyrrole, and silver nanoparticles with electroconductive and antimicrobial properties. Biomacromolecules 2014, 15, 3655–3663. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; E, Y.; Li, J.; Du, T.; Wang, K.; Yao, X.; Jiang, J.; Wang, M.; Yuan, S. Sustainable bacterial cellulose-based composite aerogels with excellent flame retardant and heat insulation. Cellulose 2023, 30, 9563–9574. [Google Scholar] [CrossRef]
- Sai, H.; Fu, R.; Xing, L.; Xiang, J.; Li, Z.; Li, F.; Zhang, T. Surface Modification of Bacterial Cellulose Aerogels’ Web-like Skeleton for Oil/Water Separation. ACS Appl. Mater. Interfaces 2015, 7, 7373–7381. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, S.; Yang, B.; Hao, M.; Chen, Z.; Liu, Y.; Wang, X.; Yao, J. Preparation of ambient-dried multifunctional cellulose aerogel by freeze-linking technique. Chem. Eng. J. 2023, 477, 147044. [Google Scholar] [CrossRef]
- Huang, Z.; Li, H.; Miao, H.; Guo, Y.; Teng, L. Modified supercritical CO2 extraction of amine template from hexagonal mesoporous silica (HMS) materials: Effects of template identity and matrix Al/Si molar ratio. Chem. Eng. Res. Des. 2014, 92, 1371–1380. [Google Scholar] [CrossRef]
- Revin, V.V.; Pestov, N.A.; Shchankin, M.V.; Mishkin, V.P.; Platonov, V.I.; Uglanov, D.A. A Study of the Physical and Mechanical Properties of Aerogels Obtained from Bacterial Cellulose. Biomacromolecules 2019, 20, 1401–1411. [Google Scholar] [CrossRef]
- Revin, V.V.; Nazarova, N.B.; Tsareva, E.E.; Liyaskina, E.V.; Revin, V.D.; Pestov, N.A. Production of Bacterial Cellulose Aerogels With Improved Physico-Mechanical Properties and Antibacterial Effect. Front. Bioeng. Biotechnol. 2020, 8, 1392. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Yang, J.; Li, Z.; Wang, H. Functionalized bacterial cellulose derivatives and nanocomposites. Carbohydr. Polym. 2014, 101, 1043–1060. [Google Scholar] [CrossRef]
- Li, H.; Ye, M.; Zhang, X.; Zhang, H.; Wang, G.; Zhang, Y. Hierarchical Porous Iron Metal–Organic Gel/Bacterial Cellulose Aerogel: Ultrafast, Scalable, Room-Temperature Aqueous Synthesis, and Efficient Arsenate Removal. ACS Appl. Mater. Interfaces 2021, 13, 47684–47695. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, X.; Hu, G.; Huang, T.; Yu, H.; Yu, B.; Zhu, M. In situ crosslinking of mechanically robust waterproof and moisture permeable cellulose diacetate nanofiber aerogels for warm clothing. Chem. Eng. J. 2022, 444, 136528. [Google Scholar] [CrossRef]
- Liao, D.; Wang, Y.; Xie, P.; Zhang, C.; Li, M.; Liu, H.; Zhou, L.; Wei, C.; Yu, C.; Chen, Y. A resilient and lightweight cellu-lose/graphene oxide/polymer-derived multifunctional carbon aerogel generated from Pickering emulsion toward a wearable pressure sensor. J. Colloid Interface Sci. 2022, 628, 574–587. [Google Scholar] [CrossRef]
- Rahmanian, V.; Pirzada, T.; Wang, S.; Khan, S.A. Cellulose-Based Hybrid Aerogels: Strategies toward Design and Functionality. Adv. Mater. 2021, 33, 2102892. [Google Scholar] [CrossRef] [PubMed]
- Meti, P.; Mahadik, D.B.; Lee, K.-Y.; Wang, Q.; Kanamori, K.; Gong, Y.-D.; Park, H.-H. Overview of organic–inorganic hybrid silica aerogels: Progress and perspectives. Mater. Des. 2022, 222, 111091. [Google Scholar] [CrossRef]
- Hu, X.; Yang, B.; Hao, M.; Chen, Z.; Liu, Y.; Ramakrishna, S.; Wang, X.; Yao, J. Preparation of high elastic bacterial cellulose aerogel through thermochemical vapor deposition catalyzed by solid acid for oil-water separation. Carbohydr. Polym. 2023, 305, 120538. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, S.; Yang, B.; Hao, M.; Chen, Z.; Liu, Y.; Ramakrishna, S.; Wang, X.; Yao, J. Bacterial cellulose composite aerogel with high elasticity and adjustable wettability for dye absorption and oil–water separation. Appl. Surf. Sci. 2023, 640, 158299. [Google Scholar] [CrossRef]
- Sai, H.; Wang, M.; Miao, C.; Song, Q.; Wang, Y.; Fu, R.; Wang, Y.; Ma, L.; Hao, Y. Robust Silica-Bacterial Cellulose Composite Aerogel Fibers for Thermal Insulation Textile. Gels 2021, 7, 145. [Google Scholar] [CrossRef]
- Clasen, C.; Sultanova, B.; Wilhelms, T.; Heisig, P.; Kulicke, W.-M. Effects of Different Drying Processes on the Material Properties of Bacterial Cellulose Membranes. Macromol. Symp. 2006, 244, 48–58. [Google Scholar] [CrossRef]
- Bueno, F.; Spivak, D.A.; Sathivel, S. Evaluation of the properties of dry bacterial cellulose synthesized from coffee kombucha fermentation dried with different drying methods. Dry. Technol. 2023, 42, 142–154. [Google Scholar] [CrossRef]
- Dey, B.; Jayaraman, S.; Balasubramanian, P. Investigating the effects of drying on the physical properties of Kombucha Bacterial Cellulose: Kinetic study and modeling approach. J. Clean. Prod. 2024, 452, 142204. [Google Scholar] [CrossRef]
- Mohamad, S.; Abdullah, L.C.; Jamari, S.S.; Al Edrus, S.S.O.; Aung, M.M.; Mohamad, S.F.S. Influence of drying method on the crystal structure and thermal property of oil palm frond juice-based bacterial cellulose. J. Mater. Sci. 2022, 57, 1462–1473. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, H.; Yu, Y.; Li, H.; Li, H.; Bai, J.; Shi, F.; Liu, J. Bacterial cellulose biomass aerogels for oil-water separation and thermal insulation. J. Environ. Chem. Eng. 2023, 11, 110403. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Jiang, Z.; Wang, H. The effect of freezing speed and hydrogel concentration on the microstructure and compressive performance of bamboo-based cellulose aerogel. J. Wood Sci. 2015, 61, 595–601. [Google Scholar] [CrossRef]
- Ruan, J.-Q.; Xie, K.-Y.; Wan, J.-N.; Chen, Q.-Y.; Zuo, X.; Li, X.; Wu, X.; Fei, C.; Yao, S. Effects of Freeze-Drying Processes on the Acoustic Absorption Performance of Sustainable Cellulose Nanocrystal Aerogels. Gels 2024, 10, 141. [Google Scholar] [CrossRef]
- Jin, H.; Nishiyama, Y.; Wada, M.; Kuga, S. Nanofibrillar cellulose aerogels. Colloids Surf. A Physicochem. Eng. Asp. 2004, 240, 63–67. [Google Scholar] [CrossRef]



| Static Culture | Agitated Culture (Stirred Condition) | Ref. | |
|---|---|---|---|
| 1 | 3D Interconnected network-like films | Pointed, uneven sphere-like cellulose particles (SCPs) | [51] |
| 2 | Carbon and air supply | Stays completely dispersed within the culture medium | [52] |
| 3 | Enhanced genetic robustness | Commercial high yield | [53] |
| 4 | Higher Young’s modulus | Very low degree of polymerization | [37] |
| 5 | Established geometrics | Lower level of crystallinity | [54] |
| 6 | Significant water retention capability | Costly production | [55] |
| 7 | Strong wet tensile strength | - | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sozcu, S.; Frajova, J.; Wiener, J.; Venkataraman, M.; Tomkova, B.; Militky, J. Effect of Drying Methods on the Thermal and Mechanical Behavior of Bacterial Cellulose Aerogel. Gels 2024, 10, 474. https://doi.org/10.3390/gels10070474
Sozcu S, Frajova J, Wiener J, Venkataraman M, Tomkova B, Militky J. Effect of Drying Methods on the Thermal and Mechanical Behavior of Bacterial Cellulose Aerogel. Gels. 2024; 10(7):474. https://doi.org/10.3390/gels10070474
Chicago/Turabian StyleSozcu, Sebnem, Jaroslava Frajova, Jakub Wiener, Mohanapriya Venkataraman, Blanka Tomkova, and Jiri Militky. 2024. "Effect of Drying Methods on the Thermal and Mechanical Behavior of Bacterial Cellulose Aerogel" Gels 10, no. 7: 474. https://doi.org/10.3390/gels10070474







