Machine Learning Techniques to Analyze the Influence of Silica on the Physico-Chemical Properties of Aerogels
Abstract
1. Introduction
2. Results and Discussion
2.1. Principal Machine Learning Model for Exploring Cross-Correlations among the Physico-Chemical Properties of Silica Aerogel
2.2. Principal Component Analysis for Silica Aerogel Composite
2.3. Principal Components vs. the Percentage of Silica in Aerogel Composites
3. Conclusions
4. Materials and Methods
4.1. Principal Machine Learning Model for Exploring Cross-Correlation among Studied Features
4.2. Data Collection and Pre-Treatment
4.3. Principal Component Analysis (PCA)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Ahmad, S.; Ahmad, S.; Sheikh, J.N. Silica Centered Aerogels as Advanced Functional Material and Their Applications: A Review. J. Non-Cryst. Solids 2023, 611, 122322. [Google Scholar] [CrossRef]
- Hrubesh, L.W.; Pekala, R.W. Thermal Properties of Organic and Inorganic Aerogels. J. Mater. Res. 1994, 9, 731–738. [Google Scholar] [CrossRef]
- Pajonk, G.M. Some Applications of Silica Aerogels. Colloid Polym. Sci. 2003, 281, 637–651. [Google Scholar] [CrossRef]
- Linhares, T.; de Amorim, M.T.P.; Durães, L. Silica Aerogel Composites with Embedded Fibres: A Review on Their Preparation, Properties and Applications. J. Mater. Chem. A 2019, 7, 22768–22802. [Google Scholar] [CrossRef]
- Kiil, S. Quantitative Analysis of Silica Aerogel-Based Thermal Insulation Coatings. Prog. Org. Coat. 2015, 89, 26–34. [Google Scholar] [CrossRef]
- Shafi, S.; Navik, R.; Ding, X.; Zhao, Y. Improved Heat Insulation and Mechanical Properties of Silica Aerogel/Glass Fiber Composite by Impregnating Silica Gel. J. Non-Cryst. Solids 2019, 503–504, 78–83. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Silva, R.F.; Durães, L. Advances in Carbon Nanostructure–Silica Aerogel Composites: A Review. J. Mater. Chem. A 2018, 6, 1340–1369. [Google Scholar] [CrossRef]
- Ul Haq, E.; Zaidi, S.F.A.; Zubair, M.; Abdul Karim, M.R.; Padmanabhan, S.K.; Licciulli, A. Hydrophobic Silica Aerogel Glass-Fibre Composite with Higher Strength and Thermal Insulation Based on Methyltrimethoxysilane (MTMS) Precursor. Energy Build. 2017, 151, 494–500. [Google Scholar] [CrossRef]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring Mechanical Properties of Aerogels for Aerospace Applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef]
- Hassan, K.T.; Wang, J.; Han, X.; Sharp, J.J.; Bhaduri, G.A.; Martis, V.; Šiller, L. Catalytic Performance of Nickel Nanowires Immobilized in Silica Aerogels for the CO2 Hydration Reaction. ACS Omega 2019, 4, 1824–1830. [Google Scholar] [CrossRef]
- He, S.; Cheng, X.; Li, Z.; Shi, X.; Yang, H.; Zhang, H. Green and Facile Synthesis of Sponge-Reinforced Silica Aerogel and Its Pumping Application for Oil Absorption. J. Mater. Sci. 2016, 51, 1292–1301. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Torres, R.B.; Vareda, J.P.; Lopes, D.; Ferreira, M.; Valente, V.; Girão, A.V.; Valente, A.J.M.; Durães, L. Amine Modification of Silica Aerogels/Xerogels for Removal of Relevant Environmental Pollutants. Molecules 2019, 24, 3701. [Google Scholar] [CrossRef]
- Rocha, H.; Lafont, U.; Semprimoschnig, C. Environmental Testing and Characterization of Fibre Reinforced Silica Aerogel Materials for Mars Exploration. Acta Astronaut. 2019, 165, 9–16. [Google Scholar] [CrossRef]
- Jadhav, S.; Sarawade, P. Recent Advances Prospective of Reinforced Silica Aerogel Nanocomposites and Their Applications. Eur. Polym. J. 2024, 206, 112766. [Google Scholar] [CrossRef]
- Cheng, E.J.; Sakamoto, J.; Salvador, J.; Wang, H.; Maloney, R.; Thompson, T. Cast-in-Place, Ambiently-Dried, Silica-Based, High-Temperature Insulation. Acta Mater. 2017, 127, 450–462. [Google Scholar] [CrossRef]
- Fricke, J.; Tillotson, T. Aerogels: Production, Characterization, and Applications. Thin Solid Film. 1997, 297, 212–223. [Google Scholar] [CrossRef]
- Capadona, L.A.; Meador, M.A.B.; Alunni, A.; Fabrizio, E.F.; Vassilaras, P.; Leventis, N. Flexible, Low-Density Polymer Crosslinked Silica Aerogels. Polymer 2006, 47, 5754–5761. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Zhang, G.; Rawashdeh, A.-M.M. Nanoengineering Strong Silica Aerogels. Nano Lett. 2002, 2, 957–960. [Google Scholar] [CrossRef]
- Yuan, B.; Ding, S.; Wang, D.; Wang, G.; Li, H. Heat Insulation Properties of Silica Aerogel/Glass Fiber Composites Fabricated by Press Forming. Mater. Lett. 2012, 75, 204–206. [Google Scholar] [CrossRef]
- Guzel Kaya, G.; Deveci, H. Synergistic Effects of Silica Aerogels/Xerogels on Properties of Polymer Composites: A Review. J. Ind. Eng. Chem. 2020, 89, 13–27. [Google Scholar] [CrossRef]
- Karamikamkar, S.; Naguib, H.E.; Park, C.B. Advances in Precursor System for Silica-Based Aerogel Production toward Improved Mechanical Properties, Customized Morphology, and Multifunctionality: A Review. Adv. Colloid Interface Sci. 2020, 276, 102101. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-C.; Horng, R.S.; Shia, R.-E. Investigation of Thermal Insulation Performance of Glass/Carbon Fiber-Reinforced Silica Aerogel Composites. J. Sol.-Gel. Sci. Technol. 2021, 97, 414–421. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Lin, Y.-J.; Lee, C.-C.; Lin, K.-Y.A.; Chung, T.-W.; Tung, K.-L. Synthesis of Mechanically Robust Epoxy Cross-Linked Silica Aerogel Membranes for CO2 Capture. J. Taiwan Inst. Chem. Eng. 2018, 87, 117–122. [Google Scholar] [CrossRef]
- Obeid, E.; Younes, K. Uncovering Key Factors in Graphene Aerogel-Based Electrocatalysts for Sustainable Hydrogen Production: An Unsupervised Machine Learning Approach. Gels 2024, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Younes, K.; Kharboutly, Y.; Antar, M.; Chaouk, H.; Obeid, E.; Mouhtady, O.; Abu-samha, M.; Halwani, J.; Murshid, N. Application of Unsupervised Machine Learning for the Evaluation of Aerogels’ Efficiency towards Ion Removal—A Principal Component Analysis (PCA) Approach. Gels 2023, 9, 304. [Google Scholar] [CrossRef]
- Younes, K.; Abdallah, M.; Hanna, E.G. The Application of Principal Components Analysis for the Comparison of Chemical and Physical Properties among Activated Carbon Models. Mater. Lett. 2022, 325, 132864. [Google Scholar] [CrossRef]
- Younes, K.; Moghrabi, A.; Moghnie, S.; Mouhtady, O.; Murshid, N.; Grasset, L. Assessment of the Efficiency of Chemical and Thermochemical Depolymerization Methods for Lignin Valorization: Principal Component Analysis (PCA) Approach. Polymers 2022, 14, 194. [Google Scholar] [CrossRef]
- Younes, K.; Mouhtady, O.; Chaouk, H.; Obeid, E.; Roufayel, R.; Moghrabi, A.; Murshid, N. The Application of Principal Component Analysis (PCA) for the Optimization of the Conditions of Fabrication of Electrospun Nanofibrous Membrane for Desalination and Ion Removal. Membranes 2021, 11, 979. [Google Scholar] [CrossRef]
- Gazo Hanna, E.; Younes, K.; Amine, S.; Roufayel, R. Exploring Gel-Point Identification in Epoxy Resin Using Rheology and Unsupervised Learning. Gels 2023, 9, 828. [Google Scholar] [CrossRef]
- Younes, K.; Kharboutly, Y.; Antar, M.; Chaouk, H.; Obeid, E.; Mouhtady, O.; Abu-samha, M.; Halwani, J.; Murshid, N. Application of Unsupervised Learning for the Evaluation of Aerogels’ Efficiency towards Dye Removal—A Principal Component Analysis (PCA) Approach. Gels 2023, 9, 327. [Google Scholar] [CrossRef]
- Mouhtady, O.; Obeid, E.; Abu-samha, M.; Younes, K.; Murshid, N. Evaluation of the Adsorption Efficiency of Graphene Oxide Hydrogels in Wastewater Dye Removal: Application of Principal Component Analysis. Gels 2022, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiang, L.; Shen, Q.; Li, X.; Wu, T.; Zhang, J.; Nie, C. Rapid Synthesis of Dual-Mesoporous Silica Aerogel with Excellent Adsorption Capacity and Ultra-Low Thermal Conductivity. J. Non-Cryst. Solids 2020, 555, 120547. [Google Scholar] [CrossRef]
- Jiang, Y.; Feng, J.; Feng, J. Synthesis and Characterization of Ambient-Dried Microglass Fibers/Silica Aerogel Nanocomposites with Low Thermal Conductivity. J. Sol.-Gel. Sci. Technol. 2017, 83, 64–71. [Google Scholar] [CrossRef]
- Wong, J.C.; Kaymak, H.; Tingaut, P.; Brunner, S.; Koebel, M.M. Mechanical and Thermal Properties of Nanofibrillated Cellulose Reinforced Silica Aerogel Composites. Microporous Mesoporous Mater. 2015, 217, 150–158. [Google Scholar] [CrossRef]
- Wang, L.-J.; Zhao, S.-Y.; Yang, M. Structural Characteristics and Thermal Conductivity of Ambient Pressure Dried Silica Aerogels with One-Step Solvent Exchange/Surface Modification. Mater. Chem. Phys. 2009, 113, 485–490. [Google Scholar] [CrossRef]
- Wei, T.; Chang, T.; Lu, S.; Chang, Y. Preparation of Monolithic Silica Aerogel of Low Thermal Conductivity by Ambient Pressure Drying. J. Am. Ceram. Soc. 2007, 90, 2003–2007. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, X.; Song, H.; Hu, Z.; Cao, B. The Influence of Thermal Treatment on the Microstructure and Thermal Insulation Performance of Silica Aerogels. J. Non-Cryst. Solids 2017, 470, 178–183. [Google Scholar] [CrossRef]
- Amonette, J.E.; Matyáš, J. Functionalized Silica Aerogels for Gas-Phase Purification, Sensing, and Catalysis: A Review. Microporous Mesoporous Mater. 2017, 250, 100–119. [Google Scholar] [CrossRef]
- Obrey, K.A.D.; Wilson, K.V.; Loy, D.A. Enhancing Mechanical Properties of Silica Aerogels. J. Non-Cryst. Solids 2011, 357, 3435–3441. [Google Scholar] [CrossRef]
- Zheng, H.; Shan, H.; Bai, Y.; Wang, X.; Liu, L.; Yu, J.; Ding, B. Assembly of Silica Aerogels within Silica Nanofibers: Towards a Super-Insulating Flexible Hybrid Aerogel Membrane. RSC Adv. 2015, 5, 91813–91820. [Google Scholar] [CrossRef]
- Xue, J.; Han, R.; Li, Y.; Zhang, J.; Liu, J.; Yang, Y. Advances in Multiple Reinforcement Strategies and Applications for Silica Aerogel. J. Mater. Sci. 2023, 58, 14255–14283. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An Overview on Silica Aerogels Synthesis and Different Mechanical Reinforcing Strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Aminoroaya, A.; Bagheri, R.; Khorasani, S.N.; Talebi, Z.; Derakhshanfar, P.; Neisiany, R.E. Mesoporous Silica Aerogel Reinforced Dental Composite: Effects of Microstructure and Surface Modification. J. Mech. Behav. Biomed. Mater. 2022, 125, 104947. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. Synthesis of Lightweight Polymer-Reinforced Silica Aerogels with Improved Mechanical and Thermal Insulation Properties for Space Applications. Microporous Mesoporous Mater. 2014, 197, 116–129. [Google Scholar] [CrossRef]
- Easaw, N.; Lee, W.S.; Lohiya, P.S.; Jalan, S.; Pradhan, P. Estimation of Correlation Matrices from Limited Time Series Data Using Machine Learning. J. Comput. Sci. 2023, 71, 102053. [Google Scholar] [CrossRef]
- Filzmoser, P.; Walczak, B. What Can Go Wrong at the Data Normalization Step for Identification of Biomarkers? J. Chromatogr. A 2014, 1362, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Hasan, B.M.S.; Abdulazeez, A.M. A Review of Principal Component Analysis Algorithm for Dimensionality Reduction. J. Soft Comput. Data Min. 2021, 2, 20–30. [Google Scholar]
- Jolliffe, I.T.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Phil. Trans. R. Soc. A. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Van Den Berg, R.A.; Hoefsloot, H.C.; Westerhuis, J.A.; Smilde, A.K.; Van Der Werf, M.J. Centering, Scaling, and Transformations: Improving the Biological Information Content of Metabolomics Data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef]
- Zien, A.; Aigner, T.; Zimmer, R.; Lengauer, T. Centralization: A New Method for the Normalization of Gene Expression Data. BIOINFORMATICS-OXFORD- 2001, 17, S323–S331. [Google Scholar] [CrossRef]
- Shahid, N.; Perraudin, N.; Kalofolias, V.; Puy, G.; Vandergheynst, P. Fast Robust PCA on Graphs. IEEE J. Sel. Top. Signal Process. 2016, 10, 740–756. [Google Scholar] [CrossRef]
- Greenacre, M.; Groenen, P.J.; Hastie, T.; d’Enza, A.I.; Markos, A.; Tuzhilina, E. Principal Component Analysis. Nat. Rev. Methods Primers 2022, 2, 100. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal Component Analysis. WIREs Comput. Stats 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Reich, D.; Price, A.L.; Patterson, N. Principal Component Analysis of Genetic Data. Nat. Genet. 2008, 40, 491–492. [Google Scholar] [CrossRef] [PubMed]
- Lenka, S.K. Measuring Financial Development in India: A PCA Approach. Theor. Appl. Econ. 2015, 22. [Google Scholar]
- Saukani, N.; Ismail, N.A. Identifying the Components of Social Capital by Categorical Principal Component Analysis (CATPCA). Soc. Indic. Res. 2019, 141, 631–655. [Google Scholar] [CrossRef]
- Ahmad, I.; Hussain, M.; Alghamdi, A.; Alelaiwi, A. Enhancing SVM Performance in Intrusion Detection Using Optimal Feature Subset Selection Based on Genetic Principal Components. Neural Comput. Appl. 2014, 24, 1671–1682. [Google Scholar] [CrossRef]
- Salam, M.A.; Azar, A.T.; Elgendy, M.S.; Fouad, K.M. The Effect of Different Dimensionality Reduction Techniques on Machine Learning Overfitting Problem. Int. J. Adv. Comput. Sci. Appl. 2021, 12, 641–655. [Google Scholar] [CrossRef]
- Dagher, I.; Hassanieh, J.; Younes, A. Face Recognition Using Voting Technique for the Gabor and LDP Features. In Proceedings of the The 2013 International Joint Conference on Neural Networks (IJCNN), Dallas, TX, USA, 4–9 August 2013; IEEE: New York, NY, USA, 2013; pp. 1–6. [Google Scholar]
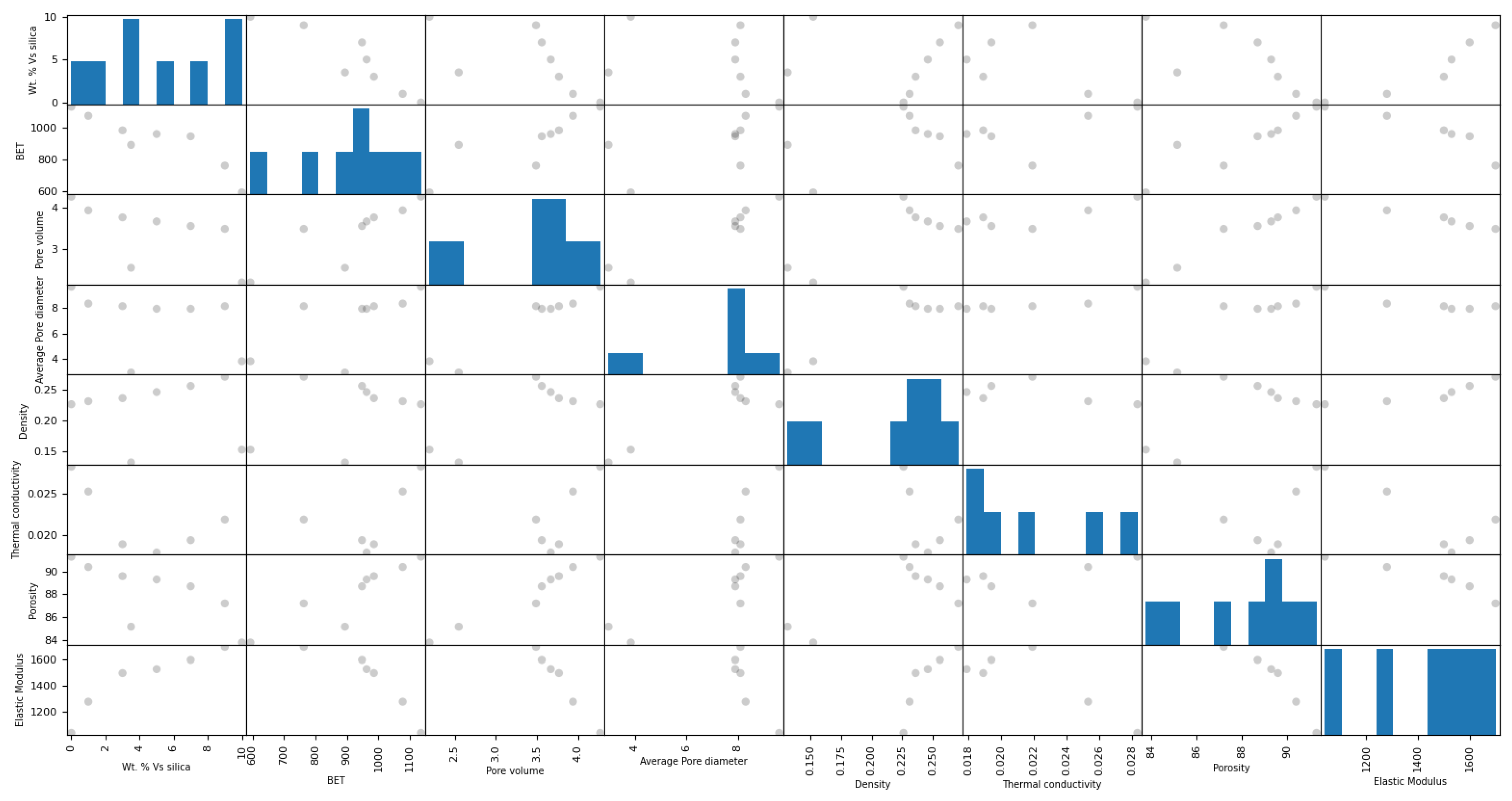
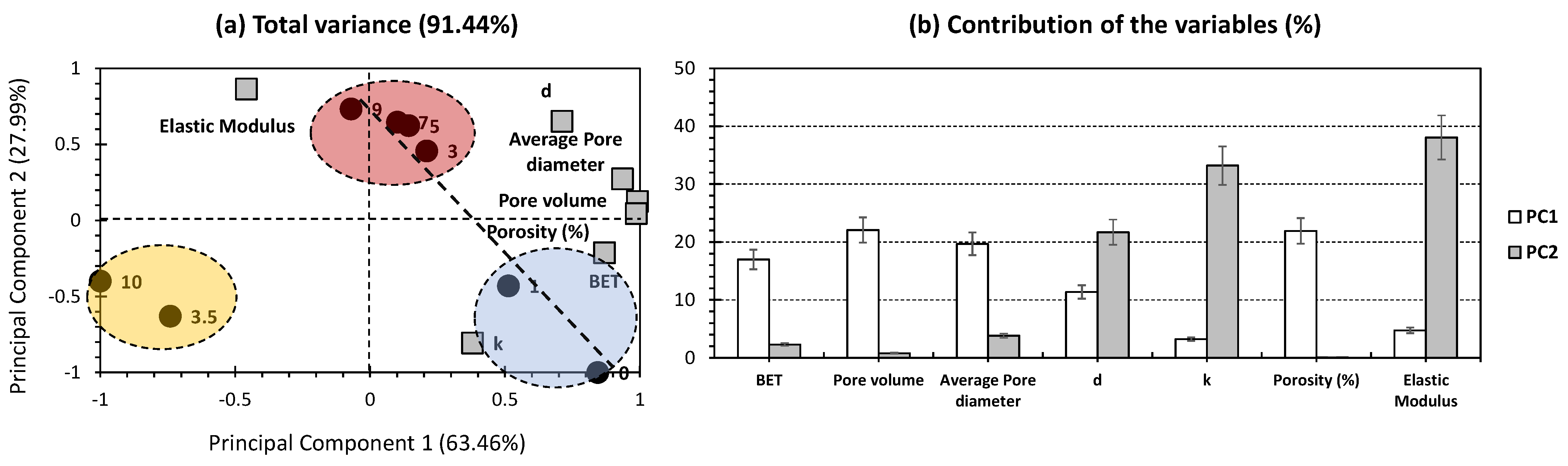
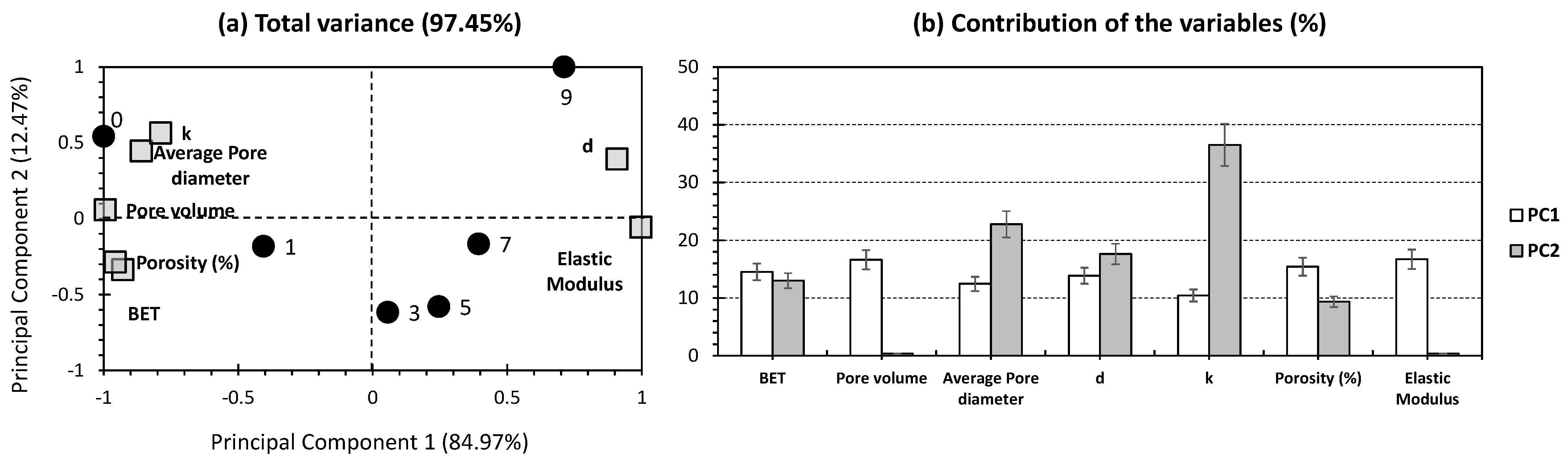
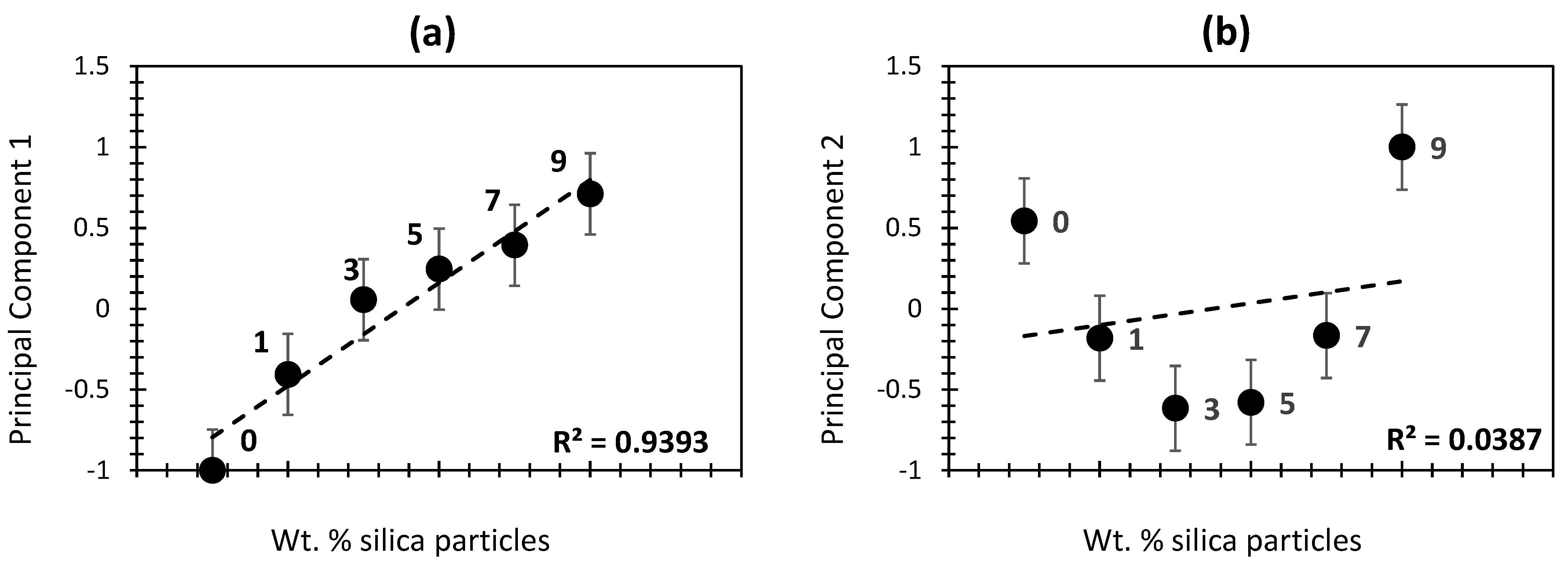

| Wt.% of Silica Particles | Drying Technique | BET Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) | Density (g/cm3) | Thermal Conductivity (W/mK) | Porosity (%) | Elastic Modulus (kPa) | References |
|---|---|---|---|---|---|---|---|---|---|
| 0 | CO2 SCD a | 1135.4 | 4.27 | 9.6 | 0.226 | 0.0283 | 91.3 | 1040 | [6] |
| 1 | CO2 SCD a | 1077.4 | 3.94 | 8.3 | 0.231 | 0.0253 | 90.4 | 1280 | [6] |
| 3 | CO2 SCD a | 985.86 | 3.77 | 8.1 | 0.236 | 0.0189 | 89.6 | 1500 | [6] |
| 3.5 | APD b | 893.773 | 2.549 | 2.984 | 0.131 | - | 85.15 | - | [22] |
| 5 | CO2 SCD a | 962.6 | 3.67 | 7.9 | 0.246 | 0.0179 | 89.3 | 1530 | [6] |
| 7 | CO2 SCD a | 947.73 | 3.56 | 7.9 | 0.256 | 0.0194 | 88.7 | 1600 | [6] |
| 9 | CO2 SCD a | 762.55 | 3.49 | 8.1 | 0.271 | 0.0219 | 87.2 | 1700 | [6] |
| 10 | APD b | 593.051 | 2.193 | 3.848 | 0.152 | - | 83.76 | - | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaouk, H.; Obeid, E.; Halwani, J.; Arayro, J.; Mezher, R.; Mouhtady, O.; Gazo-Hanna, E.; Amine, S.; Younes, K. Machine Learning Techniques to Analyze the Influence of Silica on the Physico-Chemical Properties of Aerogels. Gels 2024, 10, 554. https://doi.org/10.3390/gels10090554
Chaouk H, Obeid E, Halwani J, Arayro J, Mezher R, Mouhtady O, Gazo-Hanna E, Amine S, Younes K. Machine Learning Techniques to Analyze the Influence of Silica on the Physico-Chemical Properties of Aerogels. Gels. 2024; 10(9):554. https://doi.org/10.3390/gels10090554
Chicago/Turabian StyleChaouk, Hamdi, Emil Obeid, Jalal Halwani, Jack Arayro, Rabih Mezher, Omar Mouhtady, Eddie Gazo-Hanna, Semaan Amine, and Khaled Younes. 2024. "Machine Learning Techniques to Analyze the Influence of Silica on the Physico-Chemical Properties of Aerogels" Gels 10, no. 9: 554. https://doi.org/10.3390/gels10090554
APA StyleChaouk, H., Obeid, E., Halwani, J., Arayro, J., Mezher, R., Mouhtady, O., Gazo-Hanna, E., Amine, S., & Younes, K. (2024). Machine Learning Techniques to Analyze the Influence of Silica on the Physico-Chemical Properties of Aerogels. Gels, 10(9), 554. https://doi.org/10.3390/gels10090554










