Preparation and Characterization of Preformed Polyelectrolyte and Polyampholyte Gel Particles for Plugging of High-Permeability Porous Media
Abstract
:1. Introduction
- High-Temperature-Resistant PPGs;
- Re-Crosslinkable PPGs;
- Delayed-Swelling PPGs;
- Augmented PPGs;
- Degradable PPG.
2. Results and Discussion
2.1. FTIR Spectroscopy Results
2.2. Swelling Kinetics of Hydrogels Determined by Using Ritger–Peppas and Yavari–Azizyan Models
2.3. Temperature-, Salt-, and pH-Dependent Swelling of PPGs
2.4. Study of the Mechanical Properties of Hydrogels
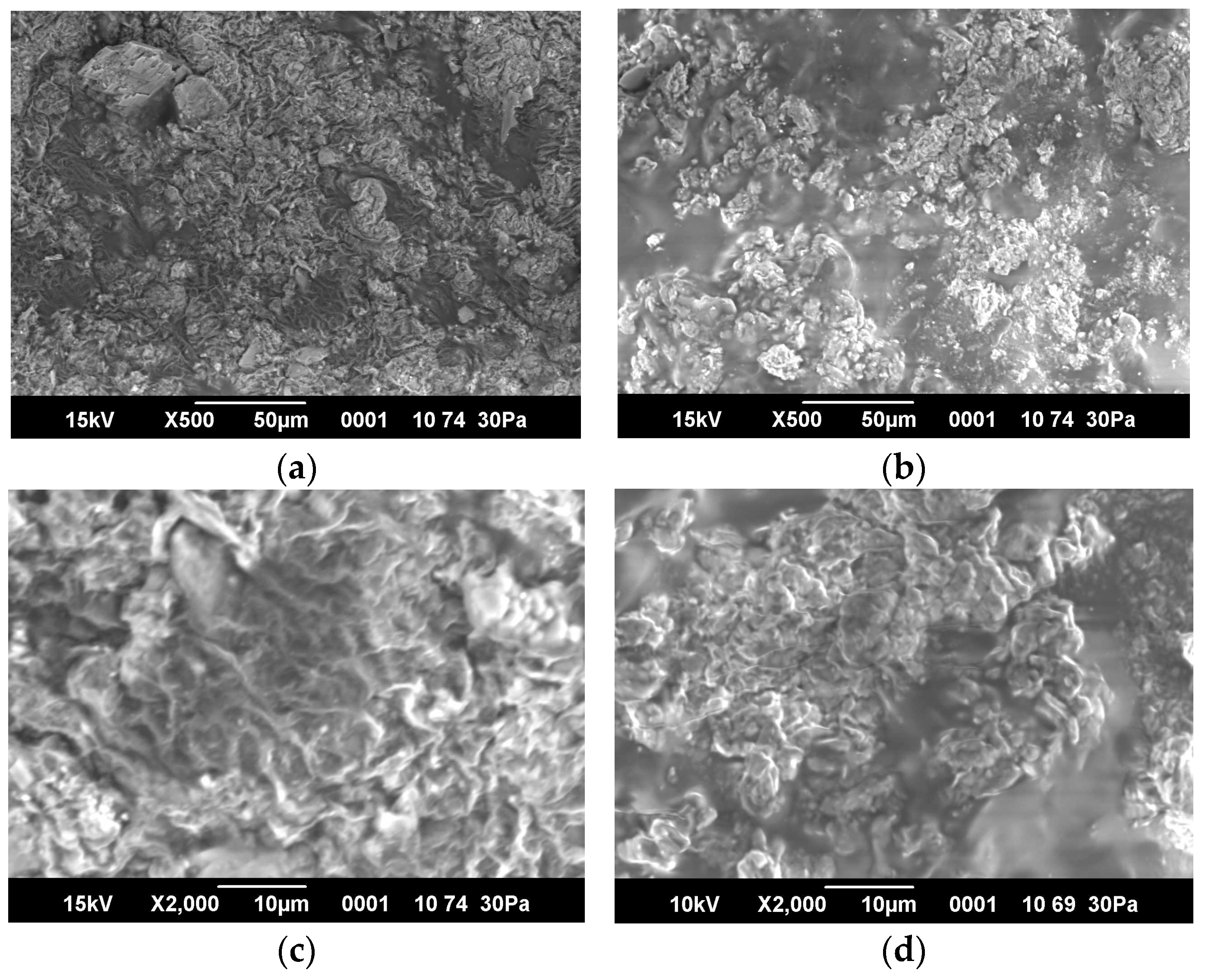
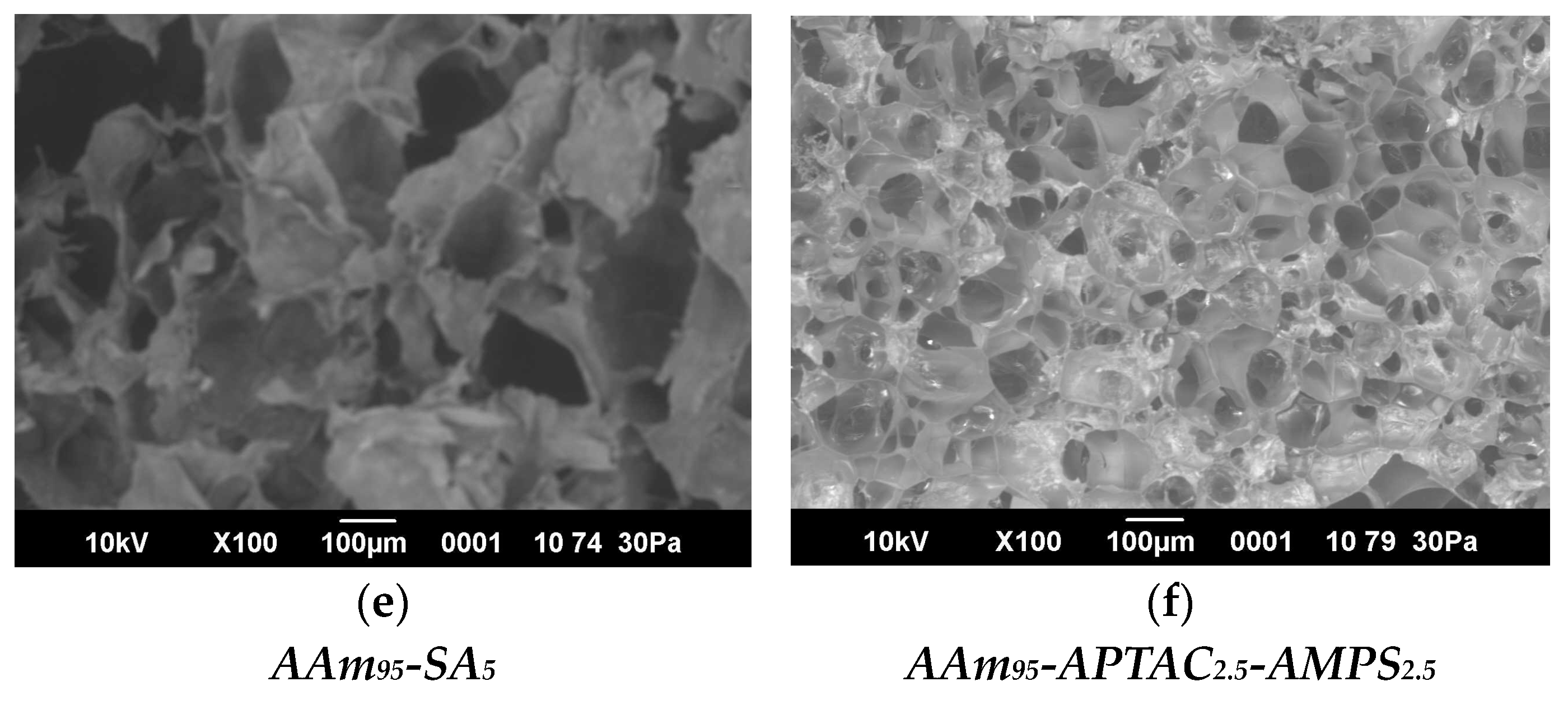
2.5. SEM Images and Porosity of Hydrogels
2.6. Sand Pack Flooding
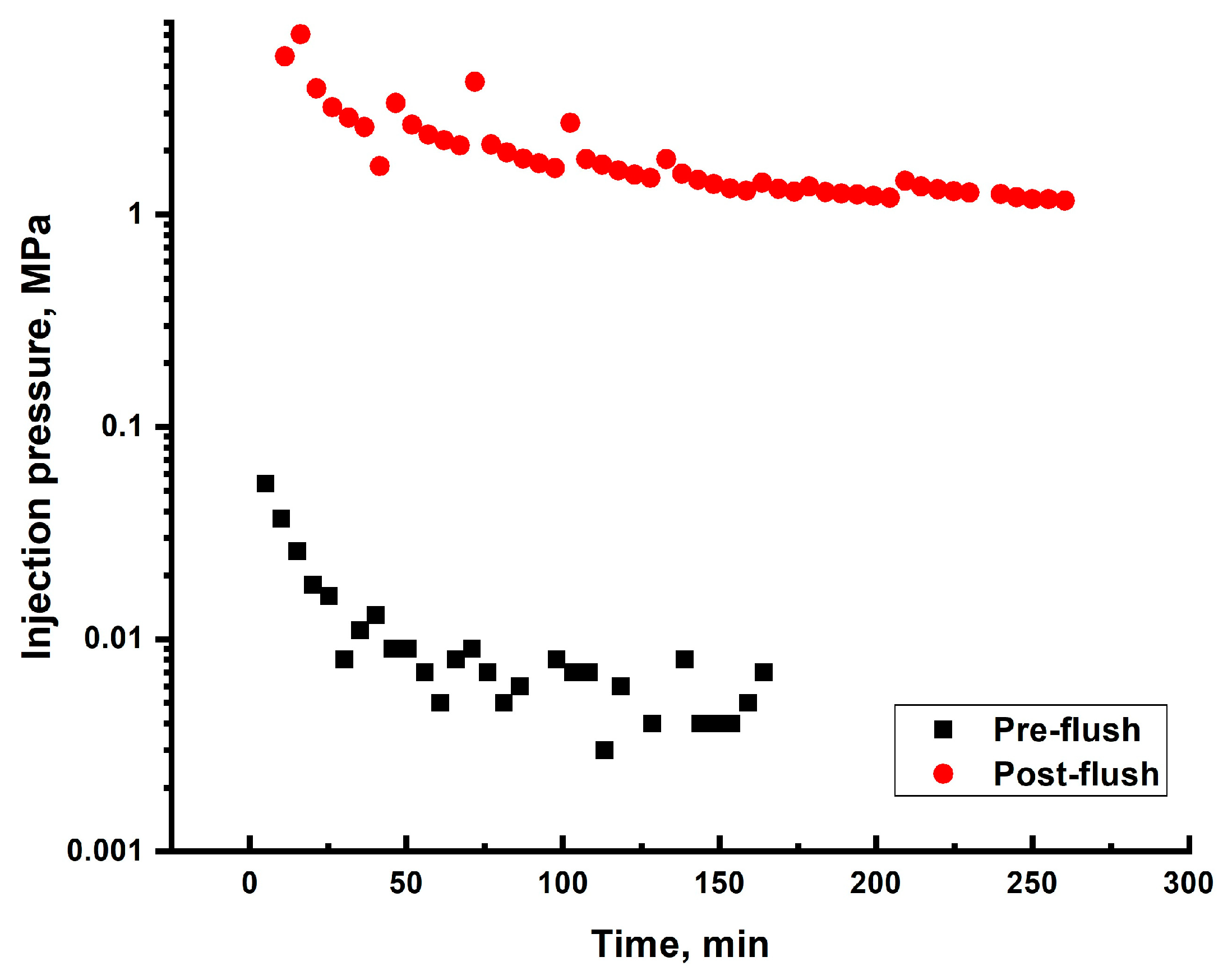
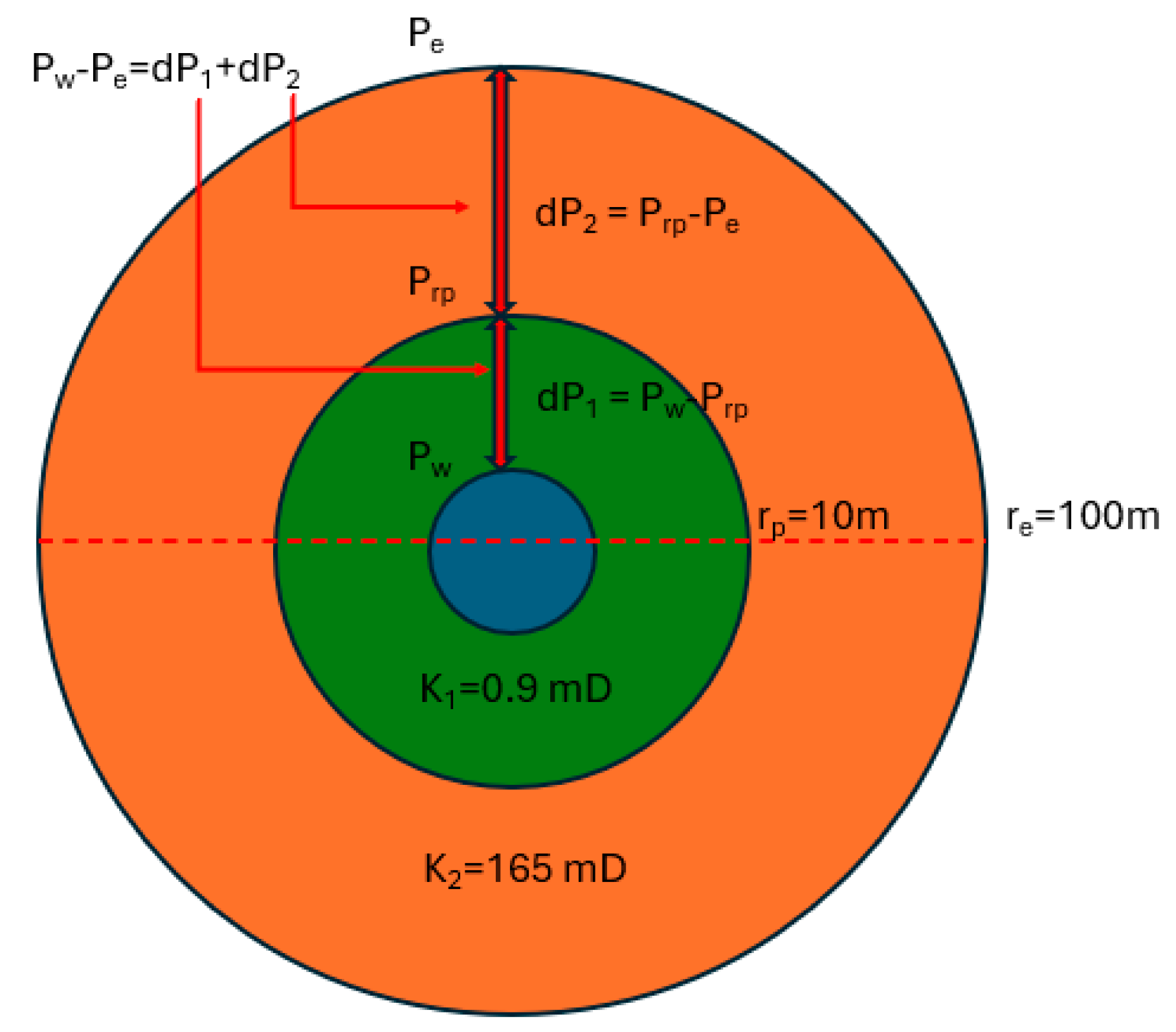
2.6.1. Darcy Sand Pack Flooding
2.6.2. >60 Darcy Sand Pack Flooding
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Synthesis of PPG
4.2.2. FTIR Spectroscopy
4.2.3. Determination of the Swelling Degree and Swelling Kinetics of Hydrogels in Dependence of Temperature, Ionic Strength, and pH Medium
4.2.4. Study of Mechanical Properties of Hydrogels
4.2.5. Study of Surface Morphology of Hydrogels
4.2.6. Study of the Porosity of Hydrogels
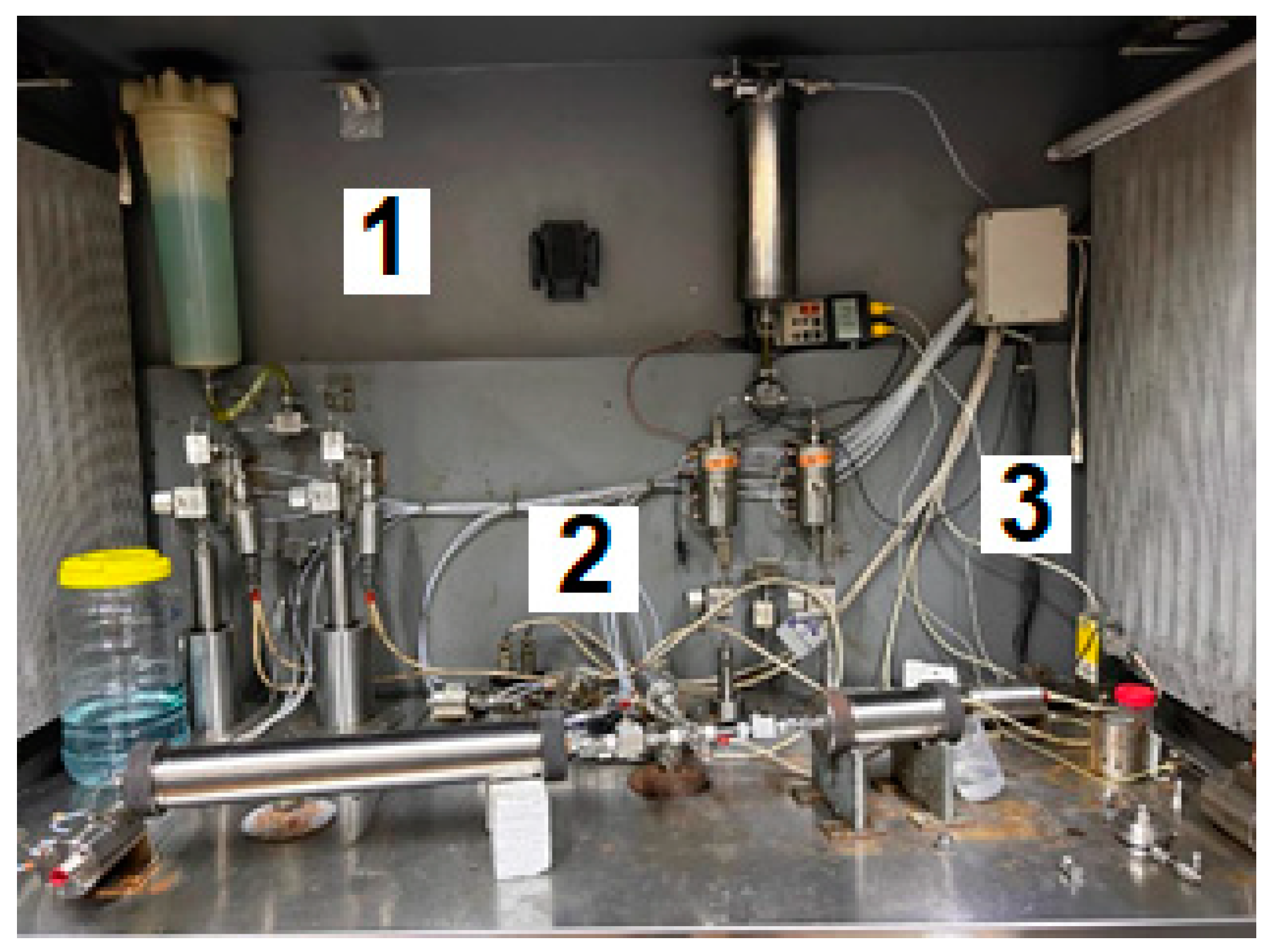
4.2.7. Sand Pack Flooding Experiments
- -
- Reservoir unconsolidated oil-wet sand was packed into a steel cylinder with a diameter of 4.3 cm and a length of 8.6 cm;
- -
- The permeability of the model was determined using 26.6 g·L−1 brine from the Karazhanbas oilfield. The brine’s composition is listed in Table 6;
- -
- The model was saturated with crude oil at 1 cm³·min−1 and 25 °C. The viscosity 796.39 The cP and density of the oil were equal to 796 cP and 0.93 g·cm-³ (20° API), respectively;
- -
- Oil was displaced using 26.6 g·L−1 brine at a rate of 1 cm³·min−1 and a temperature of 25 °C to establish the initial conditions;
- -
- Freshly prepared [0.5 wt.% 6–7 mln Da and 5% hydrolysis degree HPAM/0.5 wt.% chromium acetate] gellant was injected into the model at a rate of 1 cm3/min and a temperature of 25 °C. The gellant was prepared by using fresh low salinity brine;
- -
- The model was left for 3 days to allow the composition to gel;
- -
- After 3 days, the model was subjected to brine injection in the direction opposite to the gellant injection.
- (1)
- Vacuuming the sand pack;
- (2)
- Injecting 26.6 g∙L−1 brine at 5 cm3∙min−1 (Figure S8). Calculating pore volume and porosity;
- (3)
- Gel injection at 1 cm3∙min−1;
- (4)
- Post-flushing with 26.6 g∙L−1 brine at 1 cm3∙min−1.
4.2.8. Preparation of PPG-Impregnated Gel Samples for the Flooding Experiments
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aam | Acrylamide |
| AMPS | 2-Acrylamido-2-methyl-1-propanesulfonic acid sodium salt |
| APTAC | 3-Acrylamidopropyltrimethylammonium chloride |
| DSC | Differential scanning calorimetry |
| EOR | Enhanced oil recovery |
| GTP | Group transfer polymerization |
| HPAM | Hydrolyzed poly(acrylamide) |
| IMM | Initial monomer mixtures |
| MBAA | N,N’-methylenebisacrylamide |
| PPG | Preformed particle gels |
| SA | Sodium acrylate |
| SD | Swelling degree |
| TDS | Total dissolved solids |
| TGA | Thermogravimetric analysis |
References
- Li, Z.; Wang, Y.; He, H.; Yuan, F.; Liu, H.; Chen, Y. Insights into the Effects of Salinity on the Transport Behavior of Polymer-Enhanced Branched-Preformed Particle Gel Suspension in Porous Media. Energy Fuels 2021, 35, 1104–1112. [Google Scholar] [CrossRef]
- Afsharpour, S.; Fatemi, M.; Ghazanfari, M.H. Microfluidics investigation of the fracture/matrix interaction mechanisms during preformed particle gel (PPG) treatment in fractured porous media. Geoenergy Sci. Eng. 2024, 234, 212611. [Google Scholar] [CrossRef]
- Tao, S.; Mohamed, A.; Zh, S.; Zh, Y.; Thomas, S.; Bai, B. Evaluation of a Novel Recrosslinkable Hyperbranched Preformed Particle Gel for the Conformance Control of High-Temperature Reservoirs with Fractures. SPE J. 2022, 27, 3598–3610. [Google Scholar] [CrossRef]
- Elaf, R.; Ben Ali, A.; Saad, M.; Hussein, I.A.; Nimir, H.; Bai, B. Biodegradable Preformed Particle Gel (PPG) Made of Natural Chitosan Material for Water Shut-Off Application. Polymers 2023, 15, 1961. [Google Scholar] [CrossRef] [PubMed]
- Seidy-Esfahlan, M.; Khodapanah, E.; Tabatabaei-Nezhad, S.A. Production improvement mechanisms in combined low salinity water flooding and preformed particle gel treatment. Results Eng. 2024, 22, 102126. [Google Scholar] [CrossRef]
- Zeqiraj, D. Enhanced oil recovery (EOR) project scheduling with multiobjective of maximum net present value and minimum makespan. Results Eng. 2022, 13, 100339. [Google Scholar] [CrossRef]
- Keradeh, M.; Tabatabei-Nezhad, S. Comprehensive analysis of the effect of reservoir key parameters on the efficacy of DTPA chelating agent in minimizing interfacial tension and enhanced oil recovery. Results Eng. 2023, 19, 101316. [Google Scholar] [CrossRef]
- Xu, A.; Shan, F.; Yang, X.; Li, J.; Wang, C. Thief Zone Identification and Classification in Unconsolidated Sandstone Reservoirs: A Field Case Study. J. Pet. Explor. Prod. Technol. 2021, 11, 3451–3462. [Google Scholar] [CrossRef]
- Olsen, H.; Briedis, N.A.; Renshaw, D. Sedimentological Analysis and Reservoir Characterization of a Multi-Darcy, Billion Barrel Oil Field–The Upper Jurassic Shallow Marine Sandstones of the Johan Sverdrup Field, North Sea, Norway. Mar. Pet. Geol. 2017, 84, 102–134. [Google Scholar] [CrossRef]
- Heidari, S.; Ahmadi, M.; Esmaeilzadeh, F.; Mowla, D. Oil recovery from fractured reservoirs using in situ and preformed particle gels in micromodel structures. J. Pet. Explor. Prod. Technol. 2019, 9, 2309–2317. [Google Scholar] [CrossRef]
- Wei, B.; Mao, R.; Tian, Q.; Lei, W.; Lu, J.; Tang, J. Performance Evaluation of Nanocellulose-Engineered Robust Preformed Particle Gel upon Extrusion Through 1 to 1.5 mm Bead-Packed Porous Media. SPE Reserv. Eval. Eng. 2023, 26, 345–357. [Google Scholar] [CrossRef]
- Alhuraishawy, A.K.; Bai, B.; Wei, M. Combined ionically modified seawater and microgels to improve oil recovery in fractured carbonate reservoirs. J. Pet. Sci. Eng. 2018, 162, 434–445. [Google Scholar] [CrossRef]
- Rozhkova, Y.A.; Burin, D.A.; Galkin, S.V.; Yang, H. Review of Microgels for Enhanced Oil Recovery: Properties and Cases of Application. Gels 2022, 8, 112. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Z.-L.; Jin, Y.-X.; Tian, Y.-Q.; Liu, X.-M.; Guo, Y.-J.; Fan, L.; Wang, J.; Zhang, X.-M.; Cao, M.; et al. Heterogeneity control ability in porous media: Associative polymer versus HPAM. J. Pet. Sci. Eng. 2019, 183, 106425. [Google Scholar] [CrossRef]
- Deng, J.; Lian, H.; Zhuang, Y.; Zhao, H.; Wang, Z.; Tian, Y.; Lin, C.; Yuan, H.; Han, M.; Lu, G.; et al. Synthesis and performance evaluation of multi-crosslinked preformed particle gels with ultra-high strength and high-temperature and high-salinity resistance for conformance control. Fuel 2024, 357, 130027. [Google Scholar] [CrossRef]
- Wu, Q.-H.; Ge, J.-J.; Ding, L.; Zhang, G.-C. Unlocking the potentials of gel conformance for water shutoff in fractured reservoirs: Favorable attributes of the double network gel for enhancing oil recovery. Pet. Sci. 2023, 20, 1005–1017. [Google Scholar] [CrossRef]
- Canbolat, S.; Parlaktuna, M. Polymer gel conformance on oil recovery in fractured medium: Visualization and verification. J. Pet. Sci. Eng. 2019, 182, 106289. [Google Scholar] [CrossRef]
- Kong, R.; Guo, Y.; Barker, I.; Hamielec, T.; Hunkeler, D. Acrylamide-sodium acrylate copolymers: A comparison between copolymerization and hydrolysis. Can. J. Chem. Eng. 2023, 101, 5315. [Google Scholar] [CrossRef]
- Nakahata, R.; Yusa, S. Solution Properties of Amphoteric Random Copolymers Bearing Pendant Sulfonate and Quaternary Ammonium Groups with Controlled Structures. Langmuir 2019, 35, 1690–1698. [Google Scholar] [CrossRef]
- Gussenov, I.S.; Mukhametgazy, N.; Shakhvorostov, A.V.; Kudaibergenov, S.E. Comparative Study of Oil Recovery Using Amphoteric Terpolymer and Hydrolyzed Polyacrylamide. Polymers 2022, 14, 3095. [Google Scholar] [CrossRef]
- Sagyndikov, M.S.; Kushekov, R.M.; Seright, R.S. Review of Important Aspects and Performances of Polymer Flooding versus ASP Flooding. Bull. Univ. Karaganda Chem. Geofluids 2022, 107, 6740531. [Google Scholar] [CrossRef]
- Kang, W.; Wang, J.; Ye, Z.; Gu, G.; Li, W.; Yang, H.; Li, Z.; Xu, H.; Lv, Z.; Sarsenbekuly, B. Study on preparation and plugging effect of sawdust gel particle in fractured reservoir. J. Pet. Sci. Eng. 2022, 212, 110358. [Google Scholar] [CrossRef]
- Song, Z.; Bai, B.; Zhang, H. Preformed particle gel propagation and dehydration through semi-transparent fractures and their effect on water flow. J. Pet. Sci. Eng. 2018, 167, 549–558. [Google Scholar] [CrossRef]
- Esfahlan, M.S.; Khodapanah, E.; Tabatabaei-Nezhad, S.A. Comprehensive review on the research and field application of preformed particle gel conformance control technology. J. Pet. Sci. Eng. 2021, 202, 108440. [Google Scholar] [CrossRef]
- Paprouschi, A.; Fatemi, M.; Ghazanfari, M.H. Effect of silicate sodium and graphene nanoplatelets on morphology and rheology characteristics of new synthesized preformed particle gel (PPG) for water shut-off treatment. J. Pet. Sci. Eng. 2021, 204, 108736. [Google Scholar] [CrossRef]
- Zhai, Y.; Fang, Z.; Feng, J.; Sun, C.; Deng, W.; Wen, Y. Development and performance evaluation of re-crosslinkable preformed particle gel under high temperature and high salt conditions. Polymer 2024, 296, 126824. [Google Scholar] [CrossRef]
- Elaf, R.; Hamza, A.; Nimir, H.; Saad, M.; Ibnelwaleed, A.; Hussein, I.; Bai, B. Development of Inorganically Cross-Linked Sulfonated Polyacrylamide Preformed Particle Gels for Conformance Control: Impact of Anionic Groups. Energy Fuels 2024, 38, 2883–2897. [Google Scholar] [CrossRef]
- Farasat, A.; Sefti, M.V.; Sadeghnejad, S. Effects of reservoir temperature and water salinity on the swelling ratio performance of enhanced preformed particle gels. Korean J. Chem. Eng. 2017, 34, 1509–1516. [Google Scholar] [CrossRef]
- Fan, H.; Pu, W.; Du, D.; Xu, M.; Chen, L.; Xu, L.; Jin, F.J. Experimental study on the plugging and profile control of preformed particle gels in high temperature and salinity reservoir. Appl. Polym. Sci. 2023, 140, e54247. [Google Scholar] [CrossRef]
- Bai, B.; Zhou, J.; Yin, M. A comprehensive review of polyacrylamide polymer gels for conformance control. Pet. Explor. Dev. 2015, 42, 525–532. [Google Scholar] [CrossRef]
- Yang, J.; Sun, J.; Bai, Y.; Lv, K.; Wang, Z.; Xu, C.; Dai, L.; Wang, R. Review of the application of environmentally responsive gels in drilling and oil recovery engineering: Synthetic materials, mechanism, and application prospect. J. Pet. Sci. Eng. 2022, 215 Part A, 110581. [Google Scholar] [CrossRef]
- Wang, L.; Xia, H.; Han, P.; Cao, R.; Xu, T.; Li, W.; Zhang, H.; Zhang, S. Synthesis of new PPG and study of heterogeneous combination flooding systems. J. Dispers. Sci. Technol. 2020, 43, 164–177. [Google Scholar] [CrossRef]
- Ali, A.B.; Elaf, R.; Saad, M.; Hussein, I.A.; Bai, B. Impact of composition and salinity on swelling and gel strength of poly(acrylamide-co-acrylic acid) preformed particle gel. Emergent Mater. 2024, 7, 565–575. [Google Scholar] [CrossRef]
- Ali, A.B.; Elaf, R.; Almakimi, A.A.; Saad, M.; Hussein, I.A.; Bai, B. Development of Agar-Based Preformed Particle Gel for Water Control in High-Salinity Reservoirs. Ind. Eng. Chem. Res. 2024, 63, 8524–8541. [Google Scholar] [CrossRef]
- Rodrigues, T.Z.L.; Flor, J.C.D.; Palermo, L.C.M.; Mansur, C.R.E.J. Evaluation of the polymer hydrogels as a potential relative permeability modifier Appl. Polym. Sci. 2023, 141, e54879. Polym. Sci. 2023, 141, e54879. [Google Scholar] [CrossRef]
- Pi, Y.; Su, Z.; Cao, R.; Li, B.; Liu, J.; Fan, X.; Zhao, M. Experimental Study on Enhanced Oil Recovery of PPG/ASP Heterogeneous System after Polymer Flooding. Gels 2023, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.; Liu, J.; Cao, R.; Liu, L.; Ma, Y.; Gu, X.; Li, X.; Fan, X.; Zhao, M. Visualized Study on a New Preformed Particle Gels (PPG) + Polymer System to Enhance Oil Recovery by Oil Saturation Monitoring Online Flooding Experiment. Gels 2023, 9, 81. [Google Scholar] [CrossRef]
- Ayazbayeva, A.; Baddam, V.; Shakhvorostov, A.; Gussenov, I.; Aseyev, V.; Yermaganbetov, M.; Kudaibergenov, S. Amphoteric nano- and microgels with acrylamide backbone for potential application in oil recovery. Polym. Adv. Technol. 2023, 34, 3826–3837. [Google Scholar] [CrossRef]
- Amiri, S. Synthesis and Characterization of Acrylamide/(3-Acrylamidopropyl) Trimethylammonium Chloride Solution and Acrylamide/Na-Montmorillonite Hydrogels by Controlled Radical Polymerization for Use in High-Temperature and High-Salinity Oil Reservoirs. Polymer 2019, 76, 683–699. [Google Scholar] [CrossRef]
- Yan, L.; Xie, C.; Ao, T.; Cui, K.; Jiang, G.; Bai, B.; Zhang, Y.; Yang, J.; Wang, H.; Tian, W. Comprehensive Evaluation of Self-Healing Polyampholyte Gel Particles for Controlling Severe Drilling Fluid Losses. J. Pet. Sci. Eng. 2022, 212, 110249. [Google Scholar] [CrossRef]
- Ma, W.; Li, Y.; Liu, P.; Liu, Z.; Song, T. Progress of Research into Preformed Particle Gels for Profile Control and Water Shutoff Techniques. Gels 2024, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Mukhametgazy, N.; Gussenov, I.S.; Shakhvorostov, A.V.; Kudaibergenov, S.E. Salt tolerant acrylamide-based quenched polyampholytes for polymer flooding. Bull. Karaganda Univ. Chem. Ser. 2020, 4, 119–127. [Google Scholar] [CrossRef]
- Gussenov, I.; Mukhametgazy, N.; Shakhvorostov, A.; Kudaibergenov, S. Synthesis and characterization of high molecular weight amphoteric terpolymer based on acrylamide, 2-acrylamido-2-methyl-1-propanesulfonic acid sodium salt and (3-acrylamidopropyl)trimethylammonium chloride for oil recovery. Chem. Bull. Kazakh Natl. Univ. 2021, 4, 12–20. [Google Scholar] [CrossRef]
- Mukhametgazy, N.; Gussenov, I.S.; Shakhvorostov, A.V.; Tenhu, H. Oil recovery at high brine salinity conditions using amphoteric terpolymer. Bull. Univ. Karaganda Chem. 2022, 107, 141–149. [Google Scholar] [CrossRef]
- Gussenov, I.S.; Shakhvorostov, A.V.; Mukhametgazy, N.; Kudaibergenov, S.E. Synthetic polyampholytes based on acrylamide derivatives–new polymer for enhanced oil recovery. Kazakhstan J. Oil Gas Ind. 2022, 4, 104–116. [Google Scholar] [CrossRef]
- Heidari, A.; Vasheghani Farahani, E.; Vafaie Sefti, M. Preformed particle gels of sulfonated polyacrylamide: Preparation, characterization, and application as permeability modifier. Iran. Polym. J. 2019, 28, 1001–1013. [Google Scholar] [CrossRef]
- Rabiee, A.; Ershad-Langroudi, A.; Jamshidi, H. Polyacrylamide-based polyampholytes and their applications. Rev. Chem. Eng. 2014, 30, 525–532. [Google Scholar] [CrossRef]
- Yelemessova, G.T.; Orazzhanova, L.K.; Klivenko, A.N.; Nurgaliyev, N.N.; Ayazbayeva, A.Y.; Shakhvorostov, A.V. Synthesis and Characterization of Preformed Particle Gels (PPG) to Increase Oil Recovery. News Natl. Acad. Sci. Rep. Kazakhstan Chem. Technol. Ser. 2023, 4, 79–91. [Google Scholar] [CrossRef]
- Gussenov, I.; Shakhvorostov, A.; Ayazbayeva, A.; Gizatullina, N.; Klivenko, A.; Kudaibergenov, S. Preparation and Characterization of a Preformed Polyampholyte Particle Gel Composite for Conformance Control in Oil Recovery. Polymers 2023, 15, 4095. [Google Scholar] [CrossRef]
- Khutoryanskaya, O.; Mayeva, Z.; Mun, G.; Khutoryanskiy, V. Designing Temperature-Responsive Biocompatible Copolymers and Hydrogels Based on 2-Hydroxyethyl(meth)acrylates. Biomacromolecules 2008, 9, 1690–1698. [Google Scholar] [CrossRef]
- Ritger, P.; Peppas, N. A Simple Equation for Description of Solute Release II. Fickian and Anomalous Release from Swellable Devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Yavari, N.; Azizian, S. Mixed diffusion and relaxation kinetics model for hydrogels swelling. J. Mol. Liq. 2022, 363, 119861. [Google Scholar] [CrossRef]
- Toleutay, G.; Dauletbekova, M.; Shakhvorostov, A.; Kudaibergenov, S. Quenched Polyampholyte Hydrogels Based on (3-Acrylamidopropyl)trimethyl Ammonium Chloride and Sodium Salt of 2-Acrylamido-2-methyl-1-Propanesulfonic Acid. Macromol. Symp. 2019, 385, 1800160. [Google Scholar] [CrossRef]
- Bai, B.; Li, L.; Liu, Y.; Wang, Z.; Liu, H. Preformed particle gel for conformance control: Factors affecting its properties and applications. In Proceedings of the 2004 SPE/DOE Fourteenth Symposium on Improved Oil Recovery, Tulsa, OK, USA, 17–21 April 2004. [Google Scholar]
- Zhao, S.; Zhu, D.; Bai, B. Experimental study of degradable preformed particle gel (DPPG) as temporary plugging agent for carbonate reservoir matrix acidizing to improve oil recovery. J. Pet. Sci. Eng. 2021, 205, 108760. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Osada, Y.; Gong, J.P. Soft and Wet Materials: Polymer Gels. Adv. Mater. 1993, 5, 833–837. [Google Scholar] [CrossRef]
- Ezell, R.; McCormick, C. Electrolyte- and pH-Responsive Polyampholytes with Potential as Viscosity-Control Agents in Enhanced Petroleum Recovery. J. Appl. Polym. Sci. 2007, 104, 2812–2821. [Google Scholar] [CrossRef]
- Bai, B.; Huang, F.; Liu, Y.; Seright, R.S.; Wang, Y. Case study on preformed particle gel for in-depth fluid diversion. In Proceedings of the 2008 SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 19–23 April 2008. [Google Scholar]
- Yu, B.; Zhao, S.; Long, Y.; Bai, B.; Schuman, T. Comprehensive evaluation of a high-temperature resistant re-crosslinkable preformed particle gel for water management. Fuel 2022, 309, 122086. [Google Scholar] [CrossRef]
- Kudaibergenov, S. Polyampholytes: Past, Present, Perspectives; Center of Operative Printing: Almaty, Kazakhstan, 2011; 220p. [Google Scholar]
- Elsharaf, M.; Bai, B. Effect of weak preformed particle gel on unswept oil zones/areas during conformance control treatments. Ind. Eng. Chem. Res. 2012, 51, 11547–11554. [Google Scholar] [CrossRef]
- Beer, F.; Johnston, R.; Dewolf, J.; Mazurek, D. Mechanics of Materials; McGraw-Hill Companies: New York, NY, USA, 2009; p. 57. [Google Scholar]


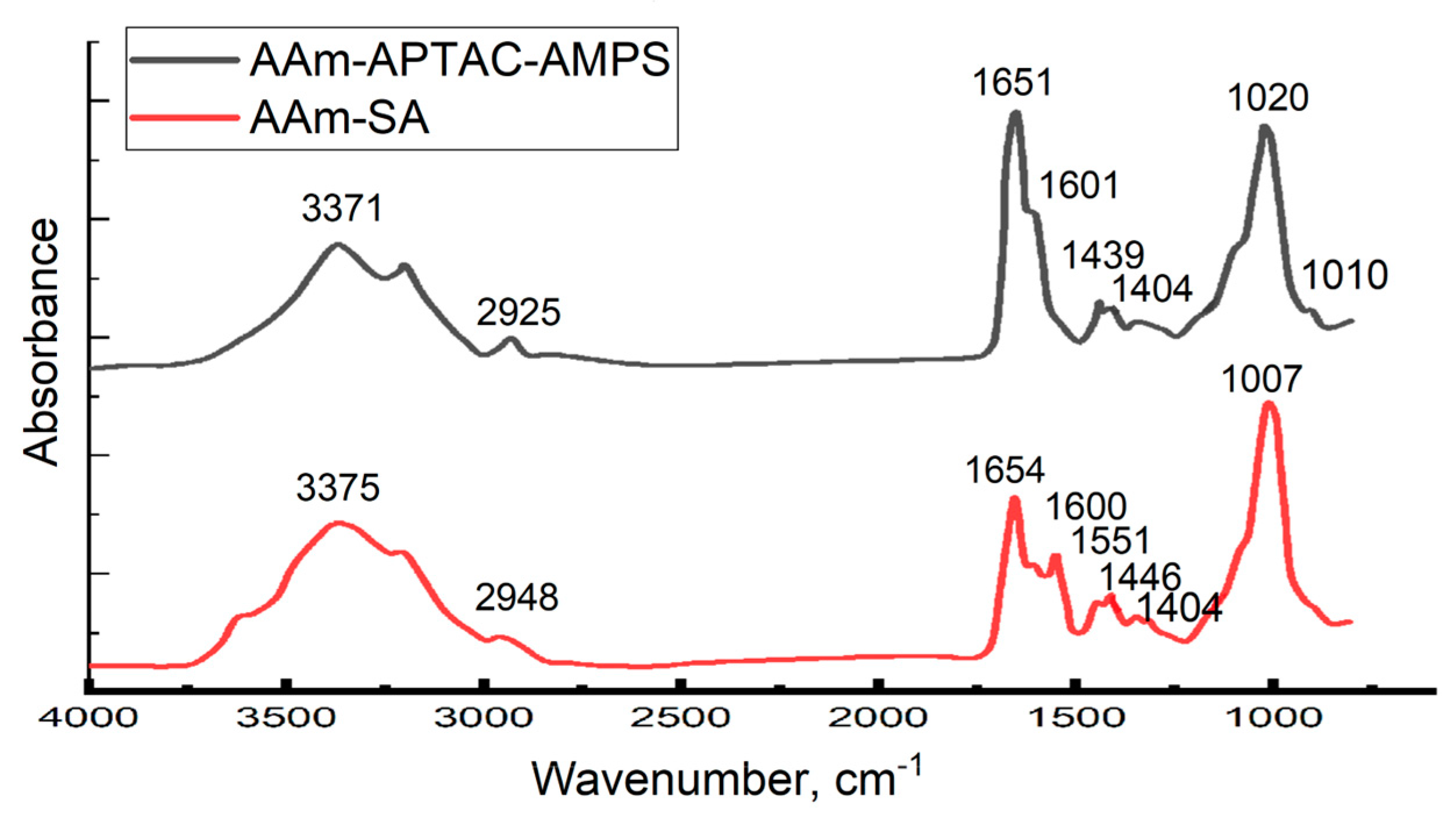
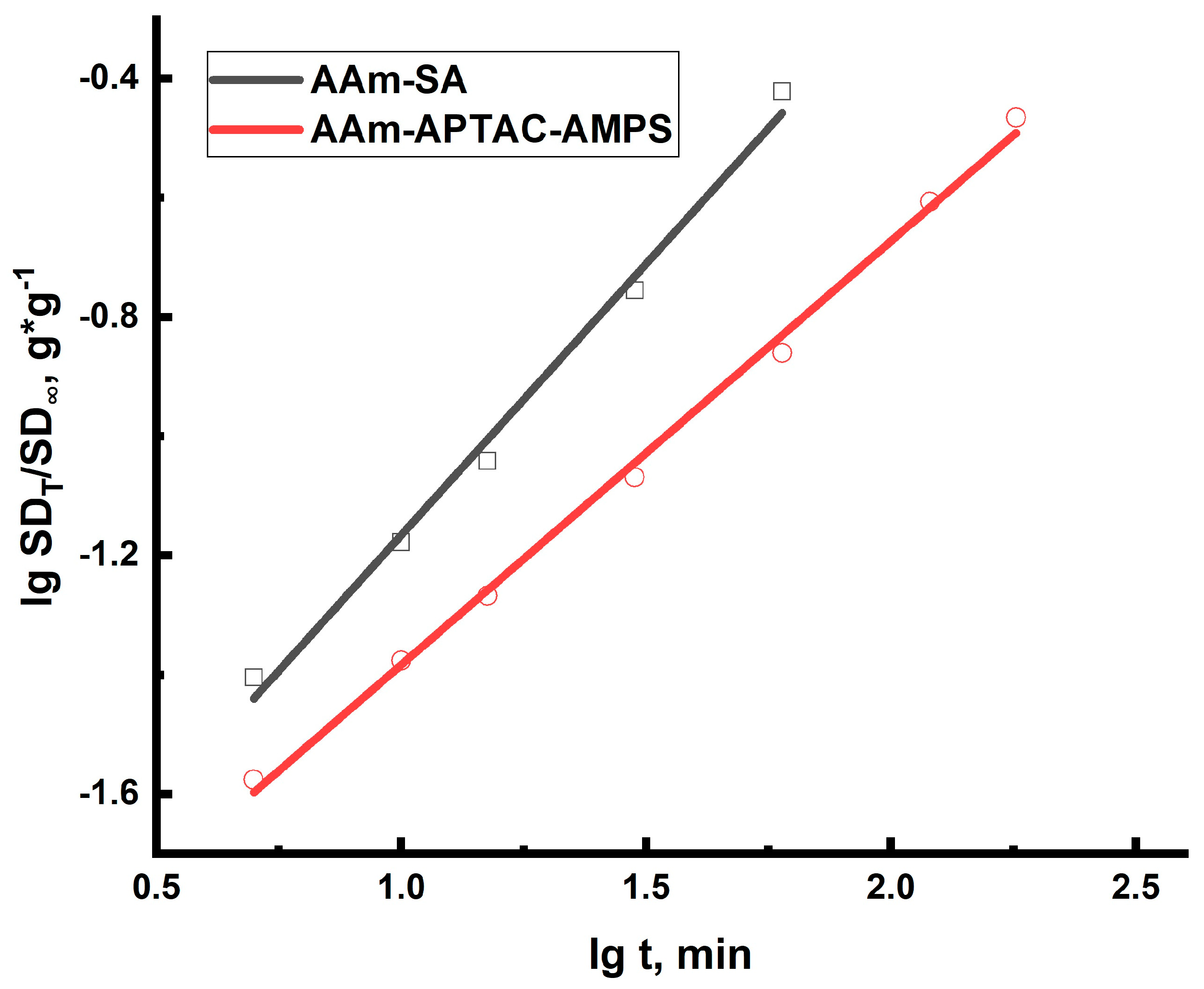
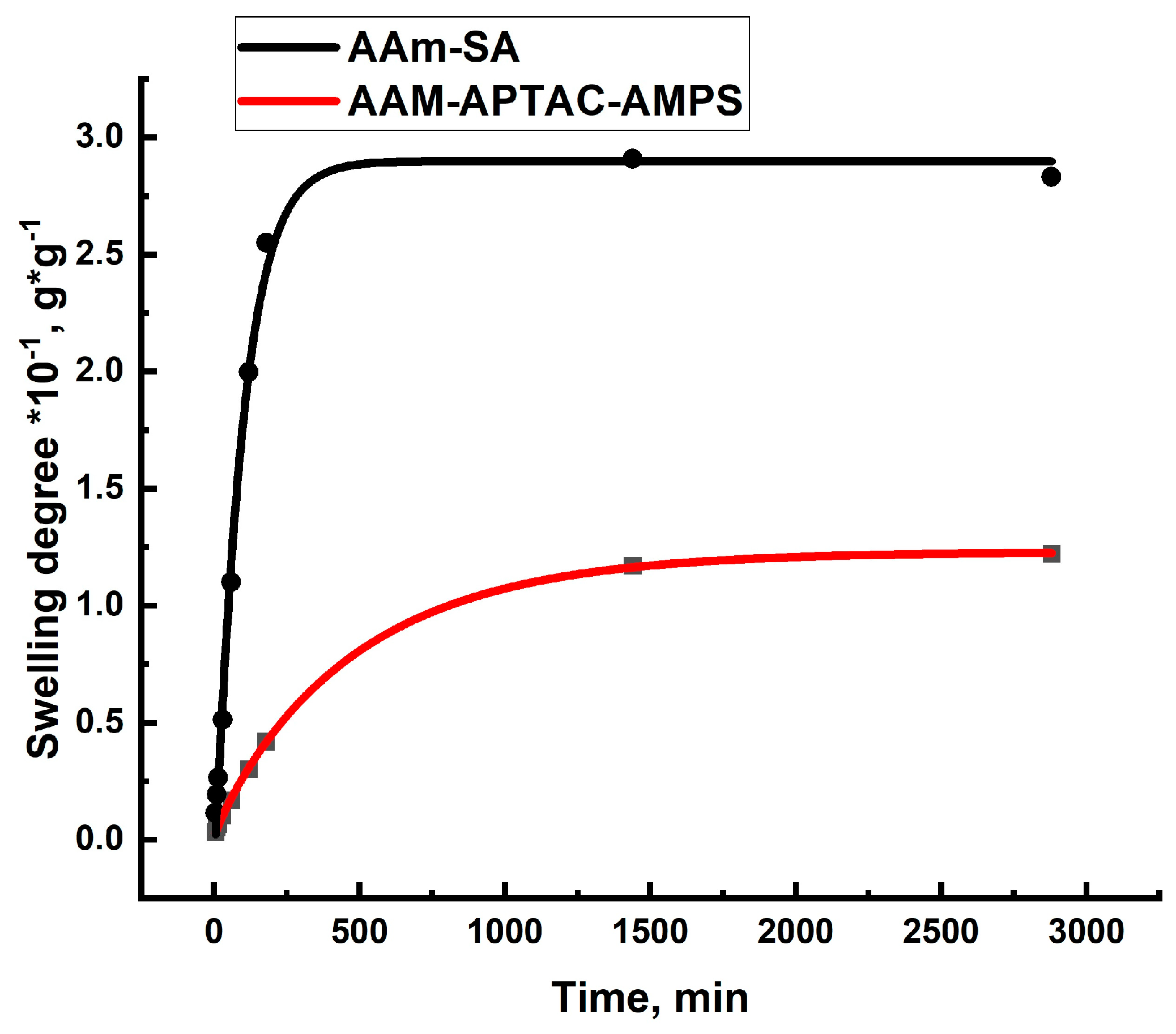
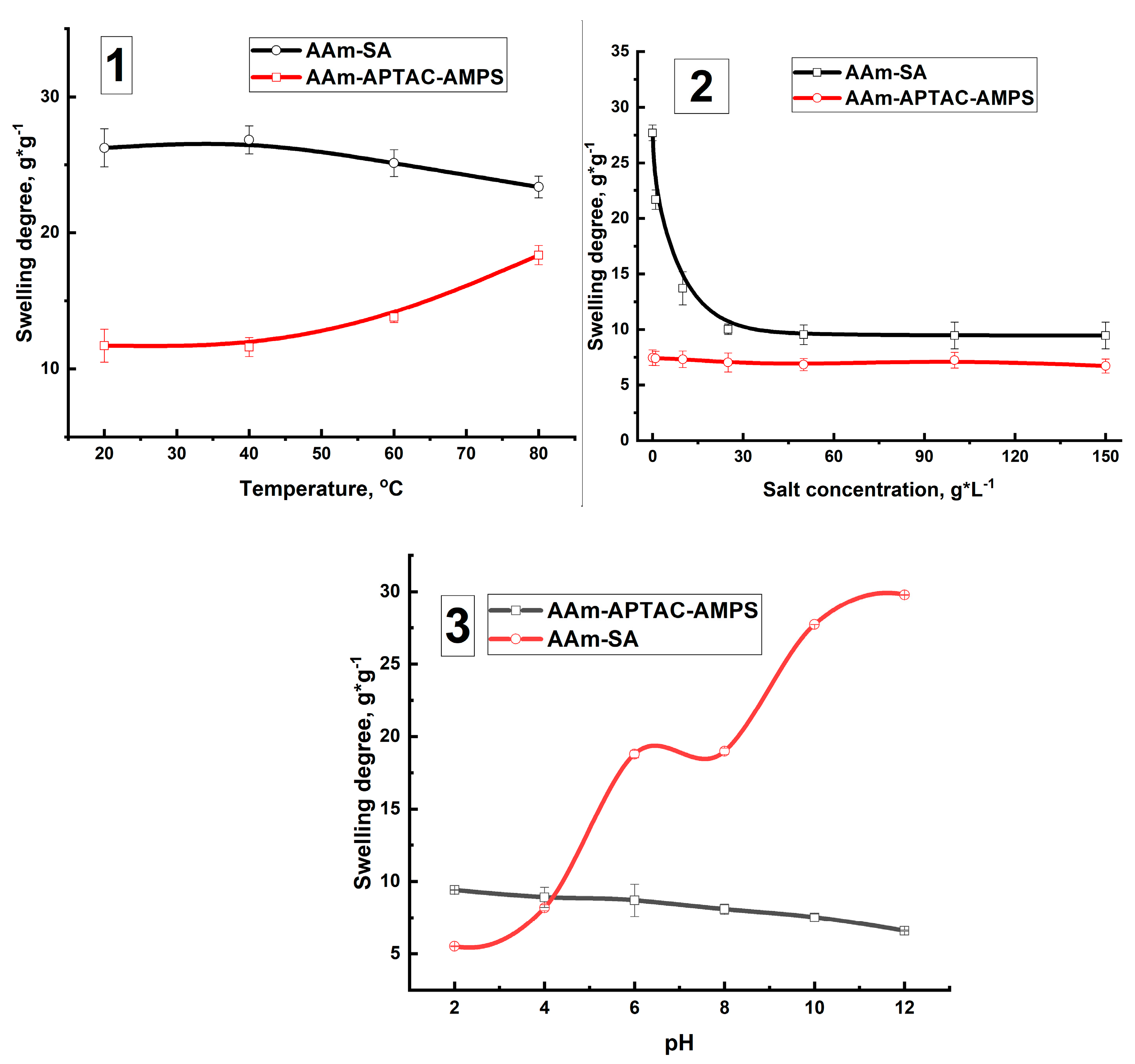

| Polymer | Key Results | Disadvantages/Problems | Reference |
|---|---|---|---|
| PAM and chitosan | Swelling capacity and storage modulus Swelling ratios vary between 5 and 107 g/g in DIW and 7 and 21 g/g in HSW. Higher temperatures and fresh water boost swelling, while more chitosan and neutral pH increase the storage modulus. | Chitosan is not soluble in water only in acidic media Mechanical properties of synthesized hydrogels is approximately weak Noncovalent interactions leads to degradation of gels under high salinity | [4] |
| Poly (acrylamide-co-acrylic acid) | Mechanical and swelling abilities Increasing acrylic acid boosts PPG swelling but weakens the network, while more crosslinker reduces swelling and strengthens the gel | What is crosslinker in these study is unclear Conventional PPGs expand too quickly in reservoir pores, making it difficult to effectively control deep profiles. Slow-swelling PPGs are easier to inject into deeper reservoirs, significantly improving plugging efficiency. | [33] |
| Agar/PAM |
| High porosity of gels can lead to reduced plugging efficiency and allow free water transport through the pores, especially since swelling decreases and pore diameter increases in saline water. | [34] |
| Poly(acrylamide-acrylic acid-2-methylpropanesulfonate) (AM-AA-AMPS) and aluminum (III) ions | The swelling behavior of these hydrogels and their interaction with rock samples were investigated.
| The anionic nature of the polymer matrix significantly influences the swelling degree of hydrogels, as it is strongly affected by the ionic strength of the solvent and the pH. | [35] |
| Alkaline surfactant polymer (ASP) |
| The necessity of using a surfactant modifier Reduction in swelling with increasing ionic strength of the solution | [36] |
| 4–6 Alkylene bisacrylamide and AAm |
| Reduction in swelling degree with increasing salt concentration | [37] |
| Sample | A | N | R2 | Main Process | |
|---|---|---|---|---|---|
| AAm95-SA5 | 28.97 | 0.13 | 0.91 | 0.99 | Relaxation and diffusion |
| AAm95-APTAC2.5-AMPS2.5 | 12.27 | 0.12 | 0.71 | 0.99 | Relaxation and diffusion |
| Sample | R2 | Main Process | |||
|---|---|---|---|---|---|
| AAm95-SA5 | 28.98 | 0.002 | −0.023 | 0.99 | Relaxation and diffusion |
| AAm95-APTAC2.5-AMPS2.5 | 12.27 | 0.005 | 0.008 | 0.99 | Relaxation and diffusion |
| # | Sand Pack Permeability to Brine, DARCY | Diameter, cm/Length, cm of the Model | Gellant/Gel Recipe | Injected Volume of Gellant/gel | Maximal Gellant/gel Injection Pressure, MPa | Maximal Post-Flush Injection Pressure, MPa | Assessed RRF |
|---|---|---|---|---|---|---|---|
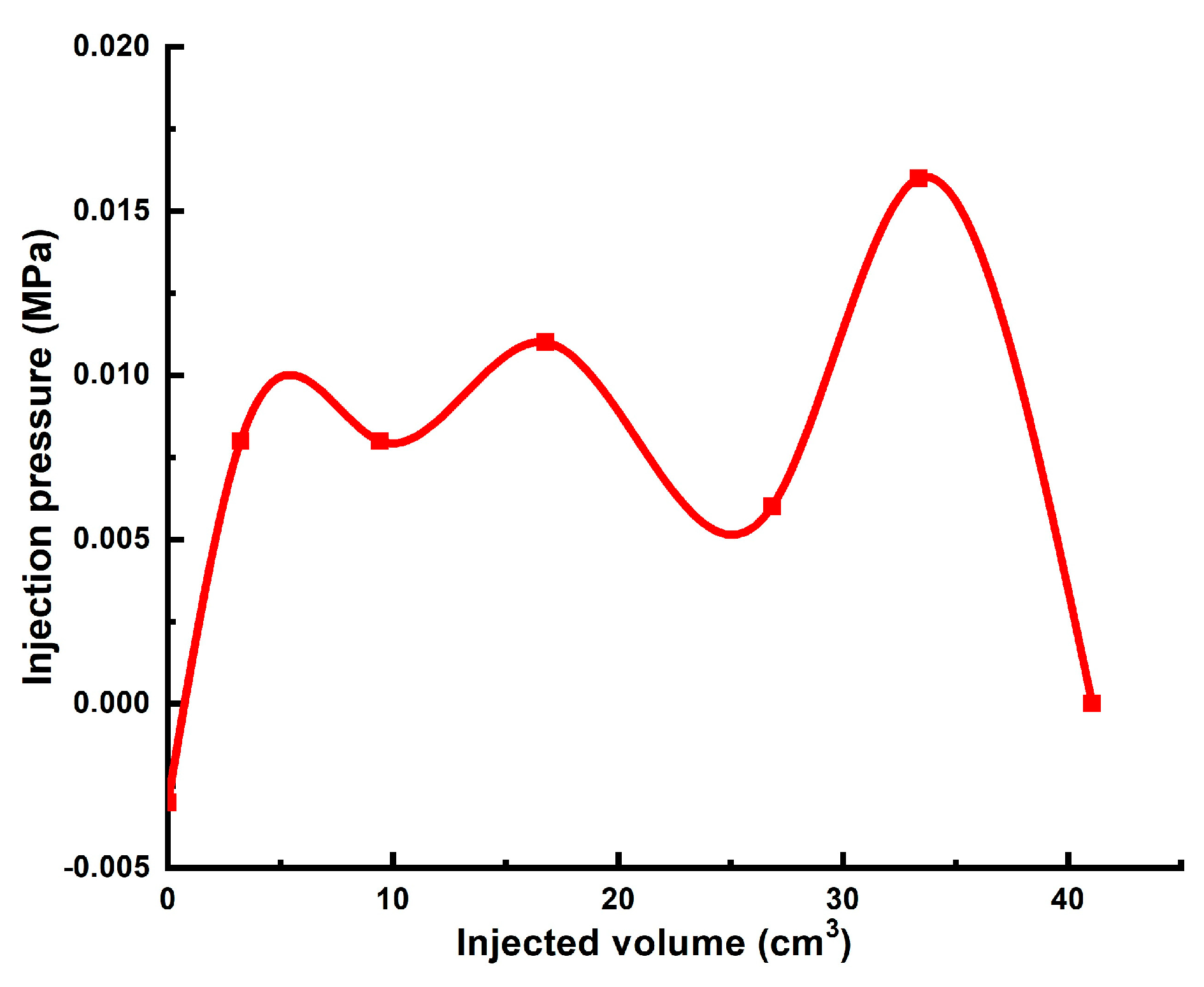
| 1 | 0.165 * | 4.3 cm/8.6 cm | Gellant [0.5 wt.% 6–7 mln Da and 5% hydroyzed HPAM/0.5 wt.% chromium acetate] | 3 PVs | 0.088 | 7 | 190 |
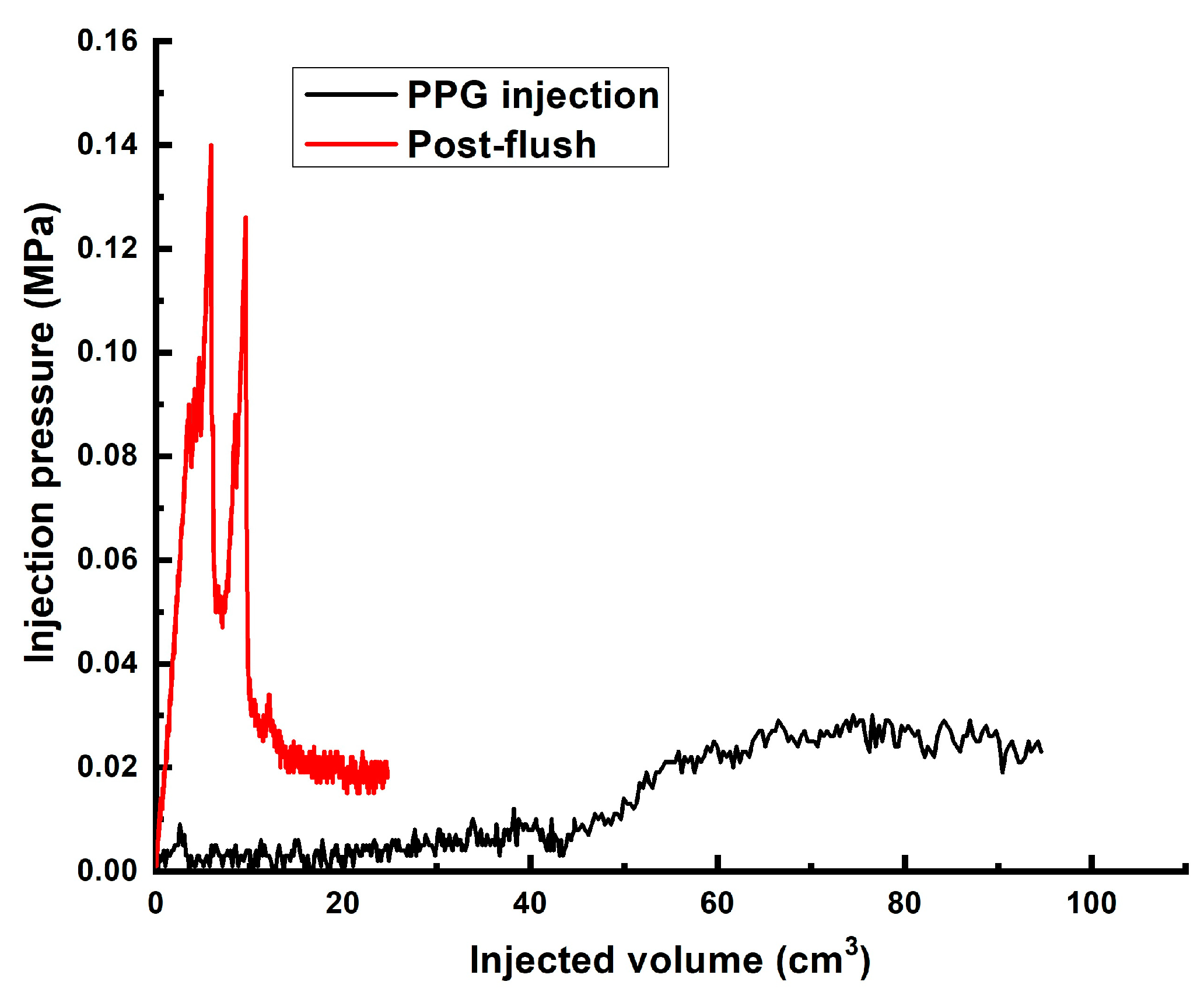
| 2 | ~60 | 4.3 cm/8.6 cm | Gellant [0.5 wt% 6–7 mln Da and 5% hydrolyzed HPAM/0.2 wt.% chromium acetate] | 1 PV | Too low to be detected | 0.016 | ~1950 |
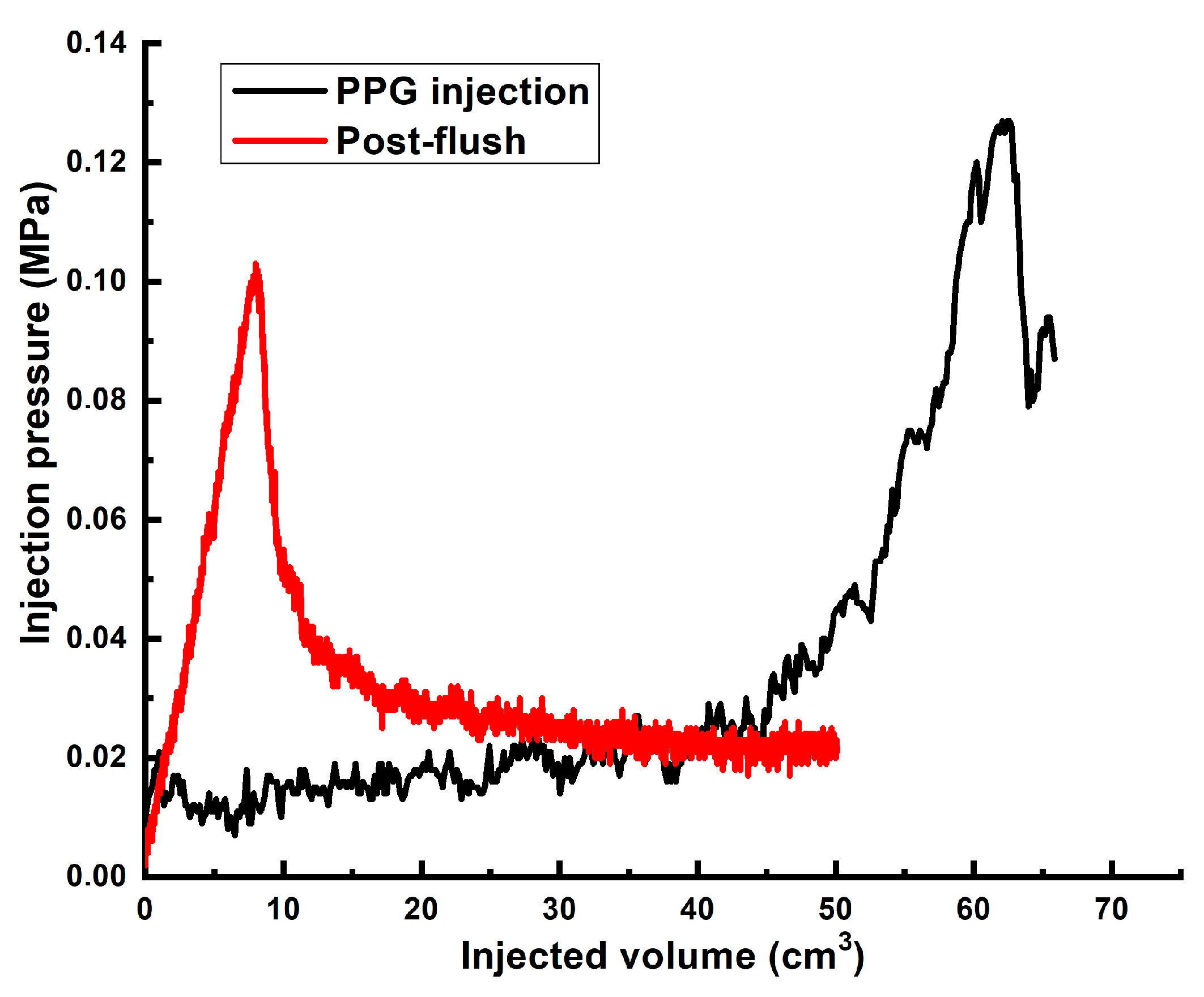
| 3 | ~60 | 4.3 cm/8.6 cm | Gel [0.5 wt.% 13–18 mln Da and 16.5% hydrolyzed HPAM/0.5 wt.% coarse (1–2.5 mm) PPG AAm95-APTAC2.5-AMPS2.5/0.05 wt.% chromium acetate] | 1 PV | 0.042 | 0.006 | ~731 |
| 4 | ~60 | 3 cm/9 cm | Gel [0.5 wt.% 13–18 mln Da and 16.5% hydrolyzed HPAM/0.5 wt.% fine PPG AAm95-SA5/0.05 wt.% chromium acetate] | 2 PVs ** | 0.028 | 0.14 | ~8000 |
| 5 | ~60 | 3 cm/9 cm | Gel [0.5 wt.% 13–18 mln Da and 16.5% hydrolyzed HPAM/0.5 wt.% fine PPG AAm95-APTAC2.5-AMPS2.5/0.05 wt.% chromium acetate] | 1.32 ** | 0.126 | 0.1 | ~5714 |
| Component | AAm-SA | AAm-APTAC-AMPS | ||
|---|---|---|---|---|
| mol.% | m, mg | mol.% | m, mg | |
| AAm | 95 | 444.2 | 95 | 733.9 |
| SA | 5 | 5 | - | - |
| APTAC | - | - | 2.5 | 106.8 |
| AMPS | - | - | 2.5 | 141.6 |
| MBAA | 1 | 10.7 | 1 | 17.7 |
| Bentonite | 5 | 0.5 | 5 | 0.5 |
| Water | 90 | 9 | 85 | 8.5 |
| Monomer concentration, % | 5 | 10 | ||
| Parameter | Value |
|---|---|
| TDS, g/L | 26.6 |
| K+, mg/L | 70.94 |
| Ca2+, mg/L | 2 |
| Mg2+, mg/L | 12.2 |
| Na+, mg/L | 12,532 |
| SO42−, mg/L | 40 |
| Cl−, mg/L | 17,373.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yelemessova, G.; Gussenov, I.; Ayazbayeva, A.; Shakhvorostov, A.; Orazzhanova, L.; Klivenko, A.; Kudaibergenov, S. Preparation and Characterization of Preformed Polyelectrolyte and Polyampholyte Gel Particles for Plugging of High-Permeability Porous Media. Gels 2024, 10, 562. https://doi.org/10.3390/gels10090562
Yelemessova G, Gussenov I, Ayazbayeva A, Shakhvorostov A, Orazzhanova L, Klivenko A, Kudaibergenov S. Preparation and Characterization of Preformed Polyelectrolyte and Polyampholyte Gel Particles for Plugging of High-Permeability Porous Media. Gels. 2024; 10(9):562. https://doi.org/10.3390/gels10090562
Chicago/Turabian StyleYelemessova, Gulnur, Iskander Gussenov, Aigerim Ayazbayeva, Alexey Shakhvorostov, Lyazzat Orazzhanova, Alexey Klivenko, and Sarkyt Kudaibergenov. 2024. "Preparation and Characterization of Preformed Polyelectrolyte and Polyampholyte Gel Particles for Plugging of High-Permeability Porous Media" Gels 10, no. 9: 562. https://doi.org/10.3390/gels10090562






