Development of Clindamycin-Releasing Polyvinyl Alcohol Hydrogel with Self-Healing Property for the Effective Treatment of Biofilm-Infected Wounds
Abstract
:1. Introduction
2. Results and Discussion
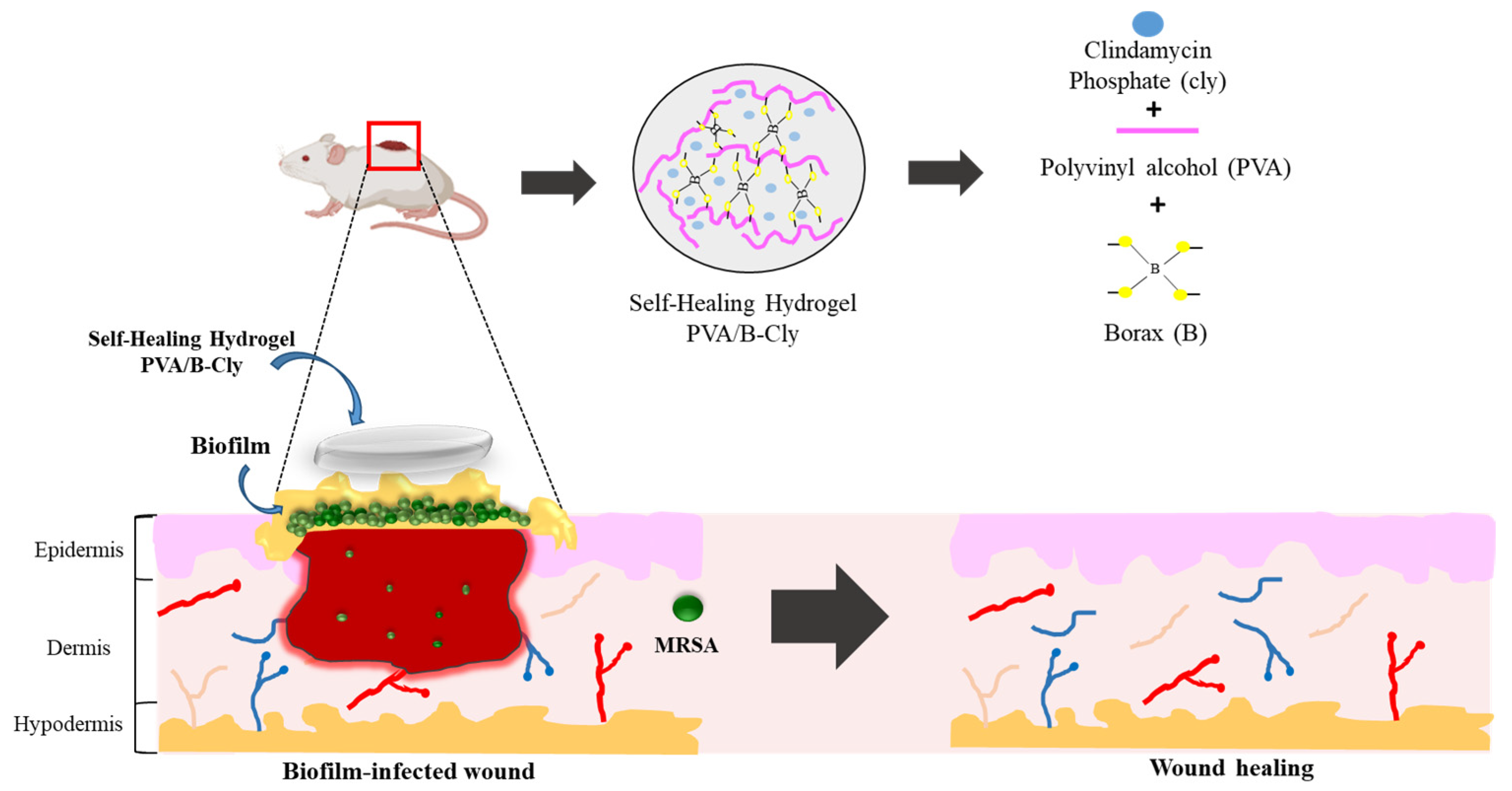
2.1. Preparation of the PVA/B and PVA/B-Cly Self-Healing Hydrogel
2.2. Physicochemical Characterization of the Self-Healing Hydrogel
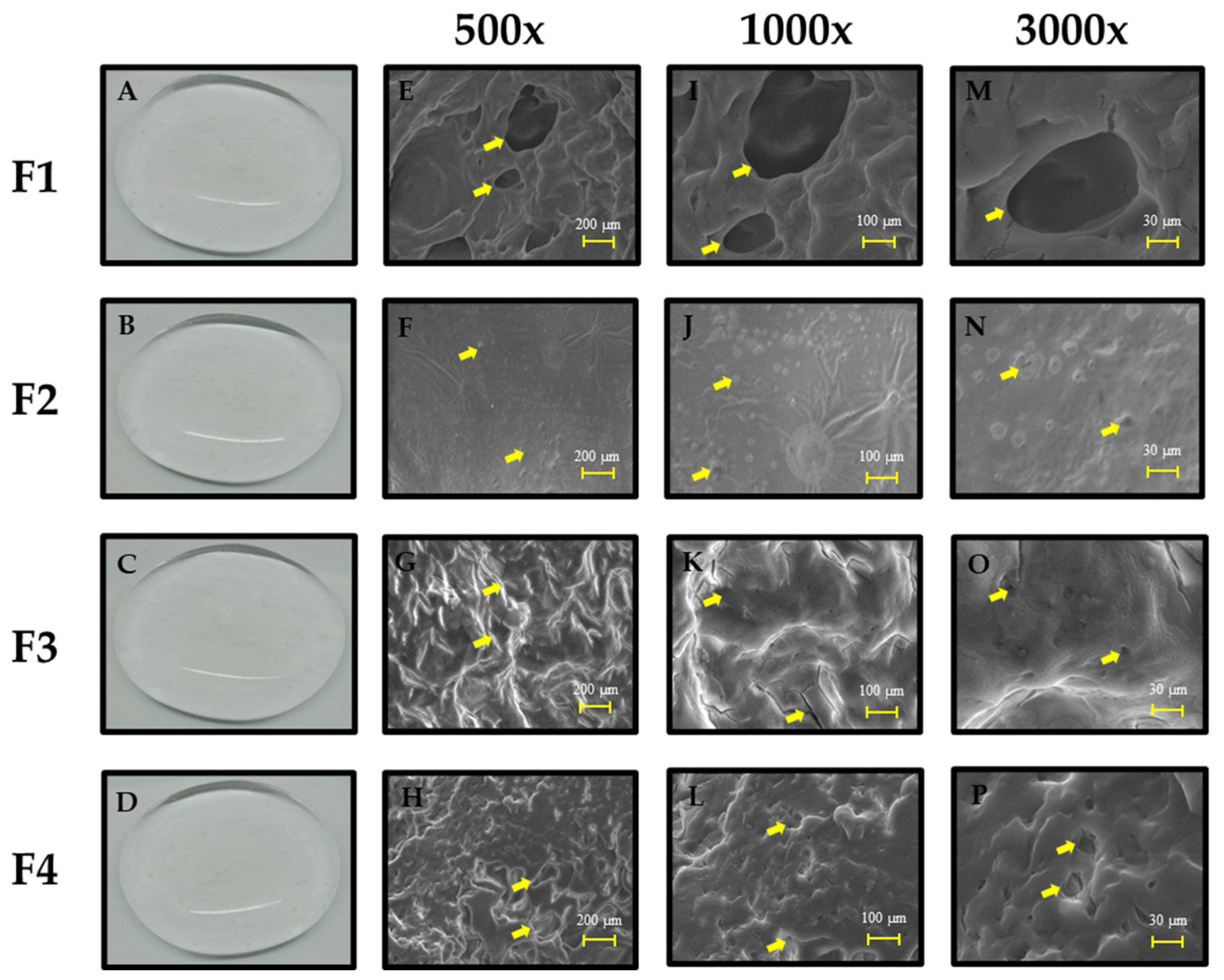
2.2.1. Morphological Analysis of Self-Healing Hydrogel PVA/B and PVA/B-Cly
2.2.2. FTIR Characterization of Self-Healing Hydrogel
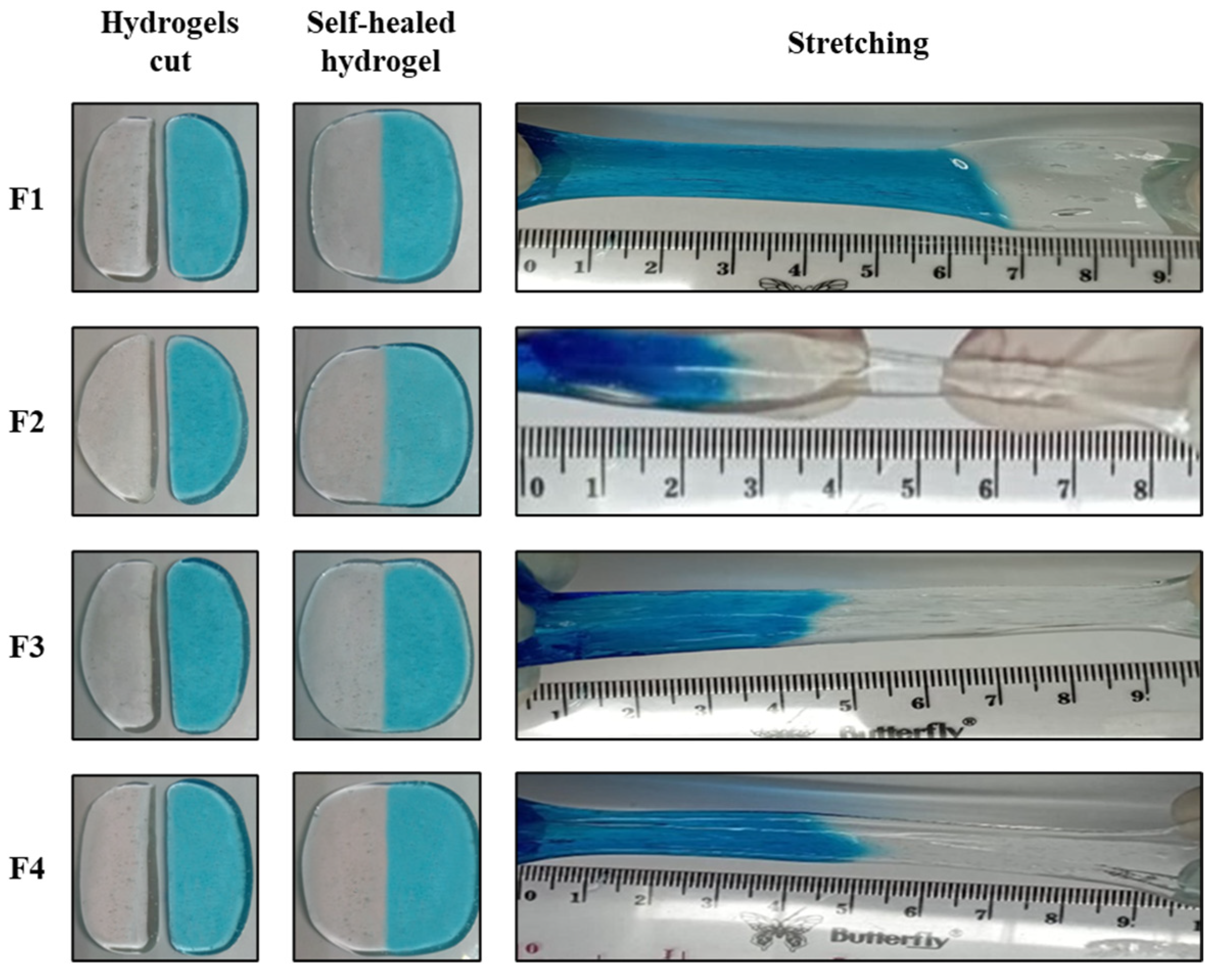
2.2.3. Self-Healing Behavior of PVA/B and PVA/B-Cly
2.2.4. pH Characterization of Self-Healing Hydrogel
2.2.5. Drug Content
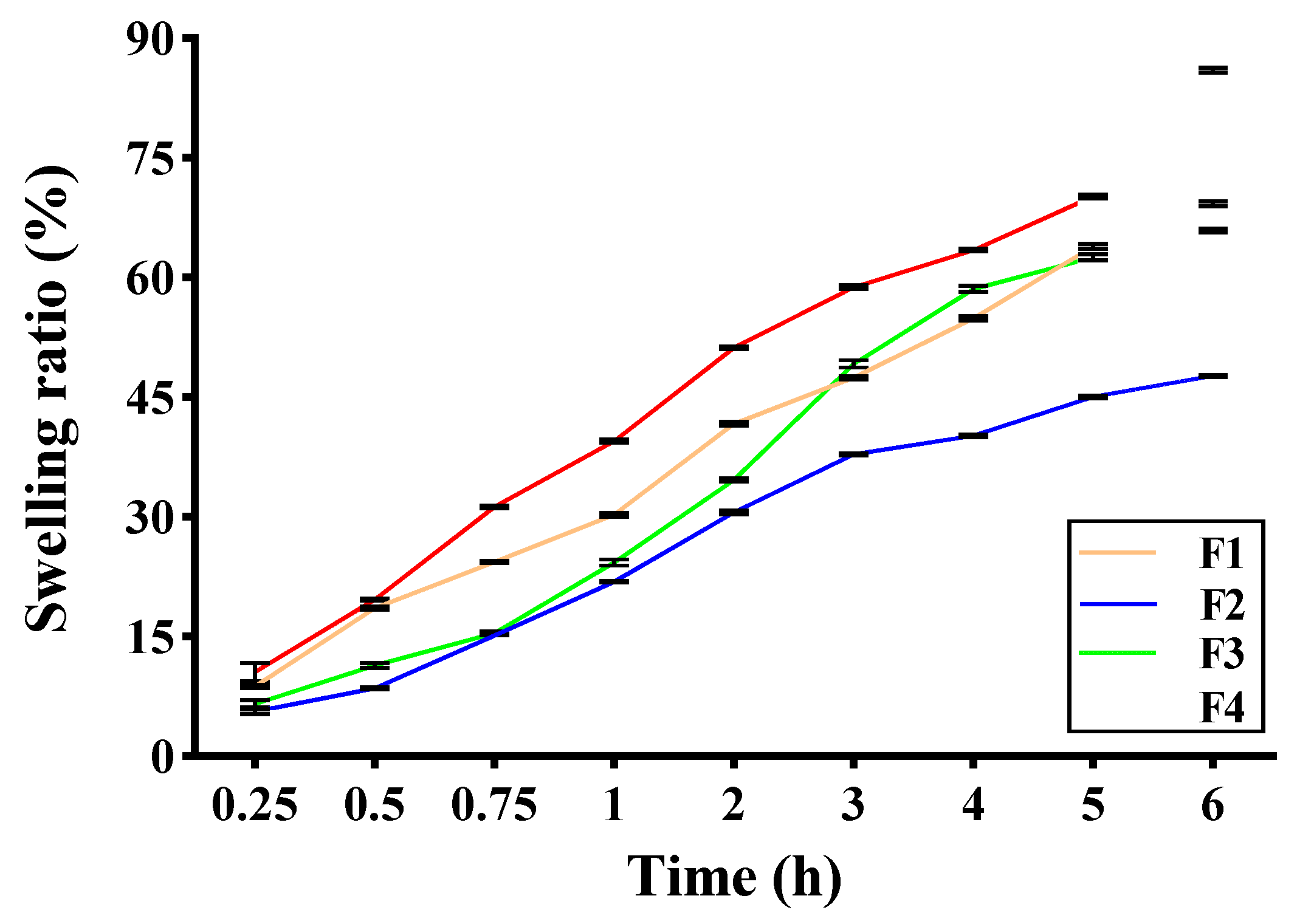
2.2.6. Swelling Ratio of Self-Healing Hydrogel
2.2.7. Stability Study
2.2.8. In Vitro Drug Release
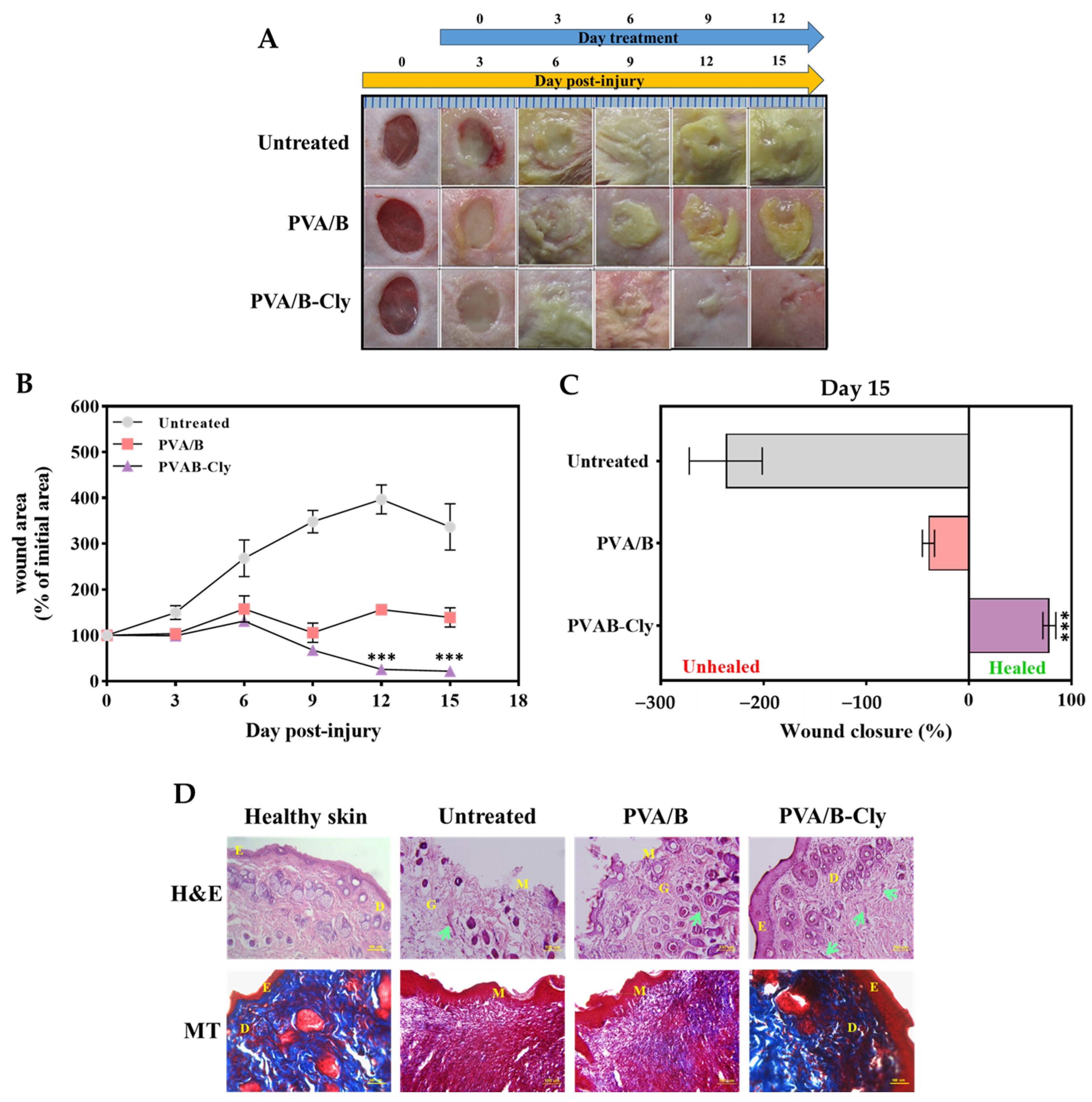
2.2.9. In Vivo Wound-Healing Activity
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Self-Healing Hydrogels
4.3. Physicochemical Characterization of the Self-Healing Hydrogels
4.3.1. Organoleptic Properties
4.3.2. Scanning Electron Microscopy (SEM)
4.3.3. Fourier Transform Infrared Spectroscopy (FTIR) Characterization
4.3.4. Self-Healing Behavior of PVA/B and PVA/B-Cly
4.3.5. pH of Self-Healing Hydrogel
4.3.6. PVA/B-Cly Self-Healing Drug Content
4.3.7. Swelling Ratio of Self-Healing Hydrogel
4.3.8. Stability Study
4.4. In Vitro Drug Release
4.5. In Vivo Wound Healing
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Fatease, A.; Abourehab, M.A.; Alqahtani, A.M.; Chidambaram, K.; Qureshi, A.A.; Venkatesan, K.; Alshahrani, S.M.; Abdelkader, H. Polymeric/Dextran Wafer Dressings as Promising Long-Acting Delivery Systems for Curcumin Topical Delivery and Enhancing Wound Healing in Male Wistar Albino Rats. Pharmaceuticals 2022, 16, 38. [Google Scholar] [CrossRef]
- Alghamdi, B.A.; Al-Johani, I.; Al-Shamrani, J.M.; Alshamrani, H.M.; Al-Otaibi, B.G.; Almazmomi, K.; Yusof, N.Y. Antimicrobial resistance in methicillin-resistant Staphylococcus aureus. Saudi J. Biol. Sci. 2023, 30, 103604. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Cao, J.; Lee, J.; Kim, H.; Yoo, J.-W. Development of clindamycin-loaded alginate/pectin/hyaluronic acid composite hydrogel film for the treatment of MRSA-infected wounds. J. Pharm. Investig. 2021, 51, 597–610. [Google Scholar] [CrossRef]
- Choi, M.; Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Yoo, J.W. Chitosan-based nitric oxide-releasing dressing for anti-biofilm and in vivo healing activities in MRSA biofilm-infected wounds. Int. J. Biol. Macromolecules 2020, 142, 680–692. [Google Scholar] [CrossRef]
- Hasan, N.; Cao, J.; Lee, J.; Naeem, M.; Hlaing, S.P.; Kim, J.; Jung, Y.; Lee, B.-L.; Yoo, J.-W. PEI/NONOates-doped PLGA nanoparticles for eradicating methicillin-resistant Staphylococcus aureus biofilm in diabetic wounds via binding to the biofilm matrix. Mater. Sci. Eng. C 2019, 103, 109741. [Google Scholar] [CrossRef] [PubMed]
- Chaiwarit, T.; Rachtanapun, P.; Kantrong, N.; Jantrawut, P. Preparation of clindamycin hydrochloride loaded de-esterified low-methoxyl mango peel pectin film used as a topical drug delivery system. Polymers 2020, 12, 1006. [Google Scholar] [CrossRef]
- Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Oshi, M.A.; Naeem, M.; Ki, M.-H.; Lee, B.L.; Jung, Y.; Yoo, J.-W. Bacteria-targeted clindamycin loaded polymeric nanoparticles: Effect of surface charge on nanoparticle adhesion to MRSA, antibacterial activity, and wound healing. Pharmaceutics 2019, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Machowska, A.; Klara, J.; Ledwójcik, G.; Wójcik, K.; Dulińska-Litewka, J.; Karewicz, A. Clindamycin-loaded halloysite nanotubes as the antibacterial component of composite hydrogel for bone repair. Polymers 2022, 14, 5151. [Google Scholar] [CrossRef]
- Frei, C.R.; Miller, M.L.; Lewis, J.S.; Lawson, K.A.; Hunter, J.M.; Oramasionwu, C.U.; Talbert, R.L. Trimethoprim-sulfamethoxazole or clindamycin for community-associated MRSA (CA-MRSA) skin infections. J. Am. Board Fam. Med. 2010, 23, 714–719. [Google Scholar] [CrossRef]
- Sadeghi, S.; Nourmohammadi, J.; Ghaee, A.; Soleimani, N. Carboxymethyl cellulose-human hair keratin hydrogel with controlled clindamycin release as antibacterial wound dressing. Int. J. Biol. Macromol. 2020, 147, 1239–1247. [Google Scholar] [CrossRef]
- Rani Raju, N.; Silina, E.; Stupin, V.; Manturova, N.; Chidambaram, S.B.; Achar, R.R. Multifunctional and Smart Wound Dressings—A Review on Recent Research Advancements in Skin Regenerative Medicine. Pharmaceutics 2022, 14, 1574. [Google Scholar] [CrossRef] [PubMed]
- Devi VK, A.; Shyam, R.; Palaniappan, A.; Jaiswal, A.K.; Oh, T.-H.; Nathanael, A.J. Self-healing hydrogels: Preparation, mechanism and advancement in biomedical applications. Polymers 2021, 13, 3782. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Fan, Y.; Deng, Y.; Hu, L.; Sun, H.; Zheng, B.; Lu, D.; Guo, C.; Zhou, L. Production and Anti-Inflammatory Performance of PVA Hydrogels Loaded with Curcumin Encapsulated in Octenyl Succinic Anhydride Modified Schizophyllan as Wound Dressings. Molecules 2023, 28, 1321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Y.; Gu, Y.; Xue, P.; Xu, X. Self-healing and highly stretchable hydrogel for interfacial compatible flexible paper-based micro-supercapacitor. Materials 2021, 14, 1852. [Google Scholar] [CrossRef] [PubMed]
- Al-Emam, E.; Soenen, H.; Caen, J.; Janssens, K. Characterization of polyvinyl alcohol-borax/agarose (PVA-B/AG) double network hydrogel utilized for the cleaning of works of art. Herit. Sci. 2020, 8, 106. [Google Scholar] [CrossRef]
- Chen, Y.-N.; Jiao, C.; Zhao, Y.; Zhang, J.; Wang, H. Self-assembled polyvinyl alcohol–tannic acid hydrogels with diverse microstructures and good mechanical properties. ACS Omega 2018, 3, 11788–11795. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Hwang, S.Y.; Oh, D.X.; Park, J. Recent progress in self-healing polymers and hydrogels based on reversible dynamic B–O bonds: Boronic/boronate esters, borax, and benzoxaborole. J. Mater. Chem. A 2021, 9, 14630–14655. [Google Scholar] [CrossRef]
- Dwynda, I.; Zainul, R. Boric Acid (H3(BO3): Recognize The Molecular Interactions in Solutions. 2018. Available online: https://osf.io/preprints/inarxiv/6wead (accessed on 6 June 2023).
- Abouzeid, R.; Shayan, M.; Wu, T.; Gwon, J.; Karki, T.A.; Wu, Q. Highly flexible, self-bonding, self-healing, and conductive soft pressure sensors based on dicarboxylic cellulose nanofiber hydrogels. ACS Appl. Polym. Mater. 2023, 5, 7009–7021. [Google Scholar] [CrossRef] [PubMed]
- Dave, H.K.; Nath, K. Synthesis, Characterization and Application of Disodium Tetraborate Cross-Linked Polyvinyl Alcohol Membranes for Pervaporation Dehydration of Ethylene Glycol. Acta Chim. Slov. 2018, 65, 902–918. [Google Scholar] [CrossRef]
- Karvinen, J.; Kellomäki, M. Characterization of self-healing hydrogels for biomedical applications. Eur. Polym. J. 2022, 181, 111641. [Google Scholar] [CrossRef]
- Spoljaric, S.; Salminen, A.; Luong, N.D.; Seppälä, J. Stable, self-healing hydrogels from nanofibrillated cellulose, poly (vinyl alcohol) and borax via reversible crosslinking. Eur. Polym. J. 2014, 56, 105–117. [Google Scholar] [CrossRef]
- Huang, M.; Hou, Y.; Li, Y.; Wang, D.; Zhang, L. High performances of dual network PVA hydrogel modified by PVP using borax as the structure-forming accelerator. Des. Monomers Polym. 2017, 20, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, P.; Jain, V.; Shende, S.; Jain, P.K. Formulation development and evaluation of emulgel of clindamycin phosphate for effective treatment of acne. J. Drug Deliv. Ther. 2019, 9, 202–207. [Google Scholar]
- Sringam, J.; Pankongadisak, P.; Trongsatitkul, T.; Suppakarn, N. Improving mechanical properties of starch-based hydrogels using double network strategy. Polymers 2022, 14, 3552. [Google Scholar] [CrossRef]
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The effect of pH on the extracellular matrix and biofilms. Adv. Wound Care 2015, 4, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Bennison, L.; Miller, C.; Summers, R.; Minnis, A.; Sussman, G.; McGuiness, W. The pH of wounds during healing and infection: A descriptive literature review. Wound Pract. Res. J. Aust. Wound Manag. Assoc. 2017, 25, 63–69. [Google Scholar]
- Goswami, A.G.; Basu, S.; Banerjee, T.; Shukla, V.K.J. Biofilm and wound healing: From bench to bedside. Eur. J. Med. Res. 2023, 28, 157. [Google Scholar] [CrossRef]
- Derwin, R.; Patton, D.; Avsar, P.; Strapp, H.; Moore, Z. The impact of topical agents and dressing on pH and temperature on wound healing: A systematic, narrative review. Int. Wound J. 2022, 19, 1397–1408. [Google Scholar] [CrossRef]
- Ganji, F.; Vasheghani, F.S.; Vasheghani, F.E. Theoretical Description of Hydrogel Swelling: A Review. Iran. Polym. J. 2010, 19, 375–398. [Google Scholar]
- Chang, K.-Y.; Chou, Y.-N.; Chen, W.-Y.; Chen, C.-Y.; Lin, H.-R. Mussel-inspired adhesive and self-healing hydrogel as an injectable wound dressing. Polymers 2022, 14, 3346. [Google Scholar] [CrossRef]
- Narayan, S.; Choudhary, M. A review on stability studies of pharmaceutical products. Int. J. Appl. Pharm. Biol. Res. 2017, 2, 67–75. [Google Scholar]
- Kostrzębska, A.; Pączek, K.; Weselak, A.; Musiał, W. Effect of hydrogel substrate components on the stability of tetracycline hydrochloride and swelling activity against model skin sebum. Int. J. Mol. Sci. 2023, 24, 2678. [Google Scholar] [CrossRef] [PubMed]
- Larrea-Wachtendorff, D.; Del Grosso, V.; Ferrari, G. Evaluation of the physical stability of starch-based hydrogels produced by high-pressure processing (HPP). Gels 2022, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Abdella, S.; Abid, F.; Youssef, S.H.; Kim, S.; Afinjuomo, F.; Malinga, C.; Song, Y.; Garg, S. pH and its applications in targeted drug delivery. Drug Discov. Today 2023, 28, 103414. [Google Scholar] [CrossRef] [PubMed]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Hydrogels as drug delivery systems; pros and cons. Trends Pharm. Sci. 2019, 5, 7–24. [Google Scholar]
- Palungan, J.; Luthfiyah, W.; Mustopa, A.Z.; Nurfatwa, M.; Rahman, L.; Yulianty, R.; Wathoni, N.; Yoo, J.-W.; Hasan, N. The Formulation and Characterization of Wound Dressing Releasing S-Nitrosoglutathione from Polyvinyl Alcohol/Borax Reinforced Carboxymethyl Chitosan Self-Healing Hydrogel. Pharmaceutics 2024, 16, 344. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Juan, J.-M.; Kolodziejczyk, E.; Acquistapace, S.; Donato-Capel, L.; Wooster, T.J. Impact of protein gel porosity on the digestion of lipid emulsions. J. Agric. Food Chem. 2015, 63, 8829–8837. [Google Scholar] [CrossRef]
- Wang, L.; Fogliano, V.; Heising, J.; Dekker, M. The effect of pore size on the diffusion of volatile antimicrobials is a key factor to preserve gelled foods. Food Chem. 2021, 351, 129316. [Google Scholar] [CrossRef] [PubMed]
- Grassi, M.; Grassi, G.; Lapasin, R.; Colombo, I. Understanding Drug Release and Absorption Mechanisms: A Physical and Mathematical Approach; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Wen, L.; Liang, Y.; Lin, Z.; Xie, D.; Zheng, Z.; Xu, C.; Lin, B. Design of multifunctional food packaging films based on carboxymethyl chitosan/polyvinyl alcohol crosslinked network by using citric acid as crosslinker. Polymer 2021, 230, 124048. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, J.; Ran, L.; Yu, K.; Lu, B.; Lan, G.; Dai, F.; Lu, F.J.C.p. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr. Polym. 2018, 201, 522–531. [Google Scholar] [CrossRef]
- Bo, Y.; Zhang, L.; Wang, Z.; Shen, J.; Zhou, Z.; Yang, Y.; Wang, Y.; Qin, J.; He, Y. Antibacterial hydrogel with self-healing property for wound-healing applications. ACS Biomater. Sci. Eng. 2021, 7, 5135–5143. [Google Scholar] [CrossRef] [PubMed]
- Medrano-David, D.; Lopera, A.M.; Londoño, M.E.; Araque-Marín, P. Formulation and characterization of a new injectable bone substitute composed PVA/borax/CaCO3 and Demineralized Bone Matrix. J. Funct. Biomater. 2021, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Lee, J.; Ahn, H.-J.; Hwang, W.R.; Bahar, M.A.; Habibie, H.; Amir, M.N.; Lallo, S.; Son, H.-J.; Yoo, J.-W.J.P. Nitric oxide-releasing bacterial cellulose/chitosan crosslinked hydrogels for the treatment of polymicrobial wound infections. Pharmaceutics 2021, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.C.; Signini, R.; Rosa, L.M.; Dias, Y.S.P.; Vinaud, M.C.; Lino, R.d.S. Carboxymethyl chitosan hydrogel formulations enhance the healing process in experimental partial-thickness (second-degree) burn wound healing. Acta Cir. Bras. 2021, 36, e360303. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Shi, X.; Li, L.; Tan, Z.; Feng, F.; Li, J.; Pang, M.; Wang, X.; He, L. An injectable and self-healing hydrogel with controlled release of curcumin to repair spinal cord injury. Bioact. Mater. 2021, 6, 4816–4829. [Google Scholar] [CrossRef] [PubMed]
- Weerawan, N.; Chalitangkoon, J.; Monvisade, P. Self-healing hydrogels based on sodium carboxymethyl cellulose/poly (vinyl alcohol) reinforced with montmorillonite. Biointerface Res. Appl. Chem 2022, 12, 4770–4779. [Google Scholar]
- Kim, J.O.; Noh, J.-K.; Thapa, R.K.; Hasan, N.; Choi, M.; Kim, J.H.; Lee, J.-H.; Ku, S.K.; Yoo, J.-W. Nitric oxide-releasing chitosan film for enhanced antibacterial and in vivo wound-healing efficacy. Int. J. Biol. Macromol. 2015, 79, 217–225. [Google Scholar] [CrossRef]
- Shafiei, F.; Ghavami-Lahiji, M.; Kashi, T.S.J.; Najafi, F. Drug release kinetics and biological properties of a novel local drug carrier system. Dent. Res. J. 2021, 18, 94. [Google Scholar]



| Formulation Code | PVA (%wt) | Borax (B, %wt) | Clindamycin Phosphate (Cly, %wt) |
|---|---|---|---|
| F1 | 4 | 1.6 | - |
| F2 | 4 | 0.8 | 1 |
| F3 | 4 | 1.2 | 1 |
| F4 | 4 | 1.6 | 1 |
| Formulation Code | Healing Time (min) | pH | Drug Loading (%) |
|---|---|---|---|
| F1 | 14.00 | 7.36 | NA |
| F2 | 6.96 | 7.56 | 98.72 |
| F3 | 9.18 | 7.65 | 97.77 |
| F4 | 12.42 | 7.63 | 98.34 |
| Kinetic Model (R2) | |||||
|---|---|---|---|---|---|
| Zero–Order | First–Order | Higuchi | Korsmeyer–Peppas | Hixson–Crowel | |
| F2 | 0.6693 | 0.9224 | 0.9738 | 0.9722 | 0.8636 |
| F3 | 0.2929 | 0.9015 | 0.9545 | 0.9848 | 0.8359 |
| F4 | 0.2719 | 0.9293 | 0.9485 | 0.9828 | 0.8774 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alifah, N.; Palungan, J.; Ardayanti, K.; Ullah, M.; Nurkhasanah, A.N.; Mustopa, A.Z.; Lallo, S.; Agustina, R.; Yoo, J.-W.; Hasan, N. Development of Clindamycin-Releasing Polyvinyl Alcohol Hydrogel with Self-Healing Property for the Effective Treatment of Biofilm-Infected Wounds. Gels 2024, 10, 482. https://doi.org/10.3390/gels10070482
Alifah N, Palungan J, Ardayanti K, Ullah M, Nurkhasanah AN, Mustopa AZ, Lallo S, Agustina R, Yoo J-W, Hasan N. Development of Clindamycin-Releasing Polyvinyl Alcohol Hydrogel with Self-Healing Property for the Effective Treatment of Biofilm-Infected Wounds. Gels. 2024; 10(7):482. https://doi.org/10.3390/gels10070482
Chicago/Turabian StyleAlifah, Nur, Juliana Palungan, Kadek Ardayanti, Muneeb Ullah, Andi Nokhaidah Nurkhasanah, Apon Zaenal Mustopa, Subehan Lallo, Rina Agustina, Jin-Wook Yoo, and Nurhasni Hasan. 2024. "Development of Clindamycin-Releasing Polyvinyl Alcohol Hydrogel with Self-Healing Property for the Effective Treatment of Biofilm-Infected Wounds" Gels 10, no. 7: 482. https://doi.org/10.3390/gels10070482
APA StyleAlifah, N., Palungan, J., Ardayanti, K., Ullah, M., Nurkhasanah, A. N., Mustopa, A. Z., Lallo, S., Agustina, R., Yoo, J.-W., & Hasan, N. (2024). Development of Clindamycin-Releasing Polyvinyl Alcohol Hydrogel with Self-Healing Property for the Effective Treatment of Biofilm-Infected Wounds. Gels, 10(7), 482. https://doi.org/10.3390/gels10070482









