Acid Hydrolysis of Quinoa Starch to Stabilize High Internal Phase Emulsion Gels
Abstract
1. Introduction
2. Results and Discussion
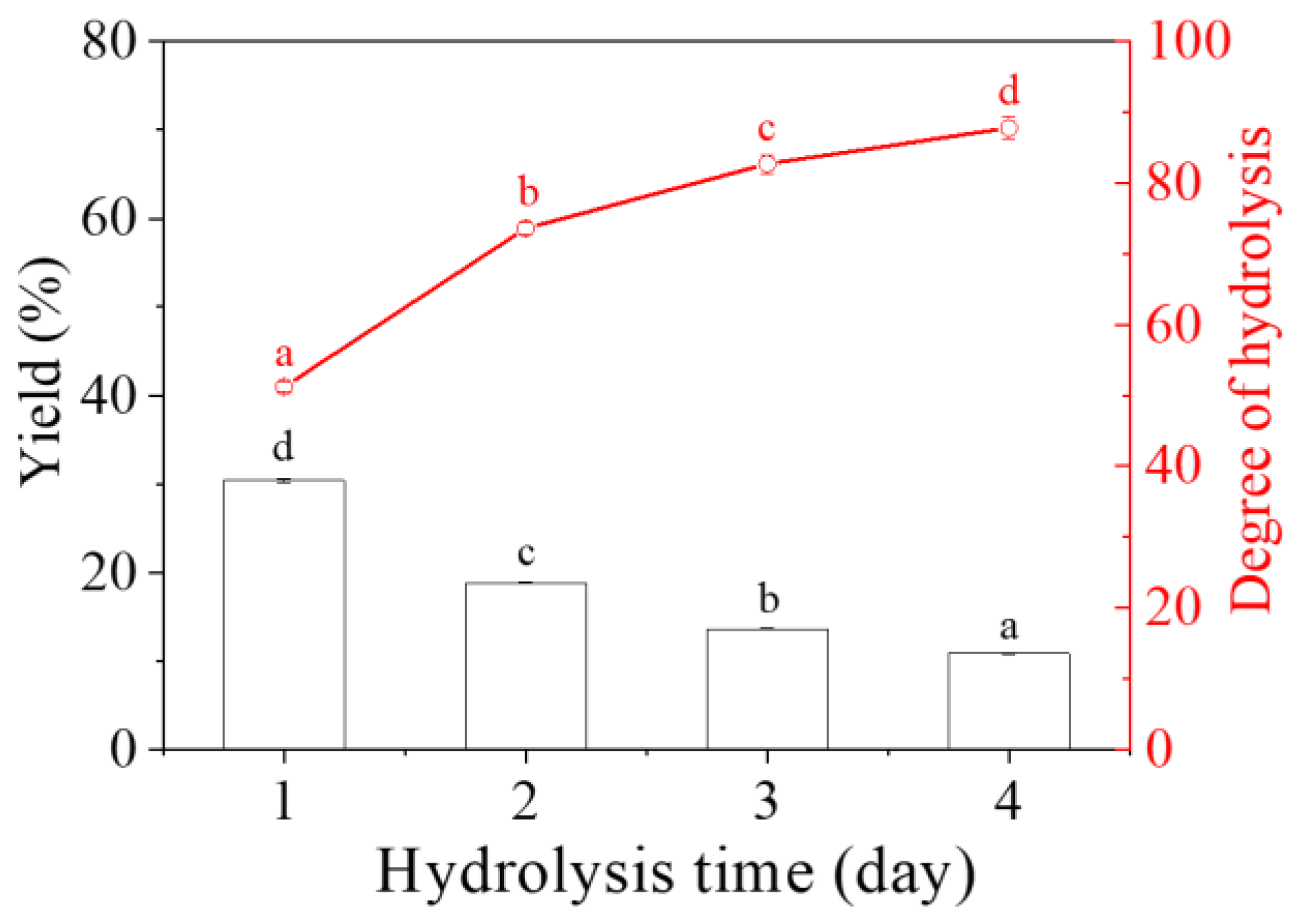
2.1. Yield and Degree of Hydrolysis (DH)
2.2. Tyndall Effect
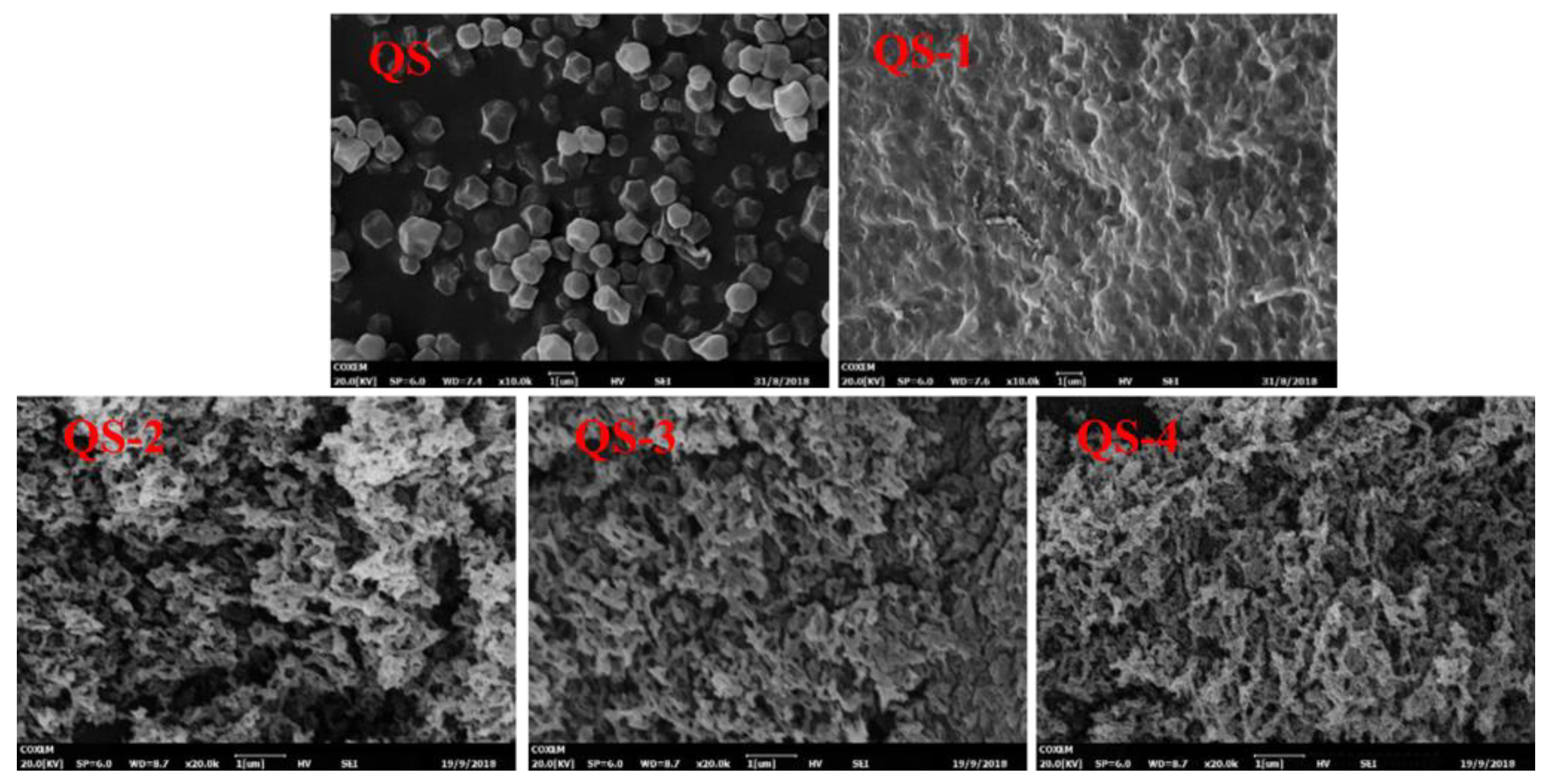
2.3. Particle Morphology
2.4. Particle Size Distribution
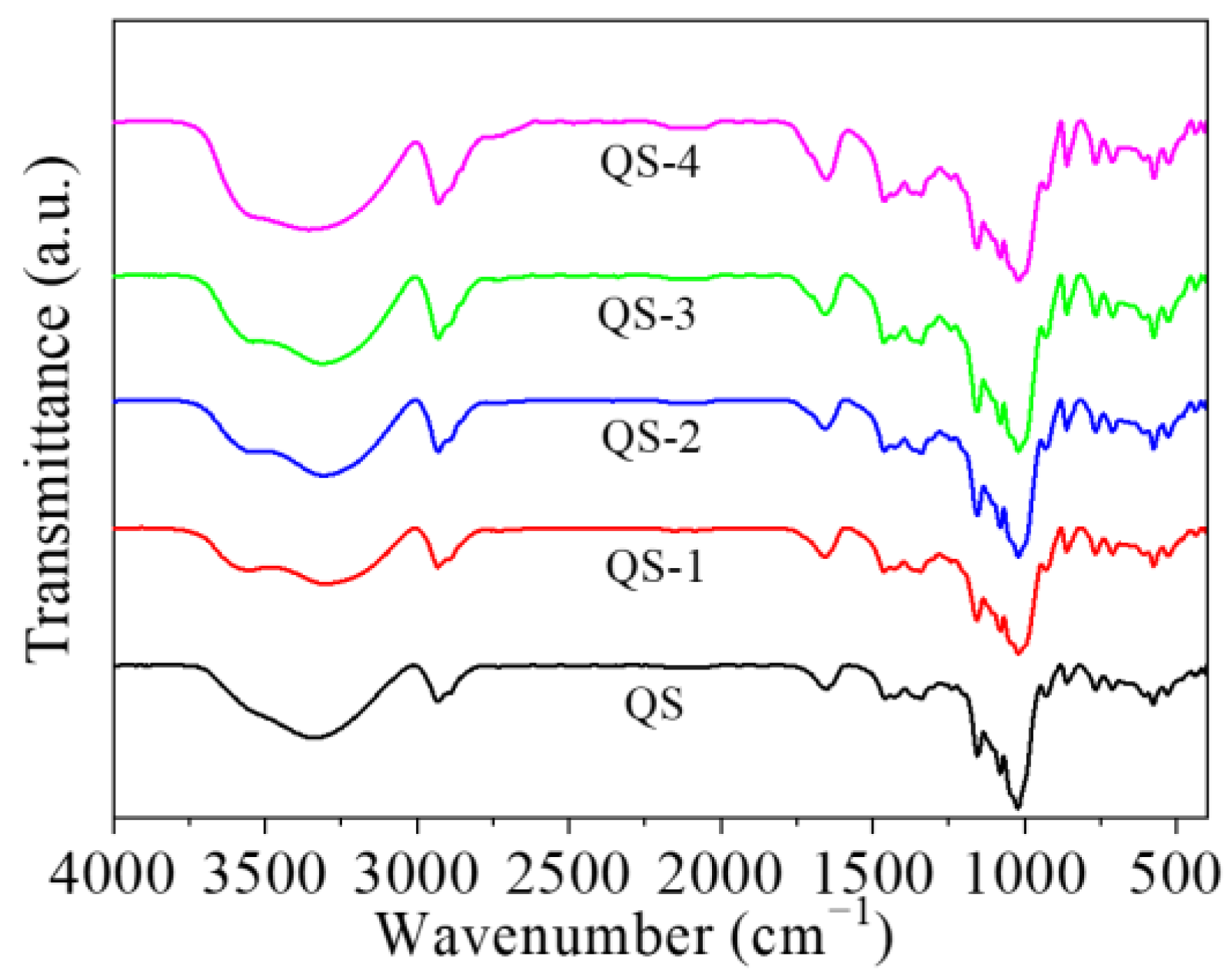
2.5. Fourier Transform Infrared (FT-IR) Pattern
2.6. X-ray Diffraction (XRD) Pattern
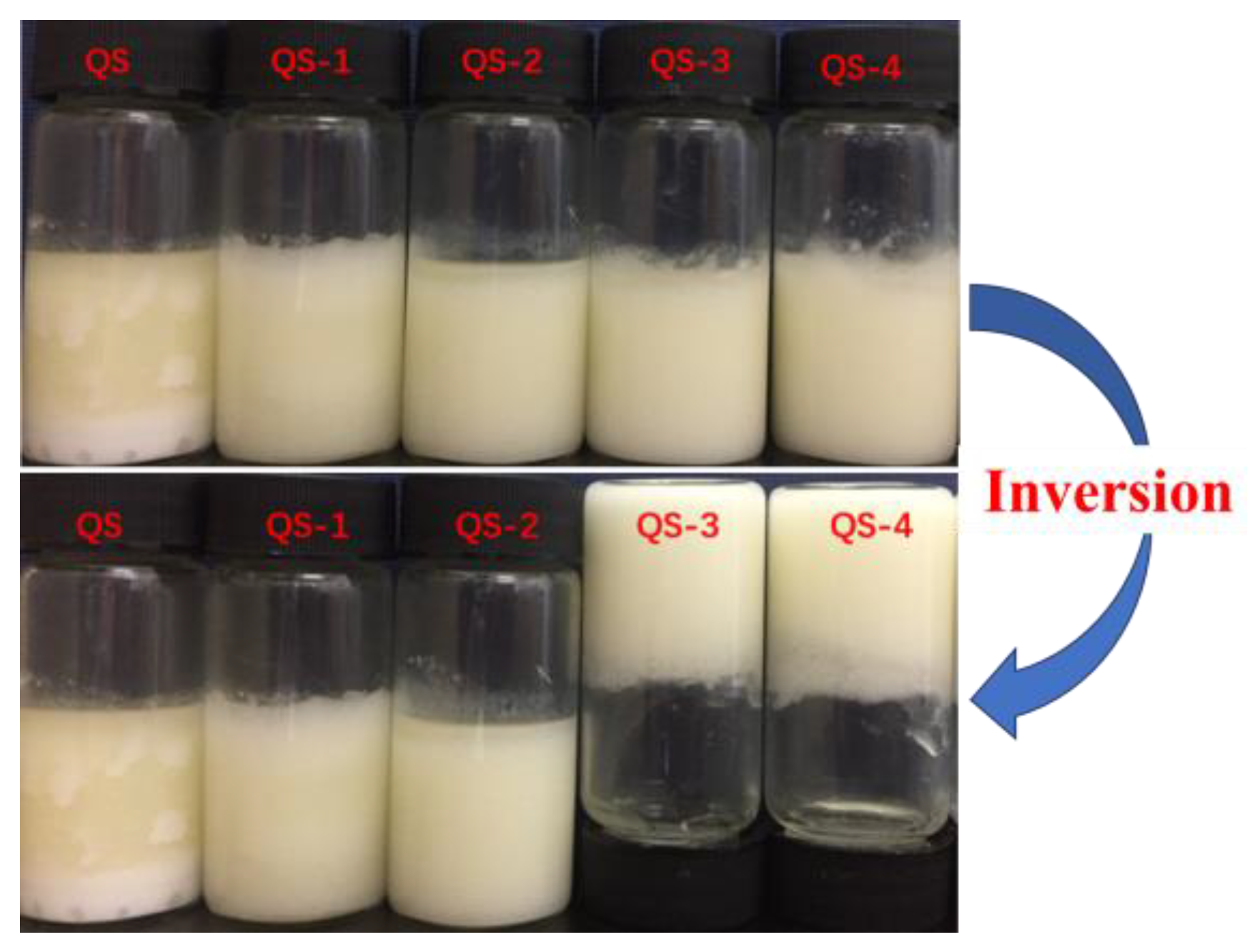
2.7. HIPE Appearance
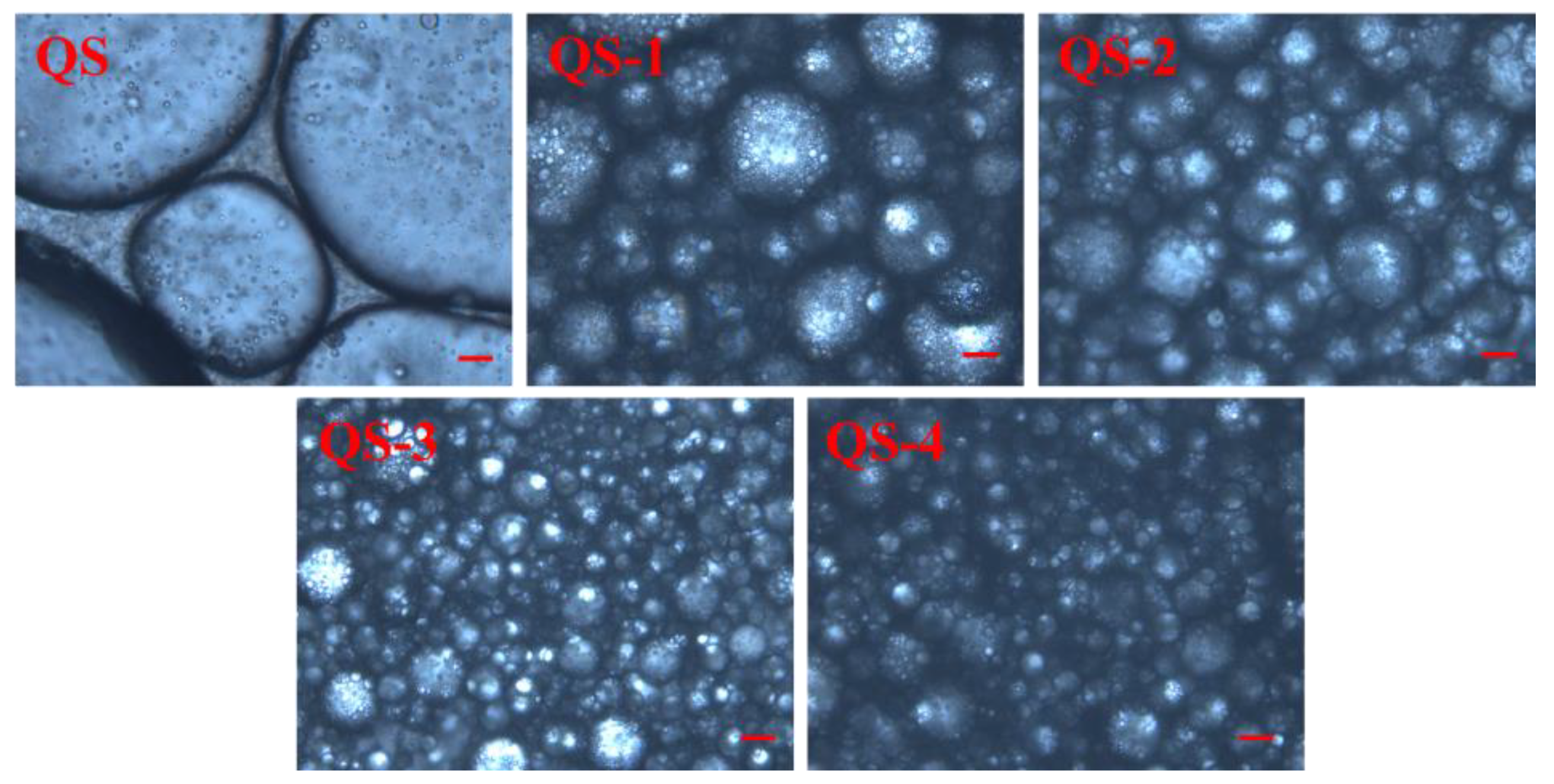
2.8. HIPE Morphology
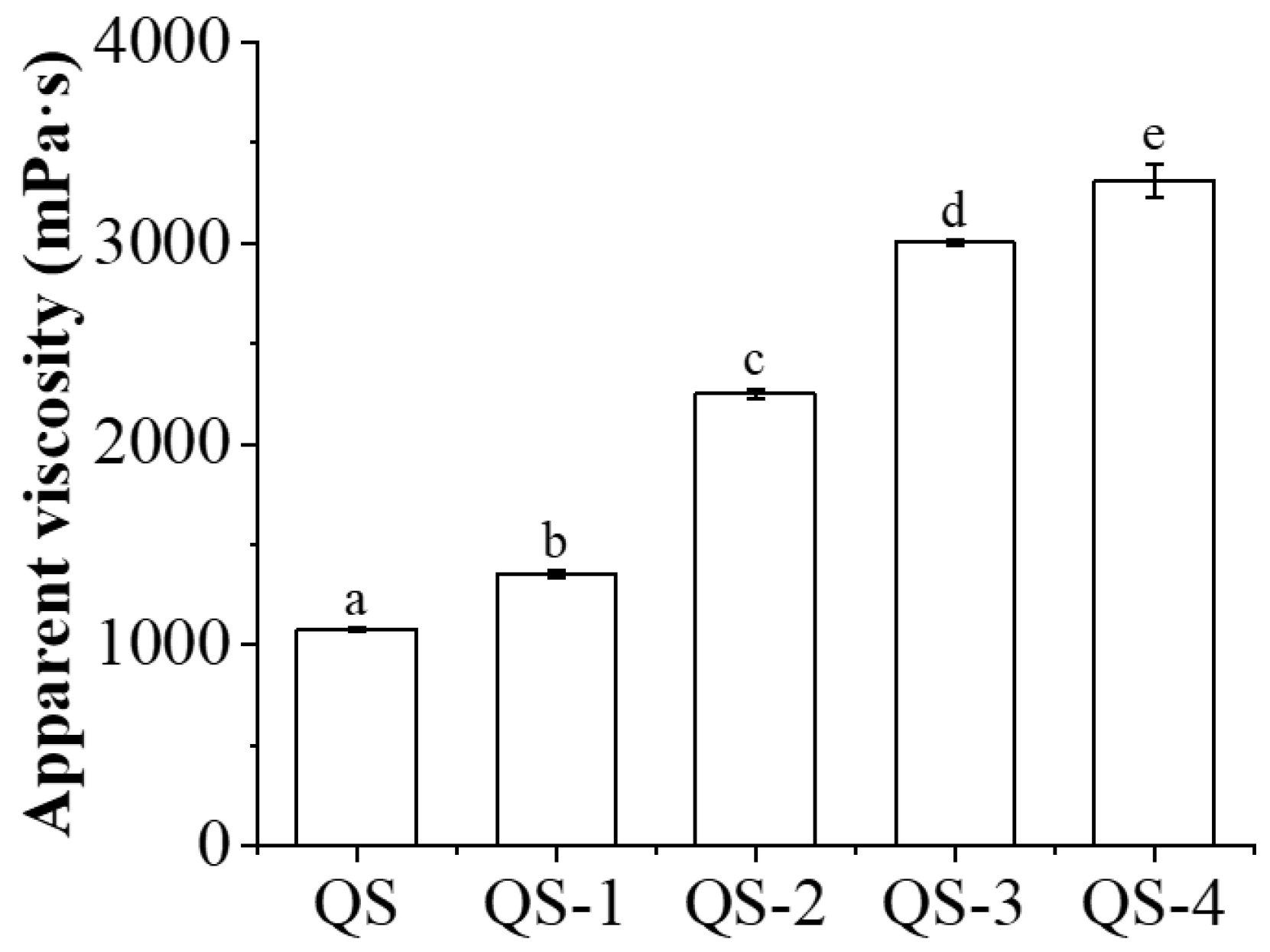
2.9. HIPE Apparent Viscosity
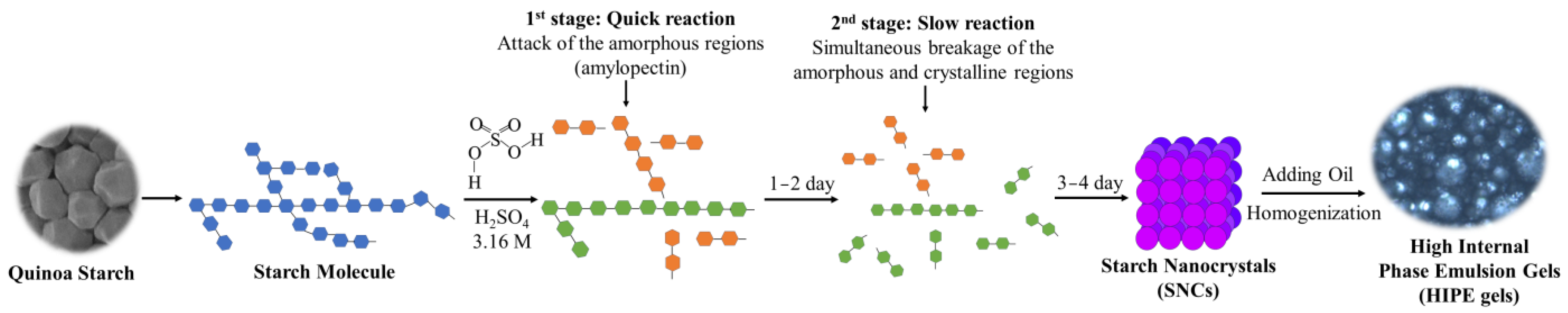
2.10. Possible Mechanism for HIPE Gels Stabilized by QS Nanocrystals
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. QS Nanocrystal Preparation
4.3. Tyndall Effect Observation
4.4. SEM Observation
4.5. Particle Size Determination
4.6. FT-IR Analysis
4.7. XRD Analysis
4.8. HIPE Preparation
4.9. Appearance and Microscopic Observation
4.10. Apparent Viscosity Measurement
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, H.; Ma, L.; Cheng, C.; Liu, J.; Liang, R.; Zou, L.; Liu, W.; McClements, D.J. Review of recent advances in the preparation, properties, and applications of high internal phase emulsions. Trends Food Sci. Technol. 2021, 112, 36–49. [Google Scholar] [CrossRef]
- Liu, X.; Guo, J.; Wan, Z.-L.; Liu, Y.-Y.; Ruan, Q.-J.; Yang, X.-Q. Wheat gluten-stabilized high internal phase emulsions as mayonnaise replacers. Food Hydrocoll. 2018, 77, 168–175. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Song, L.; Bi, A.; Wu, C.; Ma, Y.; Du, M.; Zhu, B. High internal phase emulsion for food-grade 3D printing materials. ACS Appl. Mater. Interfaces 2020, 12, 45493–45503. [Google Scholar] [CrossRef]
- Weiss, J.; Ahmad, T.; Zhang, C.; Zhang, H. A review of recent progress on high internal-phase Pickering emulsions in food science. Trends Food Sci. Technol. 2020, 106, 91–103. [Google Scholar]
- Shi, A.; Feng, X.; Wang, Q.; Adhikari, B. Pickering and high internal phase Pickering emulsions stabilized by protein-based particles: A review of synthesis, application and prospective. Food Hydrocoll. 2020, 109, 106117. [Google Scholar] [CrossRef]
- Pang, B.; Liu, H.; Zhang, K. Recent progress on Pickering emulsions stabilized by polysaccharides-based micro/nanoparticles. Adv. Colloid Interface Sci. 2021, 296, 102522. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Copeland, L. Effect of acid hydrolysis on starch structure and functionality: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1081–1097. [Google Scholar] [CrossRef]
- Li, C.; Sun, P.; Yang, C. Emulsion stabilized by starch nanocrystals. Starch-Stärke 2012, 64, 497–502. [Google Scholar] [CrossRef]
- Yang, T.; Zheng, J.; Zheng, B.-S.; Liu, F.; Wang, S.; Tang, C.-H. High internal phase emulsions stabilized by starch nanocrystals. Food Hydrocoll. 2018, 82, 230–238. [Google Scholar] [CrossRef]
- Dufresne, A. Crystalline starch based nanoparticles. Curr. Opin. Colloid Interface Sci. 2014, 19, 397–408. [Google Scholar] [CrossRef]
- Dufresne, A.; Cavaille, J.-Y.; Helbert, W. New nanocomposite materials: Microcrystalline starch reinforced thermoplastic. Macromolecules 1996, 29, 7624–7626. [Google Scholar] [CrossRef]
- Velásquez-Castillo, L.E.; Leite, M.A.; Ditchfield, C.; do Amaral Sobral, P.J.; Moraes, I.C.F. Quinoa starch nanocrystals production by acid hydrolysis: Kinetics and properties. Int. J. Biol. Macromol. 2020, 143, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sismour, E.N.; Grizzard, C.; Thomas, M.; Pestov, D.; Huba, Z.; Wang, T.; Bhardwaj, H.L. Morphological, structural, and thermal properties of starch nanocrystals affected by different botanic origins. Cereal Chem. 2014, 91, 383–388. [Google Scholar] [CrossRef]
- Li, C.; Hu, Y. Effects of acid hydrolysis on the evolution of starch fine molecular structures and gelatinization properties. Food Chem. 2021, 353, 129449. [Google Scholar] [CrossRef]
- Boufi, S.; Haaj, S.B.; Magnin, A.; Pignon, F.; Impéror-Clerc, M.; Mortha, G. Ultrasonic assisted production of starch nanoparticles: Structural characterization and mechanism of disintegration. Ultrason. Sonochem. 2018, 41, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fan, M.; Huang, Q.; Zhao, S.; Xiong, S.; Zhang, B.; Yin, T. Effect of wet-media milling on the physicochemical properties of tapioca starch and their relationship with the texture of myofibrillar protein gel. Food Hydrocoll. 2020, 109, 106082. [Google Scholar] [CrossRef]
- Hada, A.-M.; Suarasan, S.; Muntean, M.; Potara, M.; Astilean, S. Aptamer-conjugated gold nanoparticles for portable, ultrasensitive naked-eye detection of C-reactive protein based on the Tyndall effect. Anal. Chim. Acta 2024, 1307, 342626. [Google Scholar] [CrossRef]
- Junejo, S.A.; Wang, J.; Liu, Y.; Jia, R.; Zhou, Y.; Li, S. Multi-Scale Structures and Functional Properties of Quinoa Starch Extracted by Alkali, Wet-Milling, and Enzymatic Methods. Foods 2022, 11, 2625. [Google Scholar] [CrossRef]
- Li, C.; Guo, Y.; Chen, M.; Wang, S.; Gong, H.; Zuo, J.; Zhang, J.; Dai, L. Recent preparation, modification and application progress of starch nanocrystals: A review. Int. J. Biol. Macromol. 2023, 250, 126122. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B.; Tan, C.P.; Li, C.; Fu, X.; Huang, Q. Octenylsuccinate quinoa starch granule-stabilized Pickering emulsion gels: Preparation, microstructure and gelling mechanism. Food Hydrocoll. 2019, 91, 40–47. [Google Scholar] [CrossRef]
- Li, S.; Zhou, W.; Huang, C.; Hu, Y.; Gao, Q.; Chen, Y. Rapid preparation of starch nanocrystals by the mixed acid of sulfuric acid and hydrochloric acid. Int. J. Biol. Macromol. 2023, 232, 123402. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Geng, S.; Shi, Y.; Yuan, C.; Liu, B. Effect of sulfuric acid hydrolysis on the structure and Pickering emulsifying capacity of acorn starch. Food Chem. X 2024, 22, 101277. [Google Scholar] [CrossRef]
- Mukurumbira, A.; Mariano, M.; Dufresne, A.; Mellem, J.J.; Amonsou, E.O. Microstructure, thermal properties and crystallinity of amadumbe starch nanocrystals. Int. J. Biol. Macromol. 2017, 102, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Du, C.; Zhao, N.; Jiang, W.; Yu, X.; Du, S.-k. Preparation and characterization of quinoa starch nanoparticles as quercetin carriers. Food Chem. 2022, 369, 130895. [Google Scholar] [CrossRef]
- de la Concha, B.B.S.; Agama-Acevedo, E.; Nuñez-Santiago, M.C.; Bello-Perez, L.A.; Garcia, H.S.; Alvarez-Ramirez, J. Acid hydrolysis of waxy starches with different granule size for nanocrystal production. J. Cereal Sci. 2018, 79, 193–200. [Google Scholar] [CrossRef]
- Li, G.; Zhu, F. Amylopectin molecular structure in relation to physicochemical properties of quinoa starch. Carbohydr. Polym. 2017, 164, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Zhi, L.; Liu, Z.; Wu, C.; Ma, X.; Hu, H.; Liu, H.; Adhikari, B.; Wang, Q.; Shi, A. Advances in preparation and application of food-grade emulsion gels. Food Chem. 2023, 424, 136399. [Google Scholar] [CrossRef] [PubMed]
- Azfaralariff, A.; Fazial, F.F.; Sontanosamy, R.S.; Nazar, M.F.; Lazim, A.M. Food-grade particle stabilized pickering emulsion using modified sago (Metroxylon sagu) starch nanocrystal. J. Food Eng. 2020, 280, 109974. [Google Scholar] [CrossRef]
- Almeida, R.L.J.; Santos, N.C.; Monteiro, S.S.; Rios, N.S.; dos Santos, E.S. Exploring the potential of native and modified starch and starch nanocrystals in Pickering emulsions: Current advances, future perspectives, and challenges. Food Biosci. 2024, 61, 104675. [Google Scholar]
- Han, H.; Zhang, H.; Li, E.; Li, C.; Wu, P. Structural and functional properties of OSA-starches made with wide-ranging hydrolysis approaches. Food Hydrocoll. 2019, 90, 132–145. [Google Scholar] [CrossRef]
- Nilsson, L.; Zielke, C. Starch-based food emulsifiers. In Starch in Food; Elsevier: Amsterdam, The Netherlands, 2024; pp. 525–543. [Google Scholar]
- Zhu, Y.; Du, C.; Jiang, F.; Hu, W.; Yu, X.; Du, S.-k. Pickering emulsions stabilized by starch nanocrystals prepared from various crystalline starches by ultrasonic assisted acetic acid: Stability and delivery of curcumin. Int. J. Biol. Macromol. 2024, 267, 131217. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, J.H.; Kim, J.Y.; Lim, W.J.; Lim, S.T. Characterization of nanoparticles prepared by acid hydrolysis of various starches. Starch-Stärke 2012, 64, 367–373. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Bai, X.; Zhang, Y.; Wang, C. The impact of high hydrostatic pressure treatment time on the structure, gelatinization and thermal properties and in vitro digestibility of oat starch. Grain Oil Sci. Technol. 2022, 5, 1–12. [Google Scholar] [CrossRef]
- Li, S.; Huang, L.; Zhang, B.; Chen, C.; Fu, X.; Huang, Q. Fabrication and characterization of starch/zein nanocomposites with pH-responsive emulsion behavior. Food Hydrocoll. 2021, 112, 106341. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B.; Li, C.; Fu, X.; Huang, Q. Pickering emulsion gel stabilized by octenylsuccinate quinoa starch granule as lutein carrier: Role of the gel network. Food Chem. 2019, 305, 125476. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, C.; Yang, Y.; He, X.; Zhang, B.; Fu, X.; Tan, C.P.; Huang, Q. Starch granules as Pickering emulsifiers: Role of octenylsuccinylation and particle size. Food Chem. 2019, 283, 437–444. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Sun, C.; Sun, Y.; Li, E.; Li, P.; Wang, J. Acid Hydrolysis of Quinoa Starch to Stabilize High Internal Phase Emulsion Gels. Gels 2024, 10, 559. https://doi.org/10.3390/gels10090559
Li S, Sun C, Sun Y, Li E, Li P, Wang J. Acid Hydrolysis of Quinoa Starch to Stabilize High Internal Phase Emulsion Gels. Gels. 2024; 10(9):559. https://doi.org/10.3390/gels10090559
Chicago/Turabian StyleLi, Songnan, Chaohui Sun, Ye Sun, Enpeng Li, Ping Li, and Jun Wang. 2024. "Acid Hydrolysis of Quinoa Starch to Stabilize High Internal Phase Emulsion Gels" Gels 10, no. 9: 559. https://doi.org/10.3390/gels10090559
APA StyleLi, S., Sun, C., Sun, Y., Li, E., Li, P., & Wang, J. (2024). Acid Hydrolysis of Quinoa Starch to Stabilize High Internal Phase Emulsion Gels. Gels, 10(9), 559. https://doi.org/10.3390/gels10090559







