Topical Probiotic Hydrogels for Burn Wound Healing
Abstract
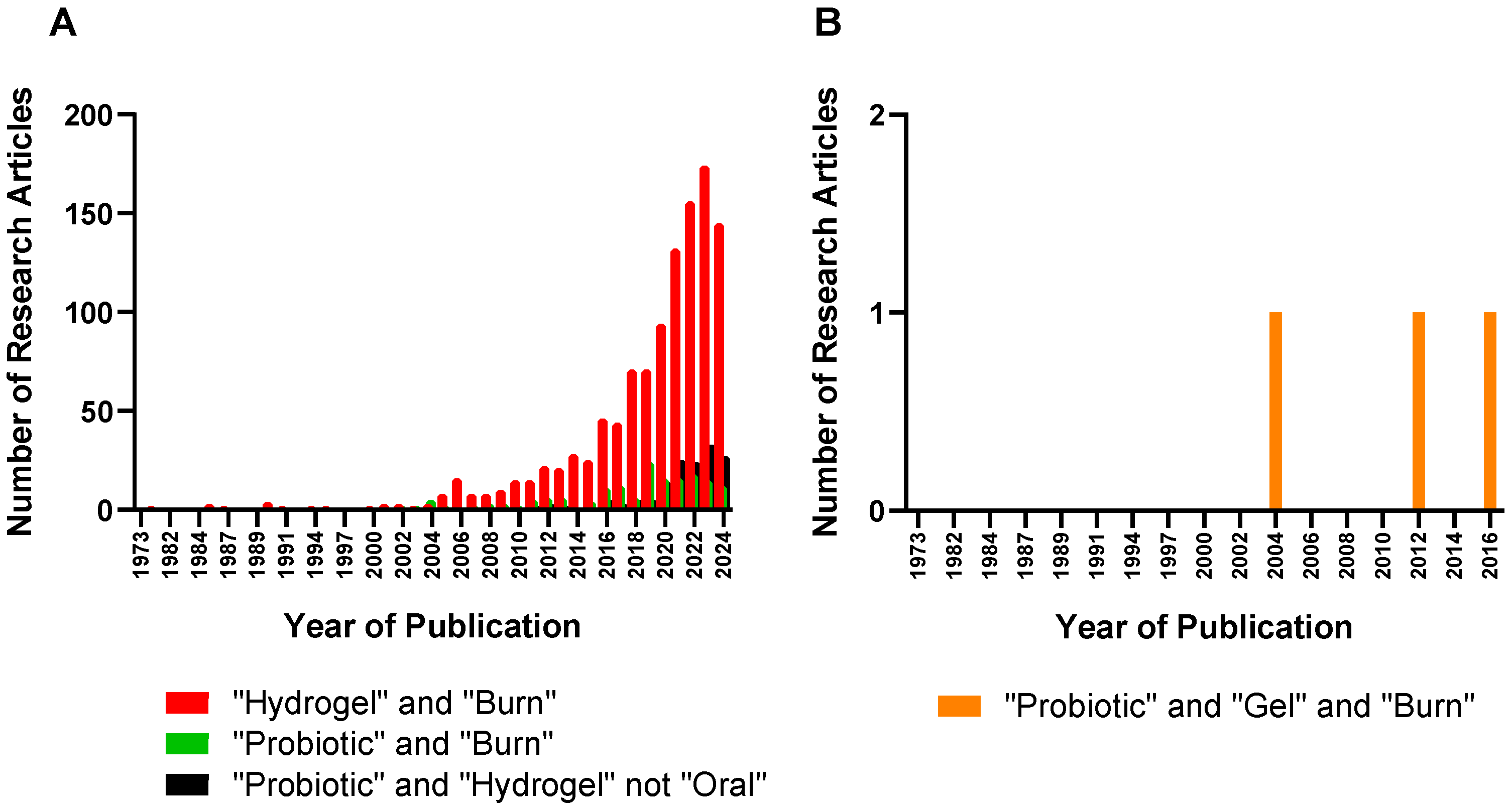
:1. Introduction
1.1. Human Skin Microbiota
1.2. Prebiotic, Probiotic, Postbiotic
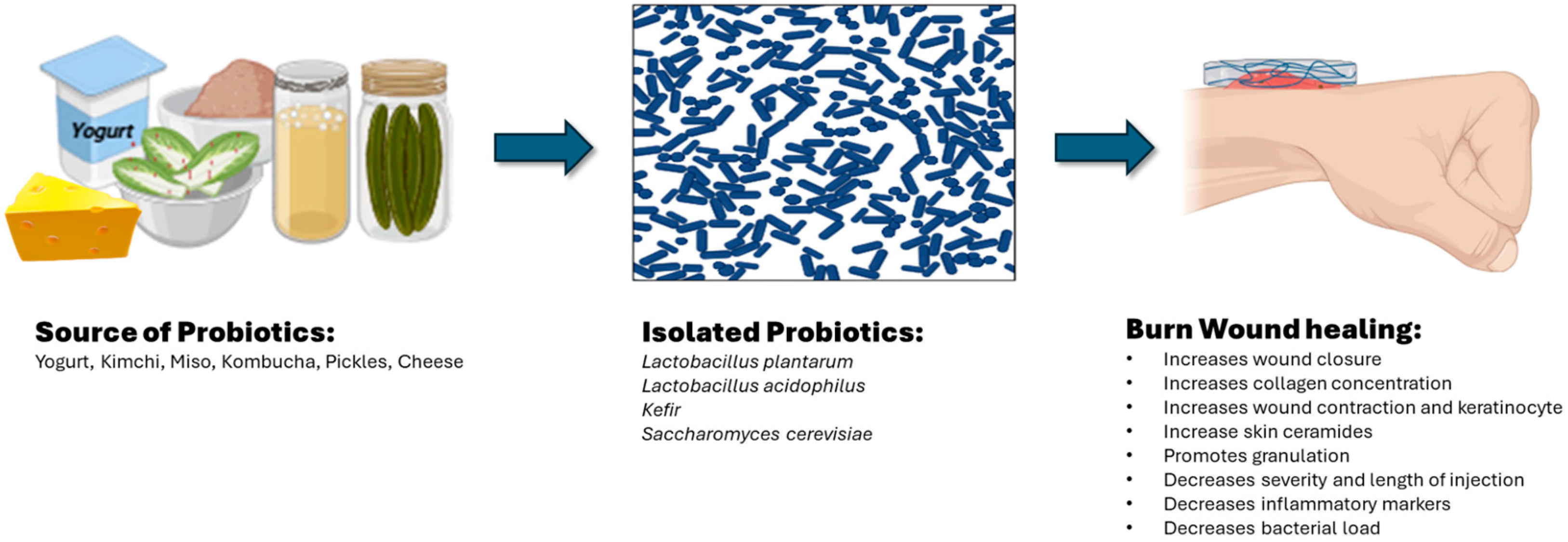
2. Topical Probiotics for Burn Wound Healing
2.1. Lactobacillus plantarum
2.2. Lactobacillus acidophilus
2.3. Kefir
2.4. Saccharomyces cerevisiae
3. Probiotic Hydrogels for Burn Wound Healing
| Form | Example | Description | Advantages | Disadvantages |
|---|---|---|---|---|
| Hydrogel | Skin-adaptable hydrogel dressing [45] | Hydrogels composed of a mixture of synthetic polymers | Skin-like, exude-absorbing, bioadhesive, cools the wounded area, offers pain-relief and moist environment, wearing comfort | Low storage stability (e.g., shrinking, swelling, syneresis), Need for preservatives |
| Ointment | Bacitracin ointment | Antimicrobial, petrolatum-based water-free | Inexpensive, can be applied to mucous membrane, occlusive | Does not penetrate eschar. May cause urticaria |
| Cream | Silver sulfadiazine (silvadene) cream | Interferes with bacterial DNA synthesis, water-based cream | Inexpensive, can be applied to mucous membrane | Need for frequent dressing changes, may need preservatives, limited eschar penetration |
| Gauze | Xeroform | Semi-occlusive, non-absorptive dressing | Non-adherent barrier, can serve as secondary dressing over absorptive dressing, clings to the body | Cannot be used for large exuding wounds, malodorous |
4. Challenges and Future Perspective
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carmona-Cruz, S.; Orozco-Covarrubias, L.; Sáez-de-Ocariz, M. The Human Skin Microbiome in Selected Cutaneous Diseases. Front. Cell. Infect. Microbiol. 2022, 12, 834135. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from Human Skin Commensal Bacteria Protect against Staphylococcus aureus and Are Deficient in Atopic Dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Song, J.; Zhou, L.; Wu, K.; Lu, X.; Zhai, X.; Wan, Z.; Gao, J. Highly Active Probiotic Hydrogels Matrixed on Bacterial EPS Accelerate Wound Healing via Maintaining Stable Skin Microbiota and Reducing Inflammation. Bioact. Mater. 2024, 35, 31–44. [Google Scholar] [CrossRef]
- Mei, L.; Zhang, D.; Shao, H.; Hao, Y.; Zhang, T.; Zheng, W.; Ji, Y.; Ling, P.; Lu, Y.; Zhou, Q. Injectable and Self-Healing Probiotics-Loaded Hydrogel for Promoting Superbacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 20538–20550. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The Human Skin Microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Rozas, M.; Hart de Ruijter, A.; Fabrega, M.J.; Zorgani, A.; Guell, M.; Paetzold, B.; Brillet, F. From Dysbiosis to Healthy Skin: Major Contributions of Cutibacterium Acnes to Skin Homeostasis. Microorganisms 2021, 9, 628. [Google Scholar] [CrossRef] [PubMed]
- Knackstedt, R.; Knackstedt, T.; Gatherwright, J. The Role of Topical Probiotics on Wound Healing: A Review of Animal and Human Studies. Int. Wound J. 2020, 17, 1687–1694. [Google Scholar] [CrossRef]
- Habeebuddin, M.; Karnati, R.K.; Shiroorkar, P.N.; Nagaraja, S.; Asdaq, S.M.B.; Anwer, K.; Fattepur, S. Topical Probiotics: More Than a Skin Deep. Pharmaceutics 2022, 14, 557. [Google Scholar] [CrossRef]
- Ji, J.; Jin, W.; Liu, S.-J.; Jiao, Z.; Li, X. Probiotics, Prebiotics, and Postbiotics in Health and Disease. MedComm 2023, 4, e420. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Koirala, S.; Anal, A.K. Probiotics-Based Foods and Beverages as Future Foods and Their Overall Safety and Regulatory Claims. Future Foods 2021, 3, 100013. [Google Scholar] [CrossRef]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef]
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The Promotion Mechanism of Prebiotics for Probiotics: A Review. Front. Nutr. 2022, 9, 1000517. [Google Scholar] [CrossRef] [PubMed]
- Bouslimani, A.; da Silva, R.; Kosciolek, T.; Janssen, S.; Callewaert, C.; Amir, A.; Dorrestein, K.; Melnik, A.V.; Zaramela, L.S.; Kim, J.-N.; et al. The Impact of Skin Care Products on Skin Chemistry and Microbiome Dynamics. BMC Biol. 2019, 17, 47. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Callewaert, C.; Knödlseder, N.; Karoglan, A.; Güell, M.; Paetzold, B. Skin Microbiome Transplantation and Manipulation: Current State of the Art. Comput. Struct. Biotechnol. J. 2021, 19, 624–631. [Google Scholar] [CrossRef]
- Żwierełło, W.; Piorun, K.; Skórka-Majewicz, M.; Maruszewska, A.; Antoniewski, J.; Gutowska, I. Burns: Classification, Pathophysiology, and Treatment: A Review. Int. J. Mol. Sci. 2023, 24, 3749. [Google Scholar] [CrossRef] [PubMed]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn Wound Infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef]
- Maitz, J.; Merlino, J.; Rizzo, S.; McKew, G.; Maitz, P. Burn Wound Infections Microbiome and Novel Approaches Using Therapeutic Microorganisms in Burn Wound Infection Control. Adv. Drug Deliv. Rev. 2023, 196, 114769. [Google Scholar] [CrossRef]
- Rowley-Conwy, G. Infection Prevention and Treatment in Patients with Major Burn Injuries. Nurs. Stand. 2010, 25, 51–60. [Google Scholar] [CrossRef]
- Macedo, J.L.S.d.; Santos, J.B. Bacterial and Fungal Colonization of Burn Wounds. Mem. Inst. Oswaldo Cruz. 2005, 100, 535–539. [Google Scholar] [CrossRef]
- Capoor, M.R.; Sarabahi, S.; Tiwari, V.K.; Narayanan, R.P. Fungal Infections in Burns: Diagnosis and Management. Indian J. Plast. Surg. 2010, 43, S37–S42. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska-Büchner, E.; Łopuszyńska, I.; Flieger, W.; Tobiasz, M.; Maciejewski, R.; Flieger, J. An Overview of Recent Developments in the Management of Burn Injuries. Int. J. Mol. Sci. 2023, 24, 16357. [Google Scholar] [CrossRef]
- Lolou, V.; Panayiotidis, M.I. Functional Role of Probiotics and Prebiotics on Skin Health and Disease. Fermentation 2019, 5, 41. [Google Scholar] [CrossRef]
- Bădăluță, V.A.; Curuțiu, C.; Dițu, L.M.; Holban, A.M.; Lazăr, V. Probiotics in Wound Healing. Int. J. Mol. Sci. 2024, 25, 5723. [Google Scholar] [CrossRef]
- Satish, L.; Gallo, P.H.; Johnson, S.; Yates, C.C.; Kathju, S. Local Probiotic Therapy with Lactobacillus plantarum Mitigates Scar Formation in Rabbits after Burn Injury and Infection. Surg. Infect. 2017, 18, 119–127. [Google Scholar] [CrossRef]
- Peral, M.C.; Huaman Martinez, M.A.; Valdez, J.C. Bacteriotherapy with Lactobacillus plantarum in Burns. Int. Wound J. 2009, 6, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Soleymanzadeh Moghaddam, S.; Momeni, M.; Mazar Atabaki, S.; Mousavi Shabestari, T.; Boustanshenas, M.; Afshar, M.; Roham, M. Topical Treatment of Second-Degree Burn Wounds with Lactobacillus plantarum Supernatant: Phase I Trial. Iran. J. Pathol. 2022, 17, 460–468. [Google Scholar] [CrossRef]
- Barzegari, A.A.; Hashemzaei, M.; Majdani, R.; Alihemmati, A.-R. Effects of Topical Treatment of Second-Degree Burn Wounds with Lactobacillus acidophilus on the Wound Healing Process in Male Rats. Pharm. Biomed. Res. 2017, 3, 23–30. [Google Scholar] [CrossRef]
- Jebur, M. Therapeutic Efficacy of Lactobacillus acidophilus against Bacterial Isolates from Burn Wounds. N. Am. J. Med Sci. 2010, 2, 586–591. [Google Scholar] [CrossRef]
- Khan, H.; Naeem, N.; Sughra, S.; Khan, S.; Soomro, S.H.; Abro, A.S. Impact of Fermented Lactobacilli Acidophilus and Antibiotics Topically during the Phase of Re-Epithelization in Wound Repair of Rats. Prof. Med J. 2023, 30, 1168–1173. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Eskandari, M.H. Kefir Accelerates Burn Wound Healing through Inducing Fibroblast Cell Migration In Vitro and Modulating the Expression of IL-1ß, TGF-SS1, and bFGF Genes In Vivo. Probiotics Antimicrob. Proteins 2019, 11, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, K.L.; Caputo, L.R.G.; Carvalho, J.C.T.; Evangelista, J.; Schneedorf, J.M. Antimicrobial and Healing Activity of Kefir and Kefiran Extract. Int. J. Antimicrob. Agents 2005, 25, 404–408. [Google Scholar] [CrossRef]
- Cetik Yildiz, S.; Demir, C.; Cengiz, M.; Ayhanci, A. Protective Properties of Kefir on Burn Wounds of Mice That Were Infected with S. aureus, P. auroginasa and E. coli. Cell. Mol. Biol. 2019, 65, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Jalili, M.; Kamali, A.; Nikahval, B. The Concurrent Use of Probiotic Microorganism and Collagen Hydrogel/Scaffold Enhances Burn Wound Healing: An In Vivo Evaluation. Burns 2018, 44, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.-D.; Gan, L.; Tian, L.-Y.; Chen, G.-H. Protein/Polysaccharide-Based Hydrogels Loaded Probiotic-Mediated Therapeutic Systems: A Review. Int. J. Biol. Macromol. 2023, 253, 126841. [Google Scholar] [CrossRef]
- Shu, W.; Wang, Y.; Zhang, X.; Li, C.; Le, H.; Chang, F. Functional Hydrogel Dressings for Treatment of Burn Wounds. Front. Bioeng. Biotechnol. 2021, 9, 788461. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, A.; Yuan, C.; Chen, X.; Liu, Y. Recent Trends on Burn Wound Care: Hydrogel Dressings and Scaffolds. Biomater. Sci. 2021, 9, 4523–4540. [Google Scholar] [CrossRef]
- Madaghiele, M.; Sannino, A.; Ambrosio, L.; Demitri, C. Polymeric Hydrogels for Burn Wound Care: Advanced Skin Wound Dressings and Regenerative Templates. Burn Trauma 2014, 2, 153–161. [Google Scholar] [CrossRef]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Hydrogel Dressings for the Treatment of Burn Wounds: An Up-to-Date Overview. Materials 2020, 13, 2853. [Google Scholar] [CrossRef]
- McKenzie, M.; Betts, D.; Suh, A.; Bui, K.; Kim, L.; Cho, H. Hydrogel-Based Drug Delivery Systems for Poorly Water-Soluble Drugs. Molecules 2015, 20, 20397–20408. [Google Scholar] [CrossRef] [PubMed]
- He, J.J.; McCarthy, C.; Camci-Unal, G. Development of Hydrogel-Based Sprayable Wound Dressings for Second- and Third-Degree Burns. Adv. NanoBiomed Res. 2021, 1, 2100004. [Google Scholar] [CrossRef]
- Huangfu, Y.; Li, S.; Deng, L.; Zhang, J.; Huang, P.; Feng, Z.; Kong, D.; Wang, W.; Dong, A. Skin-Adaptable, Long-Lasting Moisture, and Temperature-Tolerant Hydrogel Dressings for Accelerating Burn Wound Healing without Secondary Damage. ACS Appl. Mater. Interfaces 2021, 13, 59695–59707. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Bhatia, N.; Kumar, A.; Gnanamani, A.; Thilagam, R.; Shanuja, S.K.; Vadakkadath Meethal, K.; Shiji, T.M.; Suchithra, T.V. Bioinspired Gelatin Based Sticky Hydrogel for Diverse Surfaces in Burn Wound Care. Sci. Rep. 2022, 12, 13735. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Joo, Y.-J.; Kang, R.J.; Jeon, H.K.; Hong, G.S. Effect of Spray-Type Alginate Hydrogel Dressing on Burn Wounds. Gels 2024, 10, 152. [Google Scholar] [CrossRef]
- Lanham, J.S.; Nelson, N.K.; Hendren, B.; Gordon, F.; Jordan, T.S. Outpatient Burn Care: Prevention and Treatment. Am. Fam. Physician 2020, 101, 463–470. [Google Scholar]
- Tao, S.; Zhang, S.; Wei, K.; Maniura-Weber, K.; Li, Z.; Ren, Q. An Injectable Living Hydrogel with Embedded Probiotics as a Novel Strategy for Combating Multifaceted Pathogen Wound Infections. Adv Healthc. Mater. 2024, 2400921. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, M.; Sood, R.; Neelamraju, J.; Lakshmi, S.G.; Madempudi, R.S.; Rishi, P.; Kaur, I.P. Self-Preserving Gelatin Emulgel Containing Whole Cell Probiotic for Topical Use: Preclinical Safety, Efficacy, and Germination Studies. Expert Opin. Drug Deliv. 2021, 18, 1777–1789. [Google Scholar] [CrossRef]
- Ashoori, Y.; Mohkam, M.; Heidari, R.; Abootalebi, S.N.; Mousavi, S.M.; Hashemi, S.A.; Golkar, N.; Gholami, A. Development and In Vivo Characterization of Probiotic Lysate-Treated Chitosan Nanogel as a Novel Biocompatible Formulation for Wound Healing. BioMed Res. Int. 2020, 2020, 8868618. [Google Scholar] [CrossRef]
- Han, N.; Jia, L.; Su, Y.; Du, J.; Guo, L.; Luo, Z.; Liu, Y. Lactobacillus reuteri Extracts Promoted Wound Healing via PI3K/AKT/β-Catenin/TGFβ1 Pathway. Stem Cell Res. Ther. 2019, 10, 243. [Google Scholar] [CrossRef]
- Brandi, J.; Cheri, S.; Manfredi, M.; Di Carlo, C.; Vita Vanella, V.; Federici, F.; Bombiero, E.; Bazaj, A.; Rizzi, E.; Manna, L.; et al. Exploring the Wound Healing, Anti-Inflammatory, Anti-Pathogenic and Proteomic Effects of Lactic Acid Bacteria on Keratinocytes. Sci. Rep. 2020, 10, 11572. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, T.; Aljohmani, A.; Frank, N.; Zielke, L.; Mehanny, M.; Laschke, M.W.; Koch, M.; Hoppstädter, J.; Kiemer, A.K.; Yildiz, D.; et al. A Cell-Free, Biomimetic Hydrogel Based on Probiotic Membrane Vesicles Ameliorates Wound Healing. J. Control. Release 2024, 365, 969–980. [Google Scholar] [CrossRef]
- Yang, L.; Han, Z.; Chen, C.; Li, Z.; Yu, S.; Qu, Y.; Zeng, R. Novel Probiotic-Bound Oxidized Bletilla striata Polysaccharide-Chitosan Composite Hydrogel. Mater. Sci. Eng. C 2020, 117, 111265. [Google Scholar] [CrossRef]
- Gruber, J.V.; Holtz, R.; Roach, M. Examining the Genomic Influence of Topically Applied Probiotics In Vitro. Int. J. Cosmet. Sci. 2024. [Google Scholar] [CrossRef]
- Khmaladze, I.; Butler, É.; Fabre, S.; Gillbro, J.M. Lactobacillus reuteri DSM 17938—A Comparative Study on the Effect of Probiotics and Lysates on Human Skin. Exp. Dermatol. 2019, 28, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Garlet, A.; Leoty-Okombi, S.; Gault, M.; Aversa, L.; Pelletier, N.; Bonnaud-Rosaye, C.; Chan, W.; Andre, V. 524 Better Performance of Live Probiotic over Inactivated Biomass on Skin Density. J. Investig. Dermatol. 2022, 142, S89. [Google Scholar] [CrossRef]
- Gruber, J.V.; Riemer, J. Examining Skin Recovery after a 3% Aqueous Hydrogen Peroxide (H2O2) Treatment Using ATP Biofluorescence. Clin. Cosmet. Investig. Dermatol. 2022, 15, 929–937. [Google Scholar] [CrossRef]

| Skin Disorder | Probiotic Organisms |
|---|---|
| Aging | Nitrosomonas eutropha, Lactobacillus buchneri |
| Acne | Streptococcus thermophiles, Enterococcus faecalis, Streptococcus salivarlus |
| Psoriasis | Bifidobacteria infantis, Lactobacillus pentosus |
| Atopic dermatitis | Vitreoscilla filiformis, Streptococcus thermophilus, Lactobacillus johnsonii, Bifidobacterium species |
| Wound healing | Lactiplantibacillus plantarum, Kefir, Lactobacillus fermentum, Saccharomyces cerevisiae |
| Rosacea | Bifidobacterium breve BR03, Lactobacillus salivarius |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arshad, T.; Mundrathi, V.; Perez, V.E.; Nunez, J.M.; Cho, H. Topical Probiotic Hydrogels for Burn Wound Healing. Gels 2024, 10, 545. https://doi.org/10.3390/gels10090545
Arshad T, Mundrathi V, Perez VE, Nunez JM, Cho H. Topical Probiotic Hydrogels for Burn Wound Healing. Gels. 2024; 10(9):545. https://doi.org/10.3390/gels10090545
Chicago/Turabian StyleArshad, Tavinda, Varsha Mundrathi, Victoria E. Perez, Jeilyn M. Nunez, and Hyunah Cho. 2024. "Topical Probiotic Hydrogels for Burn Wound Healing" Gels 10, no. 9: 545. https://doi.org/10.3390/gels10090545





