Stimuli-Responsive Nanocomposite Hydrogels for Oral Diseases
Abstract
:1. Introduction
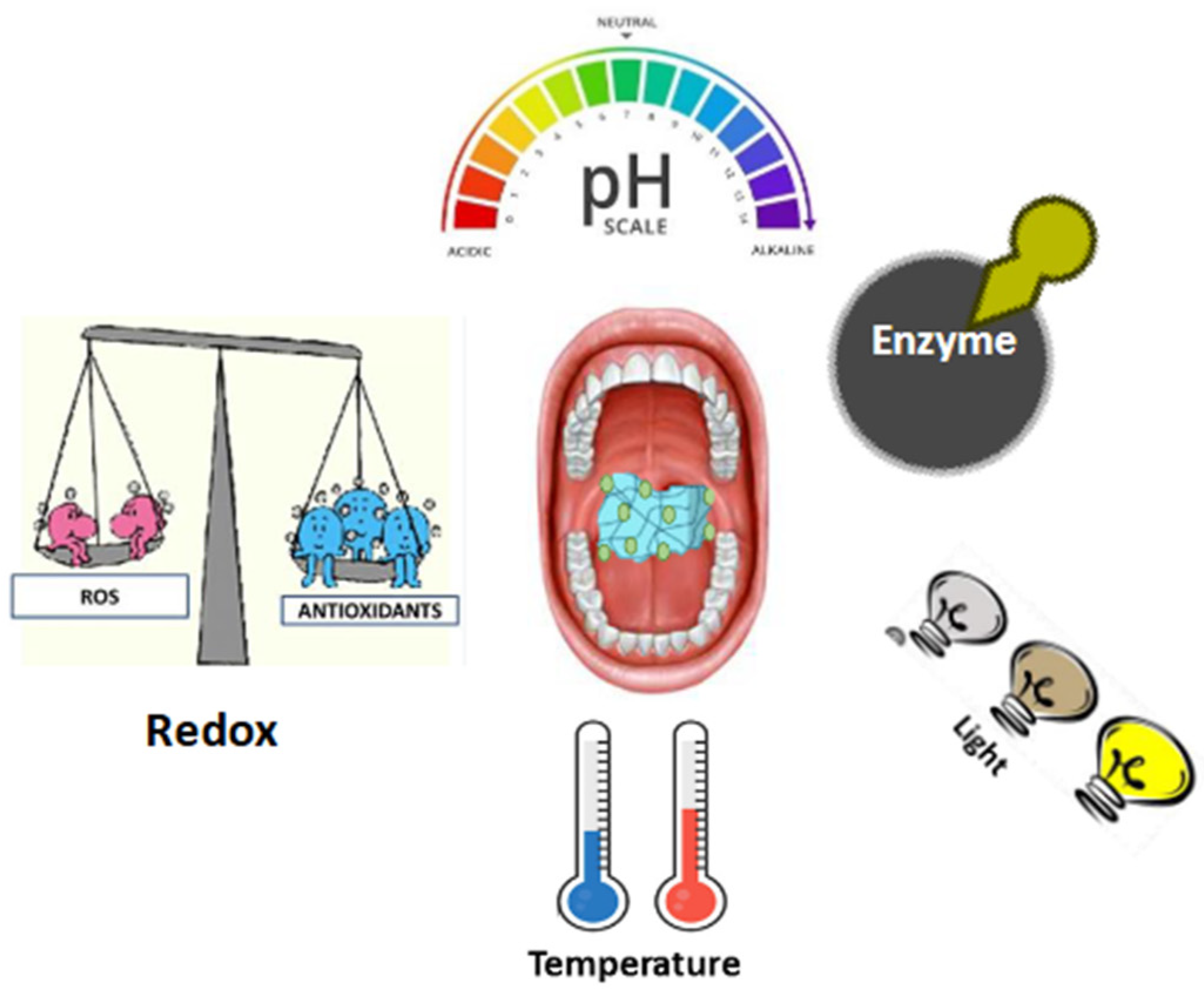
2. Classification of Stimuli-Responsive Nanocomposite Hydrogels
2.1. pH Responsive
2.2. Redox Responsive
2.3. Enzyme Responsive
2.4. Thermo Responsive
2.5. Light Responsive
3. Stimuli-Responsive Nanocomposite Hydrogels for Periodontitis Treatment
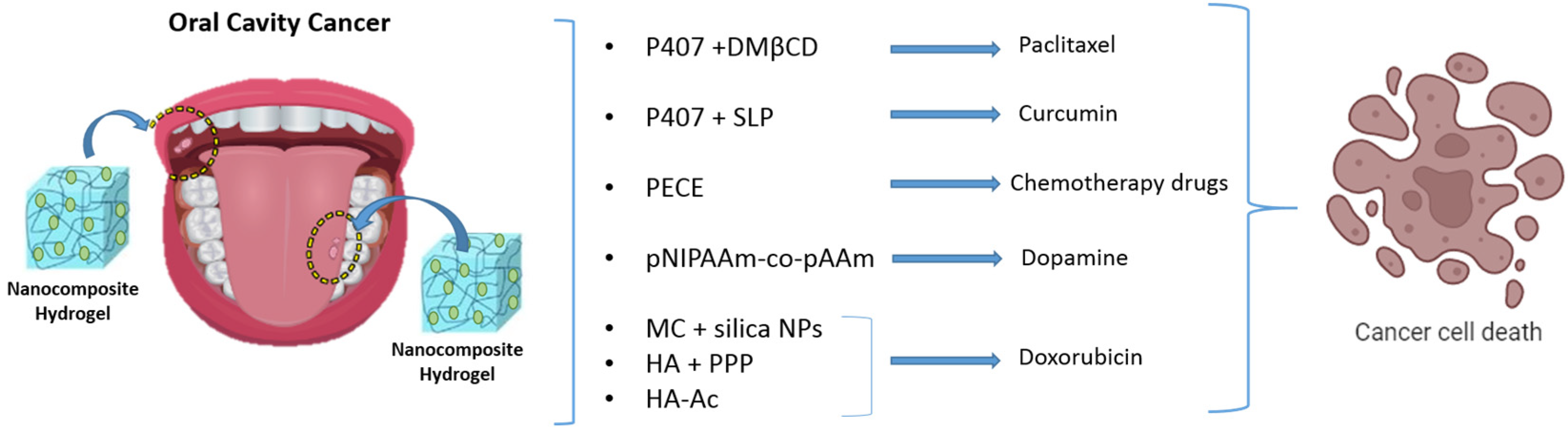
4. Stimuli-Responsive Nanocomposite Hydrogels for Oral Tumor Therapy
5. Stimuli-Responsive Nanocomposite Hydrogels for the Treatment of Endodontic Infections
6. Limitations Associated with the Utilization of Stimuli-Responsive Nanocomposite Hydrogels
7. Conclusions and Future Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Kitamoto, S.; Nagao-Kitamoto, H.; Hein, R.; Schmidt, T.M.; Kamada, N. The Bacterial Connection between the Oral Cavity and the Gut Diseases. J. Dent. Res. 2020, 99, 1021–1029. [Google Scholar] [CrossRef]
- Groeger, S.; Zhou, Y.; Ruf, S.; Meyle, J. Pathogenic Mechanisms of Fusobacterium nucleatum on Oral Epithelial Cells. Front. Oral Health 2022, 3, 831607. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Ye, F.; Jin, Y.; Luo, Y.; Zhu, H. Overexpression of DEL-1 Downregulates SH3BP2 Expression and Inhibits Porphyromonas gingivalis-induced Gingival Inflammation In Vivo and In Vitro. Oral Health Prev. Dent. 2022, 20, 199–206. [Google Scholar] [PubMed]
- Stasiewicz, M.; Karpiński, T.M. The oral microbiota and its role in carcinogenesis. Semin. Cancer Biol. 2022, 86 Pt 3, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Ricucci, D.; Siqueira, J.F., Jr.; Abdelsayed, R.A.; Lio, S.G.; Rôças, I.N. Bacterial Invasion of Pulp Blood Vessels in Teeth with Symptomatic Irreversible Pulpitis. J. Endod. 2021, 47, 1854–1864. [Google Scholar] [CrossRef] [PubMed]
- Vitkov, L.; Muñoz, L.E.; Schoen, J.; Knopf, J.; Schauer, C.; Minnich, B.; Herrmann, M.; Hannig, M. Neutrophils Orchestrate the Periodontal Pocket. Front. Immunol. 2021, 12, 788766. [Google Scholar] [CrossRef] [PubMed]
- Teles, R.; Moss, K.; Preisser, J.S.; Genco, R.; Giannobile, W.V.; Corby, P.; Garcia, N.; Jared, H.; Torresyap, G.; Salazar, E.; et al. Patterns of periodontal disease progression based on linear mixed models of clinical attachment loss. J. Clin. Periodontol. 2018, 45, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Josic, U.; Delfi, M.; Pinelli, F.; Jahed, V.; Kaya, E.; Ashrafizadeh, M.; Zarepour, A.; Rossi, F.; Zarrabi, A.; et al. Drug Delivery (Nano)Platforms for Oral and Dental Applications: Tissue Regeneration, Infection Control, and Cancer Management. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2021, 8, 2004014. [Google Scholar] [CrossRef]
- Ketabat, F.; Pundir, M.; Mohabatpour, F.; Lobanova, L.; Koutsopoulos, S.; Hadjiiski, L.; Chen, X.; Papagerakis, P.; Papagerakis, S. Controlled Drug Delivery Systems for Oral Cancer Treatment—Current Status and Future Perspectives. Pharmaceutics 2019, 11, 302. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, J.; Yang, Y.; Liang, H.; Jia, H.; Li, D. Current Trends of Targeted Drug Delivery for Oral Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 618931. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.-R.; Steinmetz, N.F.; Zhu, H. New Directions for Drug Delivery in Cancer Therapy. Mol. Pharm. 2018, 15, 3601–3602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, R.; Lei, L.; Yang, Y.; Hu, T. Drug delivery systems for oral disease applications. J. Appl. Oral Sci. Rev. FOB 2022, 30, e20210349. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.E.; Raza, A.; Gholami, M.; Giles, M.; Al-Sammak, R.; Ibrahim, A.; Ebrahimi Shahmabadi, H.; Sharma, L.A. Advanced Drug Delivery Platforms for the Treatment of Oral Pathogens. Pharmaceutics 2022, 14, 2293. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Patel, D.; Hickson, B.; DesRochers, J.; Hu, X. Recent Progress in Biopolymer-Based Hydrogel Materials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 1415. [Google Scholar] [CrossRef]
- Wei, Y.; Zheng, L.; Xie, X.; Yang, X.; Liao, J. Recent advances in stimuli responsive hydrogels for oral disease treatment. Mater. Des. 2024, 240, 112817. [Google Scholar] [CrossRef]
- Zhao, Y.; Ran, B.; Xie, X.; Gu, W.; Ye, X.; Liao, J. Developments on the Smart Hydrogel-Based Drug Delivery System for Oral Tumor Therapy. Gels 2022, 8, 741. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gu, Z.; Chen, X.; Shi, C.; Liu, C.; Liu, M.; Wang, L.; Sun, M.; Zhang, K.; Liu, Q.; et al. An injectable and thermosensitive hydrogel: Promoting periodontal regeneration by controlled-release of aspirin and erythropoietin. Acta Biomater. 2019, 86, 235–246. [Google Scholar] [CrossRef] [PubMed]
- van Dam, E.P.; Yuan, H.; Kouwer, P.H.J.; Bakker, H.J. Structure and Dynamics of a Temperature-Sensitive Hydrogel. J. Phys. Chem. B 2021, 125, 8219–8224. [Google Scholar] [CrossRef]
- Trucillo, P. Biomaterials for Drug Delivery and Human Applications. Materials 2024, 17, 456. [Google Scholar] [CrossRef]
- Han, F.; Meng, Q.; Xie, E.; Li, K.; Hu, J.; Chen, Q.; Li, J.; Han, F. Engineered biomimetic micro/nano-materials for tissue regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1205792. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Qiang, J.M.J.; Wang, C.G.; Chan, C.Y.; Zhu, Q.; Ye, E.; Li, Z.; Loh, X.J. Flexible polymeric patch based nanotherapeutics against non-cancer therapy. Bioact. Mater. 2022, 18, 471–491. [Google Scholar] [CrossRef] [PubMed]
- Alshangiti, D.M.; El-Damhougy, T.K.; Zaher, A.; Madani, M.; Ghobashy, M.M. Revolutionizing biomedicine: Advancements, applications, and prospects of nanocomposite macromolecular carbohydrate-based hydrogel biomaterials: A review. RSC Adv. 2023, 13, 35251–35291. [Google Scholar] [CrossRef] [PubMed]
- Conte, R.; De Luise, A.; Valentino, A.; Di Cristo, F.; Petillo, O.; Riccitiello, F.; Di Salle, A.; Calarco, A.; Peluso, G. Chapter 10—Hydrogel Nanocomposite Systems: Characterization and Application in Drug-Delivery Systems. In Nanocarriers for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 319–349. [Google Scholar]
- Martínez-Ballesta, M.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New “Smart-Foods” for Health. Foods 2018, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Conte, R.; Marturano, V.; Peluso, G.; Calarco, A.; Cerruti, P. Recent Advances in Nanoparticle-Mediated Delivery of Anti-Inflammatory Phytocompounds. Int. J. Mol. Sci. 2017, 18, 709. [Google Scholar] [CrossRef] [PubMed]
- Conte, R.; Luca, I.D.; Valentino, A.; Salle, A.D.; Calarco, A.; Riccitiello, F.; Peluso, G. Recent advances in “bioartificial polymeric materials” based nanovectors. Phys. Sci. Rev. 2017, 2, 20160131. [Google Scholar] [CrossRef]
- Conte, R.; Calarco, A.; Peluso, G. Nanosized Biomaterials for Regenerative Medicine. Int. J. Nano Dimens. 2018, 9, 209–214. [Google Scholar]
- Mascarenhas-Melo, F.; Mathur, A.; Murugappan, S.; Sharma, A.; Tanwar, K.; Dua, K.; Singh, S.K.; Mazzola, P.G.; Yadav, D.N.; Rengan, A.K.; et al. Inorganic nanoparticles in dermopharmaceutical and cosmetic products: Properties, formulation development, toxicity, and regulatory issues. Eur. J. Pharm. Biopharm. 2023, 192, 25–40. [Google Scholar] [CrossRef]
- Barabanova, A.I.; Afanas’ev, E.S.; Molchanov, V.S.; Askadskii, A.A.; Philippova, O.E. Unmodified Silica Nanoparticles Enhance Mechanical Properties and Welding Ability of Epoxy Thermosets with Tunable Vitrimer Matrix. Polymers 2021, 13, 3040. [Google Scholar] [CrossRef] [PubMed]
- Ferdiana, N.A.; Bahti, H.H.; Kurnia, D.; Wyantuti, S. Synthesis, characterization, and electrochemical properties of rare earth element nanoparticles and its application in electrochemical nanosensor for the detection of various biomolecules and hazardous compounds: A review. Sens. Bio-Sens. Res. 2023, 41, 100573. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-Hydrogel: A Hybrid Biomaterial System for Localized Drug Delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061. [Google Scholar] [CrossRef] [PubMed]
- Dannert, C.; Stokke, B.T.; Dias, R.S. Nanoparticle-Hydrogel Composites: From Molecular Interactions to Macroscopic Behavior. Polymers 2019, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Khizar, S.; Zine, N.; Errachid, A.; Elaissari, A. Introduction to Stimuli-Responsive Materials and Their Biomedical Applications. In Stimuli-Responsive Materials for Biomedical Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2023; Volume 1436, pp. 1–30. [Google Scholar]
- Jooken, S.; Deschaume, O.; Bartic, C. Nanocomposite Hydrogels as Functional Extracellular Matrices. Gels 2023, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.; Meehan, J.; Gray, M.E.; Murray, A.F.; Argyle, D.J.; Kunkler, I.H.; Langdon, S.P. The impact of tumour pH on cancer progression: Strategies for clinical intervention. Explor. Target. Anti-Tumor Ther. 2020, 1, 71–100. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Lavrador, P.; Esteves, M.R.; Gaspar, V.M.; Mano, J.F. Stimuli-Responsive Nanocomposite Hydrogels for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2005941. [Google Scholar] [CrossRef]
- Morey, M.; Pandit, A. Responsive triggering systems for delivery in chronic wound healing. Adv. Drug Deliv. Rev. 2018, 129, 169–193. [Google Scholar] [CrossRef]
- Dang, T.T.; Thai, A.V.; Cohen, J.; Slosberg, J.E.; Siniakowicz, K.; Doloff, J.C.; Ma, M.; Hollister-Lock, J.; Tang, K.M.; Gu, Z.; et al. Enhanced function of immuno-isolated islets in diabetes therapy by co-encapsulation with an anti-inflammatory drug. Biomaterials 2013, 34, 5792–5801. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; He, Z. ROS-responsive drug delivery systems for biomedical applications. Asian J. Pharm. Sci. 2018, 13, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, G.; Liu, S. Enzyme-responsive polymeric assemblies, nanoparticles and hydrogels. Chem. Soc. Rev. 2012, 41, 5933–5949. [Google Scholar] [CrossRef] [PubMed]
- Ooi, H.W.; Hafeez, S.; van Blitterswijk, C.A.; Moroni, L.; Baker, M.B. Hydrogels that listen to cells: A review of cell-responsive strategies in biomaterial design for tissue regeneration. Mater. Horiz. 2017, 4, 1020–1040. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Matsunaga, Y.T. Thermo-responsive polymers and their application as smart biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla-Sierra, L.; García-Arévalo, C.; Rodriguez-Cabello, J.C. Self-assembly in elastin-like recombinamers: A mechanism to mimic natural complexity. Mater. Today Bio 2019, 2, 100007. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef]
- Liras, M.; Quijada-Garrido, I.; García, O. QDs decorated with thiol-monomer ligands as new multicrosslinkers for the synthesis of smart luminescent nanogels and hydrogels. Polym. Chem. 2017, 8, 5317–5326. [Google Scholar] [CrossRef]
- Rapp, T.L.; DeForest, C.A. Visible Light-Responsive Dynamic Biomaterials: Going Deeper and Triggering More. Adv. Healthc. Mater. 2020, 9, e1901553. [Google Scholar] [CrossRef]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and Applications of Photoresponsive Hydrogels. Adv. Mater. Deerfield Beach Fla. 2019, 31, e1807333. [Google Scholar] [CrossRef]
- Wojtecki, R.J.; Meador, M.A.; Rowan, S.J. Using the dynamic bond to access macroscopically responsive structurally dynamic polymers. Nat. Mater. 2011, 10, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Kabb, C.P.; O’Bryan, C.S.; Deng, C.C.; Angelini, T.E.; Sumerlin, B.S. Photoreversible Covalent Hydrogels for Soft-Matter Additive Manufacturing. ACS Appl. Mater. Interfaces 2018, 10, 16793–16801. [Google Scholar] [CrossRef] [PubMed]
- Mertz, D.; Harlepp, S.; Goetz, J.; Bégin, D.; Schlatter, G.; Bégin-Colin, S.; Hébraud, A. Nanocomposite Polymer Scaffolds Responding under External Stimuli for Drug Delivery and Tissue Engineering Applications. Adv. Ther. 2020, 3, 1900143. [Google Scholar] [CrossRef]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Huang, J.; Wang, Z.; Sigdel, K.; Hu, Q.; Xuan, M.; Xie, H. Environment-sensitive hydrogels as potential drug delivery systems for the treatment of periodontitis. Mater. Express 2020, 10, 975–985. [Google Scholar] [CrossRef]
- Kim, A.R.; Kim, J.H.; Kim, A.; Sohn, Y.; Cha, J.H.; Bak, E.J.; Yoo, Y.J. Simvastatin attenuates tibial bone loss in rats with type 1 diabetes and periodontitis. J. Transl. Med. 2018, 16, 306. [Google Scholar] [CrossRef]
- Chen, N.; Ren, R.; Wei, X.; Mukundan, R.; Li, G.; Xu, X.; Zhao, G.; Zhao, Z.; Lele, S.M.; Reinhardt, R.A.; et al. Thermoresponsive Hydrogel-Based Local Delivery of Simvastatin for the Treatment of Periodontitis. Mol. Pharm. 2021, 18, 1992–2003. [Google Scholar] [CrossRef]
- Valentino, A.; Conte, R.; De Luca, I.; Di Cristo, F.; Peluso, G.; Bosetti, M.; Calarco, A. Thermo-Responsive Gel Containing Hydroxytyrosol-Chitosan Nanoparticles (Hyt@tgel) Counteracts the Increase of Osteoarthritis Biomarkers in Human Chondrocytes. Antioxidants 2022, 11, 1210. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.T.; Phewchan, P.; Navesit, K.; Chokamonsirikun, A.; Khemwong, T.; Tiyaboonchai, W. Development of Metronidazole-loaded In situ Thermosensitive Hydrogel for Periodontitis Treatment. Turk. J. Pharm. Sci. 2021, 18, 510–516. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Li, J.; Tang, M.; Chen, H.; Wang, G.; Guo, J.; Gui, S. Microemulsion-thermosensitive gel composites as in situ-forming drug reservoir for periodontitis tissue repair through alveolar bone and collagen regeneration strategy. Pharm. Dev. Technol. 2023, 28, 30–39. [Google Scholar] [CrossRef]
- Wang, H.; Chang, X.; Ma, Q.; Sun, B.; Li, H.; Zhou, J.; Hu, Y.; Yang, X.; Li, J.; Chen, X.; et al. Bioinspired drug-delivery system emulating the natural bone healing cascade for diabetic periodontal bone regeneration. Bioact. Mater. 2023, 21, 324–339. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Winer, D.; Goh, C.; Shrestha, A. Injectable thermosensitive hydrogel to modulate tolerogenic dendritic cells under hyperglycemic condition. Biomater. Sci. 2023, 11, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Aminu, N.; Chan, S.Y.; Yam, M.F.; Toh, S.M. A dual-action chitosan-based nanogel system of triclosan and flurbiprofen for localised treatment of periodontitis. Int. J. Pharm. 2019, 570, 118659. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.-C.; Chang, C.-Y.; Chao, Y.-C.; Jheng, Y.-H.; Yang, C.; Lee, N.; Yu, S.-H.; Yu, X.-H.; Liu, D.-M.; Chang, P.-C. pH-Responsive Hydrogel with an Anti-Glycation Agent for Modulating Experimental Periodontitis. J. Periodontol. 2016, 87, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Bako, J.; Toth, F.; Gall, J.; Kovacs, R.; Csík, A.; Varga, I.; Sculean, A.; Zelko, R.; Hegedus, C. Combined Release of Antiseptic and Antibiotic Drugs from Visible Light Polymerized Biodegradable Nanocomposite Hydrogels for Periodontitis Treatment. Pharmaceutics 2022, 14, 957. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Yang, J.; Chi, Y.; Wen, P.; Wang, Z.; Yu, S.; Xue, R.; Fan, J.; Li, H.; Chen, W.; et al. Natural polyphenol self-assembled pH-responsive nanoparticles loaded into reversible hydrogel to inhibit oral bacterial activity. Mol. Biomed. 2022, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar]
- D’Souza, S.; Addepalli, V. Preventive measures in oral cancer: An overview. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 107, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahayri, Z.N.; AlAhmad, M.M.; Ali, B.R. Current opinion on the pharmacogenomics of paclitaxel-induced toxicity. Expert. Opin. Drug Metab. Toxicol. 2021, 17, 785–801. [Google Scholar] [CrossRef]
- Choi, S.G.; Lee, S.-E.; Kang, B.-S.; Ng, C.L.; Davaa, E.; Park, J.-S. Thermosensitive and Mucoadhesive Sol-Gel Composites of Paclitaxel/Dimethyl-β-Cyclodextrin for Buccal Delivery. PLoS ONE 2014, 9, e109090. [Google Scholar] [CrossRef]
- Ortega, A.; da Silva, A.B.; da Costa, L.M.; Zatta, K.C.; Onzi, G.R.; da Fonseca, F.N.; Guterres, S.S.; Paese, K. Thermosensitive and mucoadhesive hydrogel containing curcumin-loaded lipid-core nanocapsules coated with chitosan for the treatment of oral squamous cell carcinoma. Drug Deliv. Transl. Res. 2023, 13, 642–657. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Shi, S.; Dong, P.; Kan, B.; Gou, M.; Wang, X.; Li, X.; Luo, F.; Zhao, X.; Wei, Y.; et al. Synthesis and characterization of PEG-PCL-PEG thermosensitive hydrogel. Int. J. Pharm. 2009, 365, 89–99. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; SamariKhalaj, M.; Aguilar, L.E.; Park, C.H.; Kim, C.S. pH/NIR Light-Controlled Multidrug Release via a Mussel-Inspired Nanocomposite Hydrogel for Chemo-Photothermal Cancer Therapy. Sci. Rep. 2016, 6, 33594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, M.; Zeng, Y.; Wu, L.; Wang, Q.; Han, X.; Liu, X.; Liu, J. Chlorin e6 Conjugated Poly(dopamine) Nanospheres as PDT/PTT Dual-Modal Therapeutic Agents for Enhanced Cancer Therapy. ACS Appl. Mater. Interfaces 2015, 7, 8176–8187. [Google Scholar] [CrossRef]
- Su, J.; Chen, F.; Cryns, V.L.; Messersmith, P.B. Catechol polymers for pH-responsive, targeted drug delivery to cancer cells. J. Am. Chem. Soc. 2011, 133, 11850–11853. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-J.; Cheng, Y.-J.; Zhang, X.-Z. Recent advances in nanomaterials for enhanced photothermal therapy of tumors. Nanoscale 2018, 10, 22657–22672. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, F.; Huang, N.; Li, J.; Wu, C.; Tan, B.; Liu, Y.; Li, L.; Yang, C.; Shao, D.; et al. Near-infrared light-responsive hybrid hydrogels for the synergistic chemo-photothermal therapy of oral cancer. Nanoscale 2021, 13, 17168–17182. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.P.; Shah, S.V.; Shukla, S.N.; Shah, P.M.; Patel, P.S. Clinical significance of MMP-2 and MMP-9 in patients with oral cancer. Head. Neck 2007, 29, 564–572. [Google Scholar] [CrossRef]
- Li, W.; Tao, C.; Wang, J.; Le, Y.; Zhang, J. MMP-responsive in situ forming hydrogel loaded with doxorubicin-encapsulated biodegradable micelles for local chemotherapy of oral squamous cell carcinoma. RSC Adv. 2019, 9, 31264–31273. [Google Scholar] [CrossRef]
- Wang, H.H.; Fu, Z.G.; Li, W.; Li, Y.X.; Zhao, L.S.; Wen, L.; Zhang, J.J.; Wen, N. The synthesis and application of nano doxorubicin- indocyanine green matrix metalloproteinase-responsive hydrogel in chemophototherapy for head and neck squamous cell carcinoma. Int. J. Nanomed. 2019, 14, 623–638. [Google Scholar] [CrossRef]
- Galler, K.M.; Weber, M.; Korkmaz, Y.; Widbiller, M.; Feuerer, M. Inflammatory Response Mechanisms of the Dentine-Pulp Complex and the Periapical Tissues. Int. J. Mol. Sci. 2021, 22, 1480. [Google Scholar] [CrossRef]
- Abbott, P.V. Present status and future directions: Managing endodontic emergencies. Int. Endod. J. 2022, 55 (Suppl. S3), 778–803. [Google Scholar] [CrossRef] [PubMed]
- Duncan, H.F.; Cooper, P.R.; Smith, A.J. Dissecting dentine-pulp injury and wound healing responses: Consequences for regenerative endodontics. Int. Endod. J. 2019, 52, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Riccitiello, F.; De Luise, A.; Conte, R.; D’Aniello, S.; Vittoria, V.; Di Salle, A.; Calarco, A.; Peluso, G. Effect of resveratrol release kinetic from electrospun nanofibers on osteoblast and osteoclast differentiation. Eur. Polym. J. 2018, 99, 289–297. [Google Scholar] [CrossRef]
- Albuquerque, M.T.; Valera, M.C.; Nakashima, M.; Nör, J.E.; Bottino, M.C. Tissue-engineering-based strategies for regenerative endodontics. J. Dent. Res. 2014, 93, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.S.; Bordini, E.A.F.; Ferreira, J.A.; Mei, L.; Dubey, N.; Fenno, J.C.; Piva, E.; Lund, R.G.; Schwendeman, A.; Bottino, M.C. Injectable MMP-Responsive Nanotube-Modified Gelatin Hydrogel for Dental Infection Ablation. ACS Appl. Mater. Interfaces 2020, 12, 16006–16017. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xing, X.; Peng, W.; Huang, C.; Du, Y.; Yang, H.; Zhou, J. Fabrication of an exosome-loaded thermosensitive chitin-based hydrogel for dental pulp regeneration. J. Mater. Chem. B 2023, 11, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Li, Z.; Hwang, I.N.; Huh, K.M.; Min, K.S. Glycol chitin-based thermoresponsive hydrogel scaffold supplemented with enamel matrix derivative promotes odontogenic differentiation of human dental pulp cells. J. Endod. 2013, 39, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Al-Zyoud, W.; Haddadin, D.; Hasan, S.A.; Jaradat, H.; Kanoun, O. Biocompatibility Testing for Implants: A Novel Tool for Selection and Characterization. Materials 2023, 16, 6881. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Yu, A.C.; Chen, H.; Chan, D.; Agmon, G.; Stapleton, L.M.; Sevit, A.M.; Tibbitt, M.W.; Acosta, J.D.; Zhang, T.; Franzia, P.W.; et al. Scalable manufacturing of biomimetic moldable hydrogels for industrial applications. Proc. Natl. Acad. Sci. USA 2016, 113, 14255–14260. [Google Scholar] [CrossRef] [PubMed]
- Lottes, A.E.; Cavanaugh, K.J.; Chan, Y.Y.; Devlin, V.J.; Goergen, C.J.; Jean, R.; Linnes, J.C.; Malone, M.; Peat, R.; Reuter, D.G.; et al. Navigating the Regulatory Pathway for Medical Devices-a Conversation with the FDA, Clinicians, Researchers, and Industry Experts. J. Cardiovasc. Transl. Res. 2022, 15, 927–943. [Google Scholar] [CrossRef] [PubMed]


| Nanocomposite Hydrogel Composition | Action | Main Finding | Reference |
|---|---|---|---|
| Pluronic F-127 hydrogel containing chitosan nanoparticles (Hyt-NPs) | in situ forming hydrogel for localized drug delivery of Hydroxytyrosol | Efficient and sustained Hyt delivery for osteoarthritis treatment. | [60] |
| Poloxamer 407 combined with Methylcellulose (MC), and Silk Fibroin (SF) nanostructures | Delivery of metronidazole (MTZ) in periodontal pocket | Local delivery of the drug to the oral infection site. | [61] |
| PDLLA-PEG-PDLLA (PPP) with Metformin-loaded Mesoporous Silica Nanoparticles (MSNs) | Temperature-sensitive devices for diabetic periodontal regeneration | Emulation of the mesenchymal stem cell “recruitment-osteogenesis” cascade for diabetic periodontal bone regeneration. | [63] |
| Chitosan hydrogel with Triclosan (TCS) Nanoparticles | pH-sensitive hydrogels for anti-inflammatory and antimicrobial treatment of periodontitis | Dual antibacterial and anti-inflammatory effects with an excellent therapeutic outcome | [65] |
| MPGA/Methacrylated-poly-γ-glutamic Acid Nanoparticles (PGA-MNP) | pH-sensitive hydrogels with metronidazole and chlorhexidine (CHX) for antibacterial activity | Fast photopolymerizable system which can be loaded with the required amount of medicines and can reduce the side effects of the systemic use of drugs | [67] |
| Turkish Galls Effective Constituent (TGEC) Nanoparticles (T-NPs) in in situ hydrogel | pH-sensitive and thermosensitive in-situ hydrogel for antibacterial activity | Green solution to prepare nanospheres with natural polyphenols | [68] |
| Nanocomposite Hydrogel Composition | Action | Main Finding | Reference |
|---|---|---|---|
| (2,6-di-O-methyl)-β-cyclodextrin (DMβCD) in poloxamer 407 hydrogel | Localized paclitaxel delivery to mucous membrane for extended interaction with oral tumors | Improvement of the in vitro release and cytotoxic effect of paclitaxel. | [72] |
| Curcumin-loaded lipid-core nanocapsules coated with chitosan in poloxamer 407 hydrogel | Temperature-sensitive hydrogels for improved adhesion to oral mucosa and reduced activity of epidermoid oral cancer cells | mucoadhesive system with potential to deliver buccal treatments. | [73] |
| PEG-PCL-PEG (PECE) hydrogels with nanoparticles | Long-lasting temperature-sensitive hydrogels for oral cavity application, effective for over 14 days | Excellent thermosensitivity and biodegradability | [74] |
| pNIPAAm-co-pAAm hydrogels with dopamine nanoparticles | Synergistic therapy for oral cancer through photothermal therapy (PTT) and multidrug chemotherapy | Enhanced cellular uptake and subsequently greater reactive oxygen species (ROS) production upon laser irradiation | [75,76] |
| Diselenide-bridged doxorubicin-loaded mesoporous silica nanoparticles with IR820-packaged methylcellulose hydrogels) | Photothermal agent generating thermal energy and reactive oxygen species (ROS) upon NIR light exposure to target oral cancer cells | The combination of chemotherapy and phototherapy gives a long-lasting synergistic anti-tumor effect | [79] |
| MMP-responsive peptide cross-linked hyaluronic acid hydrogels with doxorubicin-loaded PDLLA-PEG-PDLLA nano micelles | High rate of drug release and degradation in the MMP-rich microenvironment of oral cancer | Growth inhibition of oral squamous cell carcinoma without any damage to the organs | [81] |
| Doxorubicin-ICG matrix MMP-responsive nanoparticles in hyaluronic acid-acrylate (HA-Ac) hydrogels | Enhanced ROS production and tumor ablation upon laser exposure | Favorable synergistic antitumor efficacy and acceptable biosafety | [82] |
| Nanocomposite Hydrogel Composition | Action | Main Finding | Reference |
|---|---|---|---|
| MMP-sensitive GelMA hydrogels with nanotubes | Encapsulation and controlled release of chlorhexidine in response to MMP stimulation, alleviating inflammation in endodontic infections | Inhibition of bacterial growth with minimal cell toxicity | [88] |
| Chitin-based hydrogels encapsulating DPSCs-derived nano exosomes | Enhancement of pulp-like tissue formation within the root canal | Functional device that uses exosomes as biomimetic tools for tissue engineering | [89] |
| N-acetylated glycol chitosan (glycol chitin-based hydrogels) | Temperature-sensitive gel promoting odontogenic differentiation of Dental Pulp Stem Cells (DPSCs) and elevated expression levels of dentin sialophosphoprotein and dentin matrix protein-1, biomarkers of odontogenesis | This material improves the hDPCs ability to generate clonogenic adherent cell clusters. | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conte, R.; Valentino, A.; Romano, S.; Margarucci, S.; Petillo, O.; Calarco, A. Stimuli-Responsive Nanocomposite Hydrogels for Oral Diseases. Gels 2024, 10, 478. https://doi.org/10.3390/gels10070478
Conte R, Valentino A, Romano S, Margarucci S, Petillo O, Calarco A. Stimuli-Responsive Nanocomposite Hydrogels for Oral Diseases. Gels. 2024; 10(7):478. https://doi.org/10.3390/gels10070478
Chicago/Turabian StyleConte, Raffaele, Anna Valentino, Silvia Romano, Sabrina Margarucci, Orsolina Petillo, and Anna Calarco. 2024. "Stimuli-Responsive Nanocomposite Hydrogels for Oral Diseases" Gels 10, no. 7: 478. https://doi.org/10.3390/gels10070478






