Conducting Polymer-Based Gel Materials: Synthesis, Morphology, Thermal Properties, and Applications in Supercapacitors
Abstract
:1. Introduction
2. Synthesis of Conducting Polymers, Morphology, and Thermal Properties in Gel Forms
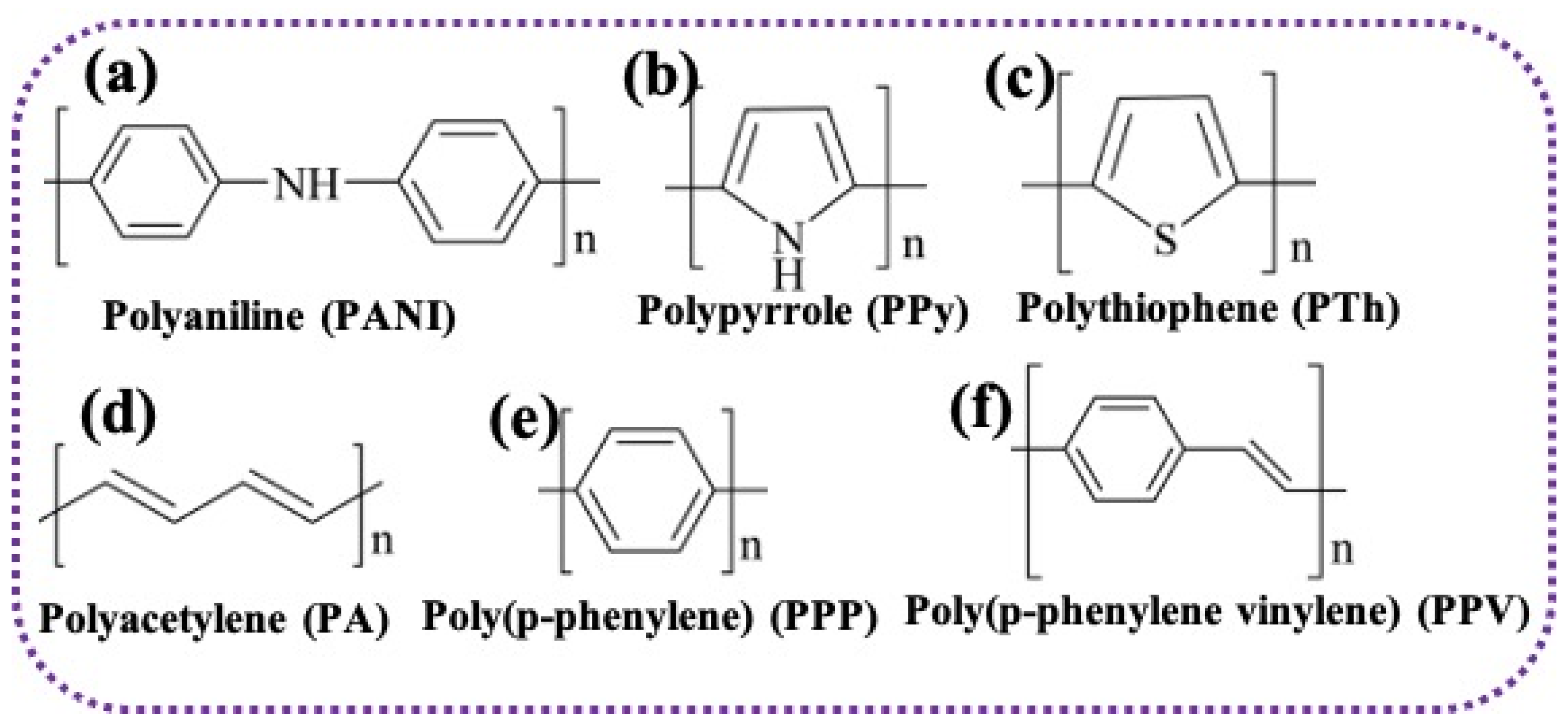
2.1. Typical Conducting Polymers
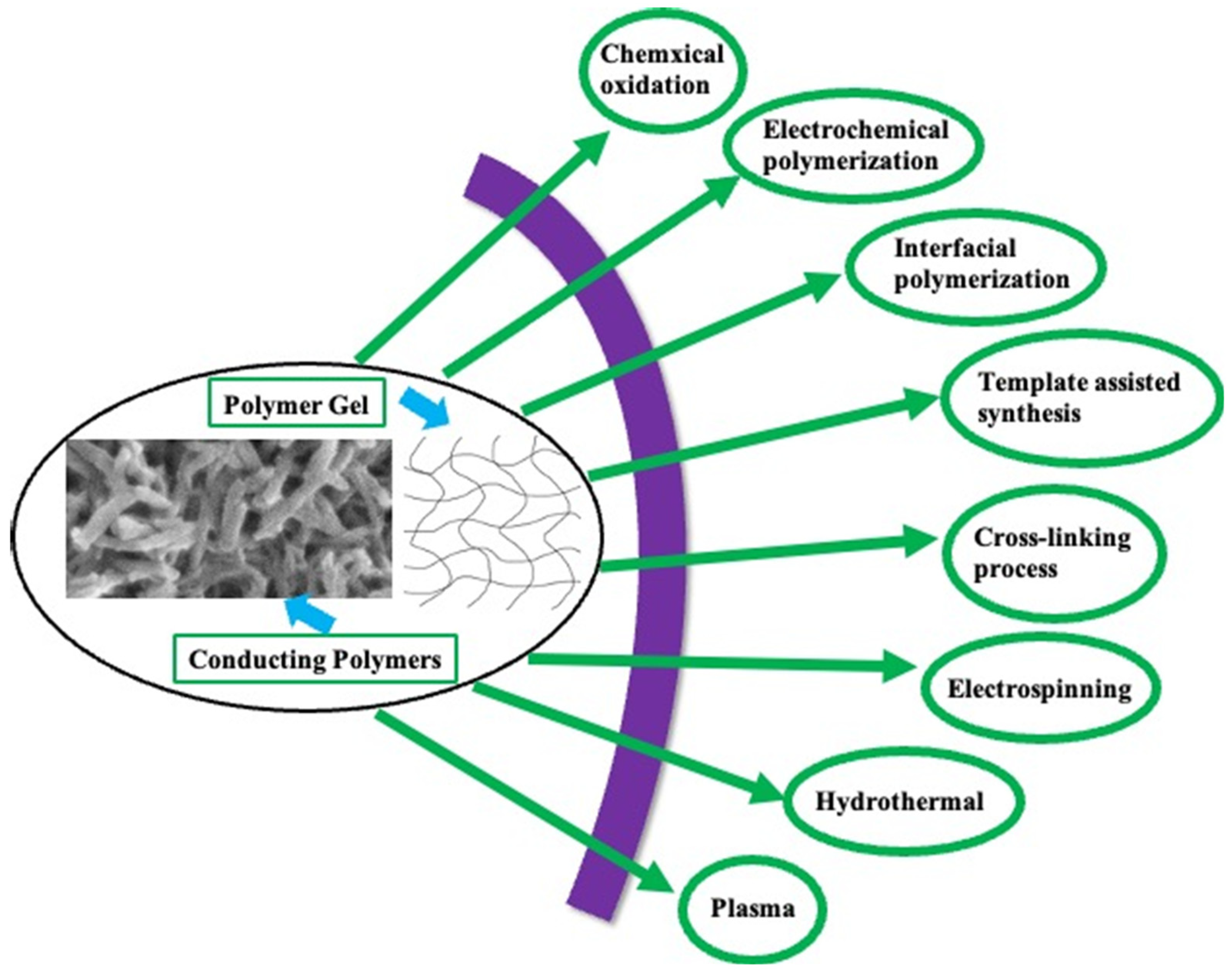
2.2. Synthesis Approaches
2.2.1. Synthesis of Conducting Polymers (CPs)
| Polymerization Approach | Advantages | Disadvantages | Significance | Refs. |
|---|---|---|---|---|
| In situ chemical oxidative | Feasible method, low cost, scalability | Complex processing | Straightforward approach | [38,39,40] |
| Electrochemical | Feasible, porosity management | Costly, complex synthesis, scalability difficulty | Autocatalytic | [39,41] |
| Interfacial | Feasible, convenient product separation | Time consuming | Controllable polymer structure | [42,43] |
| Cross-linking | Well-suited with a biological approach | Post cross-linking, low mechanical properties | Stable polymer structure | [44,45,46,47] |
| Template assisted | Polymer formation in gel forms | Both conductive and nonconductive components formation | Better solubility of monomer and template | [48,49,50] |
| Electrospinning | Simple, versatile, cost-efficient | Low solubility and brittleness of products | Depend on operating parameters | [51] |
| Hydrothermal | Simple, inexpensive | Low tensile strength | Achieve better polymer crystallinity | [52] |
| Plasma | Polymer formation in film forms, product without typical contamination | Complication of plasma processes | Solvent-free conditions, room temperature synthesis | [53] |
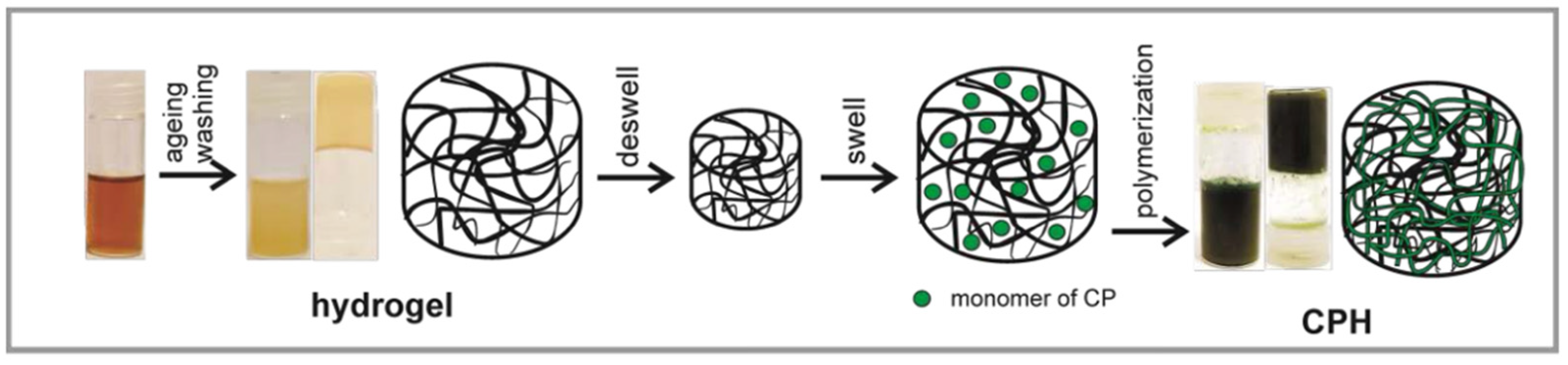
2.2.2. Synthesis of Conducting Polymer Hydrogels (CPH)
2.3. Other Synthesis Strategies for the Formation of Conducting Polymer Gels (CPGs)
2.3.1. Synthesis of Cross-Linked CPGs
2.3.2. Double Network Structured Gel Synthesis Using CPGs
2.3.3. Hybrid Gels Synthesis Based on CPGs
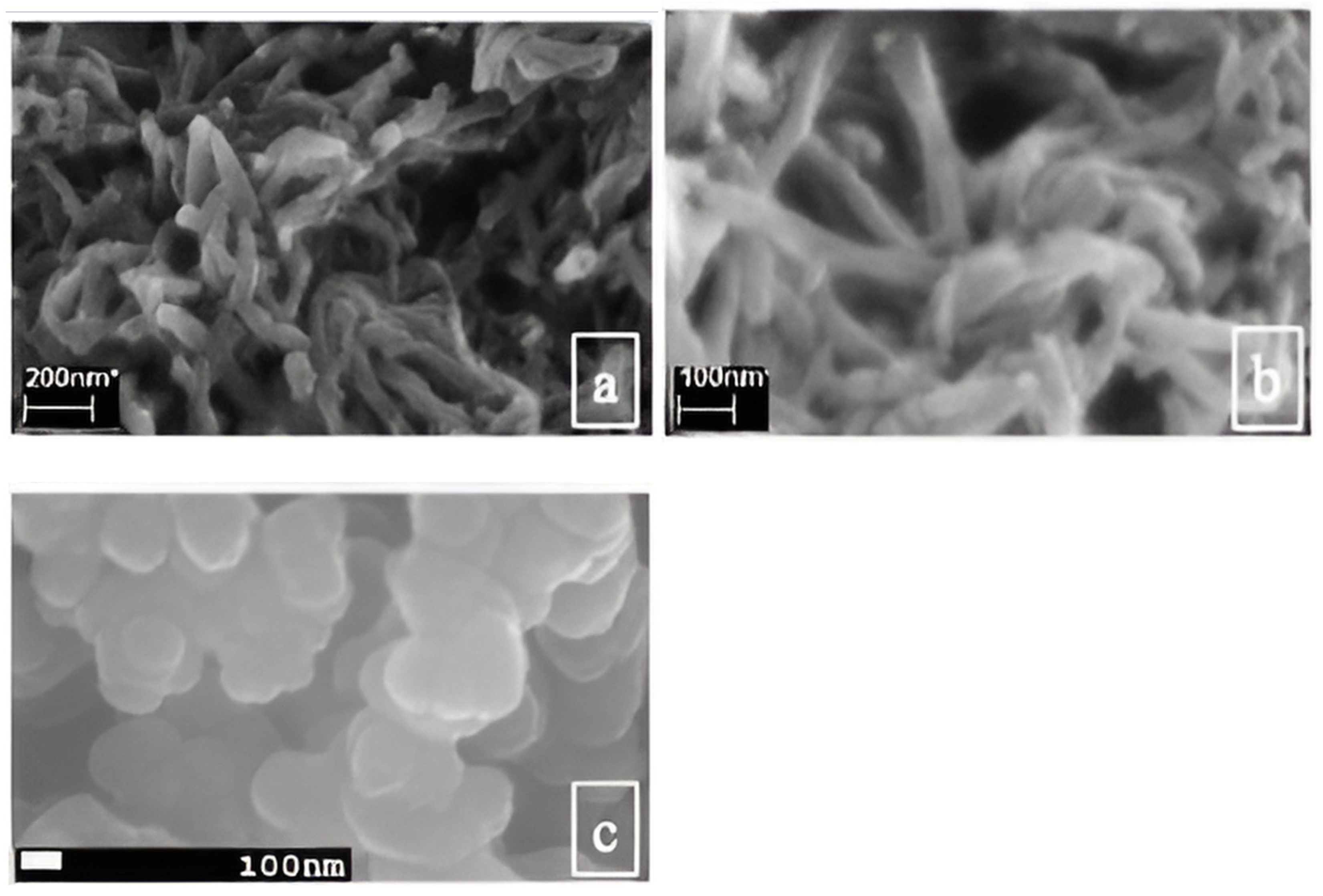
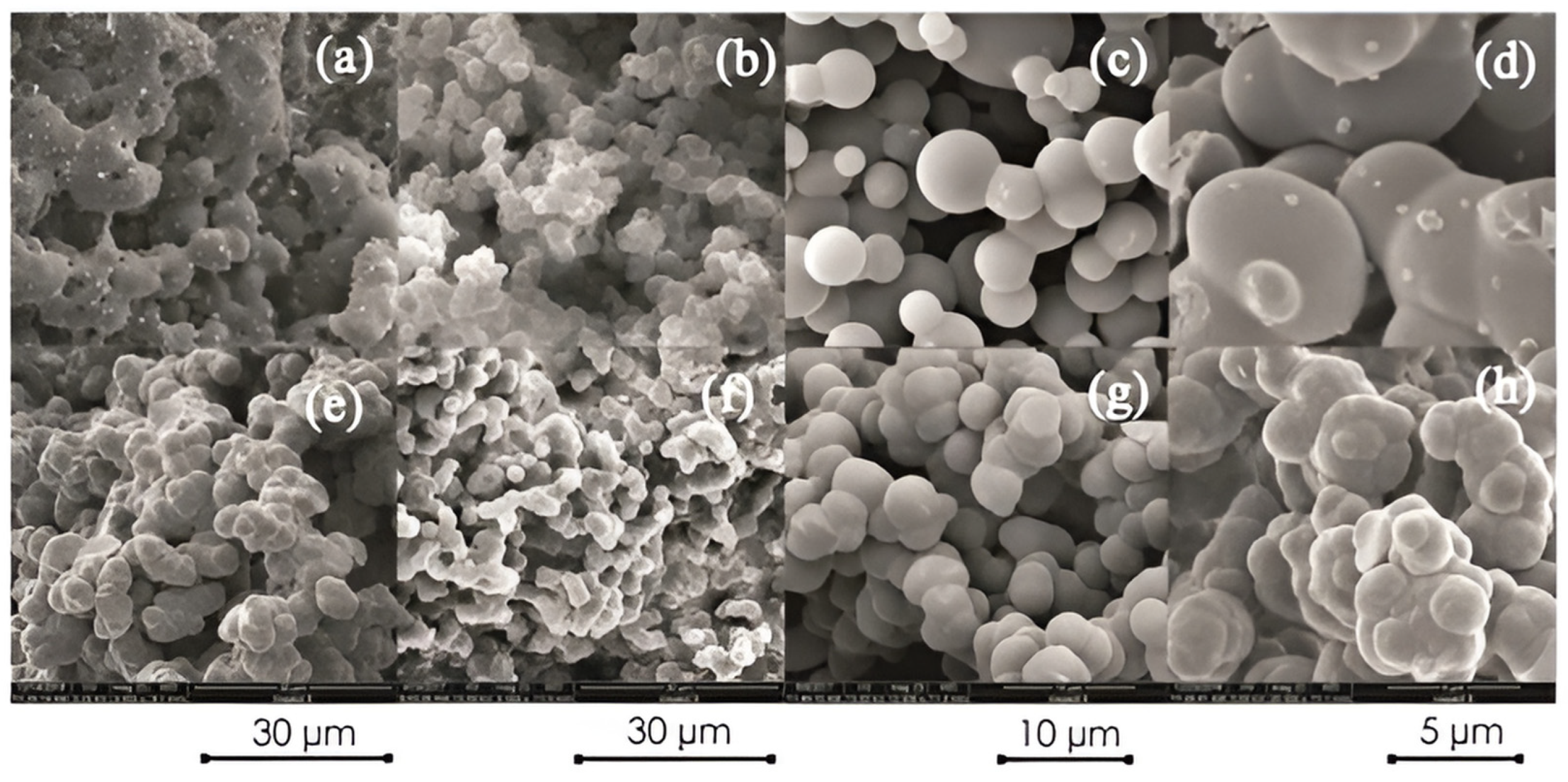
2.4. Morphology
2.5. Thermal Properties
3. Basics of Supercapacitors
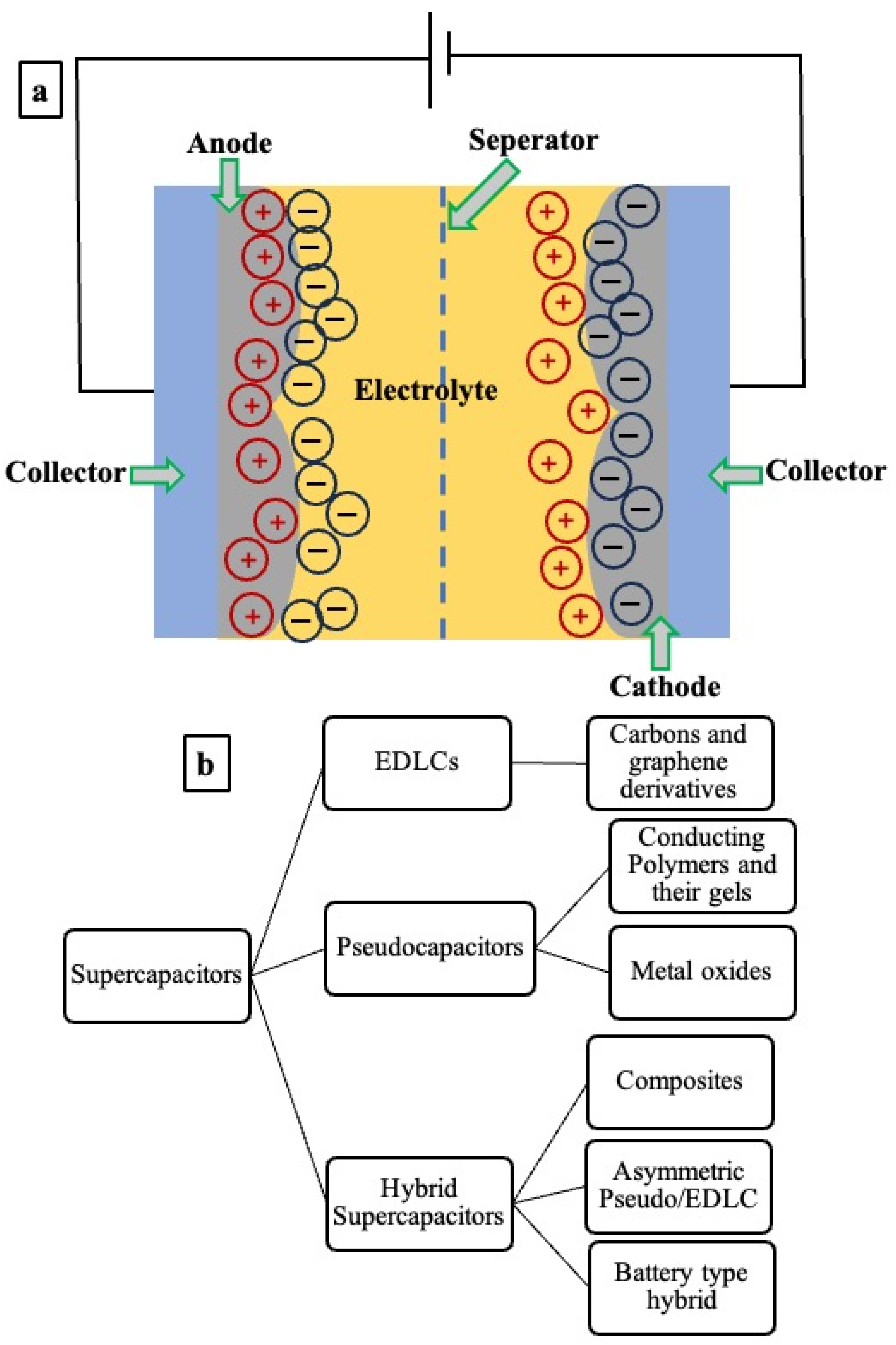
3.1. Types of Supercapacitors
- (i)
- Electric double layer capacitors (EDLCs);
- (ii)
- Pseudocapacitors;
- (iii)
- Hybrid supercapacitors.
3.2. Charge Storage Mechanism
3.2.1. Electric Double Layer Capacitors (EDLCs)
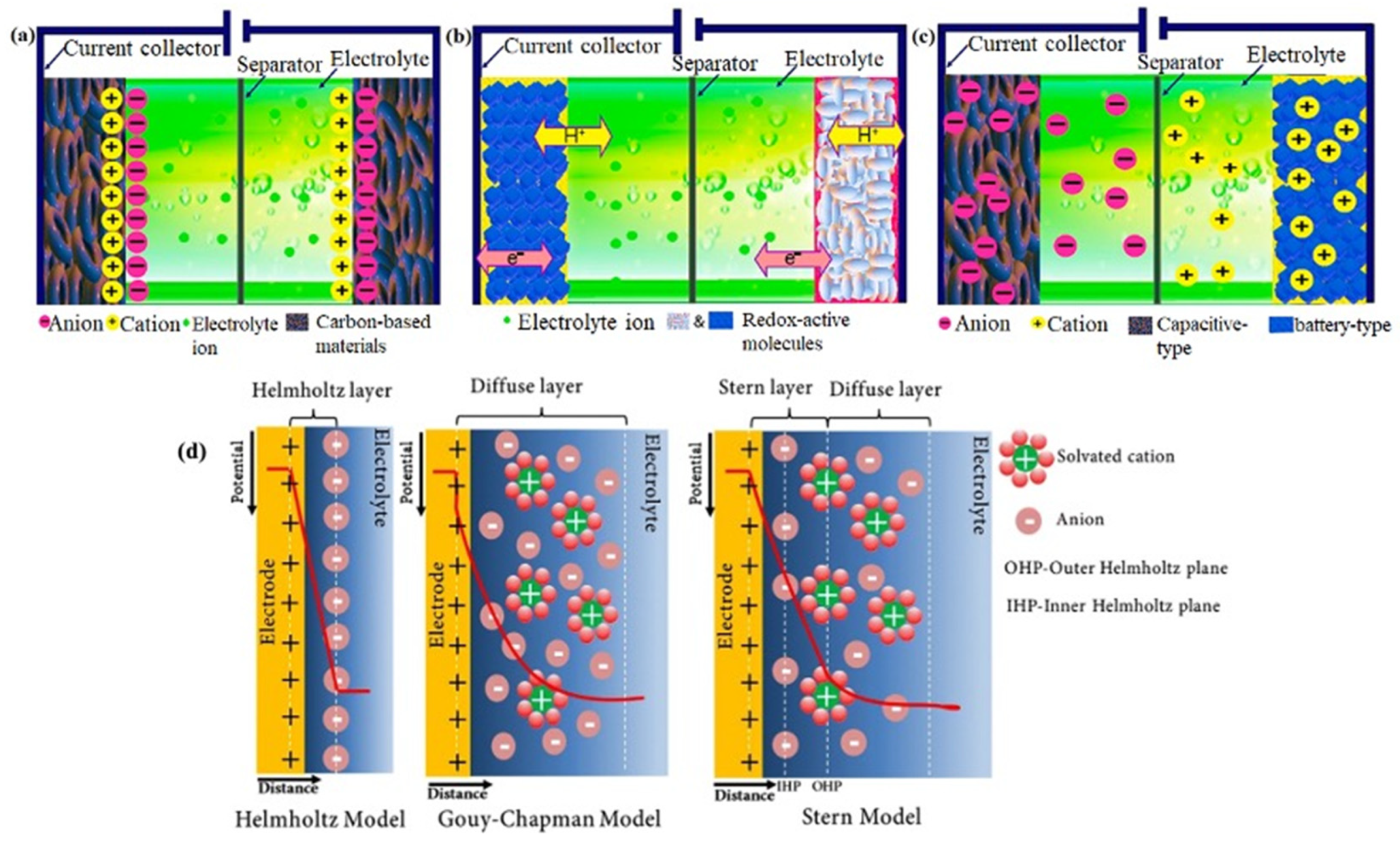
3.2.2. Pseudocapacitors
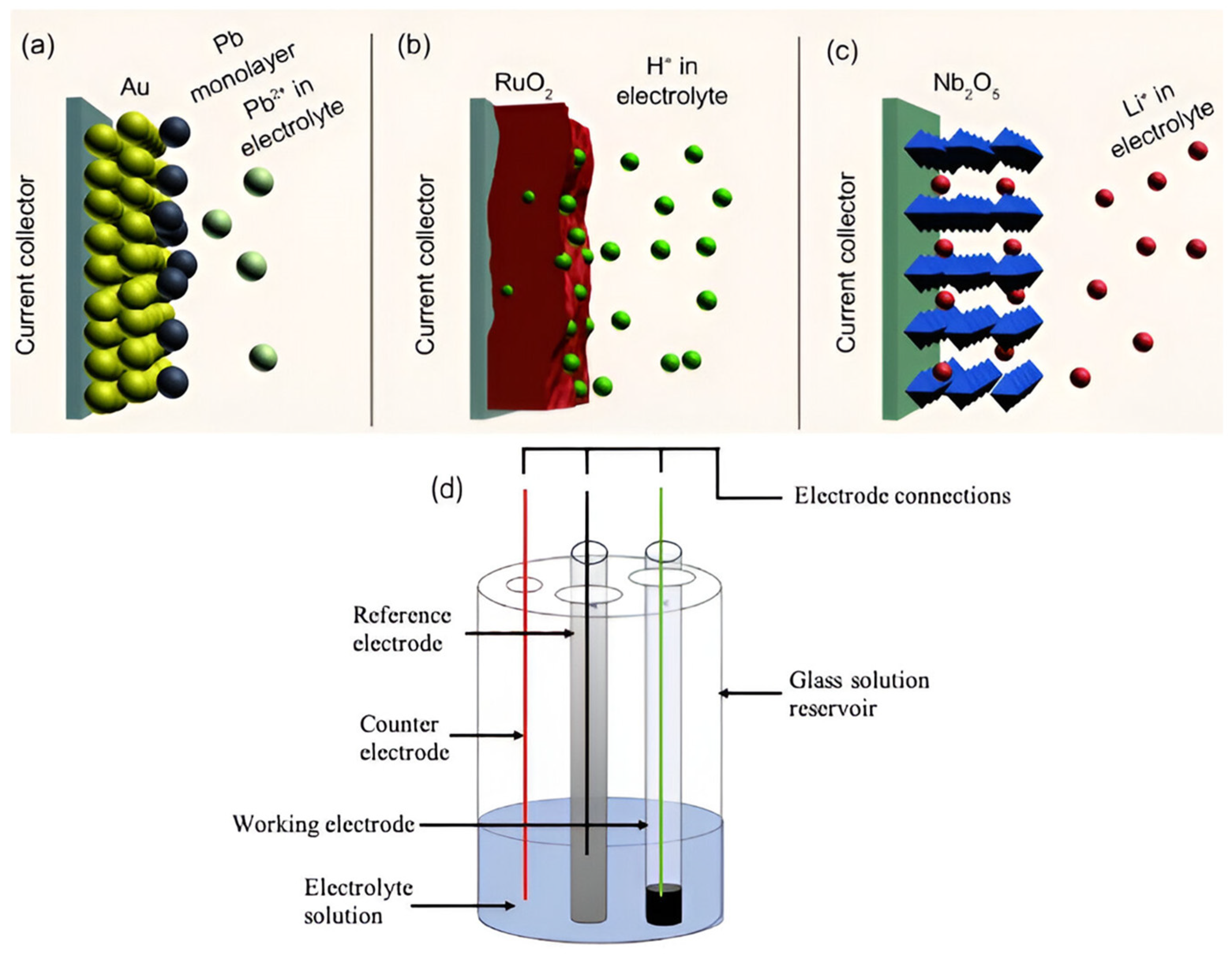
3.2.3. Hybrid Supercapacitors
3.3. Assessment of Supercapacitive Performance
3.3.1. Specific Capacitance
3.3.2. Specific Energy
3.3.3. Specific Power
3.3.4. Cycle Stability
3.4. Electrochemical Approaches for Supercapacitors
3.4.1. Cyclic Voltammetry (CV)
3.4.2. Galvanostatic Charge–Discharge (GCD)
3.4.3. Electrochemical Impedance Spectroscopy (EIS)

4. Conducting Polymer (CP) Gel-Based Electrode Materials
4.1. CP Hydrogel Electrode (CPH)
4.2. CP-Based Composite Materials
PANI-CNT Composites
4.3. PANI Metal Oxides (MO) Composites
4.4. PANI Graphene Oxide (GO) Composites
4.5. PPy-CNT
4.6. PPy-GO Composites
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sardana, S.; Gupta, A.; Singh, K.; Maan, A.S.; Ohlan, A. Conducting polymer hydrogel-based electrode materials for supercapacitor applications. J. Energy Storage 2022, 45, 103510. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, N. Supercapacitors performance evaluation. Adv. Energy Mater. 2015, 5, 1401401. [Google Scholar] [CrossRef]
- Dyatkin, B.; Presser, V.; Heon, M.; Lukatskaya, M.R.; Beidaghi, M.; Gogotsi, Y. Development of a green supercapacitor composed entirely of environmentally friendly materials. Chem. Sus. Chem. 2013, 6, 2269–2280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Li, C.; Islam, M.M.; Moore, J.; Sleppy, J.; Morrison, C.; Konstantinov, K.; Dou, S.X.; Renduchintala, C.; Thomas, J. Wearable energy-smart ribbons for synchronous energy harvest and storage. Nat. Commun. 2016, 7, 13319. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Dai, L. Flexible and wearable wire-shaped microsupercapacitors based on highly aligned titania and carbon nanotubes. Energy Storage Mater. 2016, 2, 21–26. [Google Scholar] [CrossRef]
- Zhu, T.; Wills, R.G.A.; Lot, R.; Ruan, H.; Jiang, Z. Adaptive energy management of a battery-supercapacitor energy storage system for electric vehicles based on flexible perception and neural network fitting. Appl. Energy 2021, 292, 116932. [Google Scholar] [CrossRef]
- Kouchachvili, L.; Yaïci, W.; Entchev, E. Hybrid battery/supercapacitor energy storage system for the electric vehicles. J. Power Sources 2018, 374, 237–248. [Google Scholar] [CrossRef]
- Yu, Z.N.; Tetard, L.; Zhai, L.; Thomas, J.Y. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef]
- Burke, A.; Liu, Z.; Zhao, H. Present and future applications of supercapacitors in electric and hybrid vehicles. In Proceedings of the 2014 IEEE International Electric Vehicle Conference (IEVC), Florence, Italy, 17–19 December 2014; pp. 1–8. [Google Scholar]
- Liang, R.; Du, Y.; Xiao, P.; Cheng, J.; Yuan, S.; Chen, Y.; Yuan, J.; Chen, J. Transition metal oxide electrode materials for supercapacitors: A review of recent developments. Nanomaterials 2021, 11, 1248. [Google Scholar] [CrossRef]
- Lu, Y.; He, W.; Cao, T.; Guo, H.; Zhang, Y.; Li, Q.; Shao, Z.; Cui, Y.; Zhang, X. Elastic, conductive, polymeric hydrogels and sponges. Sci. Rep. 2014, 4, 5792. [Google Scholar] [CrossRef]
- Li, P.; Yang, Y.; Shi, E.; Shen, Q.; Shang, Y.; Wu, S.; Wei, J.; Wang, K.; Zhu, H.; Yuan, Q.; et al. Core-double-shell, carbon nanotube@polypyrrole@MnO2 sponge as freestanding, compressible supercapacitor electrode. ACS Appl. Mater. Interfaces 2014, 6, 5228–5234. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Zhang, W.; Yang, Z.; Zhang, Y.; Zhang, H.; Zhang, H.; Guo, H.; Zhang, X.; Li, Q. Smart and flexible supercapacitor based on a porous carbon nanotube film and polyaniline hydrogel. RSC Adv. 2016, 6, 24946–24951. [Google Scholar] [CrossRef]
- Gupta, A.; Ohlan, A.; Singh, K. Efficient electrode material based on carbon cloth supported polyaniline/reduced graphene oxide composites for supercapacitor application. Indian J. Pure Appl. Phys. 2021, 59, 68–74. [Google Scholar]
- Ye, S.; Feng, J. Self-assembled three-dimensional hierarchical graphene/polypyrrole nanotube hybrid aerogel and its application for supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 9671–9679. [Google Scholar] [CrossRef]
- Lin, H.; Huang, Q.; Wang, J.; Jiang, J.; Liu, F.; Chen, Y.; Wang, C.; Lu, D.; Han, S. Electrochimica acta self-assembled graphene /polyaniline/Co3O4 ternary hybrid aerogels for supercapacitors. Electrochim. Acta 2016, 191, 444–451. [Google Scholar] [CrossRef]
- Selvamani, P.S.; Vijaya, J.J.; Kennedy, L.J.; Saravanakumar, B.; Bououdina, M.; Rajabathar, J.R. Design of copper (II) oxide nanoflakes decorated with molybdenum disulfide@ reduced graphene oxide composite as an electrode for high performance supercapacitor. Synth. Met. 2021, 278, 116843. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, H.; Chen, F.; Chen, L.; Zhang, N.; Ma, M. Supramolecular hydrogels for high-voltage and neutral-pH flexible supercapacitors. ACS Appl. Energy Mater. 2018, 1, 4261–4268. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Zhao, F.; Yu, G. Functional hydrogels for next-generation batteries and supercapacitors. Trends Chem. 2019, 1, 335–348. [Google Scholar] [CrossRef]
- Zhou, Y.; Wan, C.; Yang, Y.; Yang, H.; Wang, S.; Dai, Z.; Ji, K.; Jiang, H.; Chen, X.; Long, Y. Highly stretchable, elastic, and ionic conductive hydrogel for artificial soft electronics. Adv. Funct. Mater. 2019, 29, 1806220. [Google Scholar] [CrossRef]
- Lim, H.; Kim, H.S.; Qazi, R.; Kwon, Y.; Jeong, J.; Yeo, W. Advanced soft materials, sensor integrations, and applications of wearable flexible hybrid electronics in healthcare, energy, and environment. Adv. Mater. 2020, 32, 1901924. [Google Scholar] [CrossRef]
- Pavel, A.Y.; Bakary, T.; Evgeniy, M.C. Antistatic polymer materials. Nanotechnol. Construct. 2023, 15, 139–151. [Google Scholar]
- Anastasia, K.; Pavel, Y.; Alexey, O.; Oleg, L.; Nikolay, L.; Evgeniy, C. aryloxyphosphazene-modified and graphite-filled epoxy compositions with reduced flammability and electrically conductive properties. J. Compos. Sci. 2023, 7, 417. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Sun, A.; Jing, M.; Liu, X.; Wei, L.; Wu, K.; Fu, Q. A self-reinforcing and self-healing elastomer with high strength, unprecedented toughness and room-temperature reparability. Mater. Horiz. 2021, 8, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-H.; Wang, C.; Keplinger, C.; Zuo, J.-L.; Jin, L.; Sun, Y.; Zheng, P.; Cao, Y.; Lissel, F.; Linder, C.; et al. A highly stretchable autonomous self-healing elastomer. Nat. Chem. 2016, 8, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Z.; Wu, X.; Guan, Q.; Chen, S.; Sun, L.; Guo, Y.; Wang, S.; Song, J.; Jeffries, E.M.; et al. A highly efficient self-healing elastomer with unprecedented mechanical properties. Adv. Mater. 2019, 31, 1901402. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Yang, X.; Guo, L.; Wang, Y.; Shaghaleh, H.; Huang, Z.; Xu, X.; Wang, S.; Liu, H. Self-healing polyurethane with high strength and toughness based on a dynamic chemical strategy. J. Mater. Chem. A 2022, 10, 10139–10149. [Google Scholar] [CrossRef]
- Sinawang, G.; Osaki, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Supramolecular self-healing materials from non-covalent cross-linking host–guest interactions. Chem. Commun. 2020, 56, 4381–4395. [Google Scholar] [CrossRef]
- Li, C.; Zuo, J. Self-healing polymers based on coordination bonds. Adv. Mater. 2020, 32, 1903762. [Google Scholar] [CrossRef]
- Xu, J.; Wang, K.; Zu, S.Z.; Han, B.H.; Wei, Z. Hierarchical nanocomposites of polyaniline nanowire arrays on graphene oxide sheets with synergistic effect for energy storage. ACS Nano 2010, 4, 5019–5026. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Shirakawa, H. The discovery of polyacetylene film—The dawning of an era of conducting polymers. Curr. Appl. Phys. 2001, 1, 281–286. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, Y.; Yao, Z.; Liu, A.; Shi, G. Supercapacitors based on flexible graphene/polyaniline nanofiber composite films. ACS Nano 2010, 4, 1963–1970. [Google Scholar] [CrossRef]
- Huang, Y.; Tao, J.; Meng, W.; Zhu, M.; Huang, Y.; Fu, Y.; Gao, Y.; Zhi, C. Super-high rate stretchable polypyrrole-based supercapacitors with excellent cycling stability. Nano Energy 2015, 11, 518–525. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Huang, Y.; Zhi, C. Nanostructured polypyrrole as a flexible electrode material of supercapacitor. Nano Energy 2016, 22, 422–438. [Google Scholar] [CrossRef]
- Ambade, R.B.; Ambade, S.B.; Salunkhe, R.R.; Malgras, V.; Jin, S.H.; Yamauchi, Y.; Lee, S.H. Flexible-wire shaped all-solid-state supercapacitors based on facile electropolymerization of polythiophene with ultra-high energy density. J. Mater. Chem. A. 2016, 4, 7406–7415. [Google Scholar] [CrossRef]
- Li, C.; Bai, H.; Shi, G. Conducting polymer nanomaterials: Electrosynthesis and applications. Chem. Soc. Rev. 2009, 38, 2397–2409. [Google Scholar] [CrossRef]
- Rahman Khan, M.M.; Islam, M.; Amin, M.K.; Paul, S.K.; Rahman, S.; Talukder, M.M.; Rahman, M.M. Simplistic fabrication of aniline and pyrrole-based poly (Ani-co-Py) for efficient photocatalytic performance and supercapacitors. Int. J. Hydrogen Energy 2022, 47, 37860–37869. [Google Scholar] [CrossRef]
- Rahman Khan, M.M.; Wee, Y.K.; Mahmood, W.A.K. Effects of CuO on the morphology and conducting properties of PANI nanofibers. Synth. Met. 2012, 162, 1065–1072. [Google Scholar] [CrossRef]
- Chu, X.; Huang, H.; Zhang, H.; Zhang, H.; Gu, B.; Su, H.; Liu, F.; Han, Y.; Wang, Z.; Chen, N. Electrochemically building three- dimensional supramolecular polymer hydrogel for flexible solid-state micro-supercapacitors. Electrochim. Acta 2019, 301, 136–144. [Google Scholar] [CrossRef]
- Zeng, S.; Chen, H.; Cai, F.; Kang, Y.; Chen, M.; Li, Q. Electrochemical fabrication of carbon nanotube/polyaniline hydrogel film for all-solid-state flexible supercapacitor with high areal capacitance. J. Mater. Chem. A 2015, 3, 23864–23870. [Google Scholar] [CrossRef]
- Basnayaka, P.A.; Ram, M.K.; Stefanakos, L.; Kumar, A. Graphene/polypyrrole nanocomposite as electrochemical supercapacitor electrode: Electrochemical impedance studies. Graphene 2013, 2, 81–87. [Google Scholar] [CrossRef]
- Moussa, M.; El-Kady, M.F.; Dubal, D.; Tung, T.T.; Nine, M.J.; Mohamed, N.; Kaner, R.B.; Losic, D. Self-assembly and cross-linking of conducting polymers into 3D hydrogel electrodes for supercapacitor applications. ACS Appl. Energy Mater. 2020, 3, 923–932. [Google Scholar] [CrossRef]
- Valot, L.; Martinez, J.; Mehdi, A.; Subra, G. Chemical Insights into Bioinks for 3D Printing. Chem. Soc. Rev. 2019, 48, 4049–4086. [Google Scholar] [CrossRef]
- Naranjo-Alcazar, R.; Bendix, S.; Groth, T.; Gallego Ferrer, G. Research progress in enzymatically cross-Linked hydrogels as Injectable systems for bioprinting and tissue engineering. Gels 2023, 9, 230. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef]
- Abidian, M.R.; Kim, D.H.; Martin, D.C. Conducting-Polymer Nanotubes for Controlled Drug Release. Adv. Mater. 2006, 18, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.C.; Spinks, G.M.; Wallace, G.G.; John, R. Electroformation of conducting polymers in a hydrogel support matrix. Polymer 2000, 41, 1783–1790. [Google Scholar] [CrossRef]
- Zhao, F.; Shi, Y.; Pan, L.; Yu, G. Multifunctional nanostructured conductive polymer gels: Synthesis, properties, and applications. Acc. Chem. Res. 2017, 50, 1734–1743. [Google Scholar] [CrossRef]
- Xiao-Xiong, W.; Gui-Feng, Y.; Zhang, J.; Yu, M.; Ramakrishna, S.; Yun-Ze, L. Conductive polymer ultrafine fibers via electrospinning: Preparation, physical properties and applications. Progress Mater. Sci. 2021, 115, 100704. [Google Scholar]
- Melih Besir, A. Hydrothermal synthesis of polypyrrole/dye-functionalized carbon cloth electrode for wide potential window supercapacitor. Synth. Met. 2023, 293, 117275. [Google Scholar]
- Cruz, G.J.; Morales, J.; Castillo-Ortega, M.M.; Olayo, R. Synthesis of polyaniline films by plasma polymerization. Synth. Met. 1997, 88, 213–218. [Google Scholar] [CrossRef]
- Gelmi, A.; Ljunggren, M.K.; Jager, E.W.H. Influence of conductive polymer doping on the viability of cardiac progenitor cells. J. Mater. Chem. B 2014, 2, 3860–3867. [Google Scholar] [CrossRef]
- Green, R.A.; Hassarati, R.T.; Goding, J.A.; Baek, S.; Lovell, N.H.; Martens, P.J.; Poole-Warren, L.A. Conductive hydrogels: Mechanically robust hybrids for use as biomaterials. Macromol. Biosci. 2012, 12, 494–501. [Google Scholar] [CrossRef]
- Syed, A.A.; Dinesan, M.K. Review: Polyaniline—A novel polymeric material. Talanta 1991, 38, 815–837. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Wu, J.; Sun, H.; Lin, J.; Fan, S.; Hu, D. Polyaniline/polyacrylamide conducting composite hydrogel with a porous structure. Carbohydr. Polym. 2008, 74, 215–219. [Google Scholar] [CrossRef]
- Tsai, T.-S.; Pillay, V.; Choonara, Y.E.; Du Toit, L.C.; Modi, G.; Naidoo, D.; Kumar, P.A. Polyvinyl alcohol-polyaniline based electro-conductive hydrogel for controlled stimuli-actuable release of indomethacin. Polymers 2011, 3, 150–172. [Google Scholar] [CrossRef]
- Dou, P.; Liu, Z.; Cao, Z.; Zheng, J.; Wang, C.; Xu, X. Rapid synthesis of hierarchical nanostructured Polyaniline hydrogel for high power density energy storage application and three-dimensional multilayers printing. J. Mater. Sci. 2016, 51, 4274–4282. [Google Scholar] [CrossRef]
- Pan, L.; Yu, G.; Zhai, D.; Lee, H.R.; Zhao, W.; Liu, N.; Wang, H.; Tee, B.C.K.; Shi, Y.; Cui, Y.; et al. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. USA 2012, 109, 9287–9292. [Google Scholar] [CrossRef]
- Small, C.J.; Too, C.O.; Wallace, G.G. Responsive conducting polymer hydrogel composites. Polym. Gels Netw. 1997, 5, 251–265. [Google Scholar] [CrossRef]
- Mawad, D.; Stewart, E.; Officer, D.L.; Romeo, T.; Wagner, P.; Wagner, K.; Wallace, G.G. A single component conducting polymer hydrogel as a scaffold for tissue engineering. Adv. Funct. Mater. 2012, 22, 2692–2699. [Google Scholar] [CrossRef]
- Mushtaq, A.B.; Reyaz, A.R.; Aabid, H.S. PEDOT and PEDOT:PSS conducting polymeric hydrogels: A report on their emerging applications. Synth. Met. 2021, 273, 116709. [Google Scholar]
- Tang, Q.; Lin, J.; Wu, J.; Zhang, C.; Hao, S. Two-steps synthesis of a poly(acrylate–aniline) conducting hydrogel with an interpenetrated network’s structure. Carbohydr. Polym. 2007, 67, 332–336. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Bajpai, J.; Soni, S.N. Designing Polyaniline (PANI) and Polyvinyl Alcohol (PVA) Based Electrically Conductive Nanocomposites: Preparation, Characterization and Blood Compatible Study. J. Macromol. Sci. Part A 2009, 46, 774–782. [Google Scholar] [CrossRef]
- Lin, J.; Tang, Q.; Wu, J.; Li, Q. A multifunctional hydrogel with high-conductivity, pH-responsive, and release properties from polyacrylate/polyptrrole. J. Appl. Polym. Sci. 2009, 116, 1376–1383. [Google Scholar] [CrossRef]
- Dai, T.; Qing, X.; Zhou, H.; Shen, C.; Wang, J.; Lu, Y. Mechanically strong conducting hydrogels with special double-network structure. Synth. Met. 2010, 160, 791–796. [Google Scholar] [CrossRef]
- Mario Cheong, G.L.; Lim, K.S.; Jakubowicz, A.; Martens, P.J.; Poole Warren, L.A.; Green, R.A. Conductive hydrogels with tailored bioactivity for implantable electrode coatings. Acta Biomater. 2014, 10, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Sekine, S.; Ido, Y.; Miyake, T.; Nagamine, K.; Nishizawa, M. Conducting polymer electrodes printed on hydrogel. J. Am. Chem. Soc. 2010, 132, 13174–13175. [Google Scholar] [CrossRef] [PubMed]
- Monika Tomczykowa, Marta Eliza Plonska-Brzezinska, Conducting polymers, hydrogels and their composites: Preparation, properties and bioapplications. Polymers 2019, 11, 350. [CrossRef]
- Karbarz, M.; Gniadek, M.; Donten, M.; Stojek, Z. Intra-channel modification of environmentally sensitive poly(N-isopropylacrylamide) hydrogel with polyaniline using interphase synthesis. Electrochem. Commun. 2011, 13, 714–718. [Google Scholar] [CrossRef]
- Tao, Y.; Zhao, J.X.; Wu, C.X. Polyacrylamide hydrogels with trapped sulfonated polyaniline. Eur. Polym. J. 2005, 41, 1342–1349. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Kang, H.-Y.; Gwon, S.H.; Choi, G.M.; Lim, S.-M.; Sun, J.-Y.; Joo, Y.-C. A strain-insensitive stretchable electronic conductor: PEDOT:PSS/Acrylamide organogels. Adv. Mater. 2016, 28, 1636–1643. [Google Scholar] [CrossRef]
- Song, R.-B.; Wu, Y.; Lin, Z.-Q.; Xie, J.; Tan, C.H.; Loo, J.S.C.; Cao, B.; Zhang, J.-R.; Zhu, J.-J.; Zhang, Q. Living and conducting: Coating individual bacterial cells with in situ formed polypyrrole. Angew. Chem. Int. Ed. 2017, 56, 10516–10520. [Google Scholar] [CrossRef] [PubMed]
- George, P.M.; Lyckman, A.W.; LaVan, D.A.; Hegde, A.; Leung, Y.; Avasare, R.; Testa, C.; Alexander, P.M.; Langer, R.; Sur, M. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials 2005, 26, 3511–3519. [Google Scholar] [CrossRef] [PubMed]
- Grzesik, W.J.; Robey, P.G. Bone matrix RGD glycoproteins: Immunolocalization and interaction with human primary osteoblastic bone cells in vitro. J. Bone Miner. Res. 2009, 9, 487–496. [Google Scholar] [CrossRef]
- Volkov, A.; Tourillon, G.; Lacaze, P.-C.; Dubois, J.-E. Electrochemical polymerization of aromatic amines. J. Electroanal. Chem. Interfacial Electrochem. 1980, 115, 279–291. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Tang, Z.; Xiao, Y.; Huang, M.; Lin, J. Application of poly(acrylicacid-g-gelatin)/polypyrrole gel electrolyte in flexible quasi-solid-state dye-sensitized solar cell. Electrochim. Acta 2010, 55, 2777–2781. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Pan, L.; Ding, Y.; Zhao, Y.; Li, Y.; Shi, Y.; Yu, G. Dopant-enabled supramolecular approach for controlled synthesis of nanostructured conductive polymer hydrogels. Nano Lett. 2015, 15, 7736–7741. [Google Scholar] [CrossRef]
- Chen, Z.; To, J.W.F.; Wang, C.; Lu, Z.; Liu, N.; Chortos, A.; Pan, L.; Wei, F.; Cui, Y.; Bao, Z. A Three-dimensionally interconnected carbon nanotube—Conducting polymer hydrogel network for high-performance flexible battery electrodes. Adv. Energy Mater. 2014, 4, 1400207. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, L.; Liu, B.; Wang, Y.; Cui, Y.; Bao, Z.; Yu, G. Nanostructured conductive polypyrrole hydrogels as high-performance, flexible supercapacitor electrodes. J. Mater. Chem. A 2014, 2, 6086–6091. [Google Scholar] [CrossRef]
- Ma, J.; Choudhury, N.A.; Sahai, Y.; Buchheit, R.G. A high performance direct borohydride fuel cell employing cross-linked chitosan membrane. J. Power Sources 2011, 196, 8257–8264. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, D.; Wang, Y.; Cheng, C.; Zhou, K.; Ding, J.; Truong, V.-T.; Li, D. Mechanically robust, electrically conductive and stimuli- responsive binary network hydrogels enabled by superelastic graphene aerogels. Adv. Mater. 2014, 26, 3333–3337. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, C.; Peng, L.; Yu, G. Conductive “Smart” hybrid hydrogels with PNIPAM and nanostructured conductive polymers. Adv. Funct. Mater. 2015, 25, 1219–1225. [Google Scholar] [CrossRef]
- Ma, C.; Shi, Y.; Pena, D.A.; Peng, L.; Yu, G. Thermally responsive hydrogel blends: A general drug carrier model for controlled drug release. Angew. Chem. Int. Ed. 2015, 54, 7376–7380. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, Y.; Pan, L.; Shi, Y.; Yu, G. Rational design and applications of conducting polymer hydrogels as electrochemical biosensors. J. Mater. Chem. B 2015, 3, 2920–2930. [Google Scholar] [CrossRef]
- Zhai, D.; Liu, B.; Shi, Y.; Pan, L.; Wang, Y.; Li, W.; Zhang, R.; Yu, G. Highly sensitive glucose sensor based on Pt nanoparticle/polyaniline hydrogel heterostructures. ACS Nano 2013, 7, 3540–3546. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Y.X.; Yan, J.; Yang, S.; Dong, P.; Soman, P. Fabrication of conductive polyaniline hydrogel using porogen leaching and projection microstereolithography. J. Mater. Chem. B 2015, 3, 5352–5360. [Google Scholar] [CrossRef]
- Li, D.; Huang, J.; Kaner, R.B. Polyaniline Nanofibers: A Unique Polymer Nanostructure for Versatile Applications. Acc. Chem. Res. 2009, 42, 135–145. [Google Scholar] [CrossRef]
- Jang, J.; Bae, J.; Lee, K. Synthesis and characterization of polyaniline nanorods as curing agent and nanofiller for epoxy matrix composite. Polymer 2005, 46, 3677–3684. [Google Scholar] [CrossRef]
- Morsi, R.E.; Khamis, E.A.; Al-Sabagh, A.M. Polyaniline nanotubes: Facile synthesis, electrochemical, quantum chemical characteristics and corrosion inhibition efficiency. J. Taiwan Inst. Chem. Eng. 2016, 60, 573–581. [Google Scholar] [CrossRef]
- Olejnik, P.; Gniadek, M.; Echegoyen, L.; Plonska-Brzezinska, M. Nanoforest: Polyaniline nanotubes modified with carbon nano- onions as a nanocomposite material for easy-to-miniaturize high-performance solid-state supercapacitors. Polymers 2018, 10, 1408. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Chen, Z.; Ma, Y.; Zhang, Z.; Jin, A.; Gu, C.; Zhang, L.; Wei, Z.; Wan, M. Electrical conductivity of hollow polyaniline microspheres synthesized by a self-assembly method. Appl. Phys. Lett. 2004, 84, 2205–2207. [Google Scholar] [CrossRef]
- Goto, H.; Yokoo, A. Polyaniline nanospheres synthesized in the presence of polyvinyl alcohol followed by preparation of carbon nanobeads structures. J. Dispers. Sci. Technol. 2013, 34, 406–410. [Google Scholar] [CrossRef]
- Channu, V.S.R.; Holze, R.; Rambabu, B.; Kalluru, R.R. Synthesis and characterization of PANI nanostructures for supercapacitors and photoluminescence. Iran. Polym. J. 2012, 21, 457–462. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Q.; Guo, R.; Ni, Y.; Liu, K.; Ouyang, X.; Chen, L.; Huang, L.; Cao, S.; Xie, M. An integrated transparent, UV filtering organohydrogel sensor via molecular-level ion conductive channels. J. Mater. Chem. A 2019, 7, 4525–4535. [Google Scholar] [CrossRef]
- Peng, W.; Han, L.; Huang, H.; Xuan, X.; Pan, G.; Wan, L.; Lu, T.; Xu, M.; Pan, L. A direction-aware and ultrafast self-healing dual network hydrogel for a flexible electronic skin strain sensor. J. Mater. Chem. A 2020, 8, 26109–26118. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Chen, L.; Yin, Y.; Zhou, J.; Liu, M. Anti-freezing, conductive self- healing organohydrogels with stable strain- sensitivity at subzero temperatures. Angew. Chem. Int. Ed. 2017, 56, 14159–14163. [Google Scholar] [CrossRef]
- Han, S.; Liu, C.; Lin, X.; Zheng, J.; Wu, J.; Liu, C. Dual conductive network hydrogel for highly conductive, self-healing, anti- freezing, and non-drying strain sensor. ACS Appl. Polym. Mater. 2020, 2, 996–1005. [Google Scholar] [CrossRef]
- Ge, W.; Cao, S.; Yang, Y.; Rojas, O.J.; Wang, X. Nanocellulose/LiCl systems enable conductive and stretchable electrolyte hydrogels with tolerance to dehydration and extreme cold conditions. Chem. Eng. J. 2021, 408, 127306. [Google Scholar] [CrossRef]
- Xin, F.; Lyu, Q. A review on thermal properties of hydrogels for electronic devices applications. Gels 2023, 9, 7. [Google Scholar] [CrossRef]
- Njema, G.G.; Ouma, R.B.O.; Kibet, J.K. A Review on the recent advances in batterydevelopment and energy storage technologies. J. Renew. Energy 2024, 2024, 2329261. [Google Scholar]
- Czagany, M.; Hompoth, S.; Keshri, A.K.; Pandit, N.; Galambos, I.; Gacsi, Z.; Baumli, P. Supercapacitors: An efficient way for energy storage application. Materials 2024, 17, 702. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Amir, M.; Lakshmi, G.S.; Harivardhagini, S.; Ahmad, M. Fuel cell-based hybrid electric vehicles: An integrated review of current status, key challenges, recommended policies, and future prospects. Green Energy Intellig. Transport. 2023, 2, 100121. [Google Scholar] [CrossRef]
- Maddukuri, S.; Malka, D.; Chae, M.S.; Elias, Y.; Luski, S.; Aurbach, D. On the challenge of large energy storage by electrochemical devices. Electrochim. Acta 2020, 354, 136771. [Google Scholar] [CrossRef]
- Mensah-Darkwa, K.; Zequine, C.; Kahol, P.K.; Gupta, R.K. Supercapacitor energy storage device using biowastes: A sustainable approach to green Energy. Sustainability 2019, 11, 414. [Google Scholar] [CrossRef]
- Durairaj, A.; Sakthivel, T.; Ramanathan, S.; Obadiah, A.; Vasanthkumar, S. Conversion of laboratory paper waste into useful activated carbon: A potential supercapacitor material and a good adsorbent for organic pollutant and heavy metals. Cellulose 2019, 26, 3313–3324. [Google Scholar] [CrossRef]
- Durairaj, A.; Maruthapandi, M.; Saravanan, A.; Luong, J.H.T.; Gedanken, A. Cellulose nanocrystals (cnc)-based functional materials for supercapacitor applications. Nanomaterials 2022, 12, 1828. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-Based materials for supercapacitor electrodes—A review. J. Materiom. 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Libich, J.; Máca, J.; Vondrák, J.; Čech, O.; Sedlaříková, M. Supercapacitors: Properties and applications. J. Energy Storage 2018, 17, 224–227. [Google Scholar] [CrossRef]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef]
- Yu, J.; Wu, J.; Wang, H.; Zhou, A.; Huang, C.; Bai, H.; Li, L. Metallic fabrics as the current collector for high-performance graphene-based flexible solid-state supercapacitor. ACS Appl. Mater. Interfaces 2016, 8, 4724–4729. [Google Scholar] [CrossRef]
- Liu, J.; Ahmed, S.; Wang, T.; Song, S. Flexible thermotolerant zn-ion hybrid supercapacitors enabled by heat-resistant polymer electrolyte. Chem. Eng. J. 2023, 451, 138512. [Google Scholar] [CrossRef]
- Liu, M.; Turcheniuk, K.; Fu, W.; Yang, Y.; Liu, M.; Yushin, G. Scalable, safe, high-rate supercapacitor separators based on the Al2O3 nanowire polyvinyl butyral nonwoven membranes. Nano Energy 2020, 71, 104627. [Google Scholar] [CrossRef]
- Mendhe, A.; Panda, H.S. A Review on Electrolytes for Supercapacitor Device. Discov. Mater. 2023, 3, 29. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Edo, M.G.; Alemán, C. Powering the future: Application of cellulose-based materials for supercapacitors. Green Chem. 2016, 18, 5930–5956. [Google Scholar] [CrossRef]
- Tang, Z.; Tang, C.; Gong, H. A High energy density asymmetric supercapacitor from nano-architectured Ni(OH)2/carbon nanotube electrodes. Adv. Funct. Materials 2012, 22, 1272–1278. [Google Scholar] [CrossRef]
- Han, S.; Wu, D.; Li, S.; Zhang, F.; Feng, X. Porous graphene materials for advanced electrochemical energy storage and conversion devices. Adv. Mater. 2014, 26, 849–864. [Google Scholar] [CrossRef]
- Iro, Z.S.; Subramani, C.; Dash, S.S. A Brief review on electrode materials for supercapacitor. Int. J. Electrochemi. Sci. 2016, 11, 10628–10643. [Google Scholar] [CrossRef]
- Rajagopalan, B.; Chung, J.S. Reduced chemically modified graphene oxide for supercapacitor electrode. Nanoscale. Res. Lett. 2014, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ahmed, A.; Basha, D.B.; Hussain, S.; Uddin, I.; Gondal, M.A. Critical review on recent developments in conducting polymer nanocomposites fof supercapacitors. Synth. Met. 2023, 295, 117326. [Google Scholar] [CrossRef]
- Meng, Q.; Cai, K.; Chen, Y.; Chen, L. Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Saleem, A.M.; Desmaris, V.; Enoksson, P. Performance enhancement of carbon nanomaterials for supercapacitors. J. Nanomater. 2016, 2016, 1537269. [Google Scholar] [CrossRef]
- Tadesse, M.G.; Ahmmed, A.S.; Lübben, J.F. Review on conductive polymer composites for supercapacitor applications. J. Comp. Sci. 2024, 8, 53. [Google Scholar] [CrossRef]
- Lamba, P.; Singh, P.; Singh, P.; Singh, P.; Bharti; Kumar, A.; Gupta, M.; Kumar, Y. Recent advancements in supercapacitors based on different electrode materials: Classifications, synthesis methods and comparative performance. J. Energy Storage 2022, 48, 103871. [Google Scholar] [CrossRef]
- Shih, C.-C.; Lin, Y.-C.; Gao, M.; Wu, M.; Hsieh, H.-C.; Wu, N.-L.; Chen, W.-C. A rapid and green method for the fabrication of conductive hydrogels and their applications in stretchable supercapacitors. J. Power Sources 2019, 426, 205–215. [Google Scholar] [CrossRef]
- Shaheen Shah, S.; Oladepo, S.; Ali Ehsan, M.; Iali, W.; Alenaizan, A.; Nahid Siddiqui, M.; Oyama, M.; Al-Betar, A.-R.; Aziz, M.A. Recent progress in polyaniline and its composites for supercapacitors. The Chem. Record 2024, 24, e202300105. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.; Khattak, M.A.K.; Humayun, M.; Usman, M.; Shah, S.S.; Bibi, S.; Hasnain, B.S.U.; Ahmad, S.M.; Khan, A.; Shah, N.; et al. A review of supercapacitors: Materials design, modification, and applications. Energies 2021, 14, 7779. [Google Scholar] [CrossRef]
- Shaheen Shah, S.; Abu Nayem, S.M.; Sultana, N.; Saleh Ahammad, A.J.; Abdul Aziz, M. Preparation of Sulfur-Doped Carbon for Supercapacitor Applications: A Review. ChemSusChem 2022, 15, e202101282. [Google Scholar] [CrossRef]
- Hao, G.-P.; Hippauf, F.; Oschatz, M.; Wisser, F.M.; Leifert, A.; Nickel, W.; Mohamed- Noriega, N.; Zheng, Z.; Kaskel, S. Stretchable and semitransparent conductive hybrid hydrogels for flexible supercapacitors. ACS Nano 2014, 8, 7138–7146. [Google Scholar] [CrossRef]
- Zhu, J.; Kong, L.; Shen, X.; Chen, Q.; Ji, Z.; Wang, J.; Xu, K.; Zhu, G. Three-Dimensional n-doped graphene/polyaniline composite foam for high performance supercapacitors. App. Surf. Sci. 2018, 428, 348–355. [Google Scholar] [CrossRef]
- Forouzandeh, P.; Kumaravel, V.; Pillai, S.C. Electrode Materials for Supercapacitors: A Review of Recent Advances. Catalysts 2020, 10, 969. [Google Scholar] [CrossRef]
- Helmholtz, H. Ueber einige gesetze der vertheilung elektrischer ströme in körperlichen leitern, mit anwendung auf die thierisch- elektrischen versuche (Schluss.). Annalen der. Physik 1853, 165, 353–377. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on Supercapacitors:technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Balasubramaniam, S.; Mohanty, A.; Balasingam, S.K.; Kim, S.J.; Ramadoss, A. Comprehensive insight into the mechanism, material selection and performance evaluation of supercapatteries. Nano-Micro. Lett. 2020, 12, 85. [Google Scholar] [CrossRef]
- Chmiola, J.; Largeot, C.; Taberna, P.-L.; Simon, P.; Gogotsi, Y. Monolithic Carbide-derived carbon films for micro-Supercapacitors. Science 2010, 328, 480–483. [Google Scholar] [CrossRef]
- Perdana, M.Y.; Johan, B.A.; Abdallah, M.; Hossain, M.E.; Aziz, M.A.; Baroud, T.N.; Drmosh, Q.A. Understanding the behavior of supercapacitor materials via electrochemical impedance spectroscopy: A Review. Chem. Rec. 2024, 24, e202400007. [Google Scholar] [CrossRef]
- Largeot, C.; Portet, C.; Chmiola, J.; Taberna, P.-L.; Gogotsi, Y.; Simon, P. Relation between the Ion Size and Pore Size for an Electric Double-Layer Capacitor. J. Am. Chem. Soc. 2008, 130, 2730–2731. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Lin, J.; Malgras, V.; Dou, S.X.; Kim, J.H.; Yamauchi, Y. Large-scale synthesis of coaxial carbon nanotube/Ni(OH)2 composites for asymmetric supercapacitor application. Nano Energy 2015, 11, 211–218. [Google Scholar] [CrossRef]
- Tang, J.; Salunkhe, R.R.; Liu, J.; Torad, N.L.; Imura, M.; Furukawa, S.; Yamauchi, Y. Thermal Conversion of core–shell metal–organic frameworks: A new method for selectively functionalized nanoporous hybrid carbon. J. Am. Chem. Soc. 2015, 137, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Meduri, P.; Agubra, V.; Xiao, X.; Alcoutlabi, M. Graphene-based nanocomposites for energy storage. Adv. Energy Mater. 2016, 6, 1502159. [Google Scholar] [CrossRef]
- Volfkovich, Y.M. High power supercapacitors. review. J. Electroanalyt. Chem. 2024, 963, 118290. [Google Scholar] [CrossRef]
- Alipoori, S.; Mazinani, S.; Aboutalebi, S.H.; Sharif, F. Review of pva-based gel polymer electrolytes in flexible solid-state supercapacitors: Opportunities and challenges. J. Energy Storage 2020, 27, 101072. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors; Springer: Boston, MA, USA, 1999. [Google Scholar]
- Wang, J.; Dong, S.; Ding, B.; Wang, Y.; Hao, X.; Dou, H.; Xia, Y.; Zhang, X. Pseudocapacitive materials for electrochemical capacitors: From rational synthesis to capacitance optimization. Natl. Sci. Rev. 2017, 4, 71–90. [Google Scholar] [CrossRef]
- Fleischmann, S.; Mitchell, J.B.; Wang, R.; Zhan, C.; Jiang, D.; Presser, V.; Augustyn, V. Pseudocapacitance: From fundamental understanding to high power energy storage materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef]
- Tundwal, A.; Kumar, H.; Binoj, B.J.; Sharma, R.; Kumar, G.; Kumari, R.; Dhayal, A.; Yadav, A.; Singh, D.; Kumar, P. Developments in conducting polymer-, metal oxide-, and carbon nanotube-based composite electrode materials for supercapacitors: A review. RSC Adv. 2024, 14, 9406–9439. [Google Scholar] [CrossRef]
- Arbizzani, C.; Mastragostino, M.; Soavi, F. New trends in electrochemical supercapacitors. J. Power Sourc. 2001, 100, 164–170. [Google Scholar] [CrossRef]
- Eftekhari, A.; Li, L.; Yang, Y. Polyaniline supercapacitors. J. Power Sourc. 2017, 347, 86–107. [Google Scholar] [CrossRef]
- Song, Y.; Liu, T.-Y.; Xu, X.-X.; Feng, D.-Y.; Li, Y.; Liu, X.-X. Pushing the cycling stability limit of polypyrrole for supercapacitors. Adv. Funct. Mater. 2015, 25, 4626–4632. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, Y.-Z.; Zhang, J.-D.; Lai, W.-Y.; Huang, W. High-Performance free-standing pedot:pss electrodes for flexible and transparent all-solid-state supercapacitors. J. Mater. Chem. A 2016, 4, 10493–10499. [Google Scholar] [CrossRef]
- Jiang, Q.; Kurra, N.; Alhabeb, M.; Gogotsi, Y.; Alshareef, H.N. All Pseudocapacitive MXene-RuO2 asymmetric supercapacitors. Adv. Energy Mater. 2018, 8, 1703043. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Chang, Z.; Wu, X.; Liu, X.; Fu, L.; Zhu, Y.; Wu, Y.; Huang, W.A. Quasi-solid-state sodium-ion capacitor with high energy density. Adv. Mater. 2015, 27, 6962–6968. [Google Scholar] [CrossRef] [PubMed]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Lv, H.; Pan, Q.; Song, Y.; Liu, X.-X.; Liu, T. A Review on nano-/microstructured materials constructed by electrochemical technologies for supercapacitors. Nano-Micro Lett. 2020, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Reenu; Sonia; Phor, L.; Kumar, A.; Chahal, S. Electrode materials for supercapacitors: A comprehensive review of advanements and performance. J. Energy Storage 2024, 84, 110698. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A Review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar] [CrossRef]
- Alam, S.; Jadoon, S.; Iqbal, M.Z.; Hegazy, H.H.; Ahmad, Z.; Yahia, I.S. Recent progress in polypyrrole and its composites with carbon, metal oxides, sulfides and other conducting polymers as an emerging electrode material for asymmetric supercapacitors. J. Energy Storage 2024, 85, 110955. [Google Scholar] [CrossRef]
- Li, Z.; Liu, N.; Wang, J.; Xu, Y.; Bai, L.; Jiang, L.; Cui, L.; Shen, C.; Liu, X.; Zhao, F.-G. Structure-performance relationship guided design and strategic synthesis of lithiated oxa-graphene for high lithium storage. J. Colloid Interf. Sci. 2023, 635, 543–551. [Google Scholar] [CrossRef]
- Liu, J.; Khanam, Z.; Ahmed, S.; Wang, H.; Wang, T.; Song, S. A Study of low-temperature solid-state supercapacitors based on al-ion conducting polymer electrolyte and graphene electrodes. J. Power Sources 2021, 488, 229461. [Google Scholar] [CrossRef]
- Yassine, M.; Fabris, D. Performance of commercially available supercapacitors. Energies 2017, 10, 1340. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmed, A.; Rafat, M. Supercapacitor performance of activated carbon derived from rotten carrot in aqueous, organic and ionic liquid based electrolytes. J. Saudi Chem. Soc. 2018, 22, 993–1002. [Google Scholar] [CrossRef]
- Lu, Q.-L.; Zhao, S.-X.; Chen, C.-K.; Wang, X.; Deng, Y.-F.; Nan, C.-W. A Novel pseudocapacitance mechanism of elm seed-like mesoporous moo3−x nanosheets as electrodes for supercapacitors. J. Mater. Chem. A 2016, 4, 14560–14566. [Google Scholar] [CrossRef]
- Shown, I.; Ganguly, A.; Chen, L.-C.; Chen, K.-H. Conducting polymer-based flexible supercapacitor. Energy Sci. Eng. 2015, 3, 2–26. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Supercapacitors: Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2283. [Google Scholar] [CrossRef]
- Gidwani, M.; Bhagwani, A.; Rohra, N. Supercapacitors: The near future of batteries. Int. J. Eng. Invent. 2014, 4, 22–27. [Google Scholar]
- Wang, G.; Zhang, L.; Zhang, J. A Review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Pan, J.; Zhao, Y.; Liao, M.; Peng, H. Gel polymer electrolytes for electrochemical energy storage. Adv. Energy Mater. 2018, 8, 1702184. [Google Scholar] [CrossRef]
- Uno, M.; Tanaka, K. Accelerated charge–discharge cycling test and cycle life prediction model for supercapacitors in alternative battery applications. IEEE Transact. Indust. Electron. 2012, 59, 4704–4712. [Google Scholar] [CrossRef]
- Liu, T.; Finn, L.; Yu, M.; Wang, H.; Zhai, T.; Lu, X.; Tong, Y.; Li, Y. Polyaniline and polypyrrole pseudocapacitor electrodes with excellent cycling stability. Nano Lett. 2014, 14, 2522–2527. [Google Scholar] [CrossRef]
- Chee, W.K.; Lim, H.N.; Harrison, I.; Chong, K.F.; Zainal, Z.; Ng, C.H.; Huang, N.M. Performance of flexible and binderless polypyrrole/graphene oxide/zinc oxide supercapacitor electrode in a symmetrical two-electrode configuration. Electrochim. Acta 2015, 157, 88–94. [Google Scholar] [CrossRef]
- Chabi, S.; Peng, C.; Hu, D.; Zhu, Y. Ideal three-dimensional electrode structures for electrochemical energy storage. Adv. Mater. 2014, 26, 2440–2445. [Google Scholar] [CrossRef] [PubMed]
- Laheäär, A.; Przygocki, P.; Abbas, Q.; Béguin, F. Appropriate methods for evaluating the efficiency and capacitive behavior of different types of supercapacitors. Electrochem. Commun. 2015, 60, 21–25. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Zhao, J.; Burke, A.F. Electrochemical capacitors: Performance metrics and evaluation by testing and analysis. Adv. Energy Mater. 2021, 11, 2002192. [Google Scholar] [CrossRef]
- Bryan, A.M.; Santino, L.M.; Lu, Y.; Acharya, S.; D’Arcy, J.M. Conducting polymers for pseudocapacitive energy storage. Chem. Mater. 2016, 28, 5989–5998. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Penner, R.M. Energy storage in nanomaterials–capacitive, pseudocapacitive, or battery-like? ACS Nano 2018, 12, 2081–2083. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef]
- Habin, P.; Jaewon, C.; Hansol, Y.; Jongwon, J.; Cheolsoo, J. Roles of gel polymer electrolytes for high-power activated carbon supercapacitors: Ion reservoir and binder-like effects. RSC Adv. 2020, 10, 4690–4697. [Google Scholar]
- Dupont, M.F.; Hollenkamp, A.F.; Donne, S.W. Large amplitude electrochemical impedance spectroscopy for characterizing the performance of electrochemical capacitors. J. Electrochem. Soc. 2014, 161, A648. [Google Scholar] [CrossRef]
- Taberna, P.L.; Simon, P.; Fauvarque, J.F. Electrochemical characteristics and impedance spectroscopy studies of carbon-carbon supercapacitors. J. Electrochem. Soc. 2003, 150, A292. [Google Scholar] [CrossRef]
- Yang, X.; Rogach, A.L. Electrochemical techniques in battery research: A tutorial for nonelectrochemists. Adv. Energy Mater. 2019, 9, 1900747. [Google Scholar] [CrossRef]
- Ding, J.; Yang, Y.; Poisson, J.; He, Y.; Zhang, H.; Zhang, Y.; Bao, Y.; Chen, S.; Chen, Y.M.; Zhang, K. Recent advances in biopolymer-based hydrogel electrolytes for flexible supercapacitors. ACS Energy Lett. 2024, 9, 1803–1825. [Google Scholar] [CrossRef]
- Mathis, T.S.; Kurra, N.; Wang, X.; Pinto, D.; Simon, P.; Gogotsi, Y. Energy storage data reporting in perspective—Guidelines for interpreting the performance of electrochemical energy storage systems. Adv. Energy Mater. 2019, 9, 1902007. [Google Scholar] [CrossRef]
- Zhi, M.; Xiang, C.; Li, J.; Li, M.; Wu, N. Nanostructured carbon-metal oxide composite electrodes for supercapacitors: A review. Nanoscale 2013, 5, 72–88. [Google Scholar] [CrossRef]
- Cottineau, T.; Toupin, M.; Delahaye, T.; Brousse, T.; Bélanger, D. Nanostructured transition metal oxides for aqueous hybrid electrochemical supercapacitors. Appl. Phys. A 2006, 82, 599–606. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Guang, S.; Ke, F.; Xu, H. Polyaniline-graphene composites with a three-dimensional array-based nanostructure for high-performance supercapacitors. Carbon 2015, 83, 79–89. [Google Scholar] [CrossRef]
- Guo, H.; He, W.; Lu, Y.; Zhang, X. Self-crosslinked polyaniline hydrogel electrodes for electrochemical energy storage. Carbon 2015, 92, 133–141. [Google Scholar] [CrossRef]
- Ghosh, S.; Inganäs, O. Conducting polymer hydrogels as 3D electrodes:applications for supercapacitors. Adv. Mater. 1999, 11, 1214–1218. [Google Scholar] [CrossRef]
- Xu, Z.; Chu, X.; Wang, Y.; Zhang, H.; Yang, W. Three-dimensional polymer networks for solid-state electrochemical energy storage. Chem. Eng. J. 2020, 391, 123548. [Google Scholar] [CrossRef]
- Eftekhari, A.; Jafarkhani, P. Polymerization of aniline through simultaneous chemical and electrochemical routes. Polym. J. 2006, 38, 651–658. [Google Scholar] [CrossRef]
- Wu, X.; Lian, M. Highly flexible solid-state supercapacitor based on graphene/ polypyrrole hydrogel. J. Power Sources 2017, 362, 184–191. [Google Scholar] [CrossRef]
- Yu, Z.; Li, C.; Abbitt, D.; Thomas, J. Flexible, sandwich-like Ag- nanowire/PEDOT:pSS-nanopillar/MnO2 high performance supercapacitors. J. Mater. Chem. A 2014, 2, 10923–10929. [Google Scholar] [CrossRef]
- Imani, A.; Farzi, G. Facile route for multi-walled carbon nanotube coating with polyaniline: Tubular morphology nanocomposites for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2015, 26, 7438–7444. [Google Scholar] [CrossRef]
- Mi, H.Y.; Zhang, X.G.; An, S.Y.; Ye, X.G.; Yang, S.D. Microwave-assisted synthesis and electro-chemical capacitance of polyaniline/multi-wall carbon nanotubes composite. Electrochem. Commun. 2007, 9, 2859–2862. [Google Scholar] [CrossRef]
- Niu, Z.Q.; Luan, P.S.; Shao, Q.; Dong, H.B.; Li, J.Z.; Chen, J.; Zhao, D.; Cai, L.; Zhou, W.Y.; Chen, X.D.; et al. A “skeleton/skin” strategy for preparing ultrathin free-standing single-walled carbon nanotube/polyaniline films for high performance supercapacitor electrodes. Energy Environ. Sci. 2012, 5, 8726–8733. [Google Scholar] [CrossRef]
- Huang, H.; Chen, R.; Yang, S.; Li, L.; Liu, Y.; Huang, J. Facile fabrication of MnO2-embedded 3-D porous polyaniline composite hydrogel for supercapacitor electrode with high loading. High Perform. Polym. 2020, 32, 286–295. [Google Scholar] [CrossRef]
- Lei, J.Y.; Jiang, Z.Q.; Lu, X.F.; Nie, G.D.; Wang, C. Synthesis of Few-Layer MoS2 Nanosheets-Wrapped polyaniline hierarchical nanostructures for enhanced electrochemical capacitance performance. Electrochim. Acta 2015, 176, 149–155. [Google Scholar] [CrossRef]
- Zhu, J.X.; Sun, W.P.; Yang, D.; Zhang, Y.; Hoon, H.H.; Zhang, H.; Yan, Q.Y. multifunctional architectures constructing of PANI nanoneedle arrays on mos2 thin nanosheets for high-energy supercapacitors. Small 2015, 11, 4123–4129. [Google Scholar] [CrossRef]
- Sun, H.; She, P.; Xu, K.L.; Shang, Y.X.; Yin, S.Y.; Liu, Z.N. A self-standing nanocomposite foam of polyaniline@reduced graphene oxide for flexible supercapacitors. Synth. Met. 2015, 209, 68–73. [Google Scholar] [CrossRef]
- Wang, H.L.; Hao, Q.L.; Yang, X.J.; Lu, L.D.; Wang, X. Graphene oxide doped polyaniline for supercapacitors. Electrochem. Commun. 2009, 11, 1158–1161. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.L.; Zhao, X.S.; Wu, J.S. Graphene/Polyaniline nanofiber ocmposites as supercapacitor electrodes. J. Mater. Chem. A 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Wang, S.Y.; Ma, L.; Gan, M.Y.; Fu, S.N.; Dai, W.Q.; Zhou, T.; Sun, X.W.; Wang, H.H.; Wang, H.N. Free-standing 3D graphene/polyaniline composite film electrodes for high-performance supercapacitors. J. Power Sources 2015, 299, 347–355. [Google Scholar] [CrossRef]
- Jayakumar, A.; Yoon, Y.J.; Wang, R.; Lee, J.M. Novel graphene/polyaniline/MnOx 3D-hydrogels obtained by controlled morphology of MnOx in the graphene/polyaniline matrix for high performance binder-free supercapacitor electrodes. RSC Adv. 2015, 5, 94388–94396. [Google Scholar] [CrossRef]
- Mondal, S.; Rana, U.; Malik, S. Reduced graphene oxide/Fe3O4/polyaniline nanostructures as electrode materials for an all-solid- state hybrid supercapacitor. J. Phys. Chem. C. 2017, 121, 7573–7583. [Google Scholar] [CrossRef]
- Lei, W.; He, P.; Wang, Y.H.; Zhang, S.S.; Dong, F.Q.; Liu, H.T. Soft template interfacial growth of novel ultralong polypyrrole nanowires for electrochemical energy storage. Electrochim. Acta 2014, 132, 112–117. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Chen, M.M.; Yang, C.Y.; Wang, W.Q.; Bao, H.; Wang, G.C. enhancing the energy density of asymmetric stretchable supercapacitor based on wrinkled CNT@MnO2 cathode and CNT@polypyrrole anode. ACS Appl. Mater. Interface 2015, 7, 15303–15313. [Google Scholar] [CrossRef]
- Yang, L.F.; Shi, Z.; Yang, W.H. Polypyrrole directly bonded to air-plasma activated carbon nanotube as electrode materials for high-performance supercapacitor. Electrochim. Acta 2015, 153, 76–82. [Google Scholar] [CrossRef]
- Song, H.J.; Cai, K.F.; Wang, J.; Shen, S. Influence of polymerization method on the thermoelectric properties of multi-walled carbon nanotubes/polypyrrole composites. Synth. Met. 2016, 211, 58–65. [Google Scholar] [CrossRef]
- Wang, J.; Cai, K.F.; Shen, S.; Yin, J.L. Preparation and thermoelectric properties of multi-walled carbon nanotubes/polypyrrole composites. Synth. Met. 2014, 195, 132–136. [Google Scholar] [CrossRef]
- Zhou, H.H.; Han, G.Y.; Xiao, Y.M.; Chang, Y.Z.; Zhai, H.J. A comparative study on long and short carbon nanotubes-incorporated polypyrrole/poly(sodium 4-styrenesulfonate) nanocomposites as high-performance supercapacitor electrodes. Synth. Met. 2015, 209, 405–411. [Google Scholar] [CrossRef]
- Warren, R.; Sammoura, F.; Teh, K.S.; Kozinda, A.; Zang, X.N.; Lin, L.W. Electrochemically synthesized and vertically aligned carbon nanotube–polypyrrole nanolayers for high energy storage devices. Sens. Actuators A–Phys. 2015, 231, 65–73. [Google Scholar] [CrossRef]
- Jiang, L.L.; Lu, X.; Xu, J.L.; Chen, Y.Q.; Wan, G.J.; Ding, Y.H. Free-standing microporous paper-like graphene films with electrodeposited PPy coatings as electrodes for supercapacitors. J. Mater. Sci. Mater. Electron. 2015, 26, 747–754. [Google Scholar] [CrossRef]
- Zhang, J.T.; Zhao, X.S. Conducting polymers directly coated on reduced graphene oxide sheets as high-performance supercapacitor electrodes. J. Phys. Chem. C 2012, 116, 5420–5426. [Google Scholar] [CrossRef]
- Zhu, J.B.; Xu, Y.L.; Wang, J.; Wang, J.P.; Bai, Y.; Du, X.F. Morphology controllable nano sheet polypyrrole–graphene composites for high-rate supercapacitor. Phys. Chem. Chem. Phys. 2015, 17, 19885–19894. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.L.; Yang, L.Q.; Chen, S.L.; Shao, Y.M.; Jing, L.Y.; Zhao, G.H.; Wei, H. Core–shell nanospherical polypyrrole/graphene oxide composites for high performance supercapacitors. RSC Adv. 2015, 5, 91645–91653. [Google Scholar] [CrossRef]
- Shayeh, J.S.; Siadat, S.O.R.; Sadeghnia, M.; Niknam, K.; Rezaei, M.; Aghamohammadi, N. Advanced studies of coupled conductive polymer/metal oxide nano wire composite as an efficient supercapacitor by common and fast fourier electrochemical methods. J. Mol. Liq. 2016, 220, 489–494. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman Khan, M.M.; Chakraborty, N. Conducting Polymer-Based Gel Materials: Synthesis, Morphology, Thermal Properties, and Applications in Supercapacitors. Gels 2024, 10, 553. https://doi.org/10.3390/gels10090553
Rahman Khan MM, Chakraborty N. Conducting Polymer-Based Gel Materials: Synthesis, Morphology, Thermal Properties, and Applications in Supercapacitors. Gels. 2024; 10(9):553. https://doi.org/10.3390/gels10090553
Chicago/Turabian StyleRahman Khan, Mohammad Mizanur, and Nilave Chakraborty. 2024. "Conducting Polymer-Based Gel Materials: Synthesis, Morphology, Thermal Properties, and Applications in Supercapacitors" Gels 10, no. 9: 553. https://doi.org/10.3390/gels10090553





