A Novel Biomineralized Collagen Liquid Crystal Hydrogel Possessing Bone-like Nanostructures by Complete In Vitro Fabrication
Abstract
:1. Introduction
2. Results and Discussion
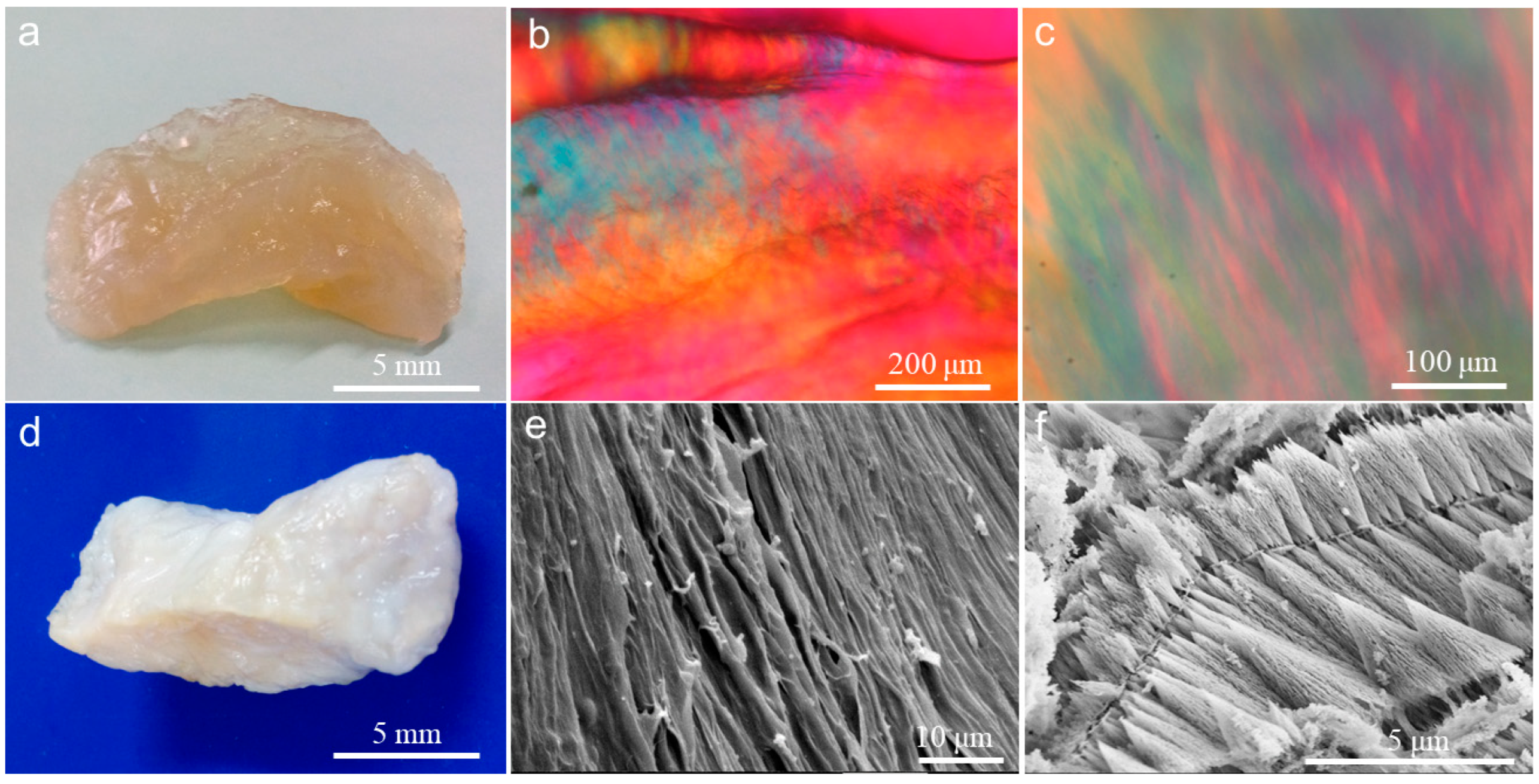
2.1. Construction of Collagen Liquid Crystal Hydrogel
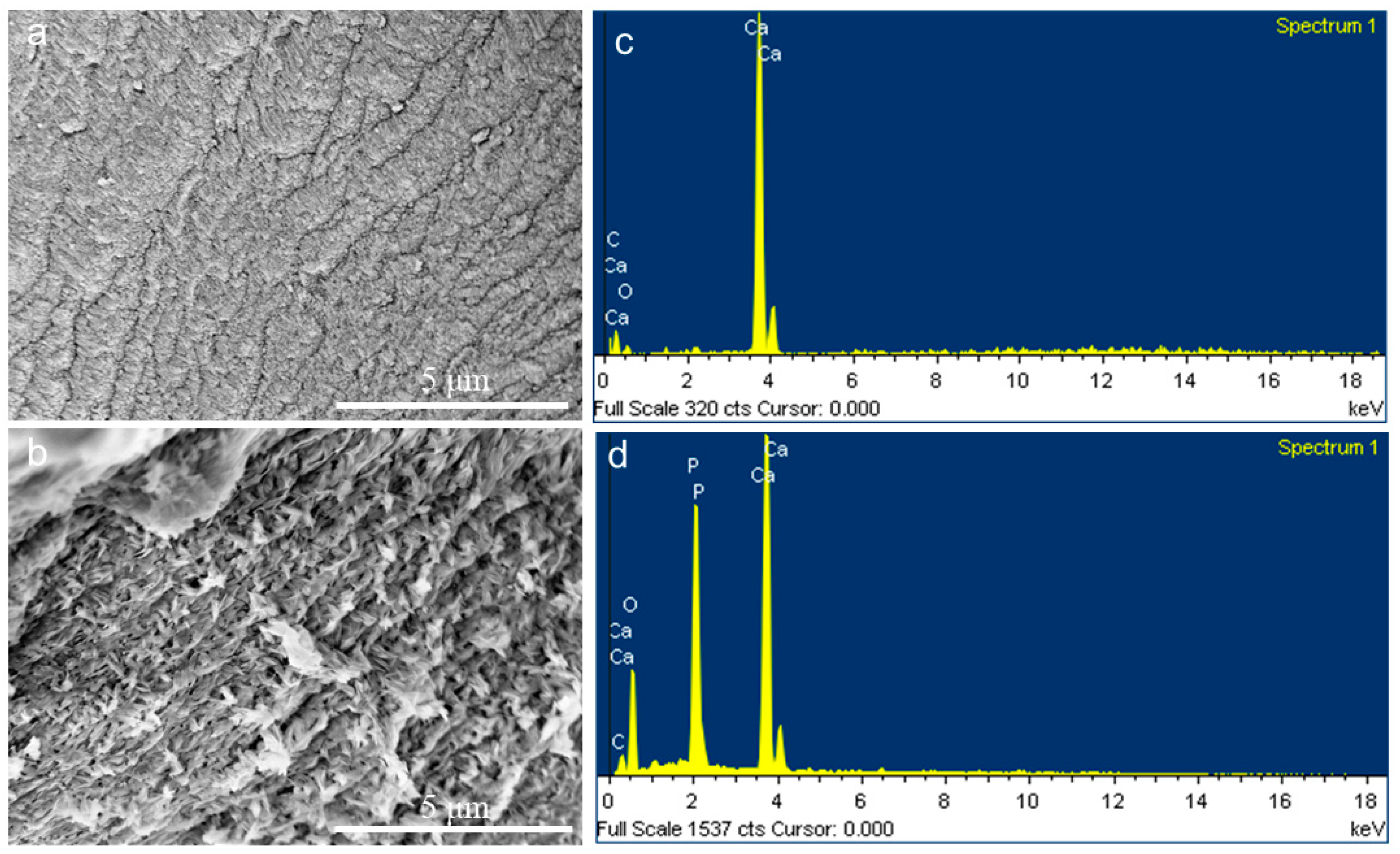
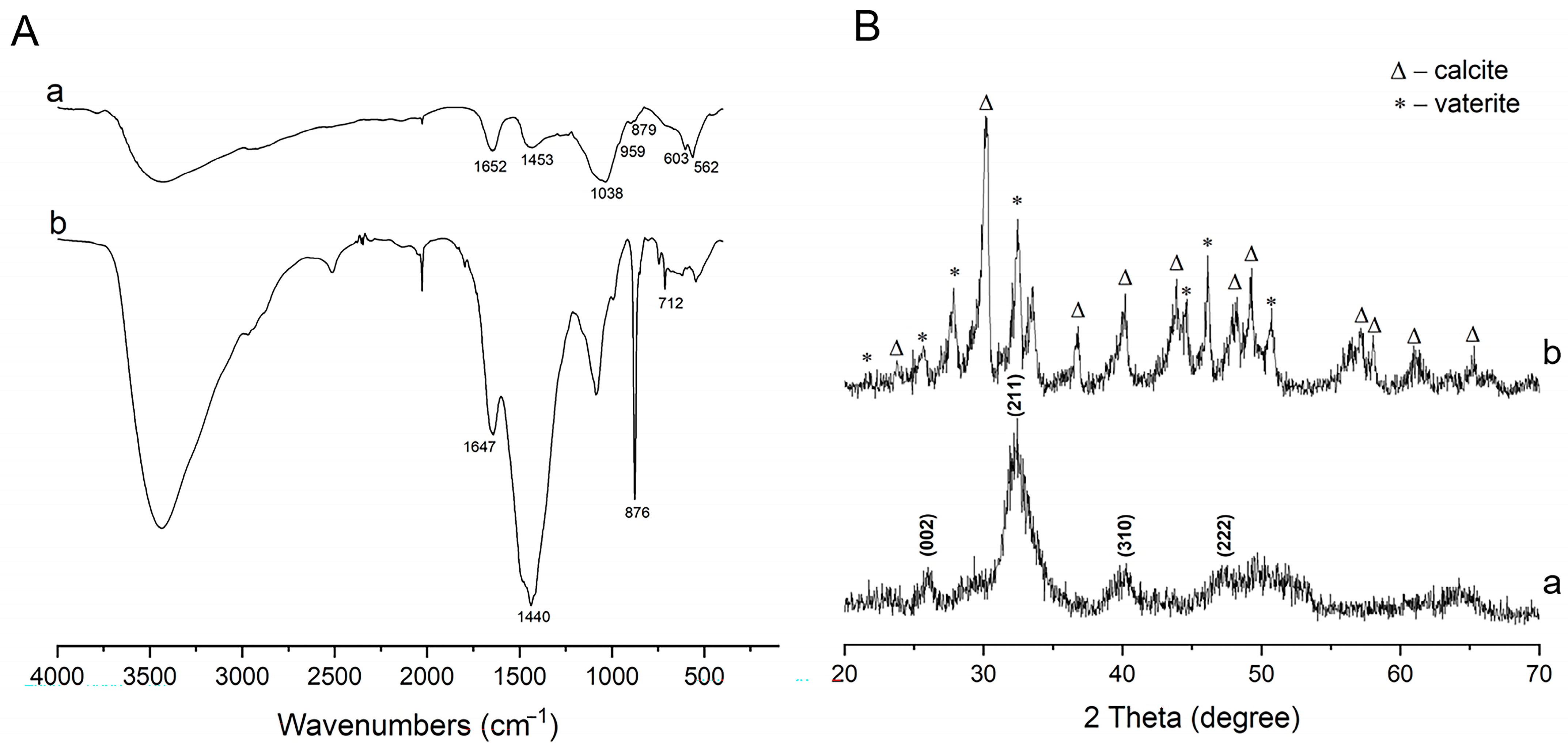
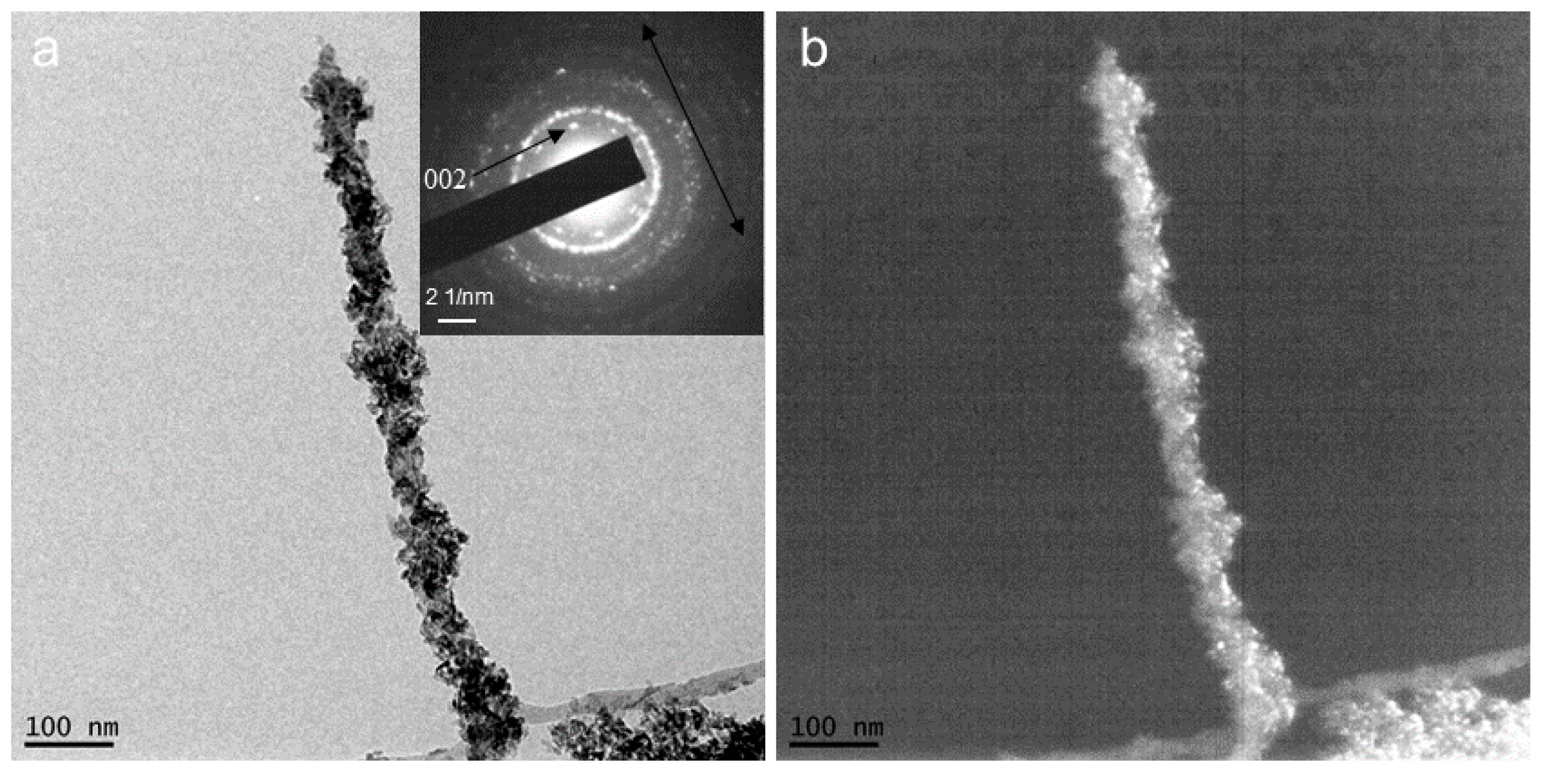
2.2. CaCO3 Pre-Mineralization and Mineralization of CLCH by Nano-HA via CaCO3 Conversion
3. Conclusions
4. Materials and Methods
4.1. Preparation of Three-Dimensional Collagen Liquid Crystal and Its Hydrogel
4.2. Pre-Mineralization for CLCH by CaCO3 via PILP Process
4.3. Intrafibrillar Mineralization of CLCH by Nano-HA Derived from CaCO3 Templet Conversion
4.4. Polarizing Light Microscopy
4.5. Differential Scanning Calorimetry
4.6. Scanning Electron Microscopy
4.7. Transmission Electron Microscopy
4.8. X-Ray Diffraction and Fourier-Transform Infrared Spectroscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weiner, S.; Wagner, H.D. The material bone: Structure mechanical function relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.-Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Landis, W.J.; Song, M.J.; Leith, A.; McEwen, L.; McEwen, B.F. Mineral and organic matrix interaction in normally calcifying tendon visualized in three dimensions by high-voltage electron microscopic tomography and graphic image reconstruction. J. Struct. Biol. 1993, 110, 39–54. [Google Scholar] [CrossRef]
- Wagermaier, W.; Gupta, H.S.; Gourrier, A.; Burghammer, M.; Roschger, P.; Fratzl, P. Spiral twisting of fiber orientation inside bone lamellae. Biointerphases 2006, 1, 1–5. [Google Scholar] [CrossRef]
- Cui, F.Z.; Li, Y.; Ge, J. Self-assembly of mineralized collagen composites. Mater. Sci. Eng. R Rep. 2007, 57, 1–27. [Google Scholar] [CrossRef]
- Gower, L.B.; Odom, D.J. Deposition of calcium carbonate films by a polymer induced liquid-precursor (PILP) process. J. Cryst. Growth 2000, 210, 719–934. [Google Scholar] [CrossRef]
- Dai, L.J.; Douglas EP Gower, L.B. Compositional analysis of a polymer-induced liquid-precursor (PILP) amorphous CaCO3 phase. J. Non-Cryst. Solids 2008, 354, 1845–1854. [Google Scholar] [CrossRef]
- Gower, L.B. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem. Rev. 2008, 108, 4551–4627. [Google Scholar]
- Cheng, X.; Gower, L.B. Molding mineral within microporous hydrogels by a polymer-induced liquid-precursor (PILP) process. Biotechnol. Prog. 2006, 22, 141–914. [Google Scholar] [CrossRef]
- Amos, F.F.; Dai, L.; Kumar, R.; Khan SR Gower, L.B. Mechanism of formation of concentrically laminated spherules: Implication to Randall’s plaque and stone formation. Urol. Res. 2009, 37, 11–17. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Gower, L.B. Patterning inorganic (CaCO3) thin films via a polymer induced-liquid-precursor (PILP) process. Langmuir 2007, 23, 4862–4870. [Google Scholar] [CrossRef]
- Li, H.; Guan, Z.Y.; Wei, L.R.; Lu, J.; Tan, Y.F.; Wei, Q.R. In situ co-deposition synthesis for collagen-Astragalus polysaccharide composite with intrafibrillar mineralization as potential biomimetic-bone repair materials. Regen. Biomater. 2024, 11, rbae070. [Google Scholar] [CrossRef]
- Amos, F.F.; Olszta, M.J.; Khan, S.R.; Gower, L.B. Relevance of a polymer-induced liquid-precursor (PILP) mineralization process to normal and pathological biomineralization. In Biomineralization—Medical Aspects of Solubility; Königsberger, E., Königsberger, L., Eds.; John Wiley & Sons, Ltd.: West Sussex, UK, 2006; pp. 125–217. [Google Scholar]
- Olszta, M.J.; Douglas, E.P.; Gower, L.B. Scanning electron microscopic analysis of the mineralization of type I collagen via a polymer-induced liquid-precursor (PILP) process. Calcif. Tissue Int. 2003, 72, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Deshpande ASBeniash, E. Bioinspired synthesis of mineralized collagen fibrils. Cryst. Growth Des. 2008, 8, 3084–3090. [Google Scholar] [CrossRef]
- Nudelman, F.; Pieterse, K.; George, A.; Bomans PH, H.; Friedrich, H.; Brylka, L.J.; Hilbers PA, J.; With, G.; Sommerdijk NA, J.M. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat. Mater. 2010, 9, 1004–1009. [Google Scholar] [CrossRef]
- Jee, S.S.; Thula TT Gower, L.B. Development of bone-like composites via the polymer-induced liquid-precursor (PILP) process: 1) influence of polymer molecular weight. Acta Biomater. 2010, 6, 3676–3686. [Google Scholar] [CrossRef] [PubMed]
- Thula, T.T.; Svedlund, F.; Rodriguez, D.E.; Podschun, J.; Pendi, L.; Gower, L.B. Mimicking the nanostructure of bone: Comparison of polymeric process-directing agents. Polymers 2011, 3, 10–35. [Google Scholar] [CrossRef] [PubMed]
- Thula, T.T.; Rodriguez, D.E.; Lee, M.H.; Pendi, L.; Podschun, J.; Gower, L.B. In vitro mineralization of dense collagen substrates: A biomimetic approach toward the development of bone-graft materials. Acta Biomater. 2011, 7, 3158–3169. [Google Scholar] [CrossRef]
- Li, L.L. Investigation on Collagen Liquid Crystal and Patterned Structure. Master’s Thesis, Sichuan University, Chengdu, China, May 2012. [Google Scholar]
- Giraud-Guille, M.M.; Mosser, G.; Belamie, E. Liquid crystallinity in collagen systems in vitro and in vivo. Curr. Opin. Colloid Interface Sci. 2008, 13, 303–313. [Google Scholar] [CrossRef]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef]
- Bonucci, E. Calcification in Biological Systems, 1st ed.; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar] [CrossRef]
- Bradt, J.H.; Mertig, M.; Teresiak, A.; Pompe, W. Biomimetic mineralization of collagen by combined fibril assembly and calcium phosphate formation. Chem. Mater. 1999, 11, 2694–2701. [Google Scholar] [CrossRef]
- Du, C.; Cui, F.Z.; Zhang, W.; Feng, Q.L.; Zhu, X.D.; Groot, K.D. Formation of calcium phosphate/collagen composites through mineralization of collagen matrix. J. Biomed. Mater. Res. 2000, 50, 518–527. [Google Scholar] [CrossRef]
- Fisher, L.W.; Termine, J.D. Non-collagenous proteins influencing the local mechanism of calcification. Clin. Orthop. 1985, 200, 362–385. [Google Scholar] [CrossRef]
- Veis, A. Mineral matrix interactions in bone and dentin. J. Bone Miner. Res. 1993, 8, S493–S497. [Google Scholar] [CrossRef] [PubMed]
- Olszta, M.J.; Odom, D.J.; Douglas, E.P.; Gower, L.B. A new paradigm for biomineral formation: Mineralization via an amorphous liquid-phase precursor. Connect. Tissue Res. 2003, 44, 326–334. [Google Scholar] [CrossRef]
- Wei, Q.R.; Lu, J.; Wang, Q.Y.; Fan, H.S.; Zhang, X.D. Novel synthesis strategy for composite hydrogel of collagen/hydroxyapatite microsphere originating from conversion of CaCO3 templates. Nanotechnology 2015, 26, 115605. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.R.; Lu, J.; Ai, H.; Jiang, B. Novel method for the fabrication of multiscale structure collagen/hydroxyapatite microsphere composites based on CaCO3 microparticle templates. Mater. Lett. 2012, 80, 91–94. [Google Scholar] [CrossRef]
- Murphy, W.L.; Mooney, D.J. Bioinspired growth of crystalline carbonate apatite on biodegradable polymer substrata. J. Am. Chem. Soc. 2002, 124, 1910–1917. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, P.X. Biomimetic polymer/apatite composite scaffolds for mineralized tissue engineering. Macromol. Biosci. 2004, 4, 100–111. [Google Scholar] [CrossRef]
- Fowler, B.O. Infrared studies of apatites. I. Vibrational assignments for calcium, strontium, and barium hydroxyapatites utilizing isotopic substitution. Inorg. Chem. 1974, 13, 194–207. [Google Scholar] [CrossRef]
- Gobeaux, F.; Mosser, G.; Anglo, A.; Panine, P.; Davidson, P.; Giraud-Guille, M.M.; Belamie, E. Fibrillogenesis in dense collagen solutions: A physicochemical study. J. Mol. Biol. 2008, 376, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Besseau, L.; Giraud-Guille, M.M. Stabilization of fluid cholesteric phases of collagen to ordered gelated matrices. J. Mol. Biol. 1995, 251, 197–202. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, Q.; Wei, Q. A Novel Biomineralized Collagen Liquid Crystal Hydrogel Possessing Bone-like Nanostructures by Complete In Vitro Fabrication. Gels 2024, 10, 550. https://doi.org/10.3390/gels10090550
Li X, Wang Q, Wei Q. A Novel Biomineralized Collagen Liquid Crystal Hydrogel Possessing Bone-like Nanostructures by Complete In Vitro Fabrication. Gels. 2024; 10(9):550. https://doi.org/10.3390/gels10090550
Chicago/Turabian StyleLi, Xiaoting, Qiaoying Wang, and Qingrong Wei. 2024. "A Novel Biomineralized Collagen Liquid Crystal Hydrogel Possessing Bone-like Nanostructures by Complete In Vitro Fabrication" Gels 10, no. 9: 550. https://doi.org/10.3390/gels10090550




