Hydrogel-Based Therapies for Ischemic and Hemorrhagic Stroke: A Comprehensive Review
Abstract
:1. Background
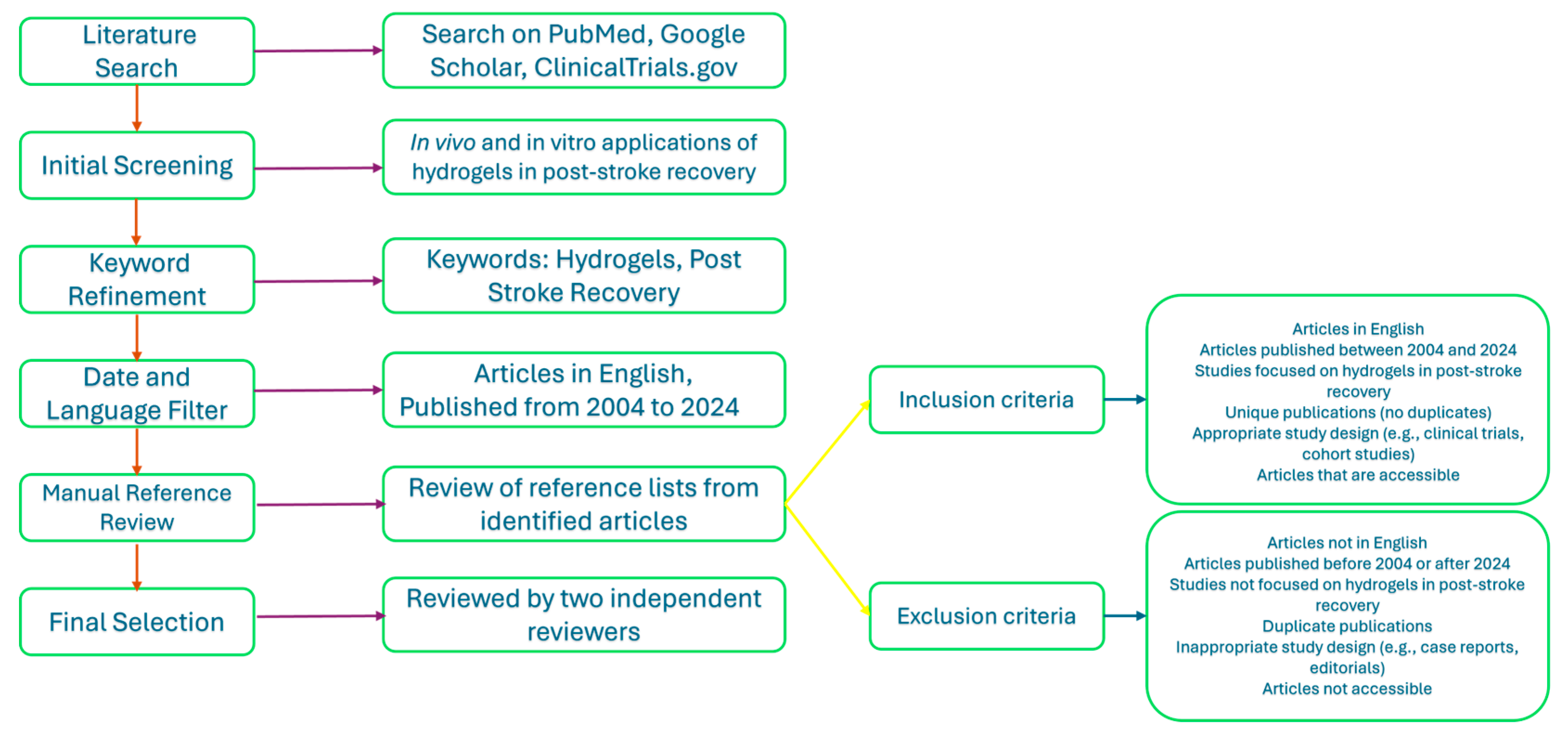
2. Method
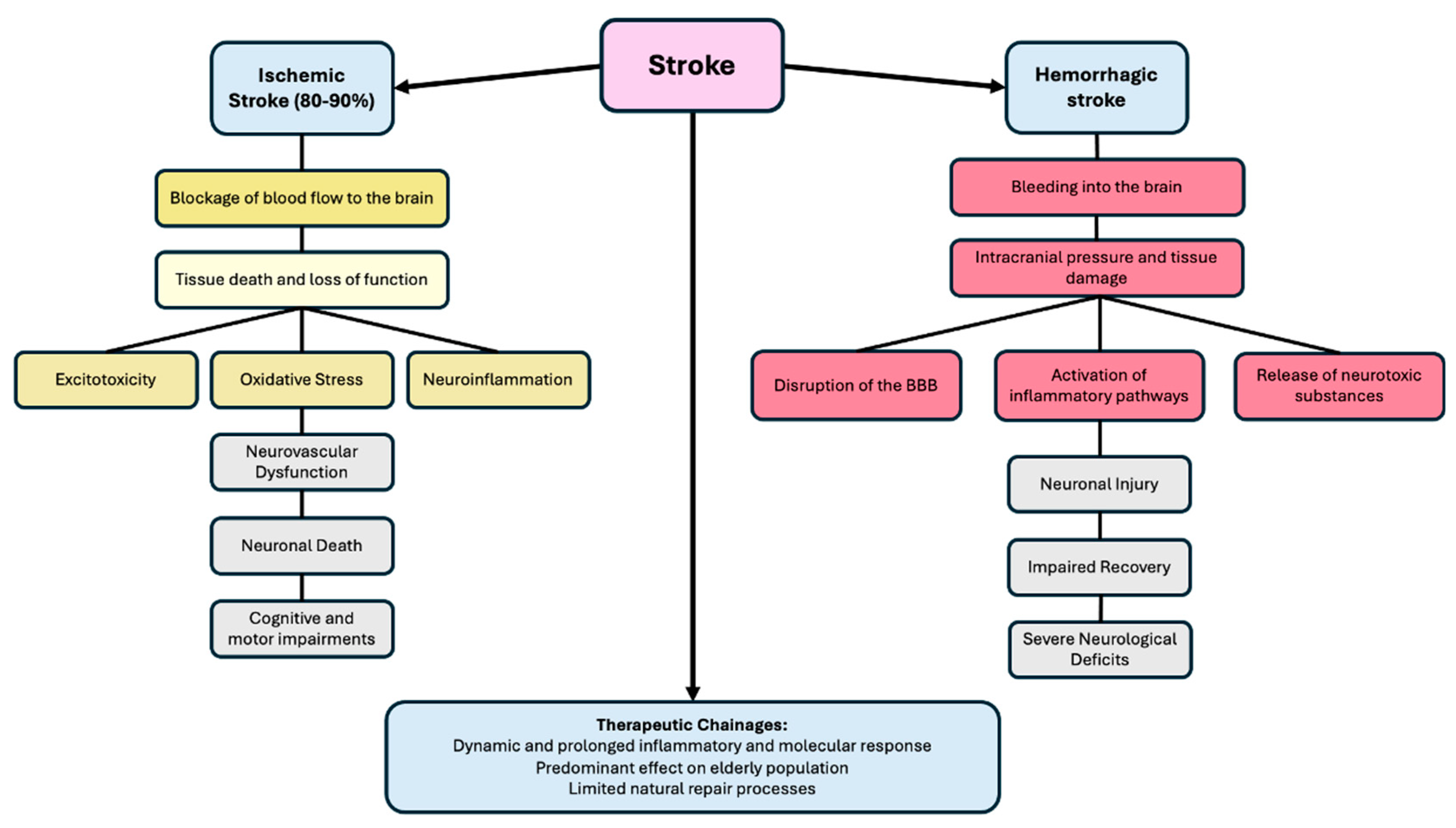
3. The Pathophysiology of Stroke
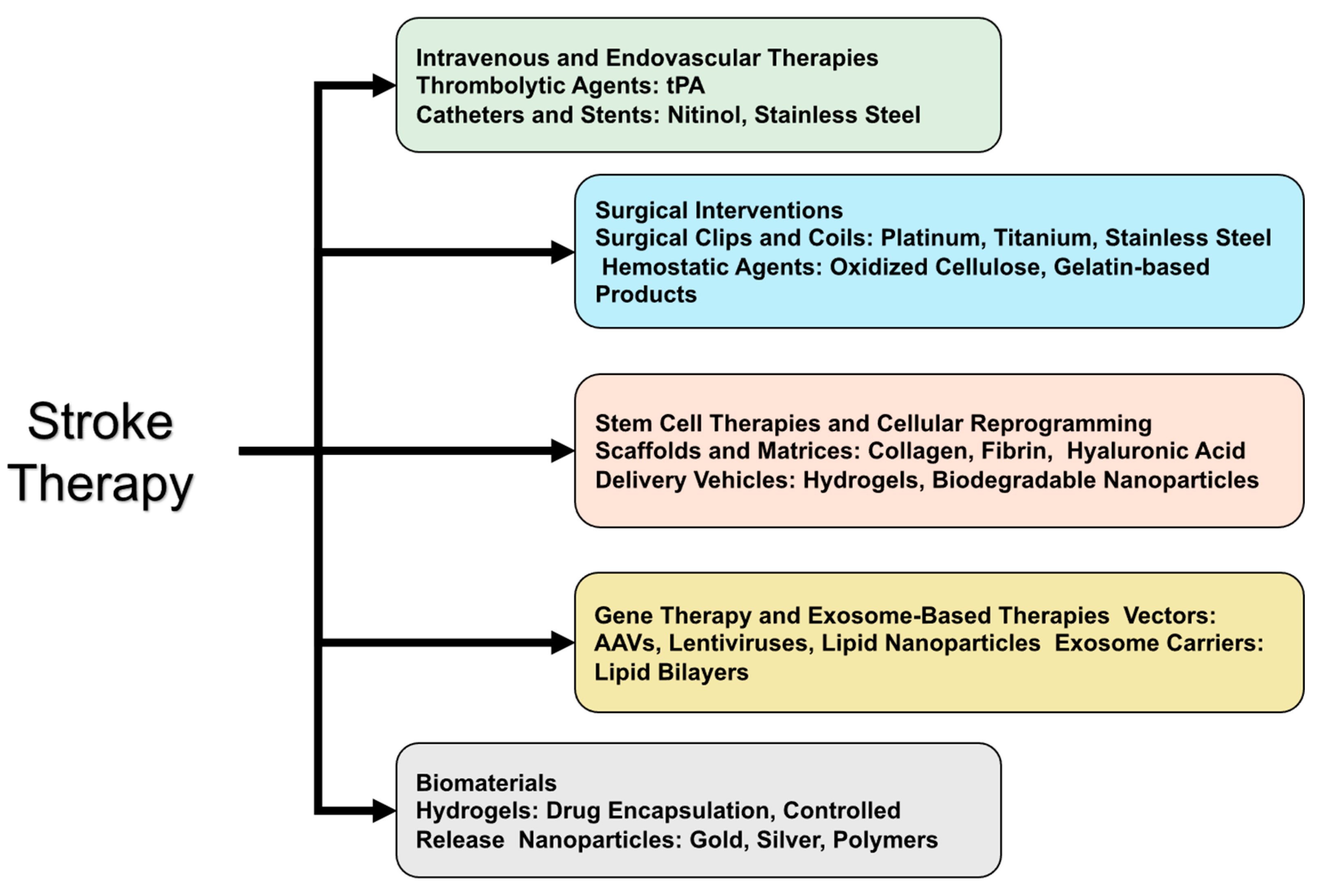
4. Current Treatment Approaches and Emerging Therapies for Stroke
4.1. Current Materials Used in Stroke Treatments
4.2. Current Treatment Approaches
4.3. Other Emerging Therapies
| Therapeutic Approach | Description | Limitations | Citations |
|---|---|---|---|
| Ischemic Stroke (IS) Treatment | |||
| Intravenous administration of tissue plasminogen activator (tPA) | The only FDA-approved pharmacological therapy for IS; used to dissolve the thrombus and restore blood flow. | Narrow treatment window (<4.5 h), risk of bleeding, and only beneficial for <5% of patients. | [63,64] |
| Endovascular thrombectomy (ET) | Mechanical removal of the thrombus through a catheter. | Short therapeutic window (<6 h), limited accessibility in some hospitals, and risk of cerebrovascular complications and hemorrhagic transformation. | [65,66] |
| Hemorrhagic Stroke Treatment | |||
| Surgical interventions (e.g., aneurysm clipping or coiling, hematoma evacuation) | Aims to repair the ruptured vessel and relieve intracranial pressure. | Limited treatment options and lower survival rates compared to ischemic stroke. | [67] |
| Pharmacological management | Use of antihypertensives, anticonvulsants, and agents to reduce cerebral edema. | Limited effectiveness in improving survival rates. | [68] |
| Emerging Therapies | |||
| Stem cell therapies | Utilizes various stem cell types (e.g., MSCs, NSCs) for neuroprotection, angiogenesis, and immunomodulation. | Challenges include poor cell survival, limited long-term viability, risk of tumor formation, and unclear optimal protocols. | [69,70,71,72] |
| Cellular reprogramming | Direct conversion of resident glial cells into functional neurons using RNA-based gene manipulation, small molecules, or transcription factors (e.g., NeuroD1, Ascl1, Neurogenin2). | Requires more research on the delivery methods and the functional integration of reprogrammed cells. | [73,74] |
| Gene therapy and exosome-based therapies | Emerging strategies to enhance stroke recovery through targeted genetic modifications or the use of exosomes to deliver therapeutic agents. | High complexity, regulatory challenges, and need for extensive preclinical and clinical validation. | [75] |
| Biomaterial-Based Interventions | |||
| Hydrogels | Biocompatible, customizable materials that can deliver, protect, and enhance therapeutic agents, potentially improving drug delivery, neuroprotection, and tissue regeneration. | Potential for immune response, difficulty in achieving precise drug release and degradation rates, scalability issues, and long-term stability concerns. | [76,77,78] |
| Other Therapies | |||
| Nanoparticle-based therapies | Using nanoparticles to deliver drugs directly to the affected brain regions, enhancing drug stability and targeting. | Potential toxicity, challenges in targeting specific brain regions, and regulatory hurdles. | [58,79] |
| Gene editing technologies (e.g., CRISPR-Cas9) | Precise genetic modifications to correct mutations or upregulate/downregulate specific genes involved in stroke pathology. | Ethical concerns, off-target effects, and delivery challenges. | [59,60] |
| Combination therapies | Combining multiple treatment modalities such as stem cells, gene therapy, and pharmacological agents to enhance therapeutic outcomes. | Complexity in treatment protocols, potential for increased side effects, and higher cost. | [61] |
| Innovative Approaches | |||
| Bioengineered scaffolds | Creating supportive structures that mimic the extracellular matrix, promoting tissue regeneration and repair. | Risk of immune response and challenges in scalability challenges and ensuring long-term stability. | [62] |
| Personalized medicine | Tailoring treatments based on individual genetic and physiological profiles to optimize efficacy and minimize side effects. | High cost, complexity of genetic profiling, and need for extensive patient data. | [60] |
5. Hydrogels in Biomedicine
5.1. The Classification, Properties, and Composition of Hydrogels
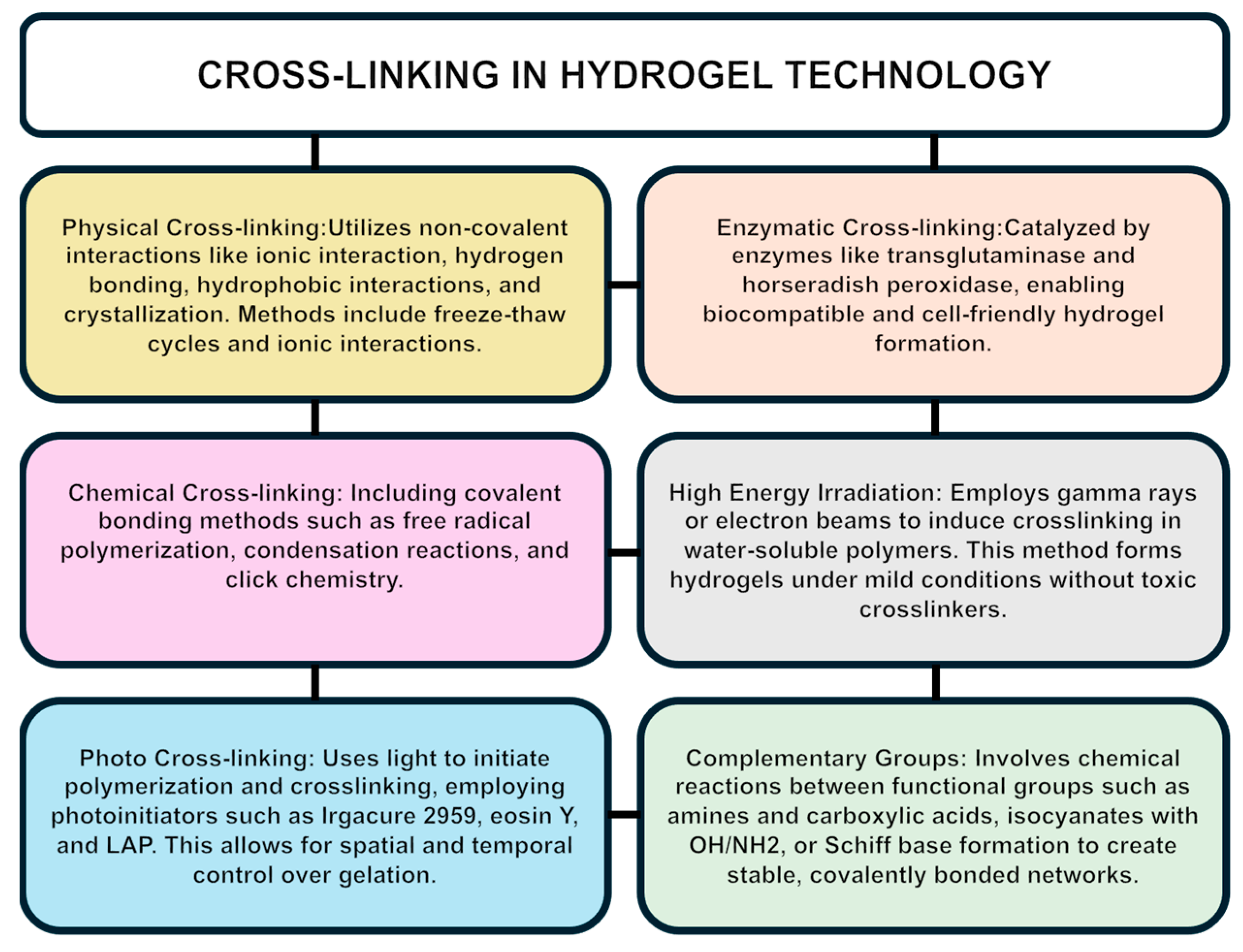
5.2. Hydrogel Crosslinking
6. Hydrogel Applications in Biomedical Research and Stroke Therapy
6.1. Hydrogels Used in Ischemic Stroke Treatment
6.1.1. Hydrogels as Drug Delivery Systems
6.1.2. Hydrogels for Neuroprotection and Tissue Repair
6.1.3. Hydrogels for Reducing Secondary Injury
6.1.4. Hydrogels’ Involvement in Blood–Brain Barrier Protection
6.1.5. Regulatory Aspects of Hydrogels in the Management of Ischemic Stroke (IS)
6.2. Hydrogels in Hemorrhagic Stroke Treatment
7. The Translational Gap
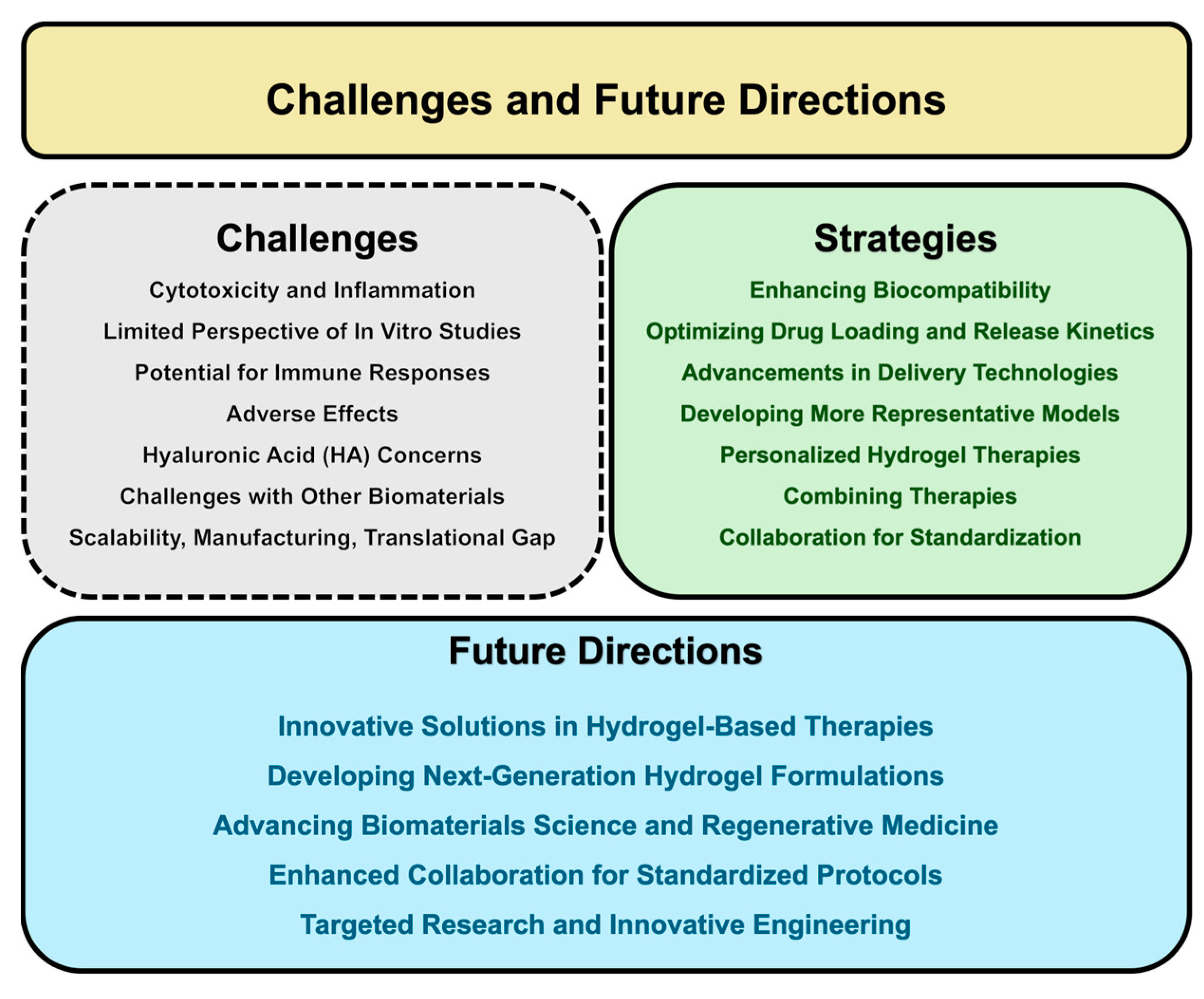
8. Challenges and Future Directions
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1. AAVs | Adeno-Associated Viruses |
| 2. AEMA | 2-aminoethyl methacrylate |
| 3. AIBN | Azobisisobutyronitrile |
| 4. ATRP | Atom Transfer Radical Polymerization |
| 5. BBB | Blood–Brain Barrier |
| 6. BDNF | Brain-Derived Neurotrophic Factor |
| 7. CC BY | Creative Commons Attribution |
| 8. DA | 3,4-dihydroxyphenylalanine |
| 9. ECM | Extracellular Matrix |
| 10. EDC | 3-ethylcarbodiimide hydrochloride |
| 11. EPO | Erythropoietin |
| 12. ESCs | Embryonic Stem Cells |
| 13. ET | Endovascular Thrombectomy |
| 14. FDA | Food and Drug Administration |
| 15. FGF | Fibroblast Growth Factor |
| 16. GDNF | Glial Cell Line-Derived Neurotrophic Factor |
| 17. GMP | Good Manufacturing Practice |
| 18. GMSs | Gelatin Microspheres |
| 19. HA | Hyaluronic Acid |
| 20. HAD | Hyaluronic Acid–Dopamine |
| 21. HAMC | Hyaluronan/Methyl Cellulose |
| 22. HMW | High-Molecular-Weight |
| 23. HSCs | Hematopoietic Stem Cells |
| 24. ICH | International Council for Harmonisation |
| 25. IL-10 | Interleukin-10 |
| 26. IND | Investigational New Drug |
| 27. iPSC | Induced Pluripotent Stem Cell |
| 28. IS | Ischemic Stroke |
| 29. LAP | Lithium Phenyl-2,4,6-Trimethylbenzoylphosphinate |
| 30. LMW | Low-Molecular-Weight |
| 31. MAP | Microporous Annealing Particle |
| 32. MC | Methyl Cellulose |
| 33. MSCs | Mesenchymal Stem Cells |
| 34. MW | Molecular Weight |
| 35. NDA | New Drug Application |
| 36. NGF | Nerve Growth Factor |
| 37. NHS | N-Hydroxy Succinimide |
| 38. NMDA | N-Methyl-D-Aspartate |
| 39. NMP | Nitroxide-Mediated Polymerization |
| 40. NPCs | Neural Progenitor Cells |
| 41. NSCs | Neural Stem Cells |
| 42. NVU | Neurovascular Unit |
| 43. PEG | Polyethylene Glycol |
| 44. PPi | Polyphosphate |
| 45. PLGA | Poly(Lactic-Co-Glycolic Acid) |
| 46. PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| 47. PVA | Polyvinyl Alcohol |
| 48. RAFT | Reversible Addition–Fragmentation Chain Transfer |
| 49. ROS | Reactive Oxygen Species |
| 50. SCs | Stem Cells |
| 51. SOD | Superoxide Dismutase |
| 52. TNF-α | Tumor Necrosis Factor-Alpha |
| 53. tPA | Tissue Plasminogen Activator |
| 54. VEGF | Vascular Endothelial Growth Factor |
References
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.J.; Werring, D.J. Stroke: Causes and clinical features. Medicine 2020, 48, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W. Update on Stroke Rehabilitation in Motor Impairment. Brain Neurorehabil 2022, 15, e12. [Google Scholar] [CrossRef] [PubMed]
- Al-Qazzaz, N.K.; Ali, S.H.; Ahmad, S.A.; Islam, S.; Mohamad, K. Cognitive impairment and memory dysfunction after a stroke diagnosis: A post-stroke memory assessment. Neuropsychiatr. Dis. Treat. 2014, 10, 1677–1691. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S. Post-stroke Mood and Emotional Disturbances: Pharmacological Therapy Based on Mechanisms. J. Stroke 2016, 18, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lu, J.; Shao, A.; Zhang, J.H.; Zhang, J. Glial Cells: Role of the Immune Response in Ischemic Stroke. Front. Immunol. 2020, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, C.V. Concise Review: Stem Cell Therapy for Stroke Patients: Are We There Yet? Stem Cells Transl. Med. 2019, 8, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Szelenberger, R.; Kostka, J.; Saluk-Bijak, J.; Miller, E. Pharmacological Interventions and Rehabilitation Approach for Enhancing Brain Self-repair and Stroke Recovery. Curr. Neuropharmacol. 2020, 18, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Morshead, C.M.; Chen, G.; Li, W. Editorial: Regulation of Cellular Reprogramming for Post-stroke Tissue Regeneration: Bridging a Gap Between Basic Research and Clinical Application. Front. Cell Dev. Biol. 2021, 9, 793900. [Google Scholar] [CrossRef]
- Nih, L.R.; Carmichael, S.T.; Segura, T. Hydrogels for brain repair after stroke: An emerging treatment option. Curr. Opin. Biotechnol. 2016, 40, 155–163. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, Y.; Zhang, J.; Liang, H.; Chen, X.; Tan, H. Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications. Pharmaceutics 2023, 15, 2514. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, K. Advances in Hydrogel-Based Drug Delivery Systems. Gels 2024, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, D.; Bertani, F.; Fusaro, L.; Clemente, N.; Carton, F.; Talmon, M.; Fresu, L.G.; Boccafoschi, F. Regenerative Potential of A Bovine ECM-Derived Hydrogel for Biomedical Applications. Biomolecules 2022, 12, 1222. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Chowdhury, S.D.; Wilson, R.L. Advancements and Challenges in Hydrogel Engineering for Regenerative Medicine. Gels 2024, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Raina, N.; Pahwa, R.; Bhattacharya, J.; Paul, A.K.; Nissapatorn, V.; de Lourdes Pereira, M.; Oliveira, S.M.R.; Dolma, K.G.; Rahmatullah, M.; Wilairatana, P.; et al. Drug Delivery Strategies and Biomedical Significance of Hydrogels: Translational Considerations. Pharmaceutics 2022, 14, 574. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Dawson, C.; Lamb, M.; Mueller, E.; Stefanek, E.; Akbari, M.; Hoare, T. Hydrogels for Tissue Engineering: Addressing Key Design Needs Toward Clinical Translation. Front. Bioeng. Biotechnol. 2022, 10, 849831. [Google Scholar] [CrossRef]
- Moskowitz, M.A.; Lo, E.H.; Iadecola, C. The science of stroke: Mechanisms in search of treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Alkahtani, R. Molecular mechanisms underlying some major common risk factors of stroke. Heliyon 2022, 8, e10218. [Google Scholar] [CrossRef]
- Mao, R.; Zong, N.; Hu, Y.; Chen, Y.; Xu, Y. Neuronal Death Mechanisms and Therapeutic Strategy in Ischemic Stroke. Neurosci. Bull. 2022, 38, 1229–1247. [Google Scholar] [CrossRef]
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-Triggered Glutamate Excitotoxicity From the Perspective of Glial Cells. Front. Cell. Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Muzio, L.; Viotti, A.; Martino, G. Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Front. Neurosci. 2021, 15, 742065. [Google Scholar] [CrossRef] [PubMed]
- Takata, F.; Nakagawa, S.; Matsumoto, J.; Dohgu, S. Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Front. Cell. Neurosci. 2021, 15, 661838. [Google Scholar] [CrossRef] [PubMed]
- StatPearls Publishing. StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Fang, Y.; Gao, S.; Wang, X.; Cao, Y.; Lu, J.; Chen, S.; Lenahan, C.; Zhang, J.H.; Shao, A.; Zhang, J. Programmed Cell Deaths and Potential Crosstalk With Blood-Brain Barrier Dysfunction After Hemorrhagic Stroke. Front. Cell. Neurosci. 2020, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Archie, S.R.; Al Shoyaib, A.; Cucullo, L. Blood-Brain Barrier Dysfunction in CNS Disorders and Putative Therapeutic Targets: An Overview. Pharmaceutics 2021, 13, 1779. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Buckwalter, M.; Soreq, H.; Vezzani, A.; Kaufer, D. Blood-brain barrier dysfunction-induced inflammatory signaling in brain pathology and epileptogenesis. Epilepsia 2012, 53 (Suppl. 6), 37–44. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Y.; Wei, R.; Khan, S.; Zhang, R.; Zhang, Y.; Yong, V.W.; Xue, M. Iron Neurotoxicity and Protection by Deferoxamine in Intracerebral Hemorrhage. Front. Mol. Neurosci. 2022, 15, 927334. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Chen, S.; Ma, L. Secondary Brain Injury by Oxidative Stress After Cerebral Hemorrhage: Recent Advances. Front. Cell. Neurosci. 2022, 16, 853589. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Lane, D.A.; Lenarczyk, R.; Boriani, G.; Doehner, W.; Benjamin, L.A.; Fisher, M.; Lowe, D.; Sacco, R.L.; Schnabel, R.; et al. Integrated care for optimizing the management of stroke and associated heart disease: A position paper of the European Society of Cardiology Council on Stroke. Eur. Heart J. 2022, 43, 2442–2460. [Google Scholar] [CrossRef]
- Zhu, A.; Rajendram, P.; Tseng, E.; Coutts, S.B.; Yu, A.Y.X. Alteplase or tenecteplase for thrombolysis in ischemic stroke: An illustrated review. Res. Pract. Thromb. Haemost. 2022, 6, e12795. [Google Scholar] [CrossRef]
- Kato, S.; Ban, Y.; Ota, T.; Miki, N. Microfabricated Nitinol Stent Retrievers with a Micro-Patterned Surface. Micromachines 2024, 15, 213. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.N.; Hwang, W.; Horn, J.; Landsman, T.L.; Boyle, A.; Wierzbicki, M.A.; Hasan, S.M.; Follmer, D.; Bryant, J.; Small, W.; et al. Design and biocompatibility of endovascular aneurysm filling devices. J. Biomed. Mater. Res. Part A 2015, 103, 1577–1594. [Google Scholar] [CrossRef]
- Irfan, N.I.; Mohd Zubir, A.Z.; Suwandi, A.; Haris, M.S.; Jaswir, I.; Lestari, W. Gelatin-based hemostatic agents for medical and dental application at a glance: A narrative literature review. Saudi Dent. J. 2022, 34, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.S. Enhancing Stem Cell-Based Therapeutic Potential by Combining Various Bioengineering Technologies. Front. Cell Dev. Biol. 2022, 10, 901661. [Google Scholar] [CrossRef]
- Ahmadi, S.E.; Soleymani, M.; Shahriyary, F.; Amirzargar, M.R.; Ofoghi, M.; Fattahi, M.D.; Safa, M. Viral vectors and extracellular vesicles: Innate delivery systems utilized in CRISPR/Cas-mediated cancer therapy. Cancer Gene Ther. 2023, 30, 936–954. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Xavier, J.; Kumar, N.; Ahmad, M.Z.; Ranjan, O.P. Exosomes as natural nanocarrier-based drug delivery system: Recent insights and future perspectives. 3 Biotech 2023, 13, 101. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Chiang, M.C.; Yang, Y.P.; Nicol, C.J.B.; Wang, C.J. Gold Nanoparticles in Neurological Diseases: A Review of Neuroprotection. Int. J. Mol. Sci. 2024, 25, 2360. [Google Scholar] [CrossRef]
- Desai, S.M.; Jha, R.M.; Linfante, I. Collateral Circulation Augmentation and Neuroprotection as Adjuvant to Mechanical Thrombectomy in Acute Ischemic Stroke. Neurology 2021, 97, S178–S184. [Google Scholar] [CrossRef]
- Hollist, M.; Morgan, L.; Cabatbat, R.; Au, K.; Kirmani, M.F.; Kirmani, B.F. Acute Stroke Management: Overview and Recent Updates. Aging Dis. 2021, 12, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2021, 97, S6–S16. [Google Scholar] [CrossRef] [PubMed]
- Phipps, M.S.; Cronin, C.A. Management of acute ischemic stroke. BMJ 2020, 368, l6983. [Google Scholar] [CrossRef]
- Hong, J.M.; Kim, D.S.; Kim, M. Hemorrhagic Transformation After Ischemic Stroke: Mechanisms and Management. Front. Neurol. 2021, 12, 703258. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Fan, J.; Han, J. Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharm. Sin. B 2015, 5, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.O.; Rymer, M.M. Hemorrhagic stroke: Aneurysmal subarachnoid hemorrhage. Mo. Med. 2011, 108, 124–127. [Google Scholar]
- Connolly, E.S.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T.; Hoh, B.L.; Kirkness, C.J.; Naidech, A.M.; Ogilvy, C.S.; et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 2012, 43, 1711–1737. [Google Scholar] [CrossRef]
- Perna, R.; Temple, J. Rehabilitation Outcomes: Ischemic versus Hemorrhagic Strokes. Behav. Neurol. 2015, 2015, 891651. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, Y.; Gao, S.; Fu, X.; Cao, Y.; Peng, Y.; Zhuang, J.; Hu, J.; Shao, A.; Wang, L. Potential Mechanisms and Perspectives in Ischemic Stroke Treatment Using Stem Cell Therapies. Front. Cell Dev. Biol. 2021, 9, 646927. [Google Scholar] [CrossRef]
- Tan, N.; Xin, W.; Huang, M.; Mao, Y. Mesenchymal stem cell therapy for ischemic stroke: Novel insight into the crosstalk with immune cells. Front. Neurol. 2022, 13, 1048113. [Google Scholar] [CrossRef]
- Panos, L.D.; Bargiotas, P.; Arnold, M.; Hadjigeorgiou, G.; Panos, G.D. Revolutionizing Stroke Recovery: Unveiling the Promise of Stem Cell Therapy. Drug Des. Dev. Ther. 2024, 18, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Reiter, S.; Zhou, X.; Chen, H.; Ou, Y.; Lenahan, C.; He, Y. Insight Into the Mechanisms and the Challenges on Stem Cell-Based Therapies for Cerebral Ischemic Stroke. Front. Cell. Neurosci. 2021, 15, 637210. [Google Scholar] [CrossRef] [PubMed]
- Savitz, S.I.; Chopp, M.; Deans, R.; Carmichael, T.; Phinney, D.; Wechsler, L.; Participants, S. Stem Cell Therapy as an Emerging Paradigm for Stroke (STEPS) II. Stroke 2011, 42, 825–829. [Google Scholar] [CrossRef]
- Talifu, Z.; Liu, J.Y.; Pan, Y.Z.; Ke, H.; Zhang, C.J.; Xu, X.; Gao, F.; Yu, Y.; Du, L.J.; Li, J.J. In vivo astrocyte-to-neuron reprogramming for central nervous system regeneration: A narrative review. Neural Regen. Res. 2023, 18, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, T.; Jia, J.; Chen, Y.; Zhang, Y.; Fang, Z.; Zhang, C.; Bai, Y.; Li, Z.; Li, Y. Perspective insights into versatile hydrogels for stroke: From molecular mechanisms to functional applications. Biomed. Pharmacother. 2024, 173, 116309. [Google Scholar] [CrossRef] [PubMed]
- Malone, K.; Amu, S.; Moore, A.C.; Waeber, C. Immunomodulatory Therapeutic Strategies in Stroke. Front. Pharmacol. 2019, 10, 630. [Google Scholar] [CrossRef]
- Jameson, J.L.; Longo, D.L. Precision medicine—Personalized, problematic, and promising. N. Engl. J. Med. 2015, 372, 2229–2234. [Google Scholar] [CrossRef]
- Suda, S.; Nito, C.; Yokobori, S.; Sakamoto, Y.; Nakajima, M.; Sowa, K.; Obinata, H.; Sasaki, K.; Savitz, S.I.; Kimura, K. Recent Advances in Cell-Based Therapies for Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 6718. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yoshida, M.; Horie, N.; Satoh, K.; Fukuda, Y.; Ishizaka, S.; Ogawa, K.; Morofuji, Y.; Hiu, T.; Izumo, T.; et al. Stem Cell Therapy for Acute/Subacute Ischemic Stroke with a Focus on Intraarterial Stem Cell Transplantation: From Basic Research to Clinical Trials. Bioengineering 2022, 10, 33. [Google Scholar] [CrossRef]
- Surugiu, R.; Olaru, A.; Hermann, D.M.; Glavan, D.; Catalin, B.; Popa-Wagner, A. Recent Advances in Mono- and Combined Stem Cell Therapies of Stroke in Animal Models and Humans. Int. J. Mol. Sci. 2019, 20, 6029. [Google Scholar] [CrossRef] [PubMed]
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Dávalos, A.; Guidetti, D.; Larrue, V.; Lees, K.R.; Medeghri, Z.; Machnig, T.; et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L.; Fonarow, G.C.; Smith, E.E.; Reeves, M.J.; Grau-Sepulveda, M.V.; Pan, W.; Olson, D.M.; Hernandez, A.F.; Peterson, E.D.; Schwamm, L.H. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA 2013, 309, 2480–2488. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.; Mitchell, P.J.; Kleinig, T.J.; Dewey, H.M.; Churilov, L.; Yassi, N.; Yan, B.; Dowling, R.J.; Parsons, M.W.; Oxley, T.J.; et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015, 372, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Sahni, R.; Weinberger, J. Management of intracerebral hemorrhage. Vasc. Health Risk Manag. 2007, 3, 701–709. [Google Scholar] [PubMed]
- Hemphill, J.C.; Greenberg, S.M.; Anderson, C.S.; Becker, K.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; Macdonald, R.L.; Mitchell, P.H.; et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2015, 46, 2032–2060. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.C.; Borlongan, C.V. Cell-based therapy in ischemic stroke. Expert. Rev. Neurother. 2008, 8, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Bersano, A.; Ballabio, E.; Lanfranconi, S.; Papadimitriou, D.; Strazzer, S.; Bresolin, N.; Comi, G.P.; Corti, S. Stem cell therapy in stroke. Cell. Mol. Life Sci. 2009, 66, 757–772. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, L.; Wu, Z.; Chen, Y.; Wang, F.; Chen, G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell 2014, 14, 188–202. [Google Scholar] [CrossRef]
- Liu, X.; Li, F.; Stubblefield, E.A.; Blanchard, B.; Richards, T.L.; Larson, G.A.; He, Y.; Huang, Q.; Tan, A.C.; Zhang, D.; et al. Direct reprogramming of human fibroblasts into dopaminergic neuron-like cells. Cell Res. 2012, 22, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Buller, B.; Katakowski, M.; Zhang, Y.; Wang, X.; Shang, X.; Zhang, Z.G.; Chopp, M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 2012, 30, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, T.L.; Zhang, H.J.; Gao, J.; Yang, P.F. A Promising Application of Injectable Hydrogels in Nerve Repair and Regeneration for Ischemic Stroke. Int. J. Nanomed. 2024, 19, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Sousa, R.A.; Salgado, A.J. Hydrogels as delivery systems for spinal cord injury regeneration. Mater. Today Bio 2021, 9, 100093. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Nhan, T.; Burgess, A.; Cho, E.E.; Stefanovic, B.; Lilge, L.; Hynynen, K. Drug delivery to the brain by focused ultrasound induced blood-brain barrier disruption: Quantitative evaluation of enhanced permeability of cerebral vasculature using two-photon microscopy. J. Control. Release 2013, 172, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, M.; Zhang, F. Applications of CRISPR-Cas systems in neuroscience. Nat. Rev. Neurosci. 2016, 17, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhai, P.; Chen, X.; Schreyer, D.J.; Sun, X.; Cui, F. Bioengineered scaffolds for spinal cord repair. Tissue Eng. Part B Rev. 2011, 17, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Horkay, F.; Basser, P.J. Hydrogel composite mimics biological tissues. Soft Matter 2022, 18, 4414–4426. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Khan, I.; Chen, J.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Emerging Fabrication Strategies of Hydrogels and Its Applications. Gels 2022, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef] [PubMed]
- Revete, A.; Aparicio, A.; Cisterna, B.A.; Revete, J.; Luis, L.; Ibarra, E.; Segura González, E.A.; Molino, J.; Reginensi, D. Advancements in the Use of Hydrogels for Regenerative Medicine: Properties and Biomedical Applications. Int. J. Biomater. 2022, 2022, 3606765. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Ucar, B. Natural biomaterials in brain repair: A focus on collagen. Neurochem. Int. 2021, 146, 105033. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.; Holloway, J.L.; Stabenfeldt, S.E. Hyaluronic Acid Biomaterials for Central Nervous System Regenerative Medicine. Cells 2020, 9, 2113. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Huang, A.P.; Lai, D.M.; Hsu, Y.H.; Tsai, H.H.; Su, C.Y.; Hsu, S.H. An anti-inflammatory gelatin hemostatic agent with biodegradable polyurethane nanoparticles for vulnerable brain tissue. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111799. [Google Scholar] [CrossRef]
- Xu, R.; Fang, Y.; Zhang, Z.; Cao, Y.; Yan, Y.; Gan, L.; Xu, J.; Zhou, G. Recent Advances in Biodegradable and Biocompatible Synthetic Polymers Used in Skin Wound Healing. Materials 2023, 16, 5459. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]
- Blaško, J.; Szekiova, E.; Slovinska, L.; Kafka, J.; Cizkova, D. Axonal outgrowth stimulation after alginate/mesenchymal stem cell therapy in injured rat spinal cord. Acta Neurobiol. Exp. 2017, 77, 337–350. [Google Scholar] [CrossRef]
- Yin, J.; Shi, C.; He, W.; Yan, W.; Deng, J.; Zhang, B.; Yin, M.; Pei, H.; Wang, H. Specific bio-functional CBD-PR1P peptide binding VEGF to collagen hydrogels promotes the recovery of cerebral ischemia in rats. J. Biomed. Mater. Res. A 2022, 110, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.S.; Ma, K.; Liang, B.; Liu, X.Y.; Xu, H.Y.; Zhang, J.; Shi, H.Y.; Sun, H.T.; Chen, X.Y.; Zhang, S. Collagen scaffold combined with human umbilical cord-mesenchymal stem cells transplantation for acute complete spinal cord injury. Neural Regen. Res. 2020, 15, 1686–1700. [Google Scholar] [CrossRef]
- Yan, J.; Huang, L.; Feng, J.; Yang, X. The Recent Applications of PLGA-Based Nanostructures for Ischemic Stroke. Pharmaceutics 2023, 15, 2322. [Google Scholar] [CrossRef]
- Lam, J.; Lowry, W.E.; Carmichael, S.T.; Segura, T. Delivery of iPS-NPCs to the Stroke Cavity within a Hyaluronic Acid Matrix Promotes the Differentiation of Transplanted Cells. Adv. Funct. Mater. 2014, 24, 7053–7062. [Google Scholar] [CrossRef] [PubMed]
- Horn, E.M.; Beaumont, M.; Shu, X.Z.; Harvey, A.; Prestwich, G.D.; Horn, K.M.; Gibson, A.R.; Preul, M.C.; Panitch, A. Influence of cross-linked hyaluronic acid hydrogels on neurite outgrowth and recovery from spinal cord injury. J. Neurosurg. Spine 2007, 6, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Ren, Y.; Cui, F.; Xu, Q. Viability and differentiation of neural precursors on hyaluronic acid hydrogel scaffold. J. Neurosci. Res. 2009, 87, 3207–3220. [Google Scholar] [CrossRef]
- Huang, H.C.; Hong, L.; Chang, P.; Zhang, J.; Lu, S.Y.; Zheng, B.W.; Jiang, Z.F. Chitooligosaccharides attenuate Cu2+-induced cellular oxidative damage and cell apoptosis involving Nrf2 activation. Neurotox. Res. 2015, 27, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Friend, N.E.; Beamish, J.A.; Margolis, E.A.; Schott, N.G.; Stegemann, J.P.; Putnam, A.J. Pre-cultured, cell-encapsulating fibrin microbeads for the vascularization of ischemic tissues. J. Biomed. Mater. Res. Part A 2024, 112, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Matsumine, H.; Sasaki, R.; Tabata, Y.; Matsui, M.; Yamato, M.; Okano, T.; Sakurai, H. Facial nerve regeneration using basic fibroblast growth factor-impregnated gelatin microspheres in a rat model. J. Tissue Eng. Regen. Med. 2016, 10, E559–E567. [Google Scholar] [CrossRef] [PubMed]
- Acciaretti, F.; Vesentini, S.; Cipolla, L. Fabrication Strategies towards Hydrogels for Biomedical Application: Chemical and Mechanical Insights. Chem. Asian J. 2022, 17, e202200797. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Recent advances in photo-crosslinkable hydrogels for biomedical applications. Biotechniques 2019, 66, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Mashabela, L.T.; Maboa, M.M.; Miya, N.F.; Ajayi, T.O.; Chasara, R.S.; Milne, M.; Mokhele, S.; Demana, P.H.; Witika, B.A.; Siwe-Noundou, X.; et al. A Comprehensive Review of Cross-Linked Gels as Vehicles for Drug Delivery to Treat Central Nervous System Disorders. Gels 2022, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Devi, V.K.A.; Shyam, R.; Palaniappan, A.; Jaiswal, A.K.; Oh, T.H.; Nathanael, A.J. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers 2021, 13, 3782. [Google Scholar] [CrossRef] [PubMed]
- Mehak;Singh, G.; Singh, R.; Stanzin, J.; Singh, H.; Kaur, G.; Singh, J. Clicking in harmony: Exploring the bio-orthogonal overlap in click chemistry. RSC Adv. 2024, 14, 7383–7413. [Google Scholar] [CrossRef] [PubMed]
- Chyzy, A.; Tomczykowa, M.; Plonska-Brzezinska, M.E. Hydrogels as Potential Nano-, Micro- and Macro-Scale Systems for Controlled Drug Delivery. Materials 2020, 13, 188. [Google Scholar] [CrossRef]
- Maiti, S.; Maji, B.; Yadav, H. Progress on green crosslinking of polysaccharide hydrogels for drug delivery and tissue engineering applications. Carbohydr. Polym. 2024, 326, 121584. [Google Scholar] [CrossRef]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Hirner, S.; Wiesbrock, F.; Fuchs, P. A Review on Modeling Cure Kinetics and Mechanisms of Photopolymerization. Polymers 2022, 14, 2074. [Google Scholar] [CrossRef] [PubMed]
- Corbin, D.A.; Miyake, G.M. Photoinduced Organocatalyzed Atom Transfer Radical Polymerization (O-ATRP): Precision Polymer Synthesis Using Organic Photoredox Catalysis. Chem. Rev. 2022, 122, 1830–1874. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, V.; Simonutti, R. New Light in Polymer Science: Photoinduced Reversible Addition-Fragmentation Chain Transfer Polymerization (PET-RAFT) as Innovative Strategy for the Synthesis of Advanced Materials. Polymers 2021, 13, 1119. [Google Scholar] [CrossRef] [PubMed]
- Audran, G.; Bagryanskaya, E.G.; Marque, S.R.A.; Postnikov, P. New Variants of Nitroxide Mediated Polymerization. Polymers 2020, 12, 1481. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.K.; Mehta, R.C.; DeLuca, P.P. Evaluation of a statistical model for the formation of poly [acryloyl hydroxyethyl starch] microspheres. Pharm. Res. 1997, 14, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.D.; Linhardt, R.J.; Dordick, J.S. Highly swelling hydrogels from ordered galactose-based polyacrylates. Biomaterials 1998, 19, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Jameela, S.R.; Jayakrishnan, A. Glutaraldehyde cross-linked chitosan microspheres as a long acting biodegradable drug delivery vehicle: Studies on the in vitro release of mitoxantrone and in vivo degradation of microspheres in rat muscle. Biomaterials 1995, 16, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, A.; Clochard, M.C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulański, P.; Al-Sheikhly, M. Polymerization Reactions and Modifications of Polymers by Ionizing Radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, M.; Borska, K.; Kubisa, P. Crosslinking of Polylactide by High Energy Irradiation and Photo-Curing. Molecules 2020, 25, 4919. [Google Scholar] [CrossRef]
- Caliceti, P.; Salmaso, S.; Lante, A.; Yoshida, M.; Katakai, R.; Martellini, F.; Mei, L.H.; Carenza, M. Controlled release of biomolecules from temperature-sensitive hydrogels prepared by radiation polymerization. J. Control. Release 2001, 75, 173–181. [Google Scholar] [CrossRef]
- Liu, J.; Su, C.; Chen, Y.; Tian, S.; Lu, C.; Huang, W.; Lv, Q. Current Understanding of the Applications of Photocrosslinked Hydrogels in Biomedical Engineering. Gels 2022, 8, 216. [Google Scholar] [CrossRef]
- Bai, Y.; Han, B.; Zhang, Y.; Cai, Y.; Shen, L.; Jia, Y. Advancements in Hydrogel Application for Ischemic Stroke Therapy. Gels 2022, 8, 777. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 1117–1150. [Google Scholar] [CrossRef] [PubMed]
- Correa, S.; Grosskopf, A.K.; Lopez Hernandez, H.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef]
- Lei, L.; Bai, Y.; Qin, X.; Liu, J.; Huang, W.; Lv, Q. Current Understanding of Hydrogel for Drug Release and Tissue Engineering. Gels 2022, 8, 301. [Google Scholar] [CrossRef]
- Guan, X.; Avci-Adali, M.; Alarçin, E.; Cheng, H.; Kashaf, S.S.; Li, Y.; Chawla, A.; Jang, H.L.; Khademhosseini, A. Development of hydrogels for regenerative engineering. Biotechnol. J. 2017, 12, 1600394. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Malcolm, D.W.; Benoit, D.S.W. Controlled and sustained delivery of siRNA/NPs from hydrogels expedites bone fracture healing. Biomaterials 2017, 139, 127–138. [Google Scholar] [CrossRef]
- Campea, M.A.; Lofts, A.; Xu, F.; Yeganeh, M.; Kostashuk, M.; Hoare, T. Disulfide-Cross-Linked Nanogel-Based Nanoassemblies for Chemotherapeutic Drug Delivery. ACS Appl. Mater. Interfaces 2023, 15, 25324–25338. [Google Scholar] [CrossRef]
- Yin, Y.; Papavasiliou, G.; Zaborina, O.Y.; Alverdy, J.C.; Teymour, F. De Novo Synthesis and Functional Analysis of Polyphosphate-Loaded Poly(Ethylene) Glycol Hydrogel Nanoparticles Targeting Pyocyanin and Pyoverdin Production in Pseudomonas aeruginosa as a Model Intestinal Pathogen. Ann. Biomed. Eng. 2017, 45, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Licht, C.; Rose, J.C.; Anarkoli, A.O.; Blondel, D.; Roccio, M.; Haraszti, T.; Gehlen, D.B.; Hubbell, J.A.; Lutolf, M.P.; De Laporte, L. Synthetic 3D PEG-Anisogel Tailored with Fibronectin Fragments Induce Aligned Nerve Extension. Biomacromolecules 2019, 20, 4075–4087. [Google Scholar] [CrossRef] [PubMed]
- Politrón-Zepeda, G.A.; Fletes-Vargas, G.; Rodríguez-Rodríguez, R. Injectable Hydrogels for Nervous Tissue Repair—A Brief Review. Gels 2024, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Ghane, N.; Beigi, M.H.; Labbaf, S.; Nasr-Esfahani, M.H.; Kiani, A. Design of hydrogel-based scaffolds for the treatment of spinal cord injuries. J. Mater. Chem. B 2020, 8, 10712–10738. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Velez, M.; Pai, S.B.; Bellamkonda, R.V. Hydrogels as carriers for stem cell transplantation. IEEE Trans. Biomed. Eng. 2014, 61, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- González-Nieto, D.; Fernández-García, L.; Pérez-Rigueiro, J.; Guinea, G.V.; Panetsos, F. Hydrogels-Assisted Cell Engraftment for Repairing the Stroke-Damaged Brain: Chimera or Reality. Polymers 2018, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Janas-Naze, A.; Zhang, W. Perioperative anaphylaxis to fibrin sealants in children with Noonan Syndrome: A retrospective study. Ann. Allergy Asthma Immunol. 2022, 129, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef]
- Hameed, H.; Faheem, S.; Paiva-Santos, A.C.; Sarwar, H.S.; Jamshaid, M. A Comprehensive Review of Hydrogel-Based Drug Delivery Systems: Classification, Properties, Recent Trends, and Applications. AAPS PharmSciTech 2024, 25, 64. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Mathew, A.P.; Uthaman, S.; Cho, K.H.; Cho, C.S.; Park, I.K. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromol. 2018, 110, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, E.; Schilling, A.L.; Little, S.R.; Decuzzi, P. Injectable thermoresponsive hydrogels as drug delivery system for the treatment of central nervous system disorders: A review. J. Control. Release 2021, 329, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Kim, I.Y.; Kim, I.D.; Lee, H.K.; Park, J.Y.; Han, P.L.; Kim, K.K.; Choi, H.; Lee, J.K. Biodegradable gelatin microspheres enhance the neuroprotective potency of osteopontin via quick and sustained release in the post-ischemic brain. Acta Biomater. 2014, 10, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Mungenast, L.; Züger, F.; Selvi, J.; Faia-Torres, A.B.; Rühe, J.; Suter-Dick, L.; Gullo, M.R. Directional Submicrofiber Hydrogel Composite Scaffolds Supporting Neuron Differentiation and Enabling Neurite Alignment. Int. J. Mol. Sci. 2022, 23, 11525. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.J.; Nguyen, C.; Chun, H.N.; L Llorente, I.; Chiu, A.S.; Machnicki, M.; Zarembinski, T.I.; Carmichael, S.T. Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J. Cereb. Blood Flow Metab. 2017, 37, 1030–1045. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cooke, M.J.; Morshead, C.M.; Shoichet, M.S. Hydrogel delivery of erythropoietin to the brain for endogenous stem cell stimulation after stroke injury. Biomaterials 2012, 33, 2681–2692. [Google Scholar] [CrossRef] [PubMed]
- Nakaguchi, K.; Jinnou, H.; Kaneko, N.; Sawada, M.; Hikita, T.; Saitoh, S.; Tabata, Y.; Sawamoto, K. Growth factors released from gelatin hydrogel microspheres increase new neurons in the adult mouse brain. Stem Cells Int. 2012, 2012, 915160. [Google Scholar] [CrossRef] [PubMed]
- Tuladhar, A.; Morshead, C.M.; Shoichet, M.S. Circumventing the blood-brain barrier: Local delivery of cyclosporin A stimulates stem cells in stroke-injured rat brain. J. Control. Release 2015, 215, 1–11. [Google Scholar] [CrossRef]
- Caicco, M.J.; Cooke, M.J.; Wang, Y.; Tuladhar, A.; Morshead, C.M.; Shoichet, M.S. A hydrogel composite system for sustained epi-cortical delivery of Cyclosporin A to the brain for treatment of stroke. J. Control. Release 2013, 166, 197–202. [Google Scholar] [CrossRef]
- González-Nieto, D.; Fernández-Serra, R.; Pérez-Rigueiro, J.; Panetsos, F.; Martinez-Murillo, R.; Guinea, G.V. Biomaterials to Neuroprotect the Stroke Brain: A Large Opportunity for Narrow Time Windows. Cells 2020, 9, 1074. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef]
- Yu, H.; Gao, R.; Liu, Y.; Fu, L.; Zhou, J.; Li, L. Stimulus-Responsive Hydrogels as Drug Delivery Systems for Inflammation Targeted Therapy. Adv Sci 2024, 11, e2306152. [Google Scholar] [CrossRef]
- Abe, K.; Yamashita, T.; Takizawa, S.; Kuroda, S.; Kinouchi, H.; Kawahara, N. Stem cell therapy for cerebral ischemia: From basic science to clinical applications. J. Cereb. Blood Flow Metab. 2012, 32, 1317–1331. [Google Scholar] [CrossRef]
- Imitola, J.; Park, K.I.; Teng, Y.D.; Nisim, S.; Lachyankar, M.; Ourednik, J.; Mueller, F.J.; Yiou, R.; Atala, A.; Sidman, R.L.; et al. Stem cells: Cross-talk and developmental programs. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2004, 359, 823–837. [Google Scholar] [CrossRef]
- Mutepfa, A.R.; Hardy, J.G.; Adams, C.F. Electroactive Scaffolds to Improve Neural Stem Cell Therapy for Spinal Cord Injury. Front. Med. Technol. 2022, 4, 693438. [Google Scholar] [CrossRef]
- Teng, Y.D.; Lavik, E.B.; Qu, X.; Park, K.I.; Ourednik, J.; Zurakowski, D.; Langer, R.; Snyder, E.Y. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc. Natl. Acad. Sci. USA 2002, 99, 3024–3029. [Google Scholar] [CrossRef]
- Bartosh, T.J.; Ylöstalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef]
- Frith, J.E.; Thomson, B.; Genever, P.G. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng. Part C Methods 2010, 16, 735–749. [Google Scholar] [CrossRef]
- Sart, S.; Tsai, A.C.; Li, Y.; Ma, T. Three-dimensional aggregates of mesenchymal stem cells: Cellular mechanisms, biological properties, and applications. Tissue Eng. Part B Rev. 2014, 20, 365–380. [Google Scholar] [CrossRef]
- Aref, A.; Horvath, R.; McColl, J.; Ramsden, J.J. Optical monitoring of stem cell-substratum interactions. J. Biomed. Opt. 2009, 14, 010501. [Google Scholar] [CrossRef]
- Kirouac, D.C.; Zandstra, P.W. The systematic production of cells for cell therapies. Cell Stem Cell 2008, 3, 369–381. [Google Scholar] [CrossRef]
- Li, R.; Xu, J.; Rao, Z.; Deng, R.; Xu, Y.; Qiu, S.; Long, H.; Zhu, Q.; Liu, X.; Bai, Y.; et al. Facilitate Angiogenesis and Neurogenesis by Growth Factors Integrated Decellularized Matrix Hydrogel. Tissue Eng. Part A 2021, 27, 771–787. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; O’Connor, M.; Wang, G.; Han, F. Brain-Derived Neurotrophic Factor and Its Potential Therapeutic Role in Stroke Comorbidities. Neural Plast. 2020, 2020, 1969482. [Google Scholar] [CrossRef]
- Obermeyer, J.M.; Tuladhar, A.; Payne, S.L.; Ho, E.; Morshead, C.M.; Shoichet, M.S. Local Delivery of Brain-Derived Neurotrophic Factor Enables Behavioral Recovery and Tissue Repair in Stroke-Injured Rats. Tissue Eng. Part A 2019, 25, 1175–1187. [Google Scholar] [CrossRef]
- Rust, R. Insights into the dual role of angiogenesis following stroke. J. Cereb. Blood Flow Metab. 2020, 40, 1167–1171. [Google Scholar] [CrossRef]
- Emerich, D.F.; Silva, E.; Ali, O.; Mooney, D.; Bell, W.; Yu, S.J.; Kaneko, Y.; Borlongan, C. Injectable VEGF hydrogels produce near complete neurological and anatomical protection following cerebral ischemia in rats. Cell Transplant. 2010, 19, 1063–1071. [Google Scholar] [CrossRef]
- Gu, C.; Li, Y.; Liu, J.; Liu, S.; Long, J.; Zhang, Q.; Duan, W.; Feng, T.; Huang, J.; Qiu, Y.; et al. Neural stem cell-derived exosomes-loaded adhesive hydrogel controlled-release promotes cerebral angiogenesis and neurological function in ischemic stroke. Exp. Neurol. 2023, 370, 114547. [Google Scholar] [CrossRef]
- Lai, T.W.; Zhang, S.; Wang, Y.T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 2014, 115, 157–188. [Google Scholar] [CrossRef]
- Buchan, A.M. Do NMDA antagonists protect against cerebral ischemia: Are clinical trials warranted? Cerebrovasc. Brain Metab. Rev. 1990, 2, 1–26. [Google Scholar]
- Chen, H.; Yoshioka, H.; Kim, G.S.; Jung, J.E.; Okami, N.; Sakata, H.; Maier, C.M.; Narasimhan, P.; Goeders, C.E.; Chan, P.H. Oxidative stress in ischemic brain damage: Mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid. Redox Signal 2011, 14, 1505–1517. [Google Scholar] [CrossRef]
- Shirley, R.; Ord, E.N.; Work, L.M. Oxidative Stress and the Use of Antioxidants in Stroke. Antioxidants 2014, 3, 472–501. [Google Scholar] [CrossRef]
- Perez-Araluce, M.; Jüngst, T.; Sanmartin, C.; Prosper, F.; Plano, D.; Mazo, M.M. Biomaterials-Based Antioxidant Strategies for the Treatment of Oxidative Stress Diseases. Biomimetics 2024, 9, 23. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Zhu, Y.X.; Bu, J.Y.; Li, G.W.; Zhou, J.H.; Zhou, S.P. Evaluation of Neuroprotective Effect of Thymoquinone Nanoformulation in the Rodent Cerebral Ischemia-Reperfusion Model. Biomed. Res. Int. 2016, 2016, 2571060. [Google Scholar] [CrossRef]
- Fang, M.; Zhong, L.; Jin, X.; Cui, R.; Yang, W.; Gao, S.; Lv, J.; Li, B.; Liu, T. Effect of Inflammation on the Process of Stroke Rehabilitation and Poststroke Depression. Front. Psychiatry 2019, 10, 184. [Google Scholar] [CrossRef]
- Shao, F.; Wang, X.; Wu, H.; Wu, Q.; Zhang, J. Microglia and Neuroinflammation: Crucial Pathological Mechanisms in Traumatic Brain Injury-Induced Neurodegeneration. Front. Aging Neurosci. 2022, 14, 825086. [Google Scholar] [CrossRef]
- Tu, Z.; Zhong, Y.; Hu, H.; Shao, D.; Haag, R.; Schirner, M.; Lee, J.; Sullenger, B.; Leong, K.W. Design of therapeutic biomaterials to control inflammation. Nat. Rev. Mater. 2022, 7, 557–574. [Google Scholar] [CrossRef]
- Fatoba, O.; Itokazu, T.; Yamashita, T. Microglia as therapeutic target in central nervous system disorders. J. Pharmacol. Sci. 2020, 144, 102–118. [Google Scholar] [CrossRef]
- Tamer, T.M.; Valachová, K.; Hassan, M.A.; Omer, A.M.; El-Shafeey, M.; Mohy Eldin, M.S.; Šoltés, L. Chitosan/hyaluronan/edaravone membranes for anti-inflammatory wound dressing: In vitro and in vivo evaluation studies. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 227–235. [Google Scholar] [CrossRef]
- Sarma, S.; Deka, D.J.; Rajak, P.; Laloo, D.; Das, T.; Chetia, P.; Saha, D.; Bharali, A.; Deka, B. Potential injectable hydrogels as biomaterials for central nervous system injury: A narrative review. Ibrain 2023, 9, 402–420. [Google Scholar] [CrossRef]
- Ju, R.; Wen, Y.; Gou, R.; Wang, Y.; Xu, Q. The experimental therapy on brain ischemia by improvement of local angiogenesis with tissue engineering in the mouse. Cell Transplant. 2014, 23 (Suppl. 1), S83–S95. [Google Scholar] [CrossRef]
- Tian, W.M.; Zhang, C.L.; Hou, S.P.; Yu, X.; Cui, F.Z.; Xu, Q.Y.; Sheng, S.L.; Cui, H.; Li, H.D. Hyaluronic acid hydrogel as Nogo-66 receptor antibody delivery system for the repairing of injured rat brain: In vitro. J. Control. Release 2005, 102, 13–22. [Google Scholar] [CrossRef]
- Dong, G.C.; Kuan, C.Y.; Subramaniam, S.; Zhao, J.Y.; Sivasubramaniam, S.; Chang, H.Y.; Lin, F.H. A potent inhibition of oxidative stress induced gene expression in neural cells by sustained ferulic acid release from chitosan based hydrogel. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 691–699. [Google Scholar] [CrossRef]
- Zhao, Y.; Gan, L.; Ren, L.; Lin, Y.; Ma, C.; Lin, X. Factors influencing the blood-brain barrier permeability. Brain Res. 2022, 1788, 147937. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Zhang, L.; Jiang, Q.; Zhang, R.; Davies, K.; Powers, C.; Bruggen, N.; Chopp, M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Investig. 2000, 106, 829–838. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Vegad, U.; Patel, M.; Khunt, D.; Zupančič, O.; Chauhan, S.; Paudel, A. pH stimuli-responsive hydrogels from non-cellulosic biopolymers for drug delivery. Front. Bioeng. Biotechnol. 2023, 11, 1270364. [Google Scholar] [CrossRef]
- Pasteuning-Vuhman, S.; de Jongh, R.; Timmers, A.; Pasterkamp, R.J. Towards Advanced iPSC-based Drug Development for Neurodegenerative Disease. Trends Mol. Med. 2021, 27, 263–279. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, Y.; Zhang, X.; Guo, S. Advances in hydrogels for stem cell therapy: Regulation mechanisms and tissue engineering applications. J. Mater. Chem. B 2022, 10, 5520–5536. [Google Scholar] [CrossRef]
- Thomas, J.M.; Louca, I.; Bolan, F.; Sava, O.R.; Allan, S.M.; Lawrence, C.B.; Pinteaux, E. Regenerative Potential of Hydrogels for Intracerebral Hemorrhage: Lessons from Ischemic Stroke and Traumatic Brain Injury Research. Adv. Healthc. Mater. 2021, 10, e2100455. [Google Scholar] [CrossRef]
- Magid-Bernstein, J.; Girard, R.; Polster, S.; Srinath, A.; Romanos, S.; Awad, I.A.; Sansing, L.H. Cerebral Hemorrhage: Pathophysiology, Treatment, and Future Directions. Circ. Res. 2022, 130, 1204–1229. [Google Scholar] [CrossRef]
- Sreekrishnan, A.; Leasure, A.C.; Shi, F.D.; Hwang, D.Y.; Schindler, J.L.; Petersen, N.H.; Gilmore, E.J.; Kamel, H.; Sansing, L.H.; Greer, D.M.; et al. Functional Improvement among Intracerebral Hemorrhage (ICH) Survivors up to 12 Months Post-injury. Neurocrit. Care 2017, 27, 326–333. [Google Scholar] [CrossRef]
- Gulati, D.; Dua, D.; Torbey, M.T. Hemostasis in Intracranial Hemorrhage. Front. Neurol. 2017, 8, 80. [Google Scholar] [CrossRef]
- Biswas, S.; Bhunia, B.K.; Janani, G.; Mandal, B.B. Silk Fibroin Based Formulations as Potential Hemostatic Agents. ACS Biomater. Sci. Eng. 2022, 8, 2654–2663. [Google Scholar] [CrossRef]
- Verbraeken, B.; Lammens, M.; Van Rompaey, V.; Ahmed, M.; Szewczyk, K.; Hermans, C.; Menovsky, T. Efficacy and histopathological effects of self-assembling peptides RADA16 and IEIK13 in neurosurgical hemostasis. Nanomedicine 2022, 40, 102485. [Google Scholar] [CrossRef]
- Ren, J.; Yin, X.; Chen, Y.; Su, H.; Wang, K.; Zhang, L.; Zhu, J.; Zhang, C. Alginate hydrogel-coated syringe needles for rapid haemostasis of vessel and viscera puncture. Biomaterials 2020, 249, 120019. [Google Scholar] [CrossRef]
- Love, C.J.; Kirschenbaum, D.; Selim, M.; Lo, E.H.; Rushing, E.; Spector, M.; Aguzzi, A. Observation of Collagen-Containing Lesions after Hematoma Resolution in Intracerebral Hemorrhage. Stroke 2021, 52, 1856–1860. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, A.; Wu, J.; Wan, Y.; You, M.; Gu, X.; Guo, H.; Tan, S.; He, Q.; Hu, B. Nanomedicine: An Emerging Novel Therapeutic Strategy for Hemorrhagic Stroke. Int. J. Nanomed. 2022, 17, 1927–1950. [Google Scholar] [CrossRef]
- Del Verme, J.; Conti, C.; Guida, F. Use of gelatin hemostatic matrices in patients with intraparenchymal hemorrhage and drug-induced coagulopathy. J. Neurosurg. Sci. 2019, 63, 737–742. [Google Scholar] [CrossRef]
- Overman, J.J.; Clarkson, A.N.; Wanner, I.B.; Overman, W.T.; Eckstein, I.; Maguire, J.L.; Dinov, I.D.; Toga, A.W.; Carmichael, S.T. A role for ephrin-A5 in axonal sprouting, recovery, and activity-dependent plasticity after stroke. Proc. Natl. Acad. Sci. USA 2012, 109, E2230–E2239. [Google Scholar] [CrossRef]
- Nih, L.R.; Sideris, E.; Carmichael, S.T.; Segura, T. Injection of Microporous Annealing Particle (MAP) Hydrogels in the Stroke Cavity Reduces Gliosis and Inflammation and Promotes NPC Migration to the Lesion. Adv. Mater. 2017, 29, 1606471. [Google Scholar] [CrossRef]
- Tesar, B.M.; Jiang, D.; Liang, J.; Palmer, S.M.; Noble, P.W.; Goldstein, D.R. The role of hyaluronan degradation products as innate alloimmune agonists. Am. J. Transplant. 2006, 6, 2622–2635. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A.; Tuohy, T.M.; Chen, H.; Wallingford, N.; Craig, A.; Struve, J.; Luo, N.L.; Banine, F.; Liu, Y.; Chang, A.; et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat. Med. 2005, 11, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Cargill, R.; Kohama, S.G.; Struve, J.; Su, W.; Banine, F.; Witkowski, E.; Back, S.A.; Sherman, L.S. Astrocytes in aged nonhuman primate brain gray matter synthesize excess hyaluronan. Neurobiol. Aging 2012, 33, 830.e813–830.e824. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.J.; Johnston, J.; Datar, I.; Sewalt, V.; Holmes, D.; Shatkin, J.A. Food safety considerations and research priorities for the cultured meat and seafood industry. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5421–5448. [Google Scholar] [CrossRef] [PubMed]
- Skop, N.B.; Calderon, F.; Cho, C.H.; Gandhi, C.D.; Levison, S.W. Improvements in biomaterial matrices for neural precursor cell transplantation. Mol. Cell. Ther. 2014, 2, 19. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Woung, L.C.; Yen, J.C.; Tseng, P.C.; Chiou, S.H.; Sung, Y.J.; Liu, K.T.; Cheng, Y.H. Thermosensitive chitosan-based hydrogels for sustained release of ferulic acid on corneal wound healing. Carbohydr. Polym. 2016, 135, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Mao, X.; Xie, L.; Greenberg, R.B.; Peng, B.; Moore, A.; Greenberg, M.B.; Greenberg, D.A. Delayed transplantation of human neural precursor cells improves outcome from focal cerebral ischemia in aged rats. Aging Cell 2010, 9, 1076–1083. [Google Scholar] [CrossRef]
- Sanz-Horta, R.; Matesanz, A.; Gallardo, A.; Reinecke, H.; Jorcano, J.L.; Acedo, P.; Velasco, D.; Elvira, C. Technological advances in fibrin for tissue engineering. J. Tissue Eng. 2023, 14, 20417314231190288. [Google Scholar] [CrossRef]
- Hofmann, S.; Foo, C.T.; Rossetti, F.; Textor, M.; Vunjak-Novakovic, G.; Kaplan, D.L.; Merkle, H.P.; Meinel, L. Silk fibroin as an organic polymer for controlled drug delivery. J. Control. Release 2006, 111, 219–227. [Google Scholar] [CrossRef]
- Thurber, A.E.; Omenetto, F.G.; Kaplan, D.L. In vivo bioresponses to silk proteins. Biomaterials 2015, 71, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P. Biomaterials for Drug Delivery and Human Applications. Materials 2024, 17, 456. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; González-Payo, J.; Fusaro, L.; Ramella, M.; Boccafoschi, F. Natural hydrogels R&D process: Technical and regulatory aspects for industrial implementation. J. Mater. Sci. Mater. Med. 2020, 31, 64. [Google Scholar] [CrossRef] [PubMed]
- Nikolakopoulou, P.; Rauti, R.; Voulgaris, D.; Shlomy, I.; Maoz, B.M.; Herland, A. Recent progress in translational engineered in vitro models of the central nervous system. Brain 2020, 143, 3181–3213. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Petcu, E.B.; Capitanescu, B.; Hermann, D.M.; Radu, E.; Gresita, A. Ageing as a risk factor for cerebral ischemia: Underlying mechanisms and therapy in animal models and in the clinic. Mech. Ageing Dev. 2020, 190, 111312. [Google Scholar] [CrossRef]
| Hydrogel Constituent Polymers | Specific Molecules Included | Role in Ischemic Stroke |
|---|---|---|
| Alginate | Alginate, calcium ions | Cell delivery, tissue repair, and targeted drug release for inflammation and oxidative stress [57,95] |
| Collagen | Collagen, growth factors (e.g., BDNF) | Cell adhesion, promotes neurogenesis and angiogenesis, supports tissue repair [96,97] |
| PLGA (poly(lactic-co-glycolic acid)) | PLGA, anti-inflammatory agents (e.g., dexamethasone) | Controlled release of anti-inflammatory and neuroprotective agents, supports neural regeneration [98] |
| Hyaluronic acid | Hyaluronic acid, extracellular matrix proteins (e.g., fibronectin) | Mimics the ECM, promotes cell proliferation and migration, delivers neuroprotective agents [99,100,101] |
| PEG (polyethylene glycol) | PEG, neurotrophic factors (e.g., VEGF) | Sustained release of therapeutic agents, enhances neuroprotection and tissue repair |
| Chitosan | Chitosan, antioxidants (e.g., Vitamin C) | Reduces oxidative stress, supports neuroprotection, and enhances tissue repair [102] |
| Fibrin | Fibrin, peptides (e.g., RGD peptide) | Enhances cell adhesion and tissue repair, supports neuroprotection [103] |
| Gelatin | Gelatin, anti-inflammatory agents | Delivers anti-inflammatory agents, supports cell proliferation and neuroprotection [104] |
| Hydrogel Type | Mechanism of Action | Advantages | Disadvantages |
|---|---|---|---|
| Alginate [197] | Hemostasis, supports tissue repair, delivers anti-inflammatory agents | Biocompatible, easy to gel, promotes wound healing | Potential immunogenicity, limited mechanical strength |
| Collagen [198] | Structural support, promotes tissue regeneration, delivers growth factors | Promotes cell adhesion, supports neurogenesis and angiogenesis | Expensive, risk of disease transmission (animal-derived) |
| PLGA (Poly(lactic-co-glycolic acid)) [98] | Controlled drug release, supports tissue healing | Biodegradable, tunable degradation rates, versatile | Acidic degradation products, potential inflammation |
| Hyaluronic Acid [191] | Mimics the extracellular matrix, reduces scarring, delivers neuroprotective agents | Hydrophilic, promotes cell proliferation and migration | Rapid degradation, requires chemical modification |
| PEG (Polyethylene Glycol) [191] | Reduces inflammation, supports tissue healing, delivers hemostatic agents | Non-immunogenic, easily modifiable, good biocompatibility | Non-biodegradable, potential for long-term persistence |
| Chitosan [199] | Structural support, reduces oxidative stress, promotes tissue regeneration | Antimicrobial, biodegradable, promotes wound healing | Variable purity, potential for allergic reactions |
| Fibrin [191] | Hemostasis, supports tissue regeneration, delivers therapeutic agents | Biocompatible, promotes cell adhesion and migration | Rapid degradation, risk of thrombosis |
| Gelatin [200] | Structural support, reduces inflammation, promotes tissue repair | Biocompatible, supports cell proliferation, inexpensive | Risk of immune response, limited mechanical strength |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotaru-Zăvăleanu, A.-D.; Dinescu, V.C.; Aldea, M.; Gresita, A. Hydrogel-Based Therapies for Ischemic and Hemorrhagic Stroke: A Comprehensive Review. Gels 2024, 10, 476. https://doi.org/10.3390/gels10070476
Rotaru-Zăvăleanu A-D, Dinescu VC, Aldea M, Gresita A. Hydrogel-Based Therapies for Ischemic and Hemorrhagic Stroke: A Comprehensive Review. Gels. 2024; 10(7):476. https://doi.org/10.3390/gels10070476
Chicago/Turabian StyleRotaru-Zăvăleanu, Alexandra-Daniela, Venera Cristina Dinescu, Madalina Aldea, and Andrei Gresita. 2024. "Hydrogel-Based Therapies for Ischemic and Hemorrhagic Stroke: A Comprehensive Review" Gels 10, no. 7: 476. https://doi.org/10.3390/gels10070476





