Deep Cleaning of Crystal Violet and Methylene Blue Dyes from Aqueous Solution by Dextran-Based Cryogel Adsorbents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sorption of CV and MB Dyes onto Dx-Based Cryogels
2.2. Sorption Isotherms
2.2.1. Sorption of CV Dye
2.2.2. Sorption of MB Dye
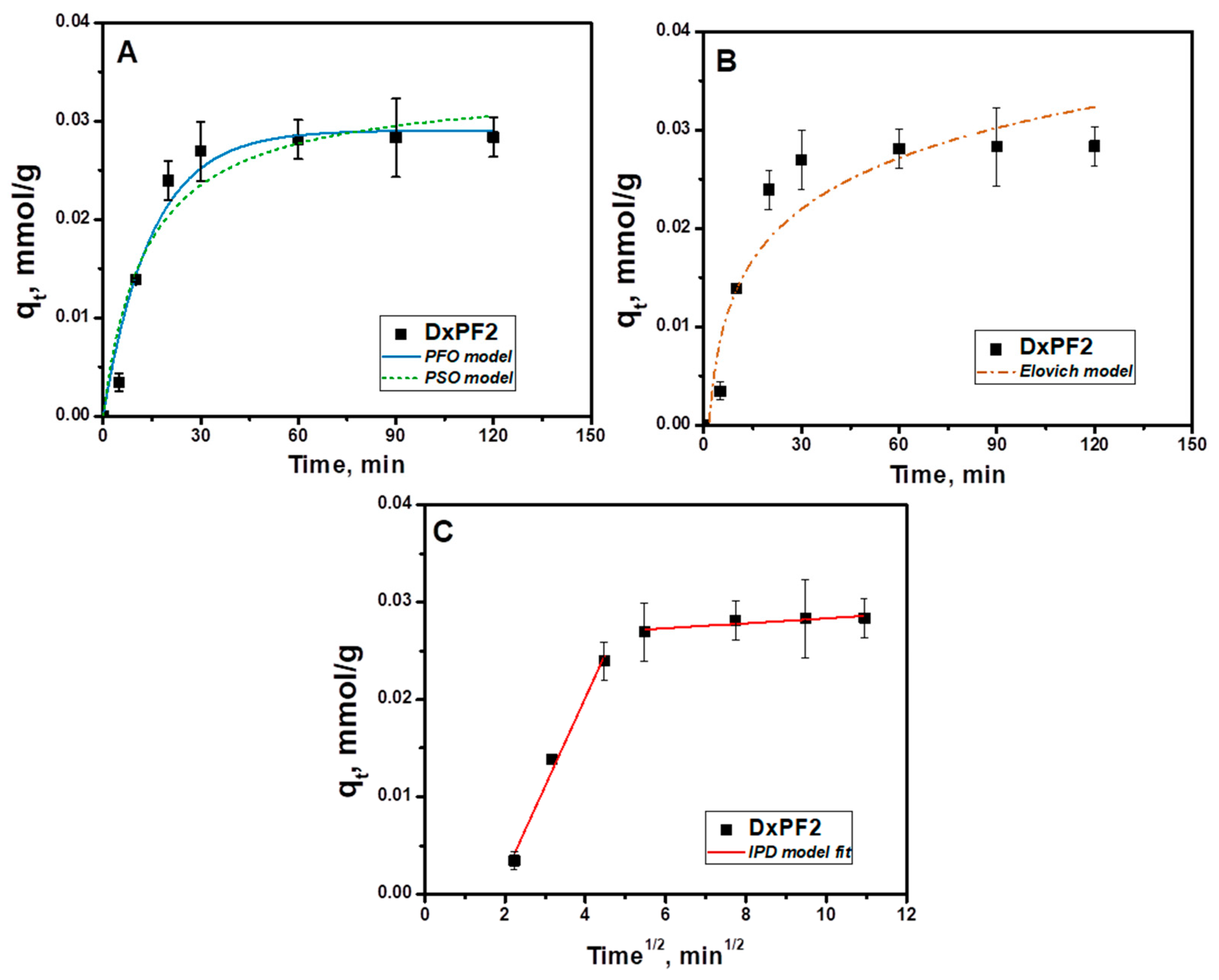
2.3. Sorption Kinetics
3. Conclusions
- ➢
- Dx, a natural polysaccharide, is biodegradable and biocompatible, making these cryogels environmentally friendly. Their use minimizes the ecological footprint and avoids the long-term pollution associated with non-biodegradable materials.
- ➢
- Cryogels are known for their high porosity and large surface area, which enhances their capacity to adsorb a wide range of pollutants, including heavy metals, organic dyes, and other toxic substances, from water and air.
- ➢
- The physicochemical properties of DxPF cryogels can be tailored by adjusting the concentration of PF and the preparation conditions. This allows for the optimization of the pore size, swelling ratio, and surface chemistry to target specific pollutants.
- ➢
- PF extracts can introduce specific functional groups that enhance the selectivity and binding affinity of cryogels for certain contaminants. This is particularly useful in selectively removing pollutants from complex mixtures.
- ➢
- The combination of dextran’s hydrophilic nature with the hydrophobic characteristics imparted by PF extracts allows these cryogels to adsorb a wide variety of contaminants, including both hydrophilic and hydrophobic compounds.
- ➢
- Both dextran and PF extract are derived from renewable natural sources, such as plants and microbial fermentation, supporting the sustainability of the material.
- ➢
- The use of natural and non-toxic materials in the preparation of these cryogels reduces the risk of secondary pollution, making them safe for use in environmental remediation processes.
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of PF Extract from Spruce Bark (Picea Abies)
4.2.2. Evaluation of PF Extracts’ Composition by HPLC
4.2.3. Preparation of DxPF Cryogel Adsorbents
4.2.4. Porosity Evaluation
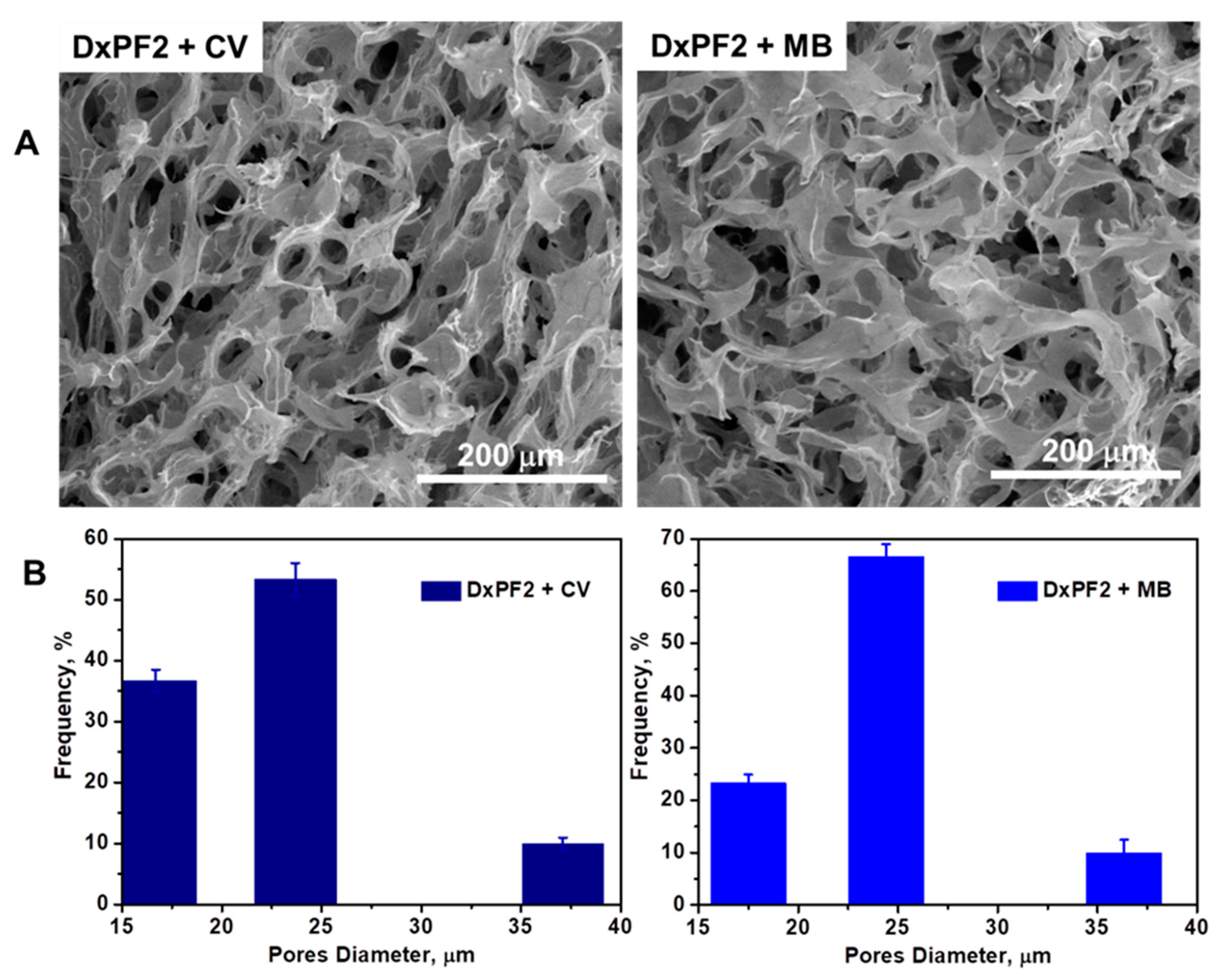
4.2.5. SEM, EDX, and Pore-Size Analysis
4.2.6. Swelling Ratio, SR
4.2.7. FTIR Spectroscopy
4.2.8. Sorption Experiments
4.2.9. Sorption Kinetics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dutta, S.; Gupta, B.; Kumar, S.S.; Kumar, G.A. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Selvaraj, V.; Karthika, T.S.; Mansiya, C.; Alagar, M. An overview on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. J. Mol. Struct. 2021, 1224, 129195. [Google Scholar] [CrossRef]
- Bentahar, S.; Dbik, A.; El Khomri, M.; El Messaoudi, N.; Lacherai, A. Adsorption of methylene blue, crystal violet and congo red from binary and ternary systems with natural clay: Kinetic, isotherm, and thermodynamic. J. Environ. Chem. Eng. 2017, 5, 5921–5932. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Peighambardoust, S.H.; Pateiro, M.; Lorenzo, J.M. Adsorption of crystal violet dye using activated carbon of lemon wood and activated carbon/Fe3O4 magnetic nanocomposite from aqueous solutions: A kinetic, equilibrium and thermodynamic study. Molecules 2021, 26, 2241. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Mittal, J.; Malviya, A.; Kaur, D.; Gupta, V.K. Adsorption of hazardous dye crystal violet from wastewater by waste materials. J. Colloid Interface Sci. 2010, 343, 463–473. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chowdhury, S.; Saha, P.D. Adsorption of Crystal Violet from aqueous solution onto NaOH-modified rice husk. Carbohydr. Polym. 2011, 86, 1533–1541. [Google Scholar] [CrossRef]
- Kaur, S.; Rani, S.; Mahajan, R.K. Adsorptive removal of dye crystal violet onto low cost carbon produced from Eichhornia plant: Kinetic, equilibrium, and thermodynamic studies. Desalin. Water Treat. 2015, 53, 543–556. [Google Scholar] [CrossRef]
- Li, S. Removal of crystal violet from aqueous solution by sorption into semi-interpenetrated networks hydrogels constituted of poly(acrylic acid-acrylamide-methacrylate) and amylose. Bioresour. Technol. 2010, 101, 2197–2202. [Google Scholar] [CrossRef]
- Pashaei-Fakhri, S.; Peighambardoust, S.J.; Foroutan, R.; Arsalani, N.; Ramavandi, B. Crystal violet dye sorption over acrylamide/graphene oxide bonded sodium alginate nanocomposite hydrogel. Chemosphere 2021, 270, 129419. [Google Scholar] [CrossRef]
- Mittal, H.; Al Alili, A.; Morajkar, P.P.; Alhassan, S.M. Graphene oxide crosslinked hydrogel nanocomposites of xanthan gum for the adsorption of crystal violet dye. J. Mol. Liq. 2021, 323, 115034. [Google Scholar] [CrossRef]
- Belkassa, K.; Khelifa, M.; Batonneau-Gener, I.; Marouf-Khelifa, K.; Khelifa, A. Understanding of the mechanism of crystal violet adsorption on modified halloysite: Hydrophobicity, performance, and interaction. J. Hazard. Mater. 2021, 415, 125656. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yan, L.; Wang, Y.; Xu, M. Freeze-thaw as a route to build manageable polysaccharide cryogel for deep cleaning of crystal violet. Chem. Eng. J. 2020, 396, 125354. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Naushad, M.; García-Peñas, A.; Al-Muhtaseb, A.H.; Ghfar, A.A.; Sharma, V.; Ahamad, T.; Stadler, F.J. Fabrication and characterization of Gum arabic-cl-poly(acrylamide) nanohydrogel for effective adsorption of crystal violet dye. Carbohydr. Polym. 2018, 202, 444–453. [Google Scholar] [CrossRef]
- Elella, M.H.A.; Sabaa, M.W.; ElHafeez, E.A.; Mohamed, R.R. Crystal violet dye removal using crosslinked grafted xanthan gum. Int. J. Biol. Macromol. 2019, 137, 1086–1101. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its properties, uses, toxicity and photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene blue dye: Toxicity and potential elimination technology from wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Sivakumar, R.; Yoon Lee, N. Adsorptive removal of organic pollutant methylene blue using polysaccharide-based composite hydrogels. Chemosphere 2022, 286, 131890. [Google Scholar] [CrossRef]
- Li, S.; Cui, Y.; Wen, M.; Ji, G. Toxic effects of Methylene Blue on the growth, reproduction and physiology of Daphnia magna. Toxics 2023, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Seera, S.D.K.; Kundu, D.; Gami, P.; Naik, P.K.; Banerjee, T. Synthesis and characterization of xylan-gelatin cross-linked reusable hydrogel for the adsorption of methylene blue. Carbohydr. Polym. 2021, 256, 117520. [Google Scholar] [CrossRef]
- Jana, S.; Ray, J.; Mondal, B.; Tripathy, T. Efficient and selective removal of cationic organic dyes from their aqueous solutions by a nanocomposite hydrogel, katira gum-cl-poly(acrylic acid-co-N,N-dimethylacrylamide)@bentonite. Appl. Clay Sci. 2019, 173, 46–64. [Google Scholar] [CrossRef]
- Xue, H.; Wang, X.; Xu, Q.; Dhaouadi, F.; Sellaoui, L.; Seliem, M.K.; Lamine, A.B.; Belmabrouk, H.; Bajahzar, A.; Bonilla-Petriciolet, A.; et al. Adsorption of methylene blue from aqueous solution on activated carbons and composite prepared from an agricultural waste biomass: A comparative study by experimental and advanced modeling analysis. Chem. Eng. J. 2022, 430, 132801. [Google Scholar] [CrossRef]
- Lazar, M.M.; Dinu, I.A.; Silion, M.; Dragan, E.S.; Dinu, M.V. Could the porous chitosan-based composite materials have a chance to a “NEW LIFE” after Cu(II) ion binding? Int. J. Biol. Macromol. 2019, 131, 134–146. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Beulah, S.S.; Muthukumaran, K. Methodologies of removal of dyes from wastewater: A Review. Int. Res. J. Pure Appl. Chem. 2020, 21, 68–78. [Google Scholar] [CrossRef]
- Singh, N.B.; Nagpal, G.; Agrawal, S.; Rachna, R. Water purification by using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Dragan, E.S.; Avram, E.; Dinu, M.V. Organic ion exchangers as beads. Synthesis, characterization and applications. Polym. Adv. Technol. 2006, 17, 571–578. [Google Scholar] [CrossRef]
- Lazar, M.M.; Ghiorghita, C.-A.; Dragan, E.S.; Humelnicu, D.; Dinu, M.V. Ion-imprinted polymeric materials for selective adsorption of heavy metal ions from aqueous solution. Molecules 2023, 28, 2798. [Google Scholar] [CrossRef] [PubMed]
- Perju, M.M.; Dinu, M.V.; Dragan, E.S. Sorption of methylene blue onto ionic composite hydrogels based on polyacrylamide and dextran sulfate: Kinetics, isotherms, and thermodynamics. Sep. Sci. Technol. 2012, 47, 1322–1333. [Google Scholar] [CrossRef]
- Dinu, M.V.; Lazar, M.M.; Dragan, E.S. Dual ionic cross-linked alginate/clinoptilolite composite microbeads with improved stability and enhanced sorption properties for methylene blue. React. Funct. Polym. 2017, 116, 31–40. [Google Scholar] [CrossRef]
- Rahmatpour, A.; Alijani, N.; Mirkani, A. Supramolecular self-assembling hydrogel film based on a polymer blend of chitosan/partially hydrolyzed polyacrylamide for removing cationic dye from water. React. Funct. Polym. 2023, 185, 105537. [Google Scholar] [CrossRef]
- Ghiorghita, C.-A.; Dinu, M.V.; Lazar, M.M.; Dragan, E.S. Polysaccharide-based composite hydrogels as sustainable materials for removal of pollutants from wastewater. Molecules 2022, 27, 8574. [Google Scholar] [CrossRef]
- Das, M.; Yadav, M.; Shukla, F.; Ansari, S.; Jadeja, R.N.; Thakore, S. Facile design of a dextran derived polyurethane hydrogel and metallopolymer: A sustainable approach for elimination of organic dyes and reduction of nitrophenols. New J. Chem. 2020, 44, 19122. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, S.; Ma, H.; Li, C.; Huang, Z. Photoresponsive hydrogel-based soft robot: A review. Mater. Today Bio 2023, 20, 100657. [Google Scholar] [CrossRef]
- Yamada, Y.; Otsuka, Y.; Mao, Z.; Maeda, S. Periodical propagation of torsion in polymer gels. Sci. Rep. 2022, 12, 16679. [Google Scholar] [CrossRef]
- Mao, Z.; Shimamoto, G.; Maeda, S. Conical frustum gel driven by the Marangoni effect for a motor without a stator. Colloids Surf. A 2021, 608, 125561. [Google Scholar] [CrossRef]
- Dinu, M.V.; Přádný, M.; Drăgan, E.S.; Michálek, J. Morphogical and swelling properties of porous hydrogels based on poly(hydroxyethyl methacrylate) and chitosan modulated by ice-templating process and porogen leaching. J. Polym. Res. 2013, 20, 285. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Brown, R.; Norton, I.T. Study of cryostructuration of polymer systems. XXI. Cryotropic gel formation of the water–maltodextrin systems. J. Appl. Polym. Sci. 2002, 83, 1658–1667. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryogels on the basis of natural and synthetic polymers: Preparation, properties and application. Russ. Chem. Rev. 2002, 71, 489–511. [Google Scholar] [CrossRef]
- Dinu, M.V.; Gradinaru, A.C.; Lazar, M.M.; Dinu, I.A.; Raschip, I.E.; Ciocarlan, N.; Aprotosoaie, A.C. Physically cross-linked chitosan/dextrin cryogels entrapping Thymus vulgaris essential oil with enhanced mechanical, antioxidant and antifungal properties. Int. J. Biol. Macromol. 2021, 184, 898–908. [Google Scholar] [CrossRef]
- Coats, T.J.; Heron, M. The effect of hypertonic saline dextran on whole blood coagulation. Resuscitation 2004, 60, 101–104. [Google Scholar] [CrossRef]
- Eckel, V.; Vogel, R.F.; Jakob, F. In situ production and characterization of cloud forming dextrans in fruit-juices. Int. J. Food Microbiol. 2019, 306, 108261. [Google Scholar] [CrossRef]
- Szafulera, K.; Wach, R.A.; Olejnik, A.K.; Rosiak, J.M.; Ularìski, P. Radiation synthesis of biocompatible hydrogels of dextran methacrylate. Radiat. Phys. Chem. 2018, 142, 115–120. [Google Scholar] [CrossRef]
- Kim, S.H.; Won, C.Y.; Chu, C.C. Synthesis and characterization of dextran-based hydrogel prepared by photocrosslinking. Carbohydr. Polym. 1999, 40, 183–190. [Google Scholar] [CrossRef]
- Reichelt, S.; Becher, J.; Weisser, J.; Prager, A.; Decker, U.; Möller, S.; Berg, A.; Schnabelrauch, M. Biocompatible polysaccharide-based cryogels. Mater. Sci. Eng. C 2014, 35, 164. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Q.; Liang, C.; Xie, T. Enzymatically degradable oxidized dextran–chitosan hydrogels with an anisotropic aligned porous structure. Soft Matter 2013, 9, 11136–11142. [Google Scholar] [CrossRef]
- Georgiev, G.L.; Trzebicka, B.; Kostova, B.; Petrov, P.D. Super-macroporous dextran cryogels via UV-induced crosslinking: Synthesis and characterization. Polym. Int. 2017, 66, 1306–1311. [Google Scholar] [CrossRef]
- Ari, B.; Yetiskin, B.; Okay, O.; Sahiner, N. Preparation of dextran cryogels for separation processes of binary dye and pesticide mixtures from aqueous solutions. Polym. Eng. Sci. 2020, 60, 1890–1901. [Google Scholar] [CrossRef]
- Ghitescu, R.L.; Volf, I.; Carausu, C.; Buhlmann, A.M.; Gilca, I.A.; Popa, V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015, 22, 535–541. [Google Scholar] [CrossRef]
- Ghitescu, R.E.; Popa, A.M.; Popa, V.I.; Rossi, R.M.; Fortunato, G. Encapsulation of polyphenols into pHEMA e-spun fibres and determination of thei antioxidant activities. Int. J. Pharm. 2015, 494, 278–287. [Google Scholar] [CrossRef]
- Cheng, W.; Wen, J. Now and future. Development and perspectives of using polyphenol nanomaterial in environmental pollution control. Coord. Chem. Rev. 2022, 473, 214825. [Google Scholar] [CrossRef]
- Tanase, C.; Berta, L.; Coman, N.A.; Rosca, I.; Man, A.; Toma, F.; Mocan, A.; Nicolescu, A.; Jakab-Farkas, L.; Biro, D.; et al. Antibacterial and antioxidant potential of silver nanoparticles biosynthesised using the spruce bark extract. Nanomaterials 2019, 9, 1541. [Google Scholar] [CrossRef]
- Koch, S.M.; Goldhahn, C.; Muller, F.J.; Yan, W.; Pilz-Allen, C.; Bidan, C.M.; Ciabattoni, B.; Sticker, L.; Fratzl, P.; Keplinger, T.; et al. Ansiotropic wood-hydrogel composites: Extending mechanical properties of wood towards soft materials’ applications. Mater. Today Bio 2023, 22, 100772. [Google Scholar]
- Raschip, I.E.; Fifere, N.; Varganici, C.-D.; Dinu, M.V. Development of antioxidant and antimicrobial xanthan-based cryogels with tuned porous morphology and controlled swelling features. Int. J. Biol. Macromol. 2020, 156, 608–620. [Google Scholar] [CrossRef]
- Memic, A.; Colombani, T.; Eggermont, L.J.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Navare, K.J.; Mohammed, H.S.; Bencherif, S.A. Latest Advances in Cryogel Technology for Biomedical Applications. Adv. Therap. 2019, 2, 1800114. [Google Scholar] [CrossRef]
- Joukhdar, H.; Seifert, A.; Jüngst, T.; Groll, J.; Lord, M.S.; Rnjak-Kovacina, J. Ice templating soft matter: Fundamental principles and fabrication approaches to tailor pore structure and morphology and their biomedical applications. Adv. Mater. 2021, 33, 2100091. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.V.; Perju, M.M.; Dragan, E.S. Porous semi-interpenetrating hydrogel networks based on dextran and polyacrylamide with superfast responsiveness. Macromol. Chem. Phys. 2011, 212, 240–251. [Google Scholar] [CrossRef]
- Saadi, R.; Saadi, Z.; Fazaeli, R.; Fard, N.E. Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J. Chem. Eng. 2015, 32, 787–799. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Sivalingam, S.; Sen, S. Efficient removal of textile dye using nanosized fly ash derived zeolite-x: Kinetics and process optimization study. J. Taiwan Inst. Chem. Eng. 2019, 96, 305–314. [Google Scholar] [CrossRef]
- Hamza, W.; Dammak, N.; Hadjltaief, H.B.; Eloussaief, M.; Benzina, M. Sono-assisted adsorption of Cristal Violet dye onto Tunisian Smectite Clay: Characterization, kinetics and adsorption isotherms. Ecotoxicol. Environ. Saf. 2018, 163, 365–371. [Google Scholar] [CrossRef]
- Alver, E.; Metin, A.Ü.; Brouers, F. Methylene blue adsorption on magnetic alginate/rice husk bio-composite. Int. J. Biol. Macromol. 2020, 154, 104–113. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Chen, L. A green composite hydrogel based on cellulose and clay as efficient absorbent of colored organic effluent. Carbohydr. Polym. 2019, 210, 314–321. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.; Cui, Y.; Dai, R.; Shan, Z.; Che, H. Fabrication of starch-based high-performance adsorptive hydrogels using a novel effective pretreatment and adsorption for cationic methylene blue dye: Behavior and mechanism. Chem. Eng. J. 2021, 405, 126953. [Google Scholar] [CrossRef]
- Somsesta, N.; Sricharoenchaikul, V.; Aht-Ong, D. Adsorption removal of methylene blue onto activated carbon/cellulose biocomposite films: Equilibrium and kinetic studies. Mater. Chem. Phys. 2020, 240, 122221. [Google Scholar] [CrossRef]
- Mahmoudi, M.M.; Nadali, A.; Arezoomand, H.R.S.; Mahvi, A.H. Adsorption of cationic dye textile wastewater using Clinoptilolite: Isotherm and kinetic study. J. Text. Inst. 2019, 110, 74–80. [Google Scholar] [CrossRef]
- Lagergren, S. Kungliga svenska vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Weber, J.W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. Proc. Am. Soc. Eng. 1963, 89, 31–60. [Google Scholar] [CrossRef]


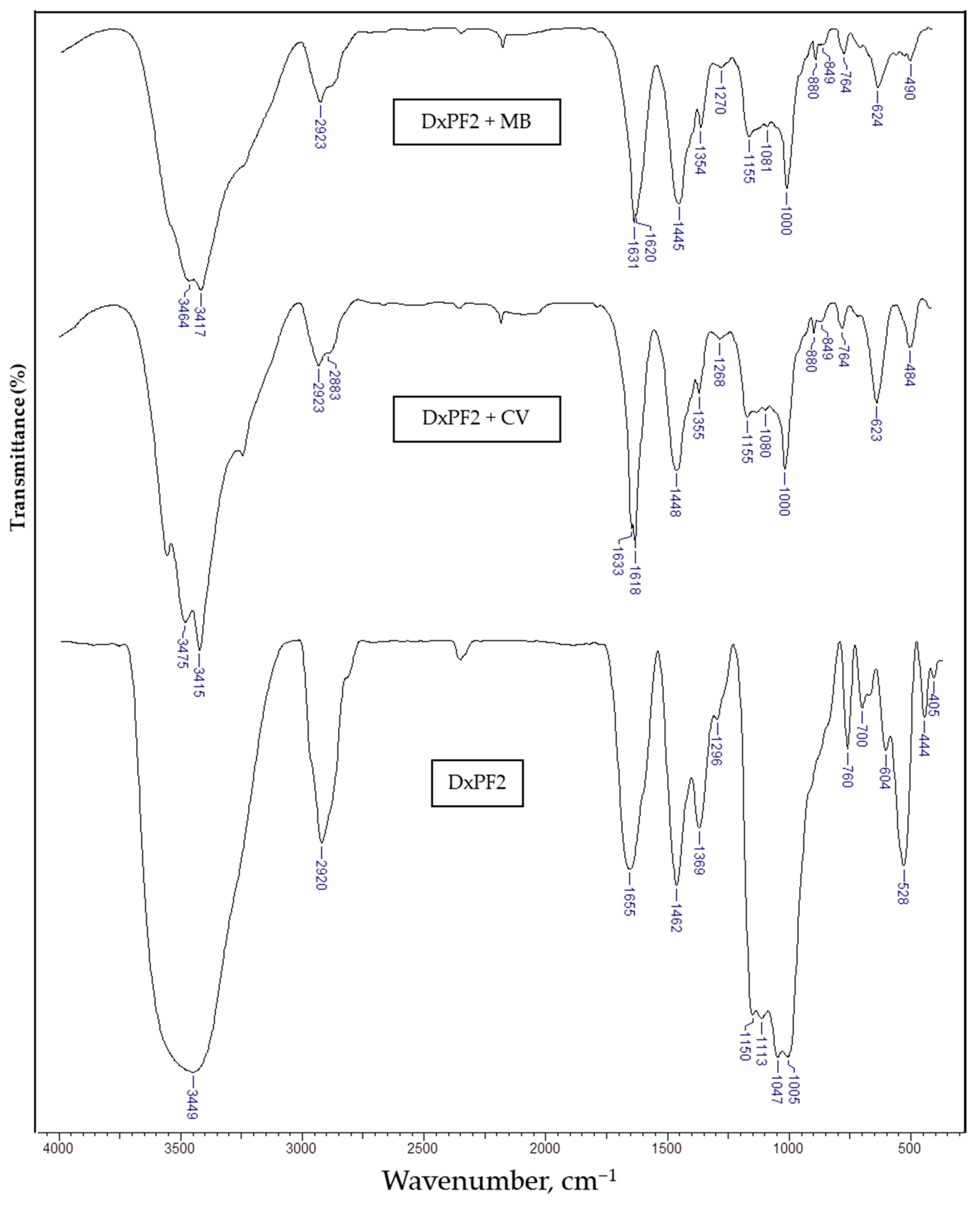



| Sample/Wavenumber (cm−1) | Assignment | ||
|---|---|---|---|
| DxPF2 | DxPF2 + CV | DxPF2 + MB | |
| 3449 | 3475 | 3464 | –OH stretching vibrations |
| — | 3415 | 3417 | C–N stretching vibrations from CV or MB |
| 1618 | 1620 | ||
| 1355 | 1354 | ||
| 2920 | 2923 | 2923 | –CH2 group vibrations |
| 1462 | 1448 | 1445 | |
| 1296 | 1268 | 1270 | Primary –OH in plane bending vibrations |
| 1150 | 1155 | 1155 | C–O–C asymmetric stretching vibrations |
| 1005 | 1000 | 1000 | C3–OH and C6–OH stretching vibrations in glucopyranose ring |
| — | 880 | 880 | –CH bending vibrations in heterocycle of dyes |
| 760 | — | — | Stretching vibration of the aromatic rings from PF |
| 700 | — | — | |
| Isotherm Model | Parameters | PF | DxPF0 | DxPF1 | DxPF2 |
|---|---|---|---|---|---|
| qm,exp, mmol/g | 0.2217 ± 0.0090 | 0.2716 ± 0.0251 | 1.1180 ± 0.0572 | 1.2779 ± 0.0703 | |
| Langmuir | qm, mmol/g | 0.2260 | 0.2649 | 1.0507 | 1.2455 |
| KL, L/mmol | 52.3785 | 64.0043 | 68.8215 | 133.2778 | |
| R2 | 0.9998 | 0.9732 | 0.9737 | 0.8326 | |
| χ2 | 1.69 × 10−6 | 2.94 × 10−4 | 5.36 × 10−3 | 4.55 × 10−2 | |
| Freundlich | KF, L/mmol | 0.2406 | 0.3206 | 1.3541 | 1.5529 |
| 1/n | 0.1316 | 0.2352 | 0.2761 | 0.2356 | |
| R2 | 0.8121 | 0.9595 | 0.9456 | 0.8520 | |
| χ2 | 1.55 × 10−4 | 4.45 × 10−4 | 1.11 × 10−2 | 4.02 × 10−2 | |
| Sips | qm, mmol/g | 0.2334 | 0.3046 | 1.1742 | 1.7694 |
| aS | 23.1089 | 10.2780 | 14.2935 | 3.6716 | |
| 1/n | 0.7298 | 0.6514 | 0.7143 | 0.4509 | |
| R2 | 0.9999 | 0.9829 | 0.9761 | 0.8455 | |
| χ2 | 1.62 × 10−9 | 1.88 × 10−4 | 4.87 × 10−3 | 4.2 × 10−2 | |
| D-R | qDR, mmol/g | 0.2167 | 0.2599 | 1.0686 | 1.2024 |
| kDR, mol2/kJ2 | 1.85∙10−4 | 1.79∙10−4 | 5.40∙10−4 | 1∙10−5 | |
| E, kJ/mol | 51.92 | 52.85 | 30.40 | 223.04 | |
| R2 | 0.9969 | 0.7965 | 0.6887 | 0.8340 | |
| χ2 | 2.6 × 10−5 | 2.24 × 10−3 | 6.34 × 10−2 | 2.45 × 10−3 |
| Isotherm Model | Parameters | PF | DxPF0 | DxPF1 | DxPF2 |
|---|---|---|---|---|---|
| qm,exp, mmol/g | 0.0577 ± 0.0031 | 0.189 ± 0.0201 | 0.3067 ± 0.0312 | 0.3238 ± 0.0121 | |
| Langmuir | qm, mmol/g | 0.0628 | 0.1790 | 0.3176 | 0.3797 |
| KL, L/mmol | 19.1645 | 25.6084 | 7.9813 | 6.7748 | |
| R2 | 0.9242 | 0.9647 | 0.9829 | 0.9940 | |
| χ2 | 4.04 × 10−5 | 1.53 × 10−4 | 2.51 × 10−4 | 1.04 × 10−4 | |
| Freundlich | KF, L/mmol | 0.0708 | 0.1924 | 0.2925 | 0.3932 |
| 1/n | 0.2925 | 0.2351 | 0.3857 | 0.4790 | |
| R2 | 0.8276 | 0.9867 | 0.9798 | 0.9804 | |
| χ2 | 9.19 × 10−5 | 5.7 × 10−5 | 2.96 × 10−4 | 3.41 × 10−4 | |
| Sips | qm, mmol/g | 0.0545 | 0.3086 | 0.4214 | 0.4222 |
| aS | 2508.9 | 1.5280 | 2.1583 | 4.1270 | |
| 1/n | 2.4630 | 0.4078 | 0.6753 | 0.8797 | |
| R2 | 0.9647 | 0.9863 | 0.9937 | 0.9940 | |
| χ2 | 1.87 × 10−5 | 5.93 × 10−5 | 1.23 × 10−4 | 1.03 × 10−4 | |
| D-R | qDR, mmol/g | 0.0548 | 0.1533 | 0.2658 | 0.2878 |
| kDR, mol2/kJ2 | 2∙10−4 | 4.38∙10−5 | 6.04∙10−4 | 3.40∙10−4 | |
| E, kJ/mol | 49.98 | 106.90 | 28.76 | 38.35 | |
| R2 | 0.9586 | 0.8408 | 0.8799 | 0.9565 | |
| χ2 | 2.2 × 10−5 | 6.92 × 10−4 | 1.7 × 10−3 | 7.56 × 10−4 |
| Sorbents | Dyes | Refs | |
|---|---|---|---|
| CV | MB | ||
| qm, mmol·g−1 | |||
| DxPF0 | 0.3046 | 0.3086 | This study |
| DxPF1 | 1.1742 | 0.4214 | |
| DxPF2 | 1.7694 | 0.4222 | |
| Nanocrystalline zeolite X | 0.5749 | [61] | |
| Tunisian smectite clay | 0.2121 | [62] | |
| Modified halloysite | 0.4767 | [11] | |
| Polysaccharide cryogel | 0.7586 | [12] | |
| Gum arabic-cl-poly(acrylamide) nanohydrogel | 0.2228 | [13] | |
| Crosslinked grafted xanthan gum | 1.5319 | [14] | |
| Magnetic alginate/rice husk bio-composite | 0.8594 | [63] | |
| Cellulose/montmorillonite hydrogels | 0.8660 | [64] | |
| Starch-based high-performance adsorptive hydrogel | 9.2782 | [65] | |
| Xylan- and gelatin-based hydrogels | 0.0814 | [19] | |
| Activated carbon/cellulose biocomposite films | 0.3242 | [66] | |
| Clinoptilolite | 0.0646 | [67] | |
| Kinetic Model | Parameters | DxPF2 |
|---|---|---|
| qe exp, mmol/g | 0.0294 ± 0.0020 | |
| PFO | qe calc, mmol/g | 0.029 |
| k1, min−1 | 0.067 | |
| R2 | 0.959 | |
| χ2 | 5.637 × 10−6 | |
| PSO | qe calc, mmol/g | 0.034 |
| k2, g/(mmol⋅min) | 2.222 | |
| R2 | 0.922 | |
| χ2 | 9.323 × 10−7 | |
| Elovich | α (mmol/g·min) | 133.44 |
| β (g/mmol) | 0.005 | |
| R2 | 0.885 | |
| χ2 | 1.602 × 10−5 | |
| IPD | kid.1, mmol/g·min0.5 | 0.009 |
| C1 | −0.016 | |
| R12 | 0.977 | |
| kid.2, mmol/g·min0.5 | 2.56 × 10−4 | |
| C2 | 0.026 | |
| R22 | 0.730 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazar, M.M.; Damaschin, R.P.; Volf, I.; Dinu, M.V. Deep Cleaning of Crystal Violet and Methylene Blue Dyes from Aqueous Solution by Dextran-Based Cryogel Adsorbents. Gels 2024, 10, 546. https://doi.org/10.3390/gels10090546
Lazar MM, Damaschin RP, Volf I, Dinu MV. Deep Cleaning of Crystal Violet and Methylene Blue Dyes from Aqueous Solution by Dextran-Based Cryogel Adsorbents. Gels. 2024; 10(9):546. https://doi.org/10.3390/gels10090546
Chicago/Turabian StyleLazar, Maria Marinela, Roxana P. Damaschin, Irina Volf, and Maria Valentina Dinu. 2024. "Deep Cleaning of Crystal Violet and Methylene Blue Dyes from Aqueous Solution by Dextran-Based Cryogel Adsorbents" Gels 10, no. 9: 546. https://doi.org/10.3390/gels10090546





