Comprehensive Biosafety Profile of Carbomer-Based Hydrogel Formulations Incorporating Phosphorus Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of Gelling Agent for the Preparation of the Hydrogels Containing a Caffeine-Phosphorus Derivative Mixture
2.2. Characterization of Carbomer-Based Hydrogel Formulations
2.2.1. Determination of Macroscopic Properties and pH
2.2.2. Rheological Measurements
2.2.3. Spreadability Test
2.2.4. Scanning Electron Microscopy Analysis
2.3. In Vitro Toxicological Screening of Carbomer-Based Hydrogel Formulations
2.3.1. Cell Viability Assay
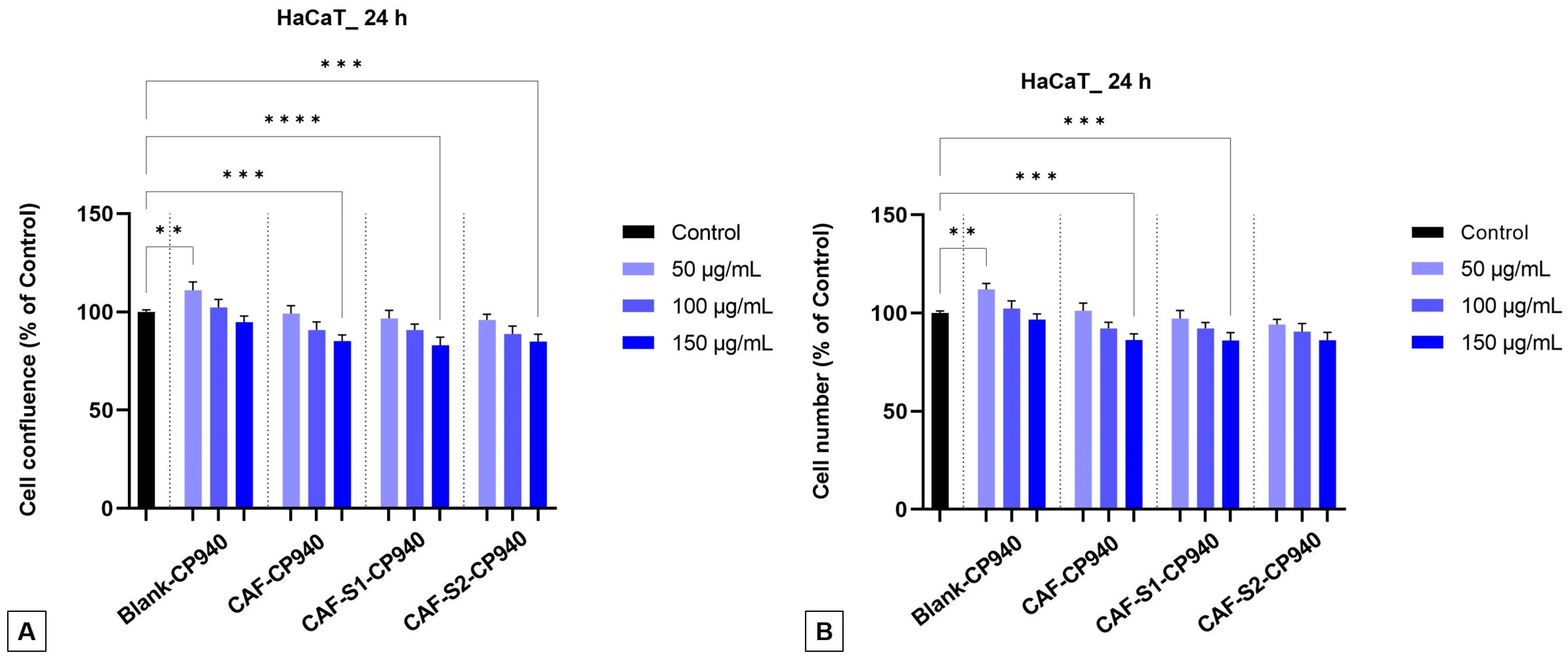
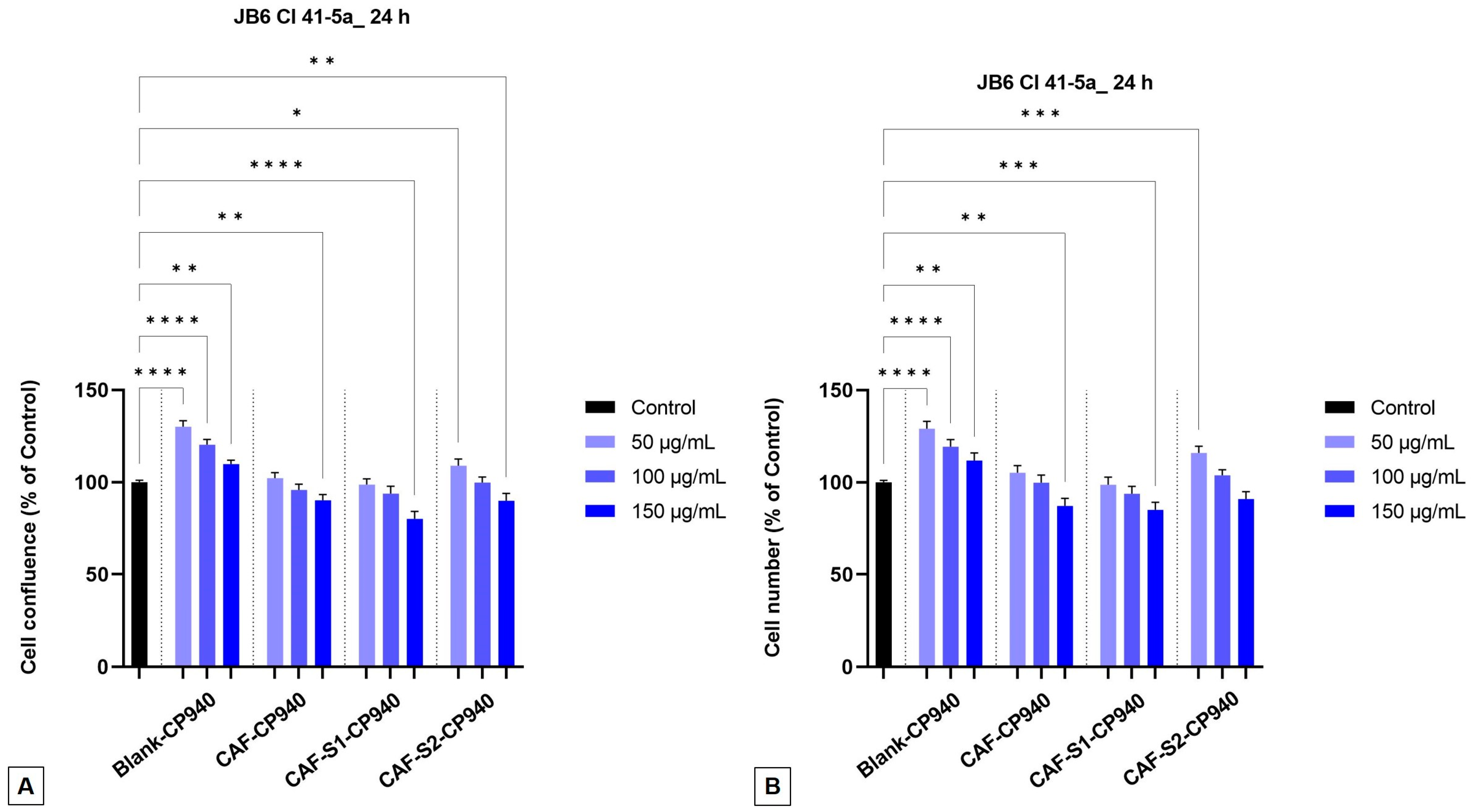
2.3.2. Cellular Morphology, Confluence, and Cell Number Evaluation
2.3.3. Cytotoxicity Assay
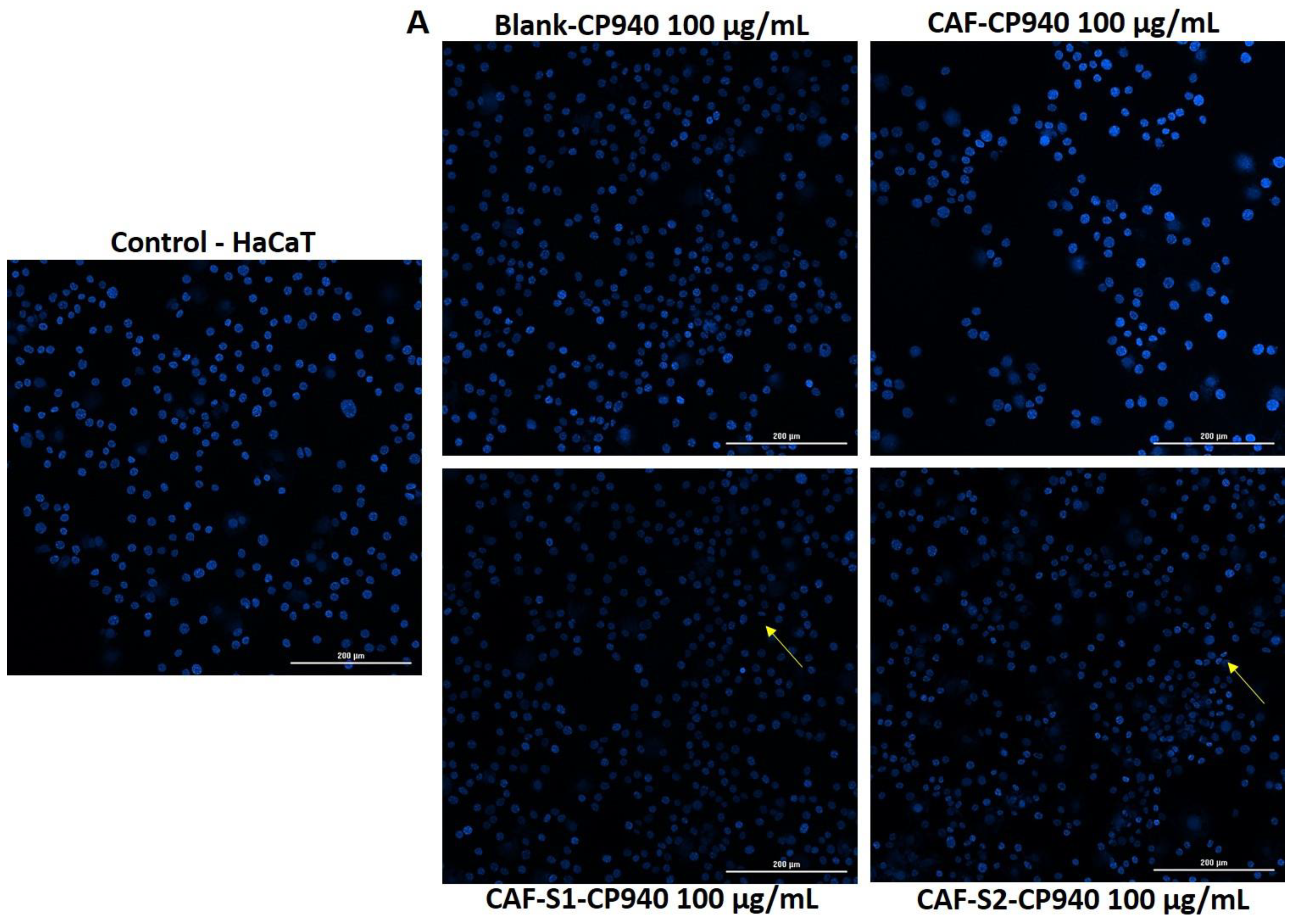
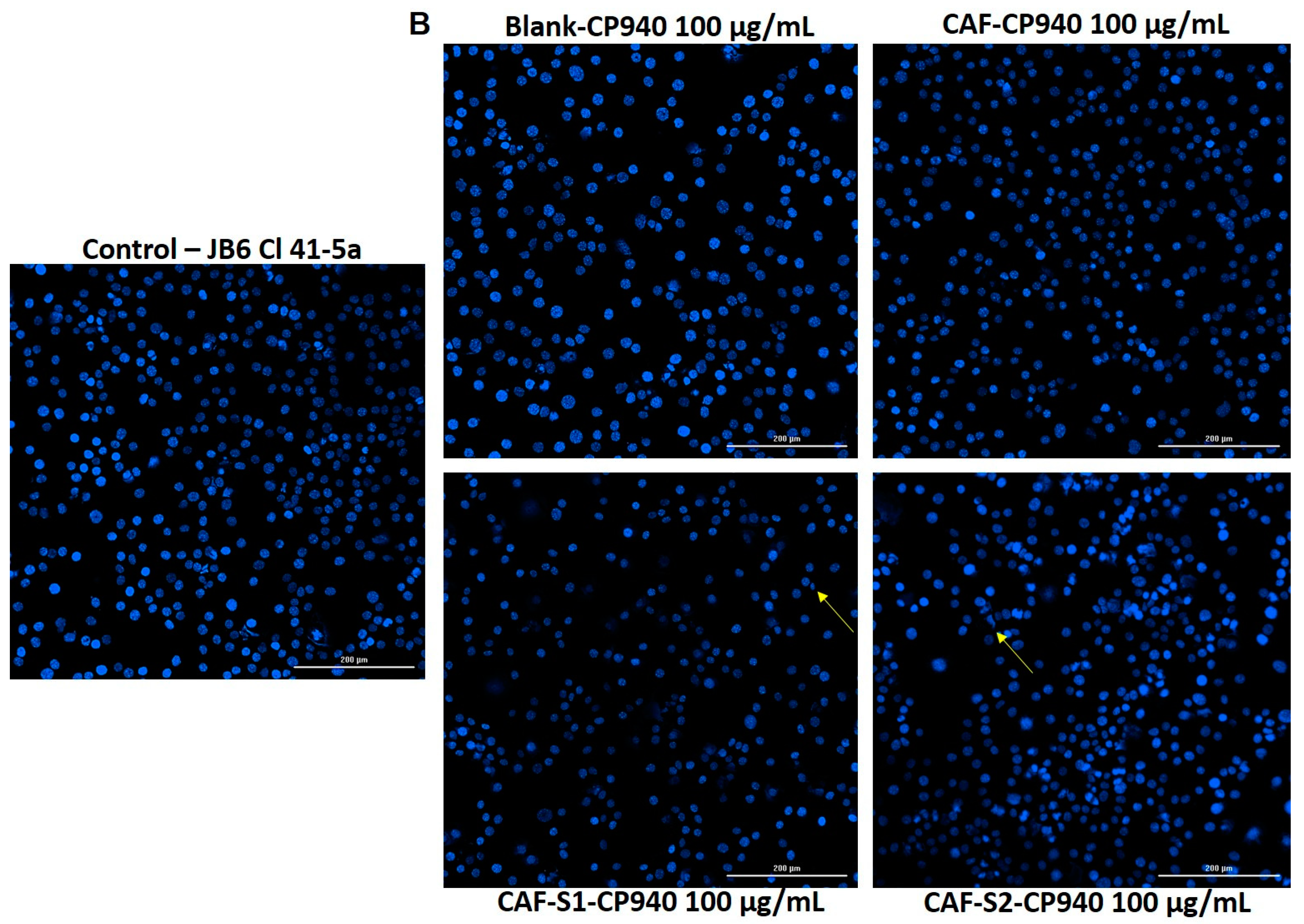
2.3.4. Hoechst Staining
2.4. Gene Expression
2.5. In Ovo Toxicological Screening of Carbomer-Based Hydrogel Formulations
2.6. In Vivo Toxicological Screening of Carbomer-Based Hydrogel Formulations
3. Conclusions
4. Materials and Methods
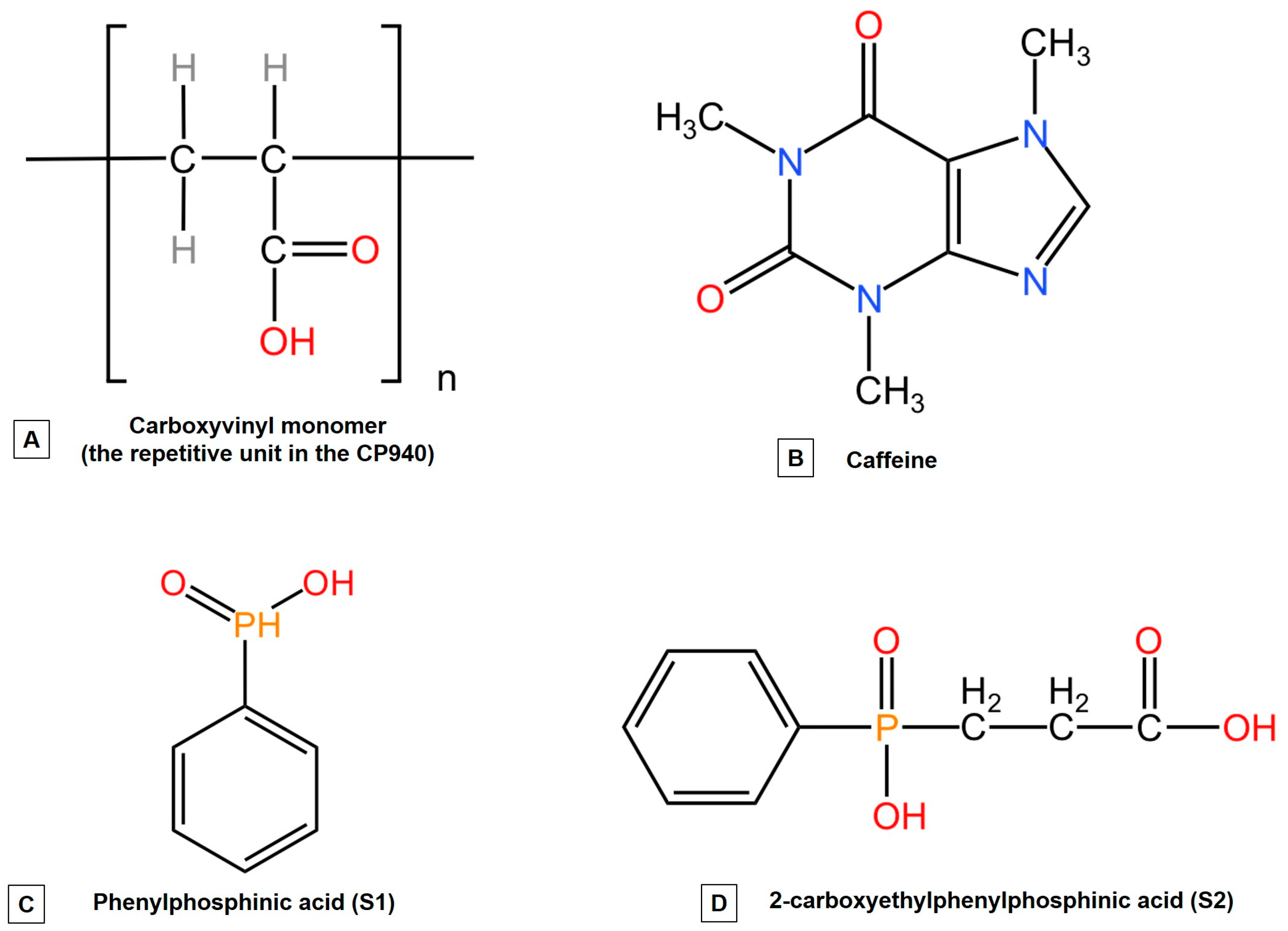
4.1. Materials
4.2. Animals
4.3. Methods
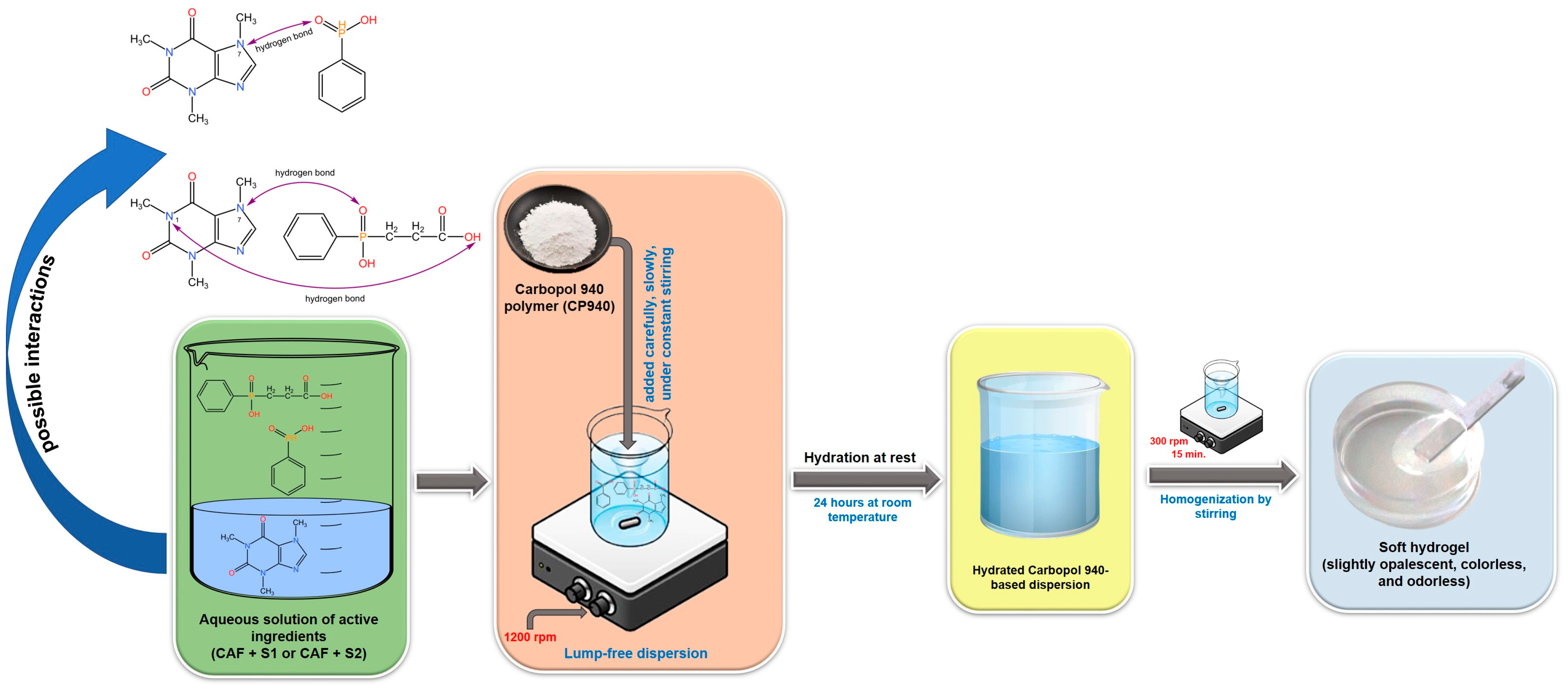
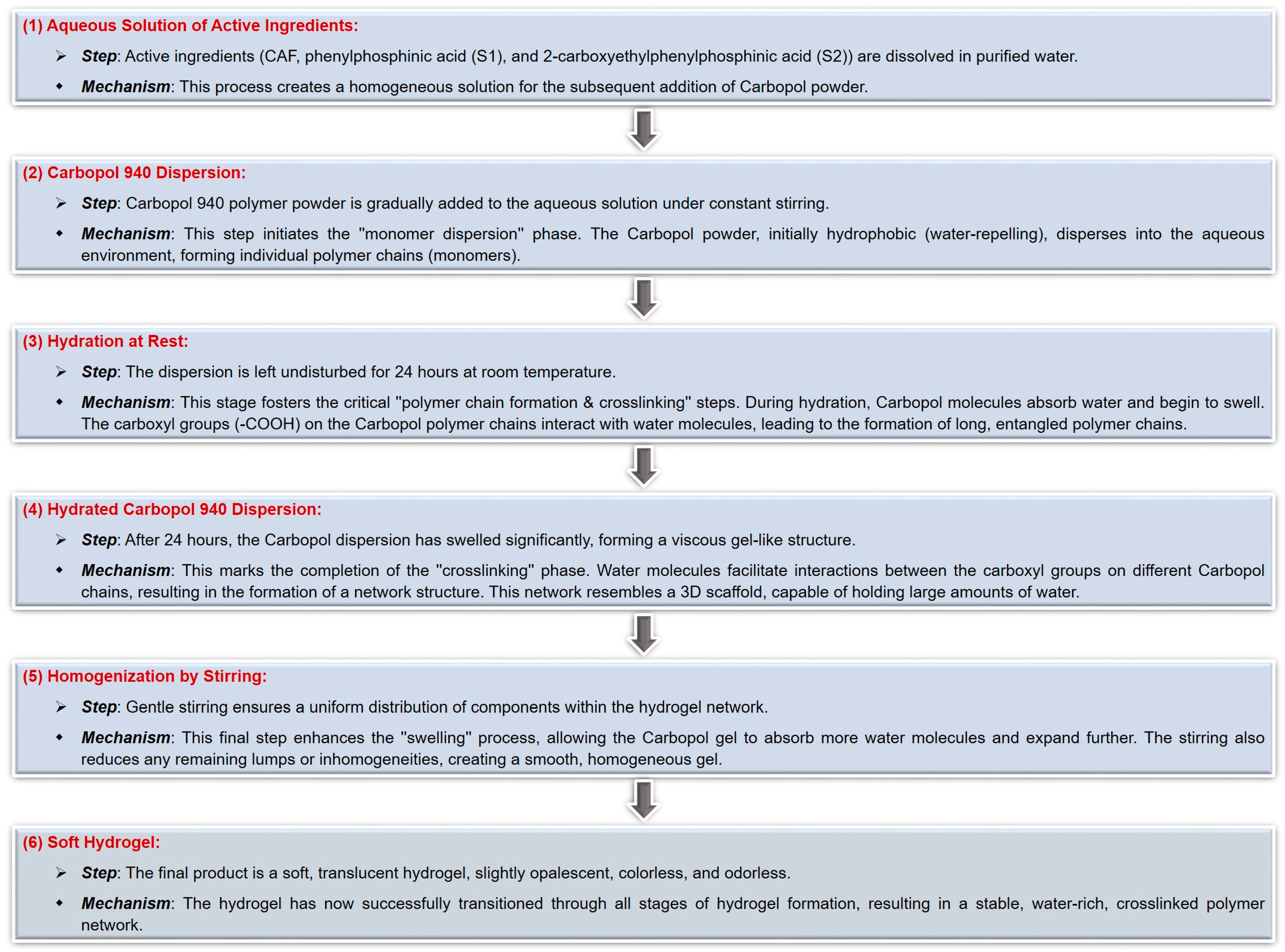
4.3.1. The Synthesis of Carbomer-Based Hydrogel Formulations
4.3.2. Characterization of Carbomer-Based Hydrogel Formulations
Determination of Macroscopic Properties and pH
Rheological Measurements
Spreadability Test
Scanning Electron Microscopy (SEM Analysis)
4.3.3. In Vitro Assays
Cell Viability Test
Cell Morphology, Confluence, and Number Evaluation
Cytotoxicity Assay via the LDH Release Method
Hoechst Nuclear Staining
4.3.4. RT-qPCR
RNA Extraction and Quantification
cDNA Synthesis and RT-qPCR
Gene Targets and Analysis
4.3.5. In Ovo Assay
4.3.6. Skin Biophysical Parameters Assessment
4.3.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartford, C.G.; Petchel, K.S.; Mickail, H.; Perez-Gutthann, S.; McHale, M.; Grana, J.M.; Marquez, P. Pharmacovigilance during the pre-approval phases: An evolving pharmaceutical industry model in response to ICH E2E, CIOMS VI, FDA, and EMEA/CHMP risk-management guidelines. Drug Saf. 2006, 29, 657–673. [Google Scholar] [CrossRef]
- ICH. E2E Pharmacovigilance Planning, Current Step 4 Version. 2004. Available online: https://database.ich.org/sites/default/files/E2E_Guideline.pdf (accessed on 15 June 2024).
- CIOMS Working Group VI. Management of Safety Information from Clinical Trials. 2005. Available online: https://cioms.ch/wp-content/uploads/2017/01/Mgment_Safety_Info.pdf (accessed on 15 June 2024).
- ICH. History. 2021. Available online: https://www.ich.org/page/history (accessed on 17 June 2024).
- CIOMS. Our History. 2021. Available online: https://cioms.ch/history/ (accessed on 17 June 2024).
- CIOMS. Pharmacovigilance. 2021. Available online: https://cioms.ch/pharmacovigilance/ (accessed on 15 June 2024).
- Krewski, D.; Acosta, D., Jr.; Andersen, M.; Anderson, H.; Bailar, J.C., III; Boekelheide, K.; Brent, R.; Charnley, G.; Cheung, V.G.; Green, S., Jr.; et al. Toxicity testing in the 21st century: A vision and a strategy. J. Toxicol. Environ. Health B Crit. Rev. 2010, 13, 51–138. [Google Scholar] [CrossRef]
- Parasuraman, S. Toxicological screening. J. Pharmacol. Pharmacother. 2011, 2, 74–79. [Google Scholar]
- Thorpe, A.A.; Freeman, C.; Farthing, P.; Callaghan, J.; Hatton, P.V.; Brook, I.M.; Sammon, C.; Le Maitre, C.L. In Vivo safety and efficacy testing of a thermally triggered injectable hydrogel scaffold for bone regeneration and augmentation in a rat model. Oncotarget 2018, 9, 18277–18295. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Engel, K.; Fowler, P.; Fürst, P.; Gürtler, R.; Gundert-Remy, U.; Husøy, T.; Manco, M.; et al. Safety evaluation of crosslinked polyacrylic acid polymers (carbomer) as a new food additive. Efsa J. 2021, 19, 6693–6719. [Google Scholar]
- Tunesi, M.; Prina, E.; Munarin, F.; Rodilossi, S.; Albani, D.; Petrini, P.; Giordano, C. Cross-linked poly(acrylic acids) microgels and agarose as semi-interpenetrating networks for resveratrol release. J. Mater. Sci. Mater. Med. 2015, 26, 5328. [Google Scholar] [CrossRef] [PubMed]
- Parente, M.E.; Andrade, A.O.; Ares, G.; Russo, F.; Jiménez-Kairuz, Á. Bioadhesive hydrogels for cosmetic applications. Int. J. Cosmet. Sci. 2015, 37, 511–518. [Google Scholar] [CrossRef]
- Yarema, I.; Fedorovska, M.; Polovko, N. Development of the emulgel for the androgenic alopecia treatment. Eureka Health Sci. 2020, 5, 82–91. [Google Scholar] [CrossRef]
- Çağlar, E.; Güven, G.; Okur, N. Preparation and characterization of carbopol-based hydrogels containing dexpanthenol. Ank. Univ. Eczaci Fak. Derg. 2023, 47, 770–783. [Google Scholar] [CrossRef]
- Martinović, M.; Stojanovic, N.; Nešić, I. Textural and sensory characterization of carbomeric gels with panthenol. Acta Fac. Med. Naissensis 2022, 39, 232–243. [Google Scholar] [CrossRef]
- Islam, M.T.; Rodríguez-Hornedo, N.; Ciotti, S.; Ackermann, C. Rheological characterization of topical carbomer gels neutralized to different ph. Pharm. Res. 2004, 21, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Osipitan, O.; Sulicz, E.; Pasqua, A. Preparation and optimization of an ultra-flexible liposomal gel for lidocaine transdermal delivery. Materials 2022, 15, 4895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, T.; Bi, J.; Zheng, Y.; Lu, C.; Hui, Q.; Wang, X.; Lin, X. Long-term toxicity study of topical administration of a highly-stable carbomer 940 hydrogel in a rabbit skin wound model. Front. Pharmacol. 2020, 11, 58. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, A.; Soto-Zarazúa, G.; Toledano-Ayala, M.; García-Trejo, J. A potential alternative for agar in In Vitro culture media based on hydrocolloids present. In Recent Research and Advances in Soilless Culture; Metin, T., Sanem, A., Ertan, Y., Adem, G., Eds.; IntechOpen: London, UK, 2023. [Google Scholar]
- Palanyandy, S.R.; Gantait, S.; Sinniah, U.R. Effects of some gelling agents and their concentrations on conversion of oil palm polyembryoids into plantlets. J. Genet. Eng. Biotechnol. 2020, 18, 5. [Google Scholar] [CrossRef]
- Du, X.; Gao, N.; Song, X. Bioadhesive polymer/lipid hybrid nanoparticles as oral delivery system of raloxifene with enhancive intestinal retention and bioavailability. Drug Deliv. 2021, 28, 252–260. [Google Scholar] [CrossRef]
- de Oliveira, É.L.; Ferreira, S.B.S.; de Castro-Hoshino, L.V.; Campanholi, K.d.S.S.; Calori, I.R.; de Morais, F.A.P.; Kimura, E.; da Silva, R.C., Jr.; Bruschi, M.L.; Sato, F.; et al. Thermoresponsive hydrogel-loading aluminum chloride phthalocyanine as a drug release platform for topical administration in photodynamic therapy. Langmuir 2021, 37, 3202–3213. [Google Scholar] [CrossRef] [PubMed]
- Rahmi, H.; Supandi, S.; Radjab, N.; Julianti, T. Tyrosinase inhibition from green tea (Camellia sinensis (l.) kuntze) gel. Indones. J. Pharm. Sci. Technol. 2021, 8, 59. [Google Scholar] [CrossRef]
- Ayuningtyas, N.; Sudarsono, A.; Yuswanti, A. Formulation of toothpaste toothpaste gel essential oil of lime leaves (citrus aurantifolia) with variations concentration of carbomer 940 as gelling agent base. J. Farm. Sains Indones. 2021, 4, 98–103. [Google Scholar] [CrossRef]
- Fitriani, L.; Afifah; Ismed, F.; Bakhtiar, A. Hydrogel formulation of usnic acid and antibacterial activity test against propionibacterium acne. Sci. Pharm. 2018, 87, 1. [Google Scholar] [CrossRef]
- Barar, J.; Aghanejad, A.; Fathi, M.; Omidi, Y. Advanced drug delivery and targeting technologies for the ocular diseases. Bioimpacts 2016, 6, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Azrifitria, A.; Purwaningsih, S.; Fatmala, A. In Vitro antimicrobial activity and formulation of herbal anti-acne gel containing rhizophora stylosa fruits extract. Pharmaciana 2022, 12, 309. [Google Scholar] [CrossRef]
- Limpongsa, E.; Tabboon, P.; Tuntiyasawasdikul, S.; Sripanidkulchai, B.; Pongjanyakul, T.; Jaipakdee, N. Formulation and In Vitro evaluation of mucoadhesive sustained release gels of phytoestrogen diarylheptanoids from Curcuma comosa for vaginal delivery. Pharmaceutics 2023, 15, 264. [Google Scholar] [CrossRef]
- Rezaei, S.; Imani, R. Highly Absorbent Egg White/Carbomer-940 Hydrofilm as a Potential Diabetic Wound Dressing. Macromol. Biosci. 2024, 24, e2300353. [Google Scholar] [CrossRef]
- Araújo, D.; Rodrigues, T.; Alves, V.; Freitas, F. Chitin-glucan complex hydrogels: Optimization of gel formation and demonstration of drug loading and release ability. Polymers 2022, 14, 785. [Google Scholar] [CrossRef]
- Barcellona, M.; Johnson, N.; Bernards, M. Characterizing drug release from non-fouling polyampholyte hydrogels. Langmuir 2015, 31, 13402–13409. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Lin, D.; Zhang, W.; Chen, S.; Ding, H.; Zhong, H.-J. Progress in the preparation and application of inulin-based hydrogels. Polymers 2024, 16, 1492. [Google Scholar] [CrossRef] [PubMed]
- Chern, J.; Lee, W.; Hsieh, M. Absorption isotherm of caffeine and release kinetics from swollen nipaam hydrogels: Experiments and modeling. Ind. Eng. Chem. Res. 2004, 43, 6150–6156. [Google Scholar] [CrossRef]
- Kodadová, A.; Vitková, Z.; Herdová, P.; Ťažký, A.; Oremusová, J.; Grančai, D.; Mikuš, P. Formulation of sage essential oil (Salvia officinalis, L.) monoterpenes into chitosan hydrogels and permeation study with gc-ms analysis. Drug Dev. Ind. Pharm. 2014, 41, 1080–1088. [Google Scholar] [CrossRef]
- Luo, L.; Lane, M. Topical and transdermal delivery of caffeine. Int. J. Pharm. 2015, 490, 155–164. [Google Scholar] [CrossRef]
- Narayanan, A.S.F.; Babu, G.; Nandana, V.; Raji, M.; Abraham, A.; Babu, N. Formulation and evaluation of skin-invigorating caffeine face mask. Int. J. Pharm. Sci. Rev. Res. 2022, 75, 86–89. [Google Scholar] [CrossRef]
- Dong, Z.; Liang, Y.; Fan, F.; Ye, J.; Zheng, X.; Lu, J. Adsorption behavior of the catechins and caffeine onto polyvinylpolypyrrolidone. J. Agric. Food Chem. 2011, 59, 4238–4247. [Google Scholar] [CrossRef] [PubMed]
- Rosado, C.; Tokunaga, V.K.; Sauce, R.; de Oliveira, C.A.; Sarruf, F.D.; Parise-Filho, R.; Maurício, E.; de Almeida, T.S.; Velasco, M.V.R.; Baby, A.R. Another reason for using caffeine in dermocosmetics: Sunscreen adjuvant. Front. Physiol. 2019, 10, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Alsamad, F.; Stamatas, G.N. Directional assessment of the skin barrier function In Vivo. Ski. Res. Technol. 2023, 29, e13346. [Google Scholar] [CrossRef] [PubMed]
- Correa, M.; Font, L. Is there a major role for adenosine a2a receptors in anxiety? Front. Biosci. Elite 2008, 13, 4058. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Szafranska, K.; Kruse, L.; Holte, C.; Wolfson, D.L.; Ahluwalia, B.S.; Whitchurch, C.B.; Cole, L.; Lockwood, G.P.; Diekmann, R.; et al. Effect of caffeine and other xanthines on liver sinusoidal endothelial cell ultrastructure. Sci. Rep. 2023, 13, 13390. [Google Scholar] [CrossRef] [PubMed]
- Quin, L.D. A Case for August Wilhelm Hofmann as the Originator of the Field of Organophosphorus Chemistry. Heteroat. Chem. 2013, 24, 243–251. [Google Scholar] [CrossRef]
- Collinsova, M.; Jiracek, J. Phosphinic Acid Compounds in Biochemistry, Biology and Medicine. Curr. Med. Chem. 2012, 7, 629–647. [Google Scholar] [CrossRef] [PubMed]
- Abdou, M.M.; O’Neill, P.M.; Amigues, E.; Matziari, M. Phosphinic Acids: Current Status and Potential for Drug Discovery. Drug Discov. Today 2019, 24, 916–929. [Google Scholar] [CrossRef]
- Kloda, M.; Ondrušová, S.; Lang, K.; Demel, J. Phosphinic Acids as Building Units in Materials Chemistry. Coord. Chem. Rev. 2021, 433, 213748. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. A Review of Recent Progress in Phosphorus-Based Flame Retardants. J. Fire Sci. 2006, 24, 345–364. [Google Scholar] [CrossRef]
- Shearan, S.J.I.; Stock, N.; Emmerling, F.; Demel, J.; Wright, P.A.; Demadis, K.D.; Vassaki, M.; Costantino, F.; Vivani, R.; Sallard, S.; et al. New Directions in Metal Phosphonate and Phosphinate Chemistry. Crystals 2019, 9, 270. [Google Scholar] [CrossRef]
- McCann, M.; Murphy, E.; Cardin, C.; Convery, M. Synthesis and Properties of Tetra-μ-Acetatodiruthenium(II,III) Phenylphosphinate and Phenylphosphonate Complexes: X-ray Crystal Structures of [Ru2(μ-O2CCH3)4(HPhPO2)2]H and [Ru2(μ-O2CCH3)4(PhPO3H)2]H·H2O. Polyhedron 1993, 12, 1725–1731. [Google Scholar] [CrossRef]
- Birum, G.H.; Jansen, R.F. Production of 2-Carboxyethyl(phenyl)phosphinic Acid. U.S. Patent 4081463, 28 March 1978. [Google Scholar]
- Bazaga-García, M.; Vílchez-Cózar, Á.; Maranescu, B.; Olivera-Pastor, P.; Marganovici, M.; Ilia, G.; Cabeza Díaz, A.; Visa, A.; Colodrero, R.M.P. Synthesis and Electrochemical Properties of Metal(Ii)-Carboxyethylphenylphosphinates. Dalton Trans. 2021, 50, 6539–6548. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Jiang, M.; Zhang, Q.; Wang, R.; Qu, X.; Zhou, G. Poly(Hexamethylene 2,5-Furandicarboxylate) Copolyesters Containing Phosphorus: Synthesis, Crystallization Behavior, Thermal, Mechanical and Flame Retardant Properties. Polym. Degrad. Stab. 2018, 153, 272–280. [Google Scholar] [CrossRef]
- Kaboudin, B.; Haghighat, H.; Yokomatsu, T. Synthesis of a new class of phosphinic acids: Synthesis of novel four-membered cyclic oxaphosphetanes by intramolecular mitsunobu reaction of bis(α-hydroxyalkyl)phosphinic acids. Synthesis 2011, 2011, 3185–3189. [Google Scholar] [CrossRef]
- Huang, J.; Chen, R. An overview of recent advances on the synthesis and biological activity of α-aminophosphonic acid derivatives. Heteroat. Chem. 2000, 11, 480–492. [Google Scholar] [CrossRef]
- Łyżwa, P.; Błaszczyk, J.; Sieroń, L.; Mikołajczyk, M. Asymmetric synthesis of structurally diverse aminophosphonic acids by using enantiopure n-(p-tolylsulfinyl) cinnamaldimines as reagents. Eur. J. Org. Chem. 2013, 2013, 2106–2115. [Google Scholar] [CrossRef]
- Palacios, F.; Retana, A.; Gil, A.; Ezpeleta, J. Simple asymmetric synthesis of 2h-azirines derived from phosphine oxides. J. Org. Chem. 2000, 65, 3213–3217. [Google Scholar] [CrossRef]
- Tran, G.; Pardo, D.; Tsuchiya, T.; Hillebrand, S.; Vors, J.; Cossy, J. Palladium-catalyzed phosphonylation: Synthesis of c3-, c4-, and c5-phosphonylated pyrazoles. Org. Lett. 2013, 15, 5550–5553. [Google Scholar] [CrossRef]
- Palácios, F.; Alonso, C.; Santos, J. Synthesis of β-aminophosphonates and -phosphinates. Chem. Rev. 2005, 105, 899–932. [Google Scholar] [CrossRef] [PubMed]
- Finkbeiner, P.; Hehn, J.; Gnamm, C. Phosphine oxides from a medicinal chemist’s perspective: Physicochemical and In Vitro parameters relevant for drug discovery. J. Med. Chem. 2020, 63, 7081–7107. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, R.; Shao, M.; Zhao, L.; Xu, W.; Guo, Y. Phosphorus-based nanomaterials for biomedical applications: A review. ACS Appl. Nano Mater. 2024, 7, 11022–11036. [Google Scholar] [CrossRef]
- Alharbi, A.; Abdel-Rahman, R. Synthesis and chemistry of phosphorus compounds substituted by 1,2,4-triazine moieties as medicinal probes. Heterocycles 2021, 102, 2247. [Google Scholar] [CrossRef]
- Kaboudin, B.; Daliri, P.; Faghih, S.; Esfandiari, H. Hydroxy- and amino-phosphonates and -bisphosphonates: Synthetic methods and their biological applications. Front. Chem. 2022, 10, 890696. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Raza, S.K.; Vijayaraghavan, R. Chemical Warfare Agents. J. Pharm. Bioallied Sci. 2010, 2, 166. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2009; pp. 110–111, 159–160, 185–187. [Google Scholar]
- Khaled, Z.; Ilia, G.; Watz, C.; Macașoi, I.; Drăghici, G.; Simulescu, V.; Merghes, P.E.; Varan, N.I.; Dehelean, C.A.; Vlaia, L.; et al. The Biological Impact of Some Phosphonic and Phos-phinic Acid Derivatives on Human Osteosarcoma. Curr. Issues Mol. Biol. 2024, 46, 4815–4831. [Google Scholar] [CrossRef] [PubMed]
- The Lubrizol Corporation. Neutralizing Carbopol®* and Pemulen™* Polymers in Aqueous and Hydroalcoholic Systems; Technical Report TDS-237; The Lubrizol Corporation: Wickliffe, OH, USA, 2009. [Google Scholar]
- The United States Pharmacopeial Convention. The United States Pharmacopoeia and National Formulary USP 43–NF 38; The United States Pharmacopeial Convention: Rockville, MD, USA, 2019; pp. 5670, 6415. [Google Scholar]
- Esteban, S.L.; Manzo, R.H.; Alovero, F.L. Azithromycin loaded on hydrogels of carbomer: Chemical stability and delivery properties. Int. J. Pharm. 2009, 366, 53–57. [Google Scholar] [CrossRef]
- Jimenez-Kairuz, A.; Allemandi, D.; Manzo, R.H. Mechanism of lidocaine release from carbomer-lidocaine hydrogels. J. Pharm. Sci. 2002, 91, 267–272. [Google Scholar] [CrossRef]
- Jimenez-Kairuz, A.F.; Allemandi, D.A.; Manzo, R.H. Equilibrium properties and mechanism of kinetic release of metoclopramide from carbomer hydrogels. Int. J. Pharm. 2003, 250, 129–136. [Google Scholar] [CrossRef]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural Skin Surface PH Is on Average below 5, Which Is Beneficial for Its Resident Flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Lukic, M.; Filipovic, M.; Pajic, N.; Lunter, D.; Bozic, D.; Savic, S. Formulation of topical acidic products and acidification of the skin—Contribution of glycolic acid. Int. J. Cosmet. Sci. 2021, 43, 419–431. [Google Scholar] [CrossRef]
- Barry, B.W.; Meyer, M.C. The rheological properties of carbopol gels I. Continuous shear and creep properties of carbopol gels. Int. J. Pharm. 1979, 2, 1–25. [Google Scholar] [CrossRef]
- Varges, R.P.; Costa, M.C.; Fonseca, S.B.; Naccache, F.M.; De Souza Mendes, P.R. Rheological Characterization of Carbopol® Dispersions in Water and in Water/Glycerol Solutions. Fluids 2019, 4, 3. [Google Scholar] [CrossRef]
- Tiossi, R.F.J.; Da Costa, J.C.; Miranda, M.A.; Praça, F.S.G.; McChesney, J.D.; Bentley, M.V.L.B.; Bastos, J.K. In Vitro and In Vivo Evaluation of the Delivery of Topical Formulations Containing Glycoalkaloids of Solanum Lycocarpum Fruits. Eur. J. Pharm. Biopharm. 2014, 88, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Kochkina, N.; Nikitina, M.; Agafonov, M.; Delyagina, E.; Terekhova, I. Iota-Carrageenan Hydrogels for Methotrexate Delivery. J. Mol. Liq. 2022, 368, 120790. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Sari, M.H.M.; Azambuja, J.H.; da Silveira, E.F.; Cervi, V.F.; Marchiori, M.C.L.; Maria-Engler, S.S.; Wink, M.R.; Azevedo, J.G.; Nogueira, C.W.; et al. Xanthan Gum-Based Hydrogel Containing Nanocapsules for Cutaneous Diphenyl Diselenide Delivery in Melanoma Therapy. Investig. New Drugs 2020, 38, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Maslii, Y.; Ruban, O.; Kasparaviciene, G.; Kalveniene, Z.; Materiienko, A.; Ivanauskas, L.; Mazurkeviciute, A.; Kopustinskiene, D.M.; Bernatoniene, J. The Influence of pH Values on the Rheological, Textural and Release Properties of Carbomer Polacril® 40P-Based Dental Gel Formulation with Plant-Derived and Synthetic Active Components. Molecules 2020, 25, 5018. [Google Scholar] [CrossRef] [PubMed]
- Dejeu, I.L.; Vicaș, L.G.; Vlaia, L.L.; Jurca, T.; Mureșan, M.E.; Pallag, A.; Coneac, G.H.; Olariu, I.V.; Muț, A.M.; Bodea, A.S.; et al. Study for evaluation of hydrogels after the incorporation of liposomes embedded with caffeic acid. Pharmaceuticals 2022, 15, 175. [Google Scholar] [CrossRef]
- Popa, E.A.; Popovici, I.; Braha, S.L. Forme Farmaceutice Bioadezive. In Tehnologie Farmaceutică; Polirom: Iasi, Romania, 2017; Volume 2, pp. 780–782. [Google Scholar]
- Coneac, G.; Vlaia, V.; Olariu, I.; Muţ, A.M.; Anghel, D.F.; Ilie, C.; Popoiu, C.; Lupuleasa, D.; Vlaia, L. Development and evaluation of new microemulsion-based hydrogel formulations for topical delivery of fluconazole. AAPS PharmSciTech 2015, 16, 889–904. [Google Scholar] [CrossRef]
- Houlleberghs, M.; Verheyden, L.; Voorspoels, F.; Chandran, C.; Duerinckx, K.; Radhakrishnan, S.; Martens, J.A.; Breynaert, E. Magneto-hydrodynamic mixing: A new technique for preparing carbomer hydrogels. Aiche J. 2022, 69, e17911. [Google Scholar] [CrossRef]
- Hui, Q.; Zhang, L.; Yang, X.; Yu, B.; Huang, Z.; Pang, S.; Zhou, Q.; Yang, R.; Li, W.; Hu, L.; et al. Higher biostability of rh-aFGF-Carbomer 940 hydrogel and its effect on wound healing in a diabetic rat model. ACS Biomater. Sci. Eng. 2018, 4, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Bezugla, O.; Liapunova, A.; Zinchenko, I.; Liapunov, O.; Lyapunov, N.; Stolper, Y. Study of factors affecting the In Vitro release of ketoprofen from carbomers-based gels. Sci. Pharm. Sci. 2022, 6, 4–20. [Google Scholar]
- Abdullah, O.; Usman Minhas, M.; Ahmad, M.; Ahmad, S.; Barkat, K.; Ahmad, A. Synthesis, optimization, and evaluation of polyvinyl alcohol-based hydrogels as controlled combinatorial drug delivery system for colon cancer. Adv. Polym. Technol. 2018, 37, 3348–3363. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, X.; Xu, B.; Shen, Y.; Ye, Z.; Chaurasiya, B.; Liu, L.; Li, Y.; Xing, X.; Chen, D. Eprinomectin nanoemulgel for transdermal delivery against endoparasites and ectoparasites: Preparation, In Vitro and In Vivo evaluation. Drug Deliv. 2019, 26, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Marković, M. Controlled release of caffeine from three-dimensional networks based on poly(methacrylic acid) and casein—Analysis of the effect of caffeine concentration on release process. In Proceedings of the 35th International Congress on Process Industry, Belgrade, Serbia, 1–3 June 2022; pp. 19–24. [Google Scholar]
- Kaberova, Z.; Karpushkin, E.; Nevoralová, M.; Vetrík, M.; Šlouf, M.; Dušková-Smrčková, M. Microscopic Structure of Swollen Hydrogels by Scanning Electron and Light Microscopies: Artifacts and Reality. Polymers 2020, 12, 578. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Sengupta, S.; Pal, A.; Sardar, S.; Sahu, N.; Lenka, N.; Panigrahi, K.C.S.; Goswami, L.; Bandyopadhyay, A. Aquasorbent Guargum Grafted Hyperbranched Poly (Acrylic Acid): A Potential Culture Medium for Microbes and Plant Tissues. Carbohydr. Polym. 2019, 222, 114983. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, O.; Minhas, M.U.; Ahmad, M.; Ahmad, S.; Ahmad, A. Synthesis of hydrogels for combinatorial delivery of 5-fluorouracil and leucovorin calcium in colon cancer: Optimization, In Vitro characterization and its toxicological evaluation. Polym. Bull. 2019, 76, 3017–3037. [Google Scholar] [CrossRef]
- Zustiak, S.; Pubill, S.; Ribeiro, A.; Leach, J. Hydrolytically degradable poly(ethylene glycol) hydrogel scaffolds as a cell delivery vehicle: Characterization of pc12 cell response. Biotechnol. Prog. 2013, 29, 1255–1264. [Google Scholar] [CrossRef]
- Gentilini, R.; Munarin, F.; Bloise, N.; Secchi, E.; Visai, L.; Tanzi, M.C.; Petrini, P. Polysaccharide-based hydrogels with tunable composition as 3D cell culture systems. Int. J. Artif. Organs 2018, 41, 213–222. [Google Scholar] [CrossRef]
- Lopez Hernandez, H.; Grosskopf, A.K.; Stapleton, L.M.; Agmon, G.; Appel, E.A. Non-Newtonian Polymer-Nanoparticle Hydrogels Enhance Cell Viability during Injection. Macromol. Biosci. 2019, 19, e1800275. [Google Scholar] [CrossRef] [PubMed]
- Tzouanas, S.; Ekenseair, A.; Kasper, F.; Mikos, A. Mesenchymal stem cell and gelatin microparticle encapsulation in thermally and chemically gelling injectable hydrogels for tissue engineering. J. Biomed. Mater. Res. Part A 2014, 102, 1222–1230. [Google Scholar] [CrossRef]
- Tamura, M.; Yanagawa, F.; Sugiura, S.; Takagi, T.; Sumaru, K.; Kanamori, T. Click-cross-linkable and photodegradable gelatin hydrogels for cytocompatible optical cell manipulation in the natural environment. Sci. Rep. 2015, 5, 15060. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging role of hydrogels in drug delivery systems, tissue engineering, and wound management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Ning, L.; Mehta, R.; Cao, C.; Theus, A.; Tomov, M.; Zhu, N.; Weeks, E.R.; Bauser-Heaton, H.; Serpooshan, V. Embedded 3d bioprinting of gelatin methacryloyl-based constructs with highly tunable structural fidelity. ACS Appl. Mater. Interfaces 2020, 12, 44563–44577. [Google Scholar] [CrossRef]
- Hayati, F.; Ghamsari, S.M.; Dehghan, M.M.; Oryan, A. Effects of carbomer 940 hydrogels on burn wounds: An In Vitro and In Vivo study. J. Dermatol. Treat. 2018, 29, 593–599. [Google Scholar] [CrossRef]
- Mair, K.; Koinig, H.; Gerner, W.; Höhne, A.; Bretthauer, J.; Kroll, J.; Roof, M.; Saalmüller, A.; Stadler, K.; Libanova, R. Carbopol improves the early cellular immune responses induced by the modified-life vaccine ingelvac Prrs® mlv. Vet. Microbiol. 2015, 176, 352–357. [Google Scholar] [CrossRef]
- Youssef, F.S.; Ismail, S.H.; ElHalim, H.F.A.; Mohamed, G.G. Synthesis, characterization, and investigation of pharmacological studies of some nanoparticles. Res. Square 2023, 1–42. [Google Scholar] [CrossRef]
- Doğan, M.; Şahbaz, S.; Uğurlu, T.; Sezer, A. Synthesis and characterization of chitosan-PVA hydrogel containing pegylated recombinant epidermal growth factor on cell culture for wound healing substitute. Braz. J. Pharm. Sci. 2022, 58, e191120. [Google Scholar] [CrossRef]
- Kraskiewicz, H.; Hinc, P.; Krawczenko, A.; Bielawska-Pohl, A.; Paprocka, M.; Witkowska, D.; Isa, I.L.M.; Pandit, A.; Klimczak, A. Hatmsc secreted factors in the hydrogel as a potential treatment for chronic wounds—In Vitro study. Int. J. Mol. Sci. 2021, 22, 12241. [Google Scholar] [CrossRef]
- Tomić, S.L.; Vuković, J.S.; Radić, M.M.B.; Filipović, V.V.; Živanović, D.P.; Nikolić, M.M.; Nikodinovic-Runic, J. Manuka honey/2-hydroxyethyl methacrylate/gelatin hybrid hydrogel scaffolds for potential tissue regeneration. Polymers 2023, 15, 589. [Google Scholar] [CrossRef]
- Zanchetta, F.; De Wever, P.; Morari, J.; Gaspar, R.C.; Prado, T.P.D.; De Maeseneer, T.; Cardinaels, R.; Araújo, E.P.; Lima, M.H.M.; Fardim, P. In Vitro and In Vivo evaluation of chitosan/hpmc/insulin hydrogel for wound healing applications. Bioengineering 2024, 11, 168. [Google Scholar] [CrossRef]
- Michalska-Sionkowska, M.; Warżyńska, O.; Kaczmarek-Szczepańska, B.; Łukowicz, K.; Osyczka, A.; Walczak, M. Characterization of collagen/beta-glucan hydrogels crosslinked with tannic acid. Polymers 2021, 13, 3412. [Google Scholar] [CrossRef]
- Makhija, P.; Kathuria, H.; Sethi, G.; Grobben, B. Polymeric Hydrogels for Controlled Release of Black Tea and Coffee Extracts for Topical Applications. Gels 2021, 7, 174. [Google Scholar] [CrossRef]
- Xu, H.; Gan, C.; Gao, Z.; Huang, Y.; Wu, S.; Zhang, D.; Wang, X.; Sheng, J. Caffeine Targets SIRT3 to Enhance SOD2 Activity in Mitochondria. Front. Cell Dev. Biol. 2020, 8, 822. [Google Scholar] [CrossRef]
- Xuan, S.H.; Lee, K.S.; Jeong, H.J.; Park, Y.M.; Ha, J.H.; Park, S.N. Cosmeceutical activities of ethanol extract and its ethyl acetate fraction from coffee silverskin. Biomater. Res. 2019, 23, 2. [Google Scholar] [CrossRef]
- Sarobo, C.; Lacorte, L.M.; Martins, M.; Rinaldi, J.C.; Moroz, A.; Scarano, W.R.; Delella, F.K.; Felisbino, S.L. Chronic caffeine intake increases androgenic stimuli, epithelial cell proliferation, and hyperplasia in rat ventral prostate. Int. J. Exp. Pathol. 2012, 93, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, J.; Zhang, R.; Ye, L.; Li, M.; Chen, W. Phloroglucinol Derivative Carbomer Hydrogel Accelerates MRSA-Infected Wounds’ Healing. Int. J. Mol. Sci. 2022, 23, 8682. [Google Scholar] [CrossRef] [PubMed]
- Osipitan, O.; Shi, Y.; Di Pasqua, A. Phenethyl Isothiocyanate-Containing Carbomer Gel for Use against Squamous Cell Carcinoma. Pharmaceutics 2021, 13, 106. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, R.; Haloi, J.; Medhi, S. Topical Delivery of Methanolic Root Extract of Annona Reticulata against Skin Cancer. S. Afr. J. Bot. 2019, 124, 484–493. [Google Scholar] [CrossRef]
- Merclin, N.; Bramer, T.; Edsman, K. Iontophoretic Delivery of 5-Aminolevulinic Acid and Its Methyl Ester Using a Carbopol Gel as Vehicle. J. Control. Release 2004, 98, 57–65. [Google Scholar] [CrossRef]
- Kirf, D.; Higginbotham, C.; Rowan, N.; Devery, S. Cyto- and genotoxicological assessment and functional characterization of n-vinyl-2-pyrrolidone–acrylic acid-based copolymeric hydrogels with potential for future use in wound healing applications. Biomed. Mater. 2010, 5, 035002. [Google Scholar] [CrossRef]
- Bustin, S.A. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 2000, 25, 169–193. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.A.; Rampy, M.A. Keratinocyte growth factor-2 accelerates wound healing in incisional wounds. J. Surg. Res. 1999, 81, 238–242. [Google Scholar] [CrossRef]
- Nolan, T.; Hands, R.E.; Bustin, S.A. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 2006, 1, 1559–1582. [Google Scholar] [CrossRef] [PubMed]
- Wasan, K.M.; Brocks, D.R.; Lee, S.D.; Sachs-Barrable, K.; Thornton, S.J. Impact of lipoproteins on the biological activity and disposition of hydrophobic drugs: Implications for drug discovery. Nat. Rev. Drug Discov. 2008, 7, 84–99. [Google Scholar] [CrossRef]
- Valasek, M.A.; Repa, J.J. The power of real-time PCR. Adv. Physiol. Educ. 2005, 29, 151–159. [Google Scholar] [CrossRef]
- Wong, M.L.; Medrano, J.F. Real-time PCR for mRNA quantitation. Biotechniques 2005, 39, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef]
- Salomonis, N.; Hanspers, K.; Zambon, A.C.; Vranizan, K.; Lawlor, S.C.; Dahlquist, K.D.; Doniger, S.W.; Stuart, J.; Conklin, B.R.; Pico, A.R. GenMAPP 2: New features and resources for pathway analysis. BMC Bioinform. 2007, 8, 217. [Google Scholar] [CrossRef]
- Chen, X.; Hu, W.; Wei, W.; Shen, Y.; Chen, Y.; Yang, S.; Gong, Y. Effects of keratinocyte growth factor 2 (KGF-2) on keratinocyte growth, migration, and on excisional wound healing. Prog. Biochem. Biophys. 2009, 36, 854–862. [Google Scholar]
- Doniger, S.W.; Salomonis, N.; Dahlquist, K.D.; Vranizan, K.; Lawlor, S.C.; Conklin, B.R. MAPPFinder: Using Gene Ontology and GenMAPP to create a global gene-expression profile from microarray data. Genome Biol. 2003, 4, R7. [Google Scholar] [CrossRef] [PubMed]
- Batista-Duharte, A.; Murillo, G.J.; Pérez, U.M.; Tur, E.N.; Portuondo, D.F.; Martínez, B.T.; Téllez-Martínez, D.; Betancourt, J.E.; Pérez, O. The hen’s egg test on chorioallantoic membrane. Int. J. Toxicol. 2016, 35, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Pârvănescu, R.D.; Watz, C.-G.; Moacă, E.-A.; Vlaia, L.; Marcovici, I.; Macașoi, I.G.; Borcan, F.; Olariu, I.; Coneac, G.; Drăghici, G.-A.; et al. Oleogel Formulations for the Topical Delivery of Betulin and Lupeol in Skin Injuries—Preparation, Physicochemical Characterization, and Pharmaco-Toxicological Evaluation. Molecules 2021, 26, 4174. [Google Scholar] [CrossRef]
- Schrage, A.; Gamer, A.; Ravenzwaay, B.; Landsiedel, R. Experience with the het-cam method in the routine testing of a broad variety of chemicals and formulations. Altern. Lab. Anim. 2010, 38, 39–52. [Google Scholar] [CrossRef]
- Luepke, N.P. Hen’s Egg Chorioallantoic Membrane Test for Irritation Potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Barel, A.; Clarys, P. Study of the Stratum corneum Barrier Function by Transepidermal Water Loss Measurements: Comparison between Two Commercial Instruments: Evaporimeter® and Tewameter®. Ski. Pharmacol. 1995, 8, 186–195. [Google Scholar] [CrossRef]
- Yamano, N.; Kunisada, M.; Nishiaki-Sawada, A.; Ohashi, H.; Igarashi, T.; Nishigori, C. Evaluation of acute reactions on mouse skin irradiated with 222 and 235 nm UV-C. Photochem. Photobiol. 2021, 97, 770–777. [Google Scholar] [CrossRef]
- Pyun, H.-B.; Kim, M.; Park, J.; Sakai, Y.; Numata, N.; Shin, J.-Y.; Shin, H.-J.; Kim, D.-U.; Hwang, J.-K. Effects of collagen tripeptide supplement on photoaging and epidermal skin barrier in UVB-exposed hairless mice. Prev. Nutr. Food Sci. 2012, 17, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, J.; Kopcewicz, M.; Kur-Piotrowska, A.; Szóstek-Mioduchowska, A.; Walendzik, K.; Gawrońska-Kozak, B. Effect of tgfβ1, tgfβ3, and keratinocyte conditioned media on functional characteristics of dermal fibroblasts derived from reparative (BALB/c) and regenerative (foxn1 deficient; nude) mouse models. Cell Tissue Res. 2018, 374, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Borcan, L.C.; Dudas, Z.; Len, A.; Fuzi, J.; Borcan, F.; Tomescu, M.C. Synthesis and characterization of a polyurethane carrier used for a prolonged transmembrane transfer of a chili pepper extract. Int. J. Nanomed. 2018, 13, 7155–7166. [Google Scholar] [CrossRef]
- Ra, J.; Lee, D.E.; Kim, S.H.; Jeong, J.-W.; Ku, H.K.; Kim, T.-Y.; Choi, I.-D.; Jeung, W.; Sim, J.-H.; Ahn, Y.-T. Effect of oral administration of lactobacillus plantarum hy7714 on epidermal hydration in ultraviolet b-irradiated hairless mice. J. Microbiol. Biotechnol. 2014, 24, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Baba, H.; Masuyama, A.; Yoshimura, C.; Aoyama, Y.; Takano, T.; Ohki, K. Oral intake of lactobacillus helveticus-fermented milk whey decreased transepidermal water loss and prevented the onset of sodium dodecyl sulfate-induced dermatitis in mice. Biosci. Biotechnol. Biochem. 2010, 74, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Yumnam, S.; Kim, S. Oral intake of collagen peptide attenuates ultraviolet b irradiation-induced skin dehydration In Vivo by regulating hyaluronic acid synthesis. Int. J. Mol. Sci. 2018, 19, 3551. [Google Scholar] [CrossRef] [PubMed]
- Avila Acevedo, J.G.; Espinosa González, A.M.; De Maria y Campos, D.M.; Benitez Flores, J.d.C.; Hernández Delgado, T.; Flores Maya, S.; Campos Contreras, J.; Muñoz López, J.L.; García Bores, A.M. Photoprotection of Buddleja cordata extract against UVB-induced skin damage in SKH-1 hairless mice. BMC Complement. Altern. Med. 2014, 14, 281. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Hiramoto, K.; Koyama, M.; Ooi, K. Skin disruption is associated with indomethacin-induced small intestinal injury in mice. Exp. Dermatol. 2014, 23, 659–663. [Google Scholar] [CrossRef] [PubMed]
- The Lubrizol Corporation. Dispersion Techniques for Carbopol Polymers; Technical Report TDS-103; The Lubrizol Corporation: Wickliffe, OH, USA, 2007. [Google Scholar]
- Piao, Y.; Chen, B. Self-assembled graphene oxide–gelatin nanocomposite hydrogels: Characterization, formation mechanisms, and pH-sensitive drug release behavior. J. Polym. Sci. Part B Polym. Phys. 2014, 53, 356–367. [Google Scholar] [CrossRef]
- Directorate for the Quality of Medicines and Healthcare of the Council of Europe. Potentiometric Determination of pH. In European Pharmacopeia, 11th ed.; EDQM Council of Europe: Strasbourg, France, 2022. [Google Scholar]
- Pan, T.; Song, W.; Cao, X.; Wang, Y. 3D Bioplotting of Gelatin/Alginate Scaffolds for Tissue Engineering: Influence of Crosslinking Degree and Pore Architecture on Physicochemical Properties. J. Mater. Sci. Technol. 2016, 32, 889–900. [Google Scholar] [CrossRef]
- Farcas, C.G.; Dehelean, C.; Pinzaru, I.A.; Mioc, M.; Socoliuc, V.; Moaca, E.A.; Avram, S.; Ghiulai, R.; Coricovac, D.; Pavel, I.; et al. Thermosensitive Betulinic Acid-Loaded Magnetoliposomes: A Promising Antitumor Potential for Highly Aggressive Human Breast Adenocarcinoma Cells Under Hyperthermic Conditions. Int. J. Nanomed. 2020, 15, 8175–8200. [Google Scholar] [CrossRef] [PubMed]
- Khaled, Z.; Vlaia, L.; Semenescu, A.D.; Dumitrescu, C.; Coneac, G.; Kis, A.M.; Moaca, A.; Vlaia, V.; Iurciuc, S.; Stefanescu, E.H. In Vitro study of topical caffeine and ascorbyl magnesium phosphate formulations—Preparation, physicochemical characterization, and pharmacotoxicological assessment. Farmacia 2024, 72, 381–396. [Google Scholar]
- Gheran, C.V.; Voicu, S.N.; Galateanu, B.; Callewaert, M.; Moreau, J.; Cadiou, C.; Chuburu, F.; Dinischiotu, A. In Vitro Studies Regarding the Safety of Chitosan and Hyaluronic Acid-Based Nanohydrogels Containing Contrast Agents for Magnetic Resonance Imaging. Int. J. Mol. Sci. 2022, 23, 3258. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.; Beneš, V.; Nolan, T.; Pfaffl, M. Quantitative real-time RT-PCR—A perspective. J. Mol. Endocrinol. 2005, 34, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Moacă, E.A.; Farcaş, C.; Coricovac, D.; Avram, S.; Mihali, C.V.; Drăghici, G.A.; Loghin, F.; Păcurariu, C.; Dehelean, C. Oleic Acid Double Coated Fe3O4 Nanoparticles as Anti-Melanoma Compounds with a Complex Mechanism of Activity-In Vitro and In Ovo Assessment. J. Biomed. Nanotechnol. 2019, 15, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Coricovac, D.-E.; Moacă, E.-A.; Pinzaru, I.; Cîtu, C.; Soica, C.; Mihali, C.-V.; Păcurariu, C.; Tutelyan, V.A.; Tsatsakis, A.; Dehelean, C.-A. Biocompatible Colloidal Suspensions Based on Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Toxicological Profile. Front. Pharmacol. 2017, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Mohd Ariffin, N.H.; Hasham, R. Assessment of non-invasive techniques and herbal-based products on dermatological physiology and intercellular lipid properties. Heliyon 2020, 6, e03955. [Google Scholar] [CrossRef]
- Gonzalez-Bravo, A.; Montero-Vilchez, T.; Arias-Santiago, S.; Buendia-Eisman, A. The Effect of Sunscreens on the Skin Barrier. Life 2022, 12, 2083. [Google Scholar] [CrossRef]

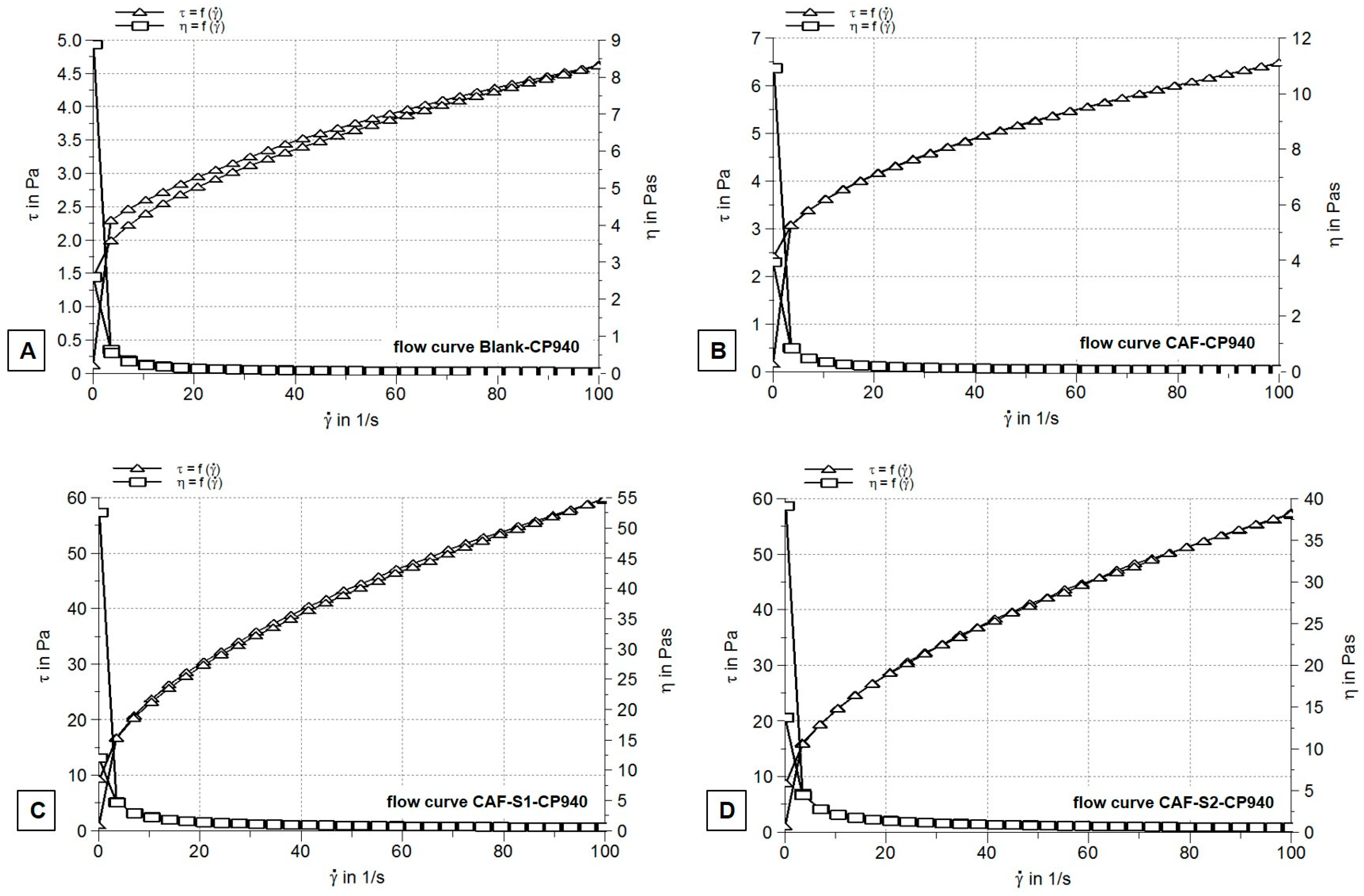

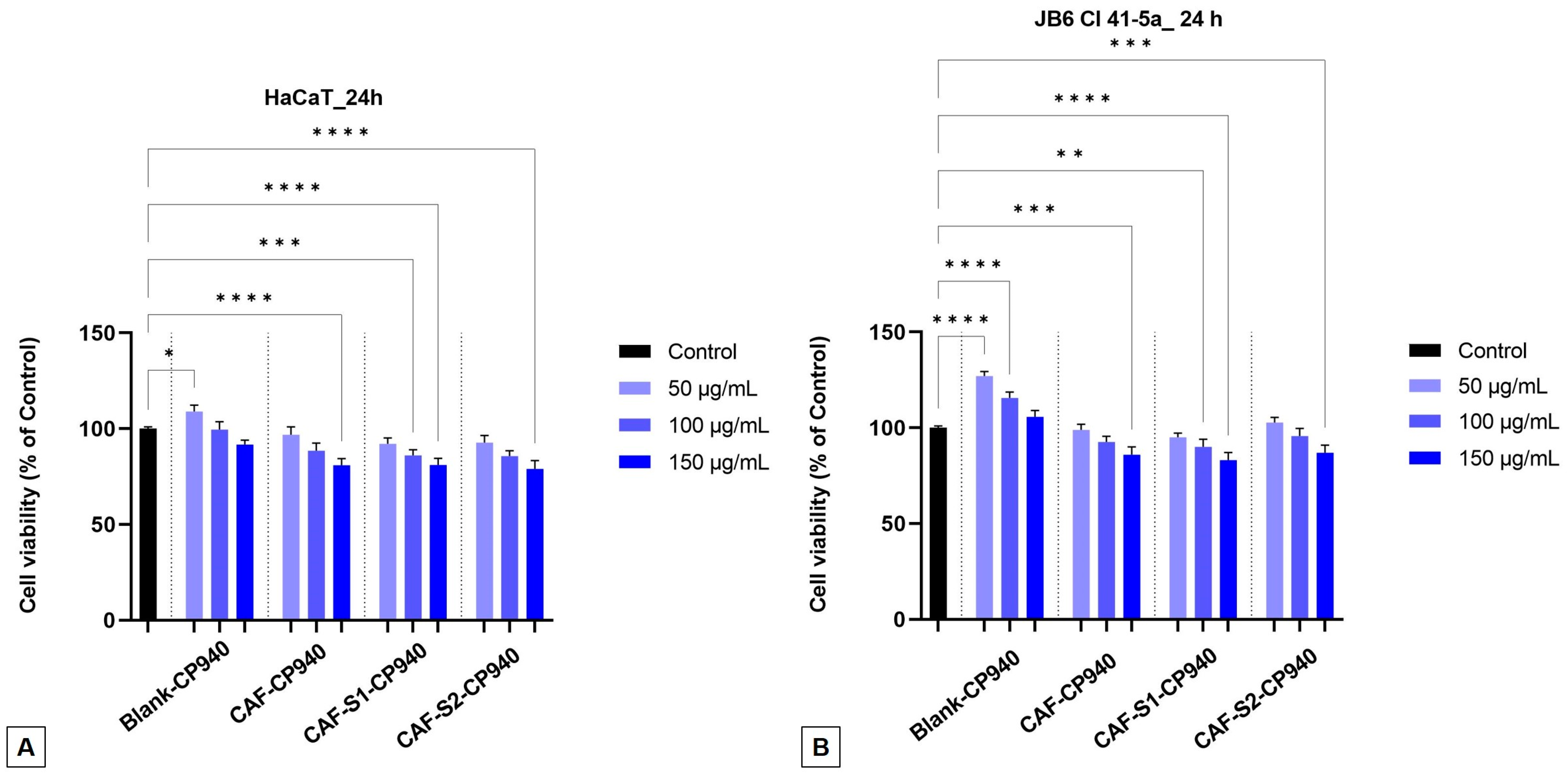
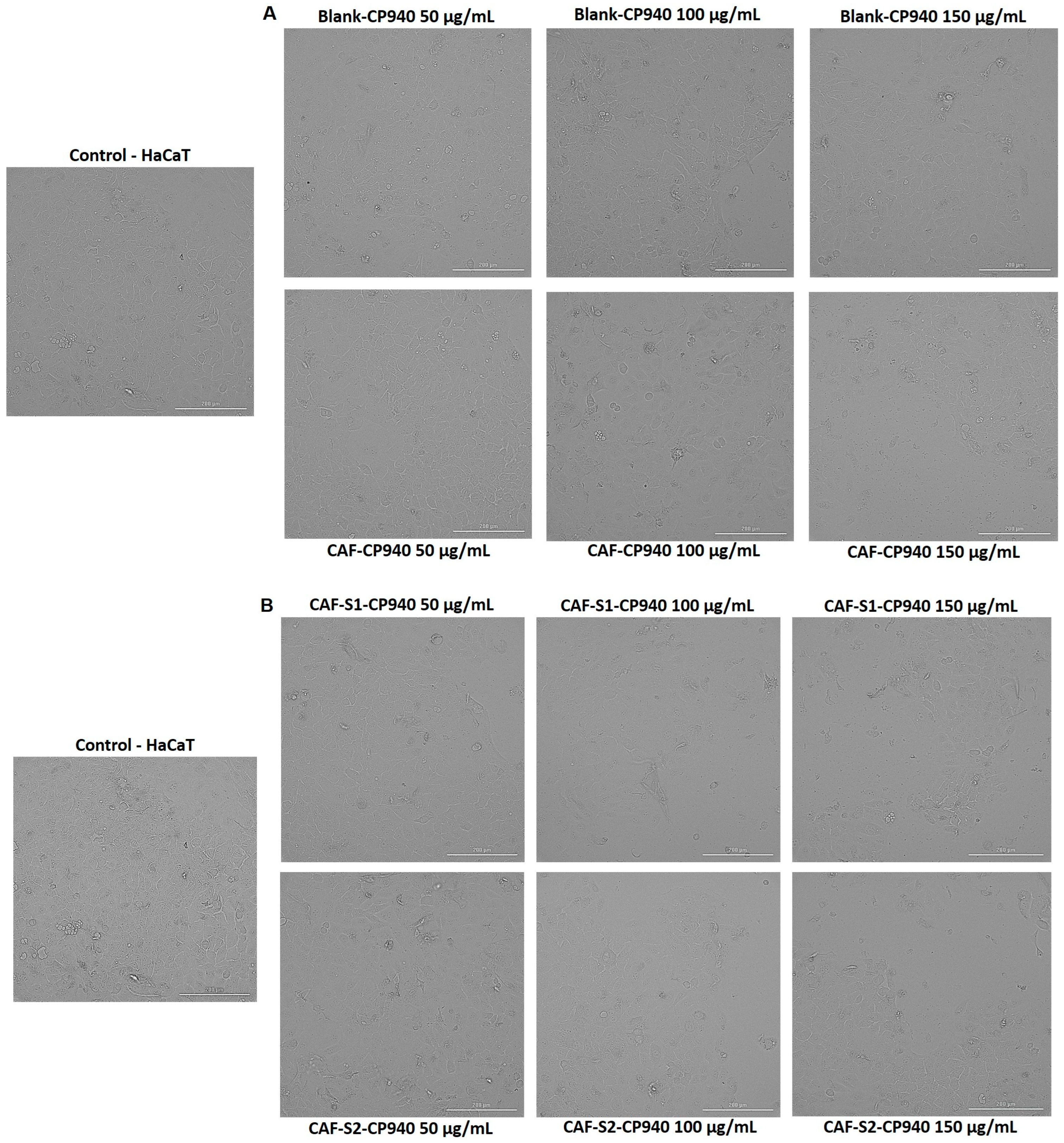
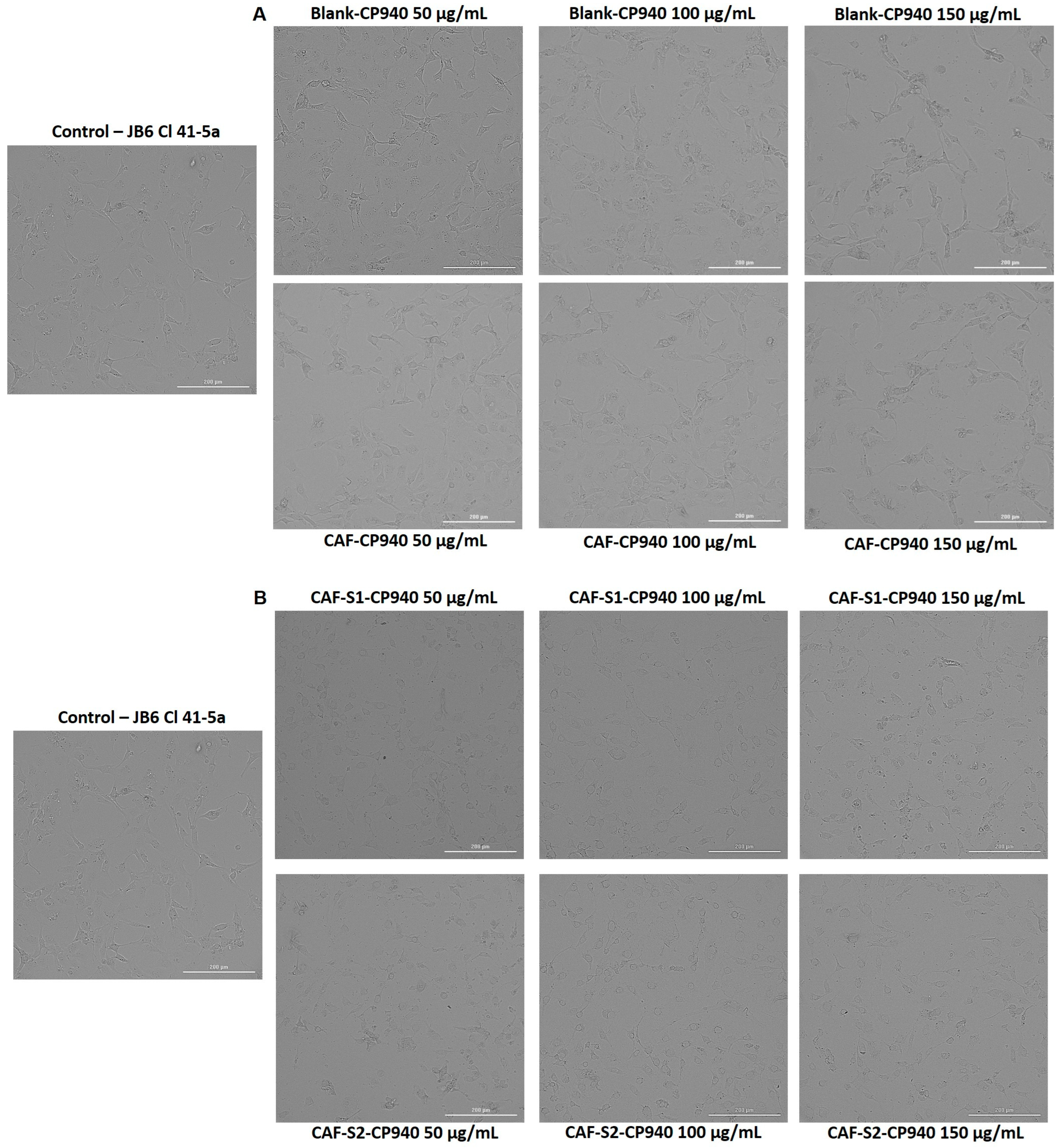


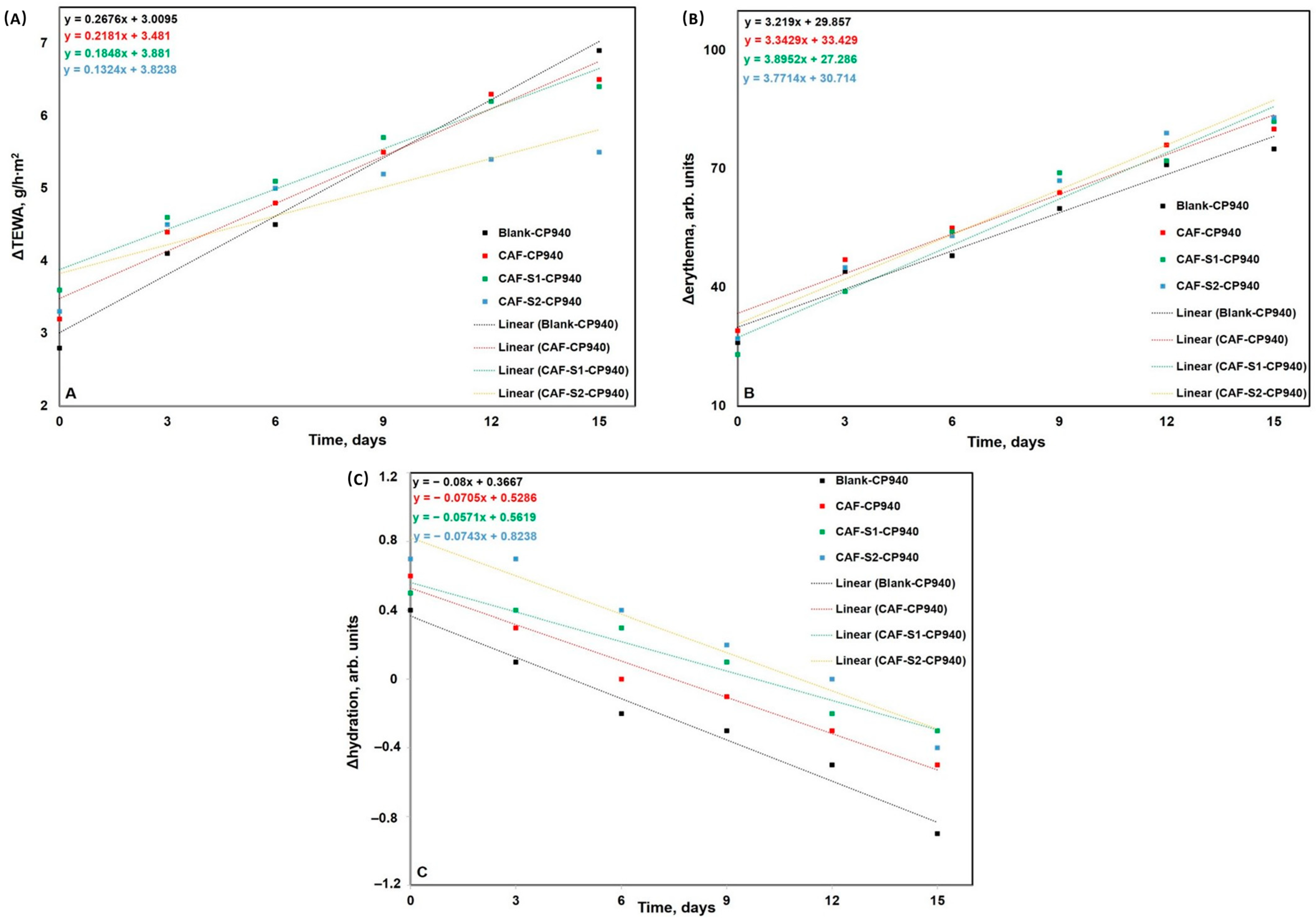
| Hydrogels Annotation | pH | Apparent Viscosity (Pa.s) | Spreadability (mm2) |
|---|---|---|---|
| Blank-CP940 | 3.96 ± 0.029 | 0.046 ± 0.16 | 4654.27 ± 3.091 |
| CAF-CP940 | 4.83 ± 0.037 | 0.065 ± 0.34 | 5671.63 ± 3.898 |
| CAF-S1-CP940 | 3.32 ± 0.048 | 0.597 ± 0.52 | 3846.50 ± 5.477 |
| CAF-S2-CP940 | 3.71 ± 0.052 | 0.573 ± 0.81 | 3957.19 ± 7.001 |
| Gene | CAF-S1-CP940 | p-Value | CAF-S2-CP940 | p-Value |
|---|---|---|---|---|
| Bcl-XL | −0.241 | 0.818 | 1.749 | 0.134 |
| BAD | −0.255 | 0.806 | 1.505 | 0.185 |
| Caspase-3 | −0.065 | 0.95 | 2.518 | 0.048 |
| Bcl-2 | −0.866 | 0.428 | 1.748 | 0.134 |
| Caspase-8 | −0.587 | 0.586 | 1.744 | 0.135 |
| Bax | −0.157 | 0.878 | 2.927 | 0.03 |
| Sample/Concentration | Time [seconds] | Irritation Score (IS) | ||
|---|---|---|---|---|
| Hemorrhage (tH) | Lysis (tL) | Coagulation (tC) | ||
| H2Od | 300 | 300 | 300 | 0.070 |
| SLS 1% | 14 | 50 | 20 | 18.070 |
| CAF-S1-CP940 100 μg/mL | 300 | 300 | 300 | 0.070 |
| CAF-S2-CP940 100 μg/mL | 300 | 290 | 300 | 0.304 |
| Genes | Forward | Reverse |
|---|---|---|
| 18S | 5′ GTACCCGTTGAACCCCATT 3′ | 5′CCATCCAATCGGTAGTAGCG3′ |
| BAD | 5′ CCCAGAGTTTGAGCCGAGTG 3′ | 5′CCCATCCCTTCGTCCT3′ |
| Caspase 3 | 5′ GCGGTTGTAGAAGAGTTTCGTG 3′ | 5′CTCACGGCCTGGGATTTCAA 3′ |
| Caspase 8 | 5′ AGAGTCTGTGCCCAAATCAAC 3′ | 5′GCTGCTTCTCTCTTTGCTGAA 3′ |
| BCL-XL | 5′ GATCCCCATGGCAGCAGTAAAGCAAG 3′ | 5′CCCCATCCCGGAAGAGTTCATTCACT 3′ |
| Bax | 5′ GCCGGGTTGTCGCCCTTTT 3′ | 5′CCGCTCCCGGAGGAAGTCCA 3′ |
| Bcl-2 | 5′-CGGGAGATGTCGCCCCTGGT-3′ | 5′-GCATGCTGGGGCCGTACAGT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakzak, K.; Semenescu, A.-D.; Moacă, E.-A.; Predescu, I.; Drăghici, G.; Vlaia, L.; Vlaia, V.; Borcan, F.; Dehelean, C.-A. Comprehensive Biosafety Profile of Carbomer-Based Hydrogel Formulations Incorporating Phosphorus Derivatives. Gels 2024, 10, 477. https://doi.org/10.3390/gels10070477
Zakzak K, Semenescu A-D, Moacă E-A, Predescu I, Drăghici G, Vlaia L, Vlaia V, Borcan F, Dehelean C-A. Comprehensive Biosafety Profile of Carbomer-Based Hydrogel Formulations Incorporating Phosphorus Derivatives. Gels. 2024; 10(7):477. https://doi.org/10.3390/gels10070477
Chicago/Turabian StyleZakzak, Khaled, Alexandra-Denisa Semenescu, Elena-Alina Moacă, Iasmina Predescu, George Drăghici, Lavinia Vlaia, Vicenţiu Vlaia, Florin Borcan, and Cristina-Adriana Dehelean. 2024. "Comprehensive Biosafety Profile of Carbomer-Based Hydrogel Formulations Incorporating Phosphorus Derivatives" Gels 10, no. 7: 477. https://doi.org/10.3390/gels10070477






