The Effect of High Hydrostatic Pressure (HHP) Induction Parameters on the Formation and Properties of Inulin–Soy Protein Hydrogels
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Effect of High Hydrostatic Pressure Induction Parameters on the Formation and Stability of the Obtained Hydrogels
2.1.1. Volumetric Gel Index (VGI)
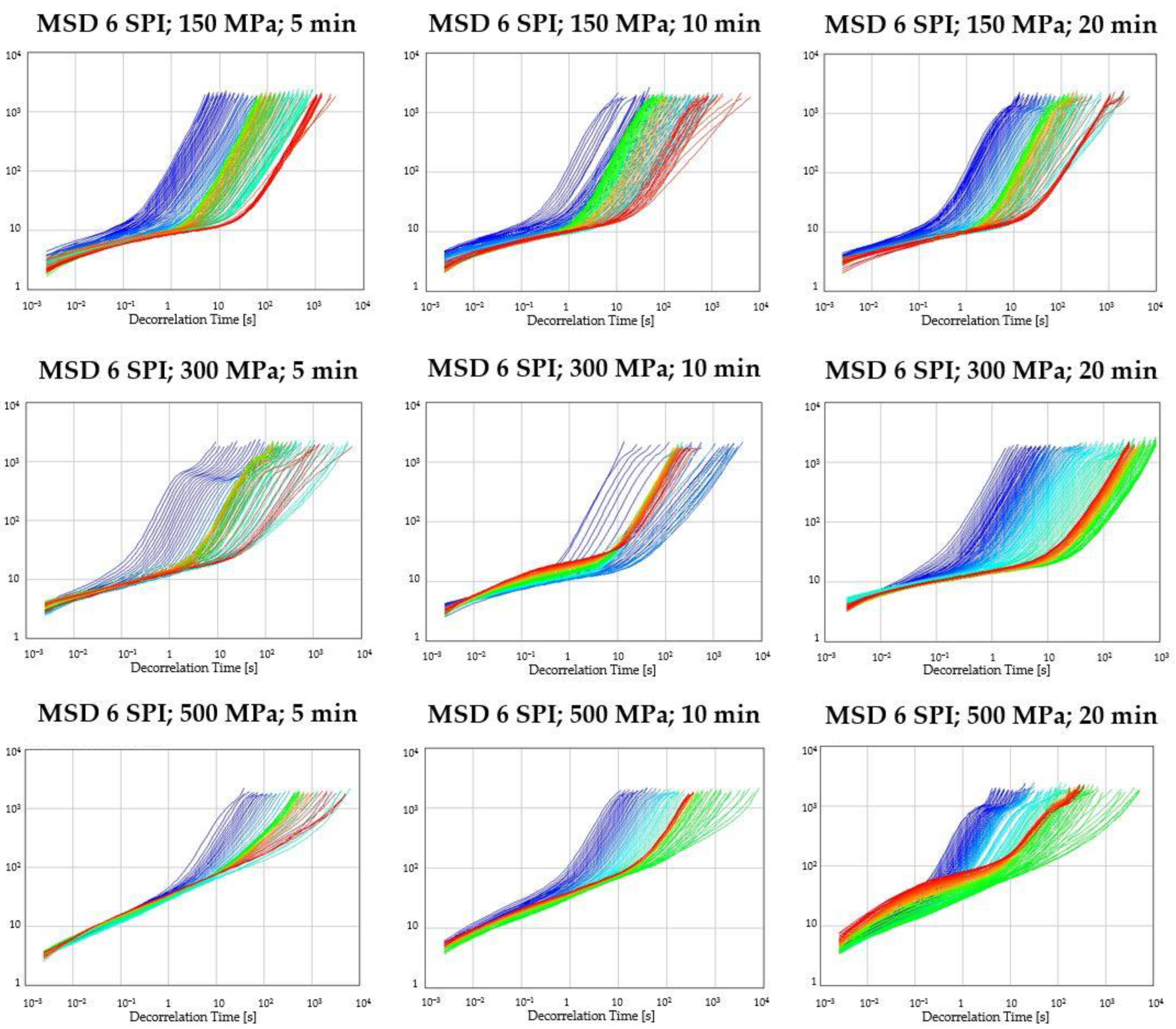
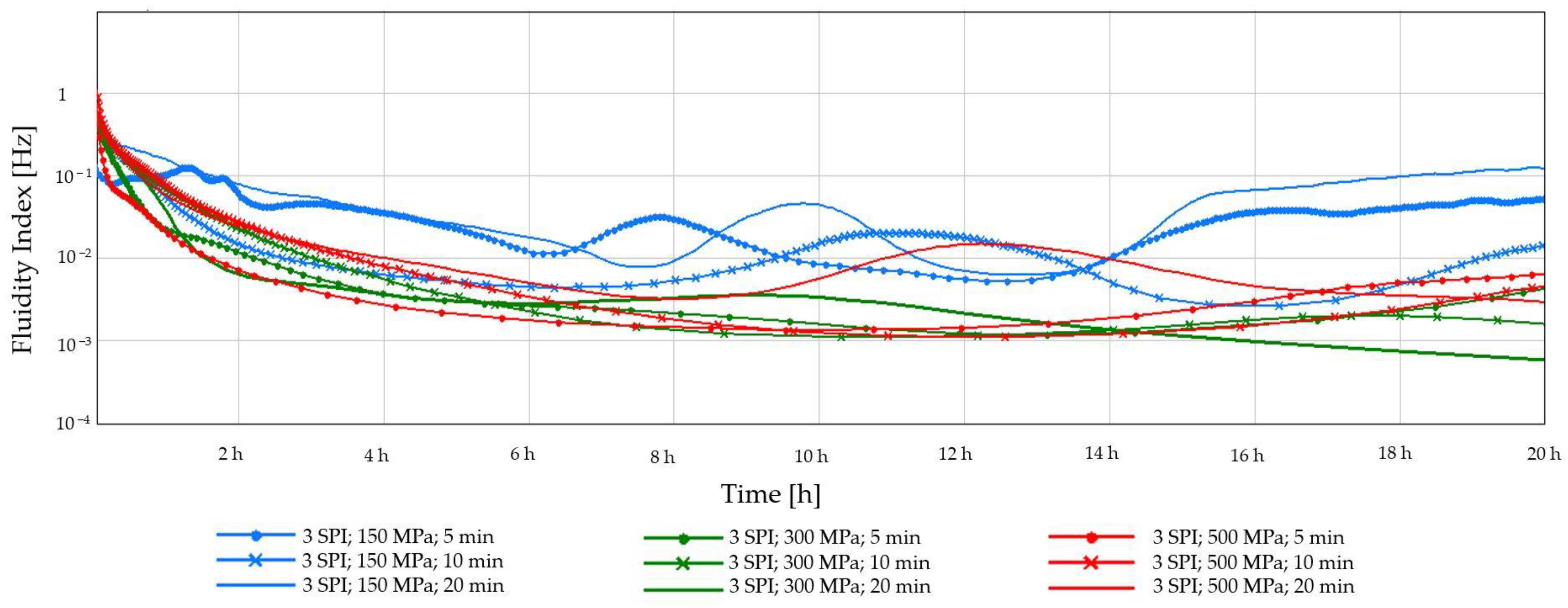
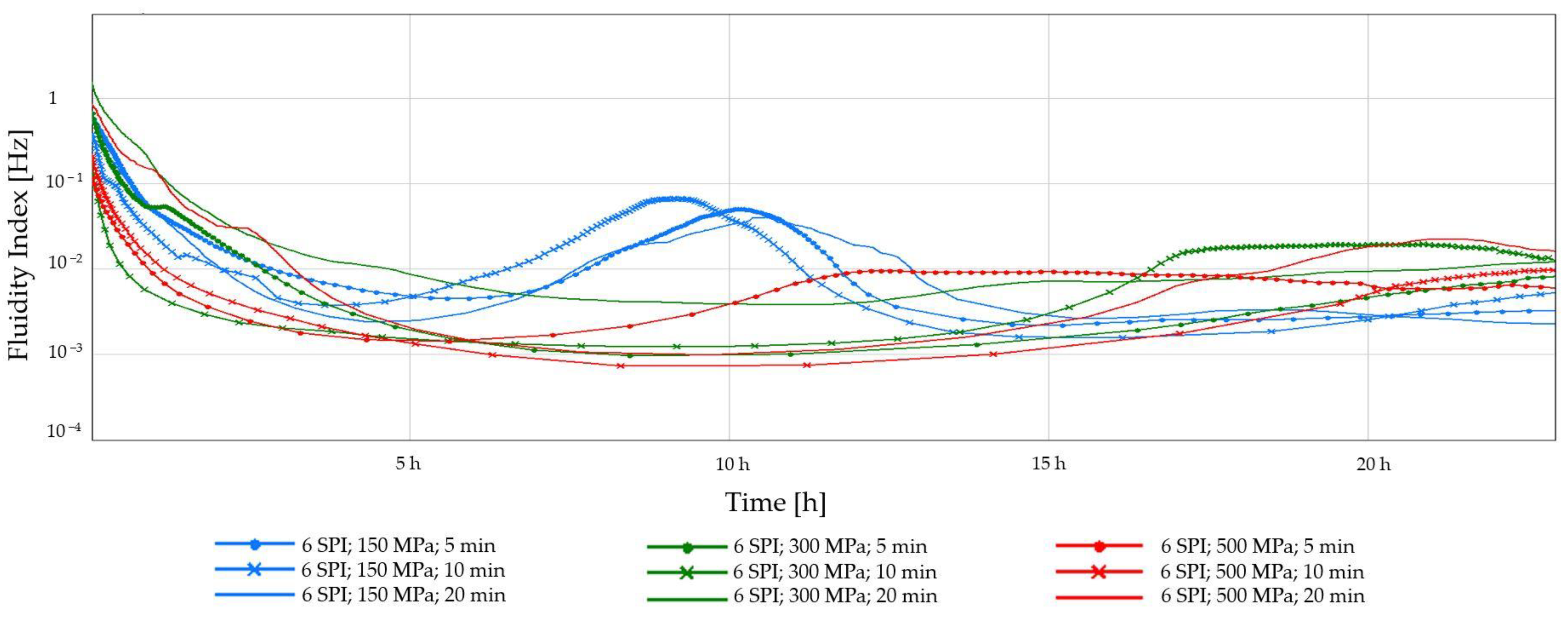
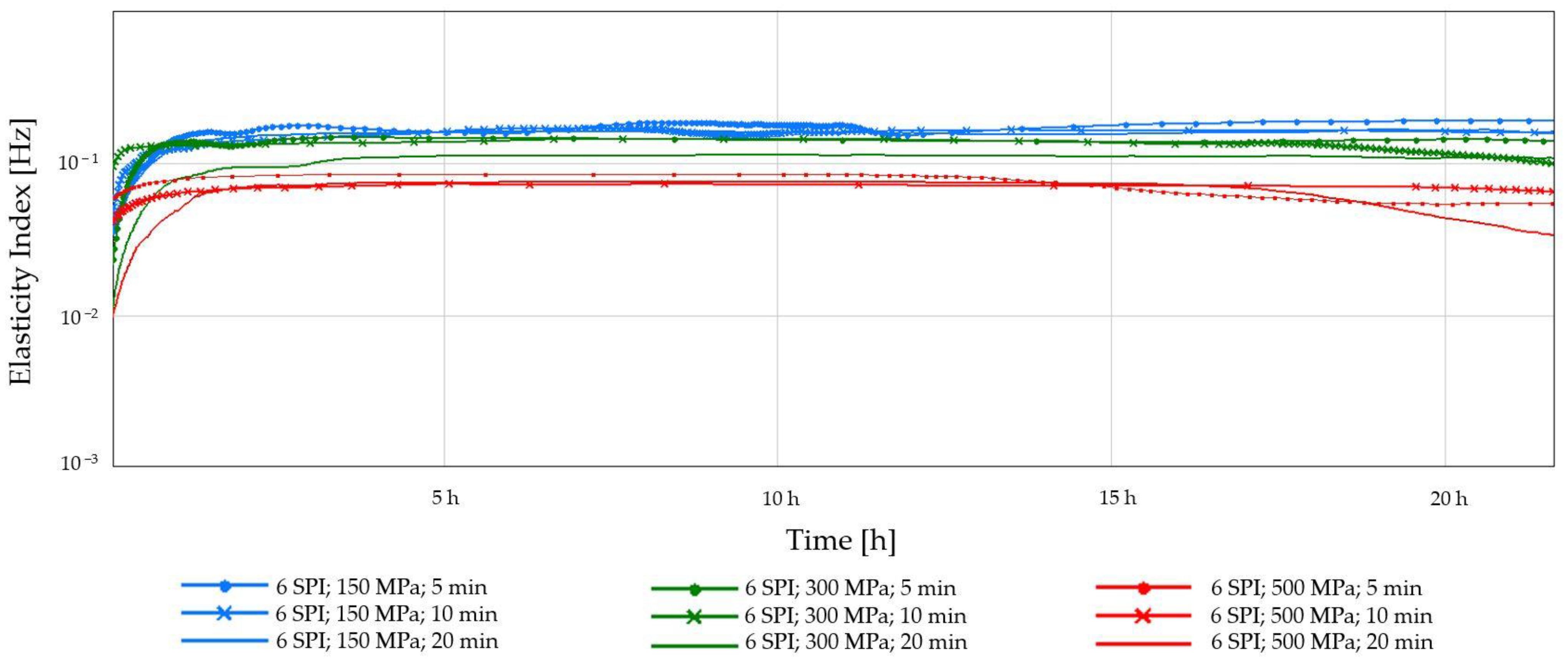
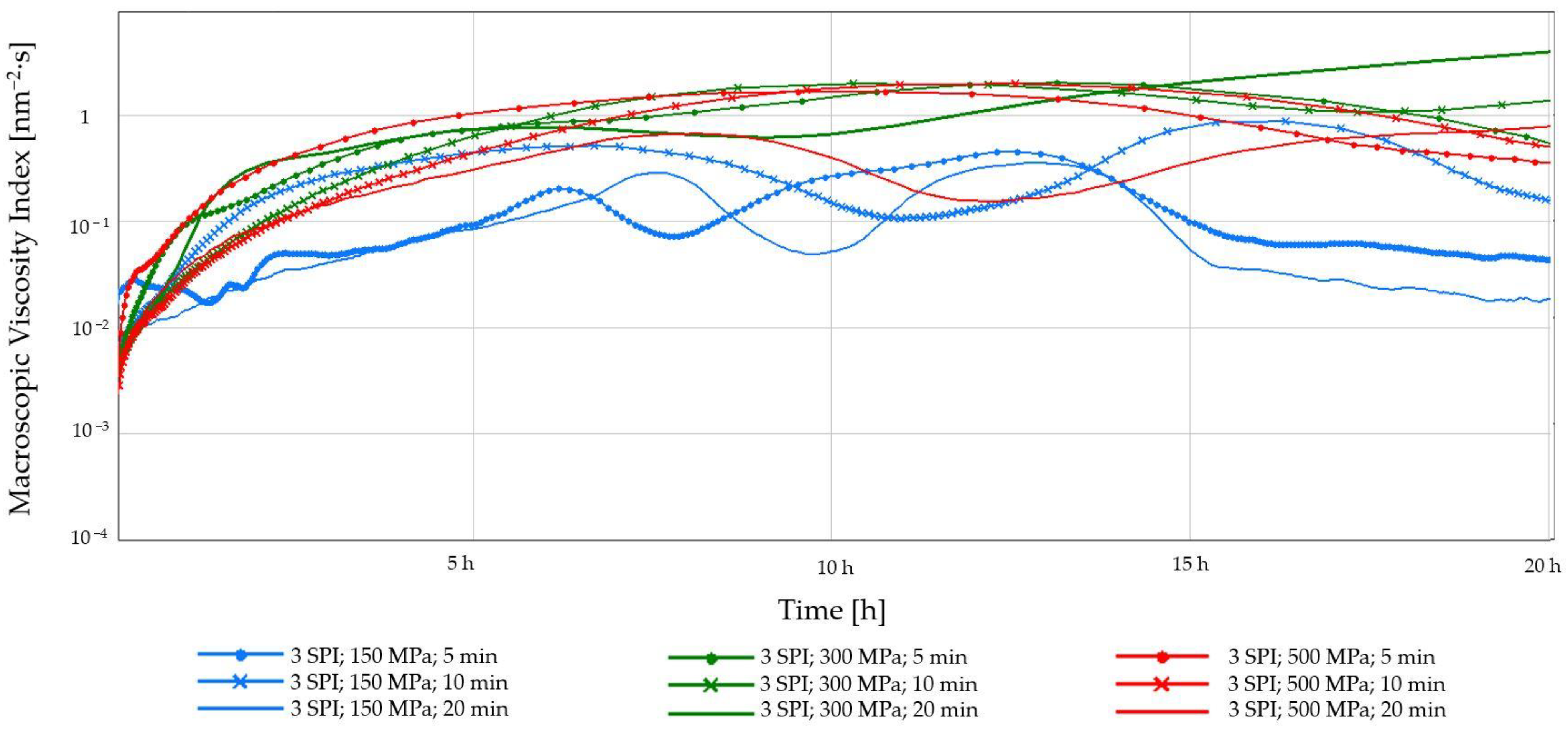
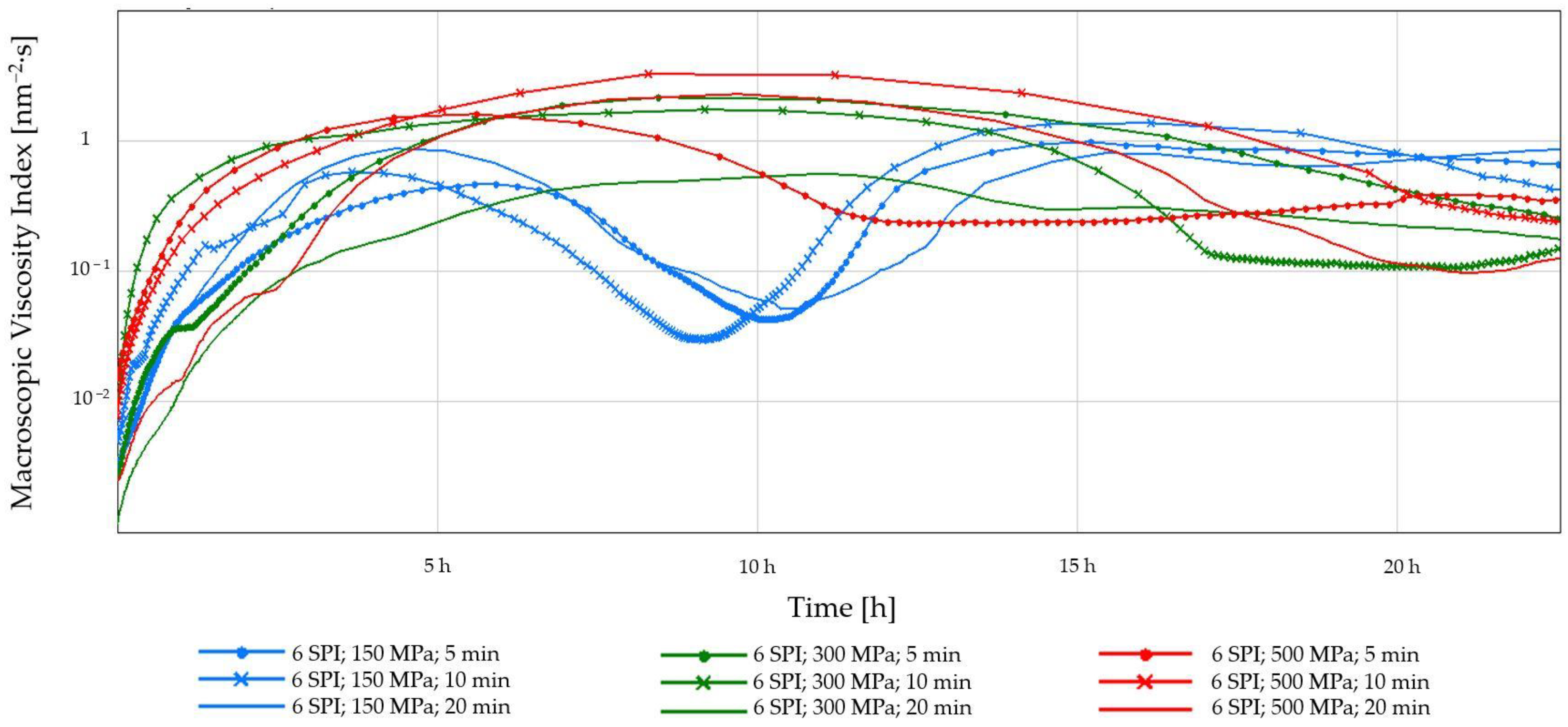
2.1.2. Diffusing-Wave Spectroscopy (DWS)
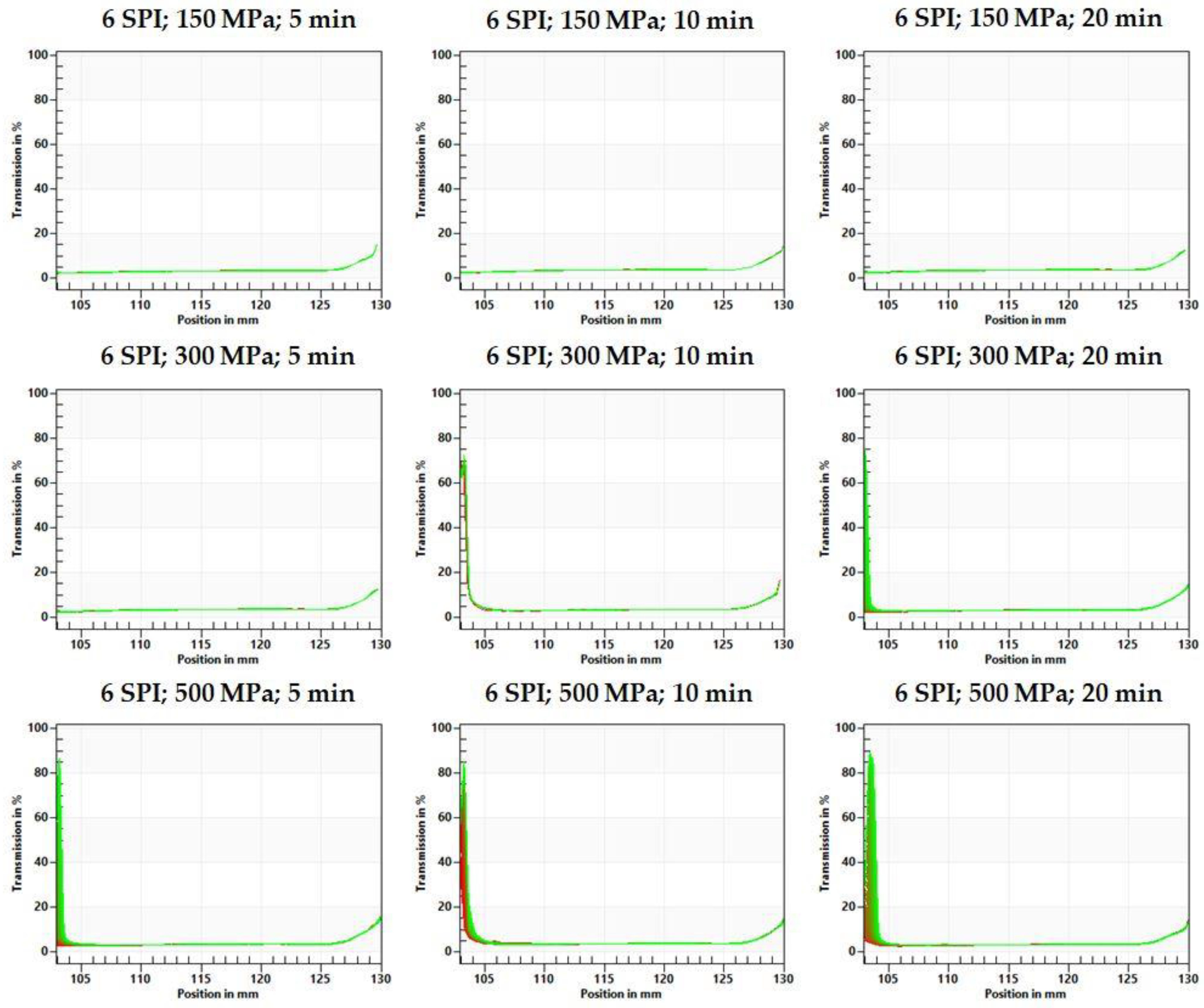
2.1.3. Centrifugal Stability Analysis Method (CSA)
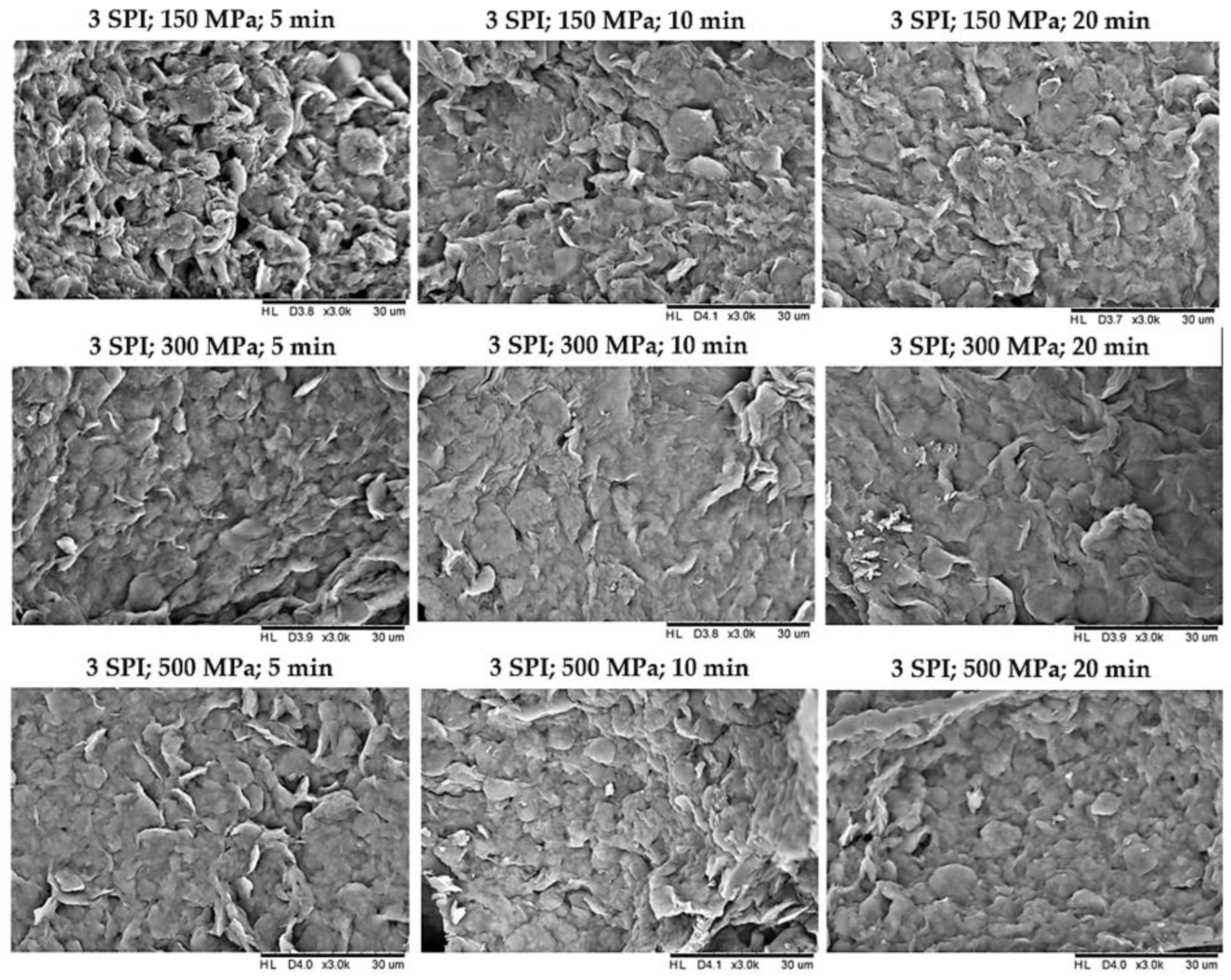
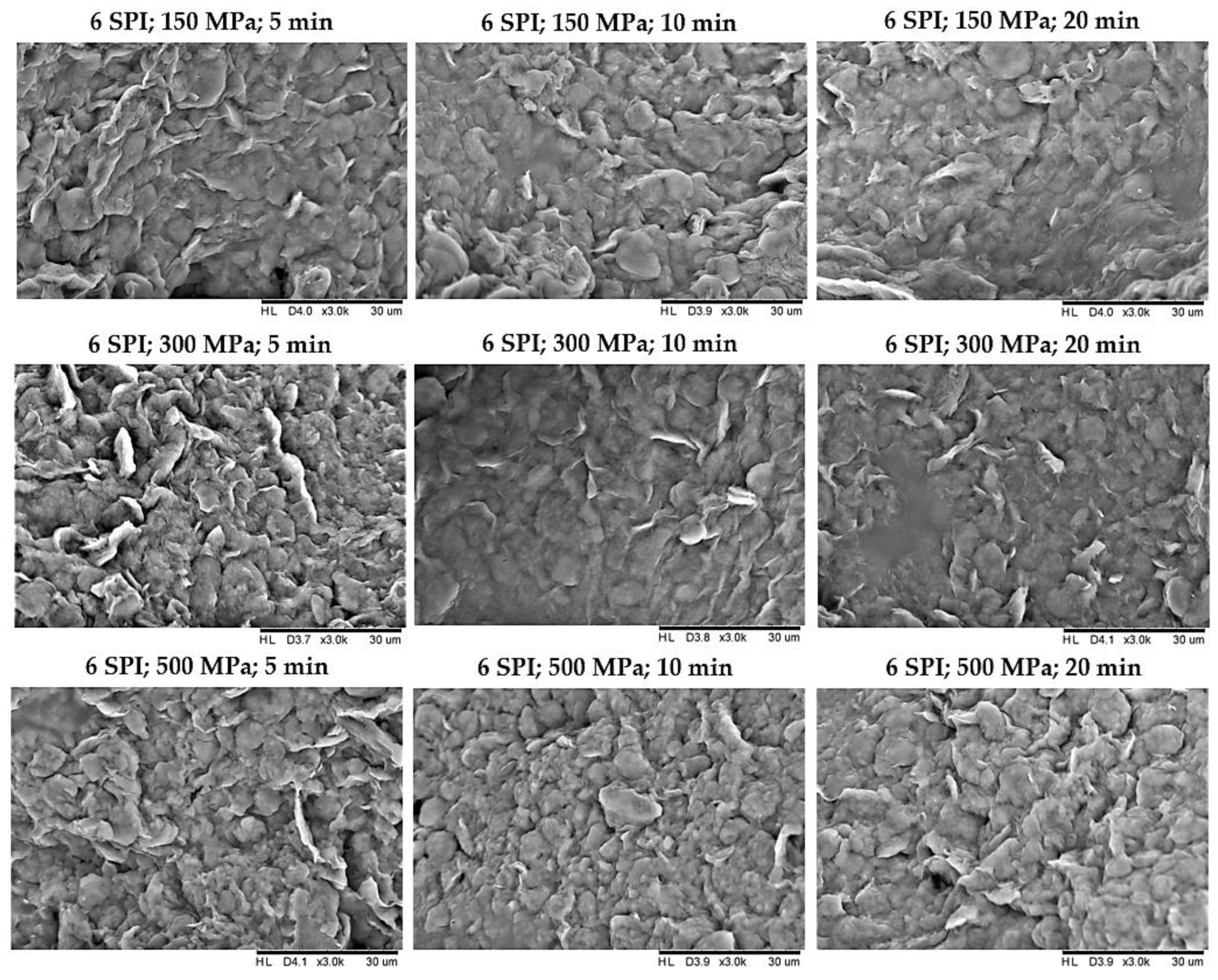
2.2. The Effect of High Hydrostatic Pressure Induction Parameters on the Microstructure of the Obtained Hydrogels
2.3. The Effect of High Hydrostatic Pressure Induction Parameters on the Yield Stress and Texture Parameters of the Obtained Hydrogels
2.3.1. Yield Stress
2.3.2. Firmness
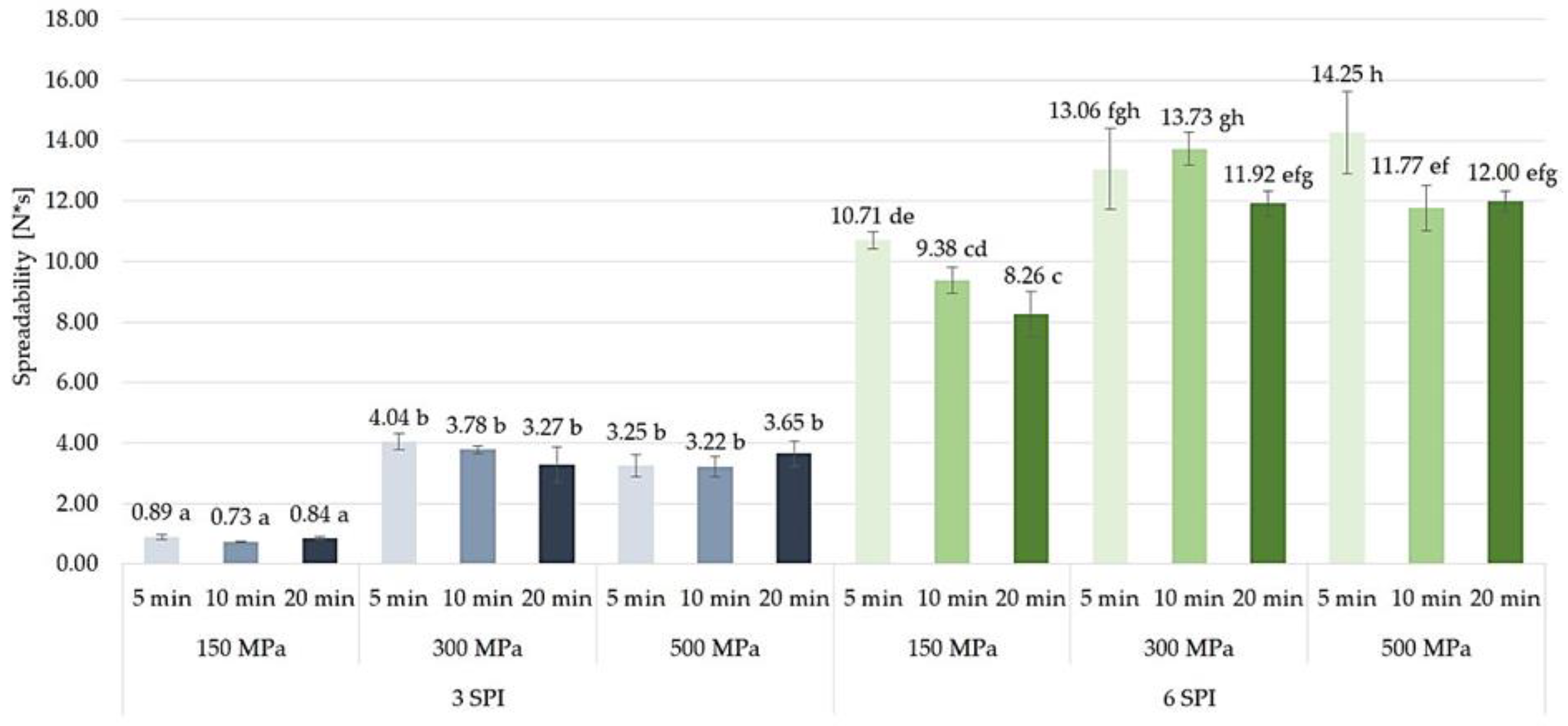
2.3.3. Spreadability
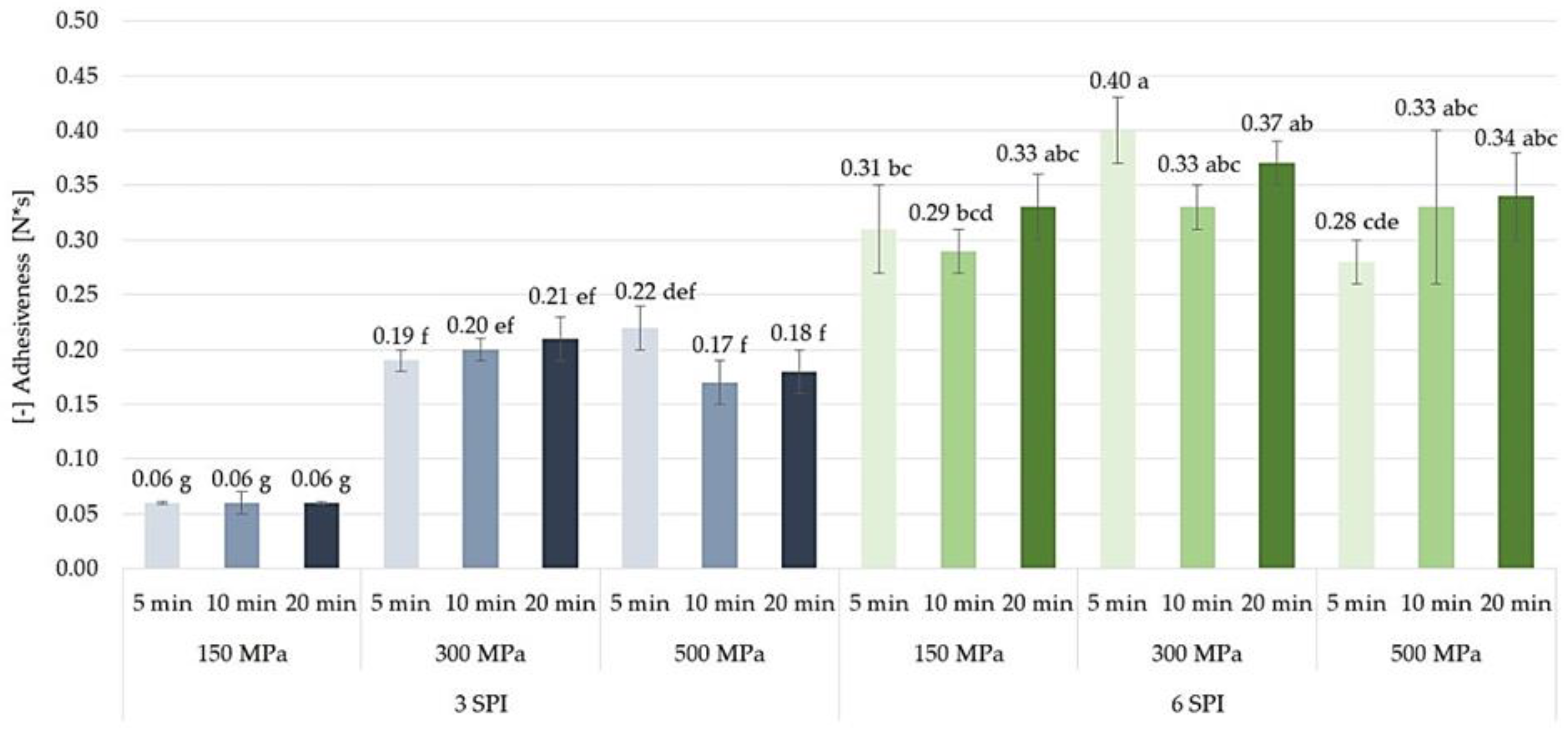
2.3.4. Adhesiveness
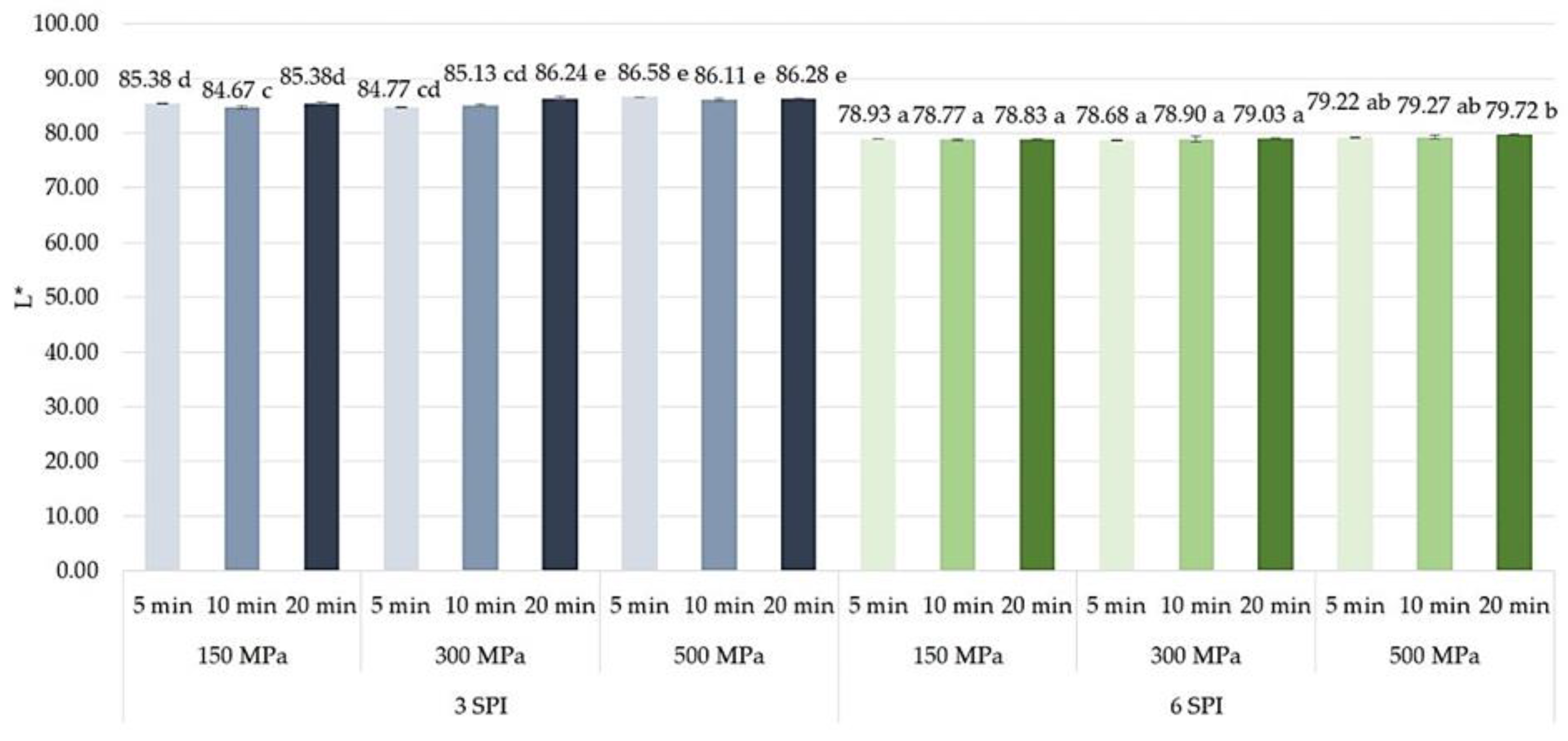
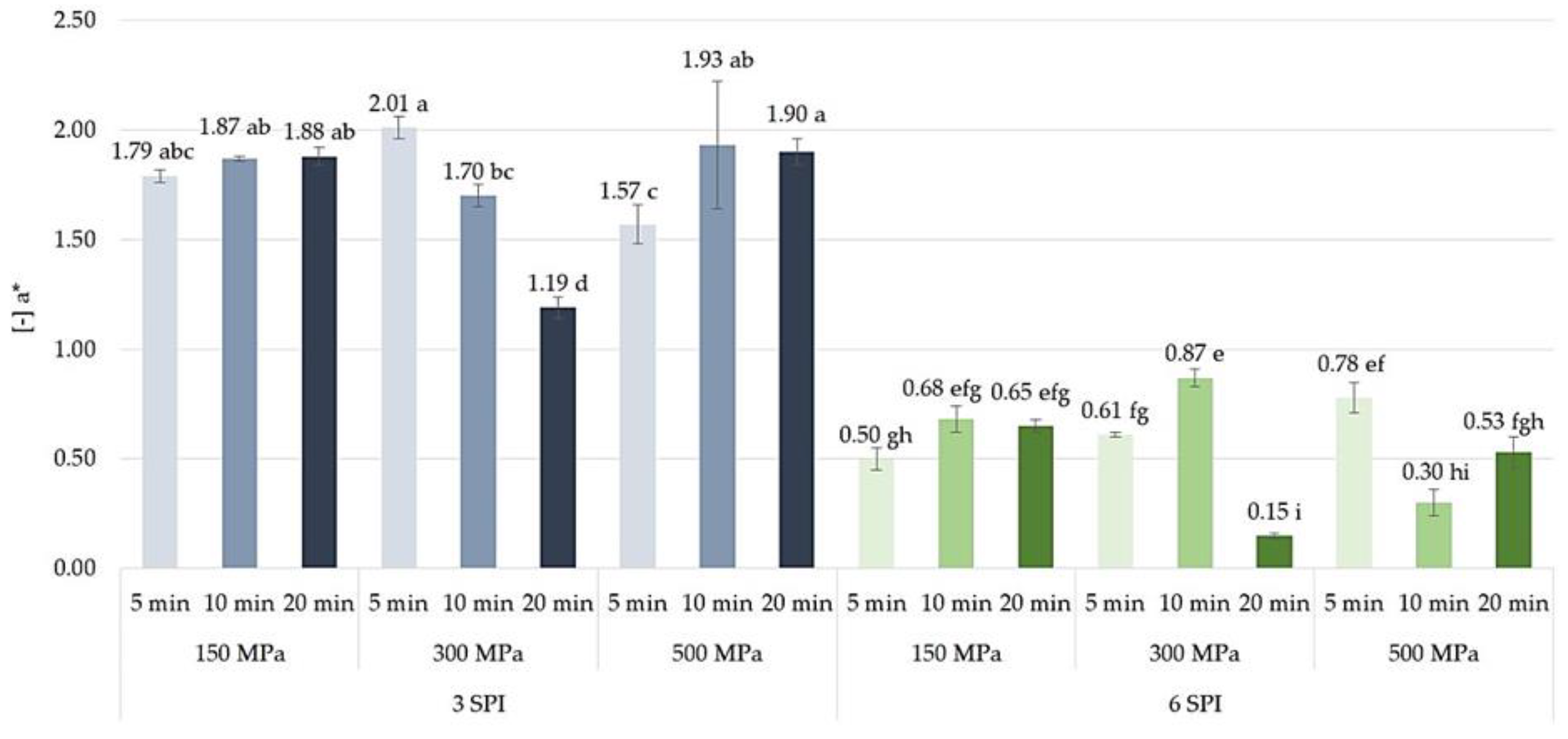
2.4. The Effect of High Hydrostatic Pressure Induction Parameters on the Color and the Total Color Difference Parameters of the Obtained Hydrogels
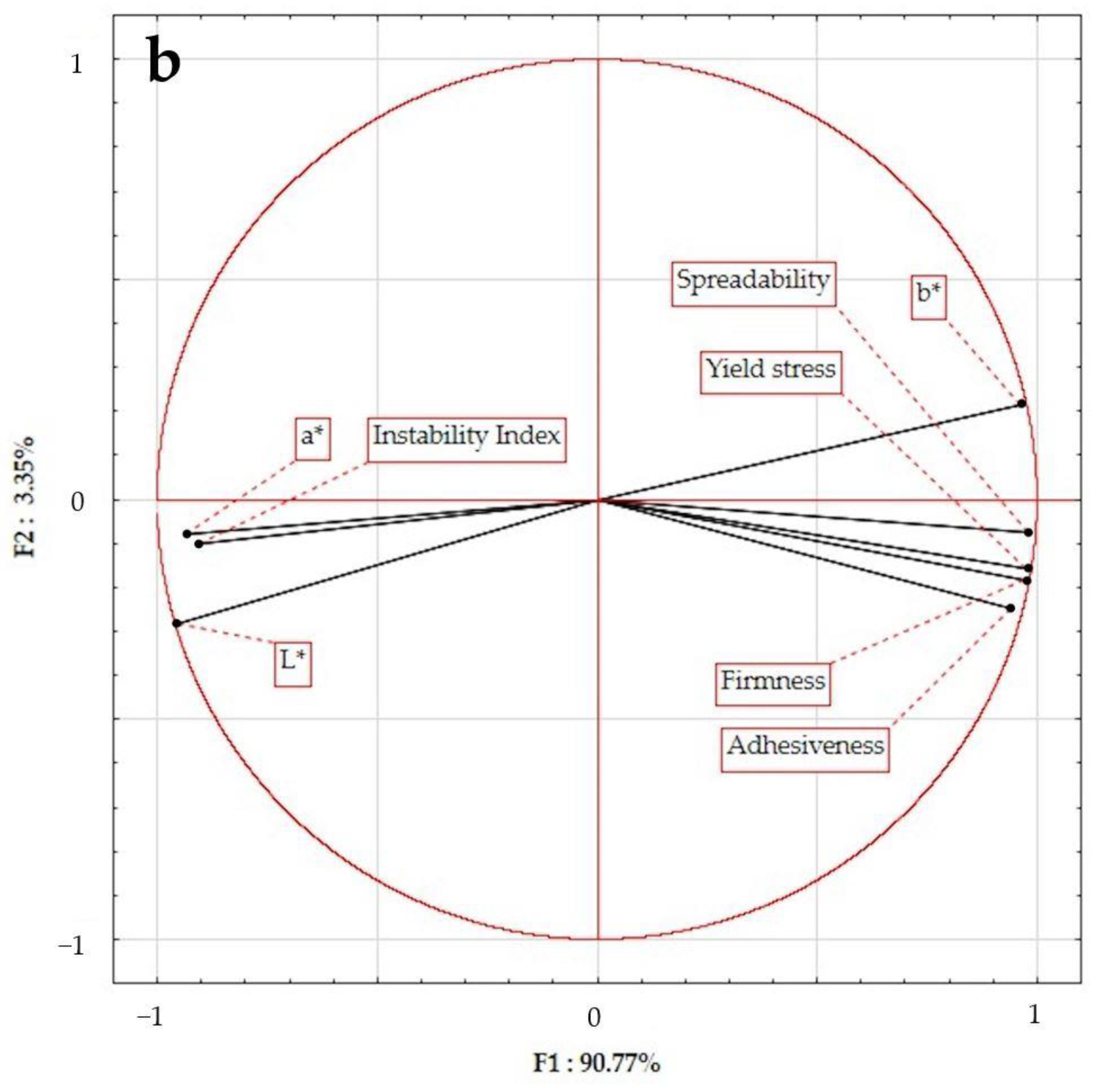
2.5. Principal Component Analysis (PCA)
3. Conclusions
4. Materials and Methods
4.1. Materials and Preparation of Hydrogels
- -
- Preparation of an aqueous solution of inulin by mixing it with water;
- -
- Addition of the appropriate protein concentration, and preparing the suspension;
- -
- Pouring the samples into plastic bottles used in the high-pressure chamber for pressurization;
- -
- Subjecting samples to high-pressure treatments (apparatus U 5000/120 Unipress, Warsaw, Poland), using pressures of 150, 300, or 500 MPa and time treatments of 5, 10, or 20 min.
4.2. Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guilherme, M.G.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef]
- Habib, M.A.; Akter, M.A.; Alam, M.E.; Karim, Z.; Joarder, M.T.A. Hydrogel: A noble approach to healthcare. Int. J. Innovat. Pharmaceut. Sci. Res. 2015, 72, 1372–1388. [Google Scholar]
- Bahram, M.; Mohseni, N.; Moghtader, M. An Introduction to hydrogels and some recent applications. In Emerging Concepts in Analysis and Applications of Hydrogels; Majee, S.B., Ed.; IntechOpen: London, UK, 2016; pp. 9–38. [Google Scholar]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef]
- Farrukh, A.; Nayab, S. Shape Memory Hydrogels for Biomedical Applications. Gels 2024, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Chen, W.; Zhong, Y.; Ma, X.; Wang, B. Biomedical Applications of Deformable Hydrogel Microrobots. Micromachines 2023, 14, 1824. [Google Scholar] [CrossRef]
- Le, X.T.; Rioux, L.E.; Turgeon, S.L. Formation and functional properties of protein-polysaccharide electrostatic hydrogels in comparison to protein or polysaccharide hydrogels. Adv. Colloid Interface Sci. 2017, 239, 127–135. [Google Scholar] [CrossRef]
- Larrea-Wachtendorff, D.; Di Nobile, G.; Ferrari, G. Effects of processing conditions and glycerol concentration on rheological and texture properties of starch-based hydrogels produced by high pressure processing (HHP). Int. J. Biol. Macromol. 2020, 159, 590–597. [Google Scholar] [CrossRef]
- Huang, M.; Kennedy, J.F.; Li, B.; Xu, X.; Xie, B.J. Characters of rice starch gel modified by gellan, carrageenan, and glucomannan: A texture profile analysis study. Carbohydr Polym. 2007, 69, 411–418. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V. Polysaccharides constructed hydrogels as vehicles for proteins and peptides. A review. Carbohydr Polym. 2019, 225, 115210. [Google Scholar] [CrossRef]
- Florowska, A.; Florowski, T.; Sokołowska, B.; Janowicz, M.; Adamczak, L.; Pietrzak, D. Effect of high hydrostatic pressure on formation and rheological properties of inulin gels. LWT 2020, 121, 108995. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, R.; Sabapathy, S.N.; Bawa, A.S. Functional and edible uses of soy protein products. Compr. Rev. Food Sci. Food Saf. 2008, 7, 14–28. [Google Scholar] [CrossRef]
- Boeckner, L.S.; Schnepf, M.I.; Tungland, B.C. Inulin: A review of nutritional and health implications. Adv. Food Nutr. Res. 2001, 43, 1–63. [Google Scholar] [PubMed]
- Franck, A. Inulin. In Food Polysaccharides and Their Applications, 2nd ed.; Stephen, A.M., Phillips, G.O., Williams, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2006; Volume 10, pp. 335–352. [Google Scholar]
- Mensink, M.A.; Frijlink, H.W.; van der Voort Maarschalk, K.; Hinrichs, W.L. Inulin, a flexible oligosaccharide. II: Review of its pharmaceutical applications. Carbohydr. Polym. 2015, 134, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Piao, X.; Huang, J.; Sun, Y.; Zhao, Y.; Zheng, B.; Zhou, Y.; Yu, H.; Zhou, R.; Cullen, P.J. Inulin for surimi gel fortification: Performance and molecular weight-dependent effects. Carbohydr. Polym. 2023, 305, 120550. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G. Inulin-type prebiotics--a review: Part 1. Altern. Med. Rev. 2008, 13, 315–329. [Google Scholar] [PubMed]
- Glibowski, P. Effect of thermal and mechanical factors on rheological properties of high performance inulin gels and spreads. J. Food Eng. 2010, 99, 106–113. [Google Scholar] [CrossRef]
- Yu, J.Y.; Li, X.H.; Lu, F.; Wang, D.; Qin, N.; Wang, L. Comparative research on basic composition and processing properties of Inulin and Inulin Granules. Adv. Mater. Res. 2014, 850, 86–91. [Google Scholar] [CrossRef]
- Luo, D.; Liu, J.; Xu, W.; Xu, B.; Li, P. Gelatin properties of inulin in acidic solution. Trans. Chin. Soc. Agric. Mach. 2014, 45, 185–190. [Google Scholar]
- Florowska, A.; Hilal, A.; Florowski, T.; Wroniak, M. Addition of Selected Plant-Derived Proteins as Modifiers of Inulin Hydrogels Properties. Foods 2020, 9, 845. [Google Scholar] [CrossRef]
- Endres, J.G. Soy Protein Products Characteristics, Nutritional Aspects, and Utilization; AOCS Press: Champaign, IL, USA, 2001; Volume 29. [Google Scholar]
- Chen, H.; Gan, J.; Ji, A.; Song, S.; Yin, L. Development of double network gels based on soy protein isolate and sugarbeet pectin induced by thermal treatment and laccase catalysis. Food Chem. 2019, 292, 188–196. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, J.; Liu, Y.; Peng, F.; Wang, X.; Wang, C.; Li, M.; Xu, H. Gel properties and formation mechanism of soy protein isolate gels improved by wheat bran cellulose. Food Chem. 2020, 324, 126876. [Google Scholar] [CrossRef]
- Liu, M.; Chen, G.; Zhang, H.; Yu, Q.; Mei, X.; Kan, J. Heat-induced inulin-gluten gel: Insights into the influences of inulin molecular weight on the rheological and structural properties of gluten gel to molecular and physicochemical characteristics. Food Hydrocoll. 2021, 111, 106397. [Google Scholar] [CrossRef]
- Kim, Y.; Faqih, M.N.; Wang, S.S. Factors affecting gel formation of inulin. Carbohydr. Polym. 2001, 46, 135–145. [Google Scholar] [CrossRef]
- Alvarez-Sabatel, S.; de Marañón, I.M.; Arboleya, J.C. Impact of high pressure homogenisation (HPH) on inulin gelling properties, stability and development during storage. Food Hydrocoll. 2015, 44, 333–344. [Google Scholar] [CrossRef]
- Peyrano, F.; de Lamballerie, M.; Avanza, M.V.; Speroni, F. Gelation of cowpea proteins induced by high hydrostatic pressure. Food Hydrocoll. 2021, 11, 106191. [Google Scholar] [CrossRef]
- Florowska, A.; Florowski, T.; Sokołowska, B.; Adamczak, L.; Szymańska, I. Effects of Pressure Level and Time Treatment of High Hydrostatic Pressure (HHP) on Inulin Gelation and Properties of Obtained Hydrogels. Foods 2021, 10, 2514. [Google Scholar] [CrossRef]
- Nakul, U.; Roy, S.; Nalupurackal, G.; Chakraborty, S.; Siwach, P.; Goswami, J.; Privita, E.; Saumendra Kumar, B.; Rajesh, S.; Roy, B. Studying fluctuating trajectories of optically confined passive tracers inside cells provides familiar active forces. Biomed. Opt. Express 2023, 14, 5440–5450. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; de la Fuente, J.L.; Jover, E. Microrheology of food biopolymers: A review. Food Rev. Int. 2017, 28, 122–147. [Google Scholar]
- Abu-Thabit, N.H.; Khalil, I.A.; Mahmoud, Y.A. Inulin-based hydrogels: A review of recent progress in biocompatibility and biomedical applications. Carbohydr. Polym. 2014, 112, 407–417. [Google Scholar]
- Pasqua, L.; Aprea, P.; Avagliano, D.; Pepe, F.; Sorrentino, R. Hybrid gels for the removal of organic contaminants from water. J. Mater. Chem. A 2014, 2, 14436–14444. [Google Scholar]
- Guo, M.; Liu, G.; Liu, Y.; Xu, X. Effect of inulin on the physicochemical and structural properties of whey protein gels. Food Hydrocoll. 2018, 85, 162–171. [Google Scholar]
- Kim, Y.S.; Randolph, T.W.; Seefeldt, M.B.; Carpenter, J.F. High-Pressure Studies on Protein Aggregates and Amyloid Fibrils, Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2006; Volume 413, pp. 237–253. [Google Scholar]
- Masiá, C.; Ong, L.; Logan, A.; Stockmann, R.; Gambetta, J.; Jensen, P.E.; Yazdi, S.R.; Gras, S. Enhancing the textural and rheological properties of fermentation-induced pea protein emulsion gels with transglutaminase. Soft Matter 2024, 20, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Larrea-Wachtendorff, D.; Tabilo-Munizaga, G.; Moreno-Osorio, L.; Villalobos-Carvajal, R.; Pérez-Won, M. Protein changes caused by high hydrostatic pressure (HHP): A study using differential scanning calorimetry (DSC) and Fourier transform infrared (FTIR) spectroscopy. Food Eng. Rev. 2015, 7, 222–230. [Google Scholar] [CrossRef]
- Wang, W.; Yang, P.; Rao, L.; Zhao, L.; Wu, X.; Wang, Y.; Liao, X. Effect of high hydrostatic pressure processing on the structure, functionality, and nutritional properties of food proteins: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4640–4682. [Google Scholar] [CrossRef]
- Sun, X.D.; Holley, R.A. High hydrostatic pressure effects on the texture of meat and meat products. J. Food Sci. 2010, 75, R17–R23. [Google Scholar] [CrossRef]
- Cando, D.; Moreno, H.M.; Tovar, C.A.; Herranz, B.; Borderias, A.J. Effect of high pressure and/or temperature over gelation of isolated hake myofibrils. Food Bioprocess Technol. 2014, 7, 3197–3207. [Google Scholar] [CrossRef]
- Shinko, A. Structure and Morphology Control in Polymer Aerogels with Low Crosslink Density. Ph.D. Thesis, The University of Akron, Akron, OH, USA, 2015. [Google Scholar]
- De Maria, S.; Ferrari, G.; Maresca, P. Effects of high hydrostatic pressure on the conformational structure and the functional properties of bovine serum albumin. Innov. Food Sci. Emerg. Technol. 2016, 33, 67–75. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Tang, X.; Chen, Y.; You, Y. Chemical forces and water holding capacity study of heat-induced myofibrillar protein gel as affected by high pressure. Food Chem. 2015, 188, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, K.; Li, J.; Ma, L.; Chen, F.; Hu, X.; Ji, J. Effects of different high hydrostatic pressure treatments on gel formation of pea 7S and 11S globulins. Food Sci. 2024, 45, 153–160. [Google Scholar]
- Omar, A.K.; Wang, Z.-G. Shear-induced heterogeneity in associating polymer gels: Role of network structure and dilatancy. Phys. Rev. Lett. 2017, 119, 17801. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Hashemnejad, S.M.; Zabet, M.; Mishra, S. Self-assembly and mechanical properties of a triblock copolymer gel in a midblock selective solvent. ACS Symp. Ser. 2018, 1296, 157–197. [Google Scholar]
- Yang, C.-H.; Lin, Y.-Y. Surface wrinkles of swelling gels under arbitrary lateral confinements. Eur. J. Mech. A/Solids 2014, 45, 90–100. [Google Scholar] [CrossRef]
- Nelson, A.Z.; Wang, Y.; Wang, Y.; Margotta, A.S.; Sammler, R.L.; Izmitli, A.; Katz, J.S.; Curtis-Fisk, J.; Li, Y.; Ewoldt, R.H. Gelation under stress: Impact of shear flow on the formation and mechanical properties of methylcellulose hydrogels. Soft Matter 2022, 18, 1554–1565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Fan, Q.; Li, H.; Zheng, J. Preparation of konjac glucomannan/casein blending gels optimized by response surface methodology and assessment of the effects of high-pressure processing on their gel properties and structure. J. Sci. Food Agric. 2018, 98, 4160–4167. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Powers, J.R. Effects of high pressure on food proteins. In High Pressure Processing of Food; Balasubramaniam, V., Barbosa-Cánovas, G., Lelieveld, H., Eds.; Food Engineering Series; Springer: New York, NY, USA, 2016; pp. 353–389. [Google Scholar]
- Sun, B.; Gu, X.; Wang, F.; Liu, L.; Huang, Y.; Gao, Y.; Lü, M.; Zhu, Y.; Shi, Y.; Zhu, X. Effect of high-pressure homogenization on Ca2+-induced gel formation of soybean 11 S globulin. J. Sci. Food Agric. 2023, 103, 2057–2069. [Google Scholar] [CrossRef]
- Liu, P.-L.; Ren, R.-L.; Bao, Y.-L.; Ning, H.-M.; Wang, X.-L.; Li, Y.-J. Effect of high hydrostatic pressure as a physical modification method on waxy maize starch gelatinization and retrogradation. Chin. J. High Press. Phys. 2014, 28, 247–256. [Google Scholar]
- Ji, Z.; Yu, L.; Liu, H.; Bao, X.; Wang, Y.; Chen, L. Effect of pressure with shear stress on gelatinization of starches with different amylose/amylopectin ratios. Food Hydrocoll. 2017, 72, 331–337. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Mao, L.; Gao, Y.; Yuan, F. Modification of the structural and rheological properties of β-lactoglobulin/κ-carrageenan mixed gels induced by high pressure processing. J. Food Eng. 2020, 274, 109851. [Google Scholar] [CrossRef]
- Zhang, S.; Han, J.; Chen, L. Fabrication of pea protein gels with modulated rheological properties using high pressure processing. Food Hydrocoll. 2023, 144, 109002. [Google Scholar] [CrossRef]
- Wang, L.; Moraru, C.I. High-pressure structuring of milk protein concentrate: Effect of pH and calcium. J. Dairy Sci. 2021, 104, 4074–4083. [Google Scholar] [CrossRef]
- Li, X.; Mao, L.; He, X.; Ma, P.; Gao, Y.; Yuan, F. Characterization of β-lactoglobulin gels induced by high pressure processing. Innov. Food Sci. Emerg. Technol. 2018, 47, 335–345. [Google Scholar] [CrossRef]
- Ramaswamy, H.S.; Gundurao, A. Effect of soluble solids and high pressure treatment on rheological properties of protein enriched mango puree. Foods 2019, 8, 39. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Qiu, Y.; Fan, X.; Zhang, L.; Yu, Q. Valorization of cattle slaughtering industry by-products: Modification of the functional properties and structural characteristics of cowhide gelatin induced by high hydrostatic pressure. Gels 2022, 8, 243. [Google Scholar] [CrossRef]
- Shim, G.-S.; Kim, H.-J.; Bartkowiak, M. Curing behavior and impact of crosslinking agent variation in stepwise UV/UV cured acrylic pressure-sensitive adhesives. J. Mater. Res. Technol. 2021, 15, 1622–1629. [Google Scholar] [CrossRef]
- Yagi, A.; Honma, M.; Ito, R.; Nose, T. Optical Properties of Nematic Liquid Crystal/ Polyfluorene Gel Devices. Proc. Int. Disp. Workshops 2022, 29, 101–103. [Google Scholar] [CrossRef]
- Truong, B.Q.; Buckow, R.; Nguyen, M. Mechanical and Functional Properties of Unwashed Barramundi (Lates calcarifer) Gels as Affected by High-Pressure Processing at three Different Temperatures and Salt Concentrations. J. Aquat. Food Prod. Technol. 2020, 29, 373–382. [Google Scholar] [CrossRef]
- Wenqiong, W.; Pei-pei, Y.; Ji-yang, Z.; Zhi-hang, G. Effect of temperature and pH on the gelation, rheology, texture, and structural properties of whey protein and sugar gels based on Maillard reaction. J. Food Sci. 2021, 86, 1228–1242. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Colour Difference ∆E—A Survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Wang, X.S.; Tang, C.H.; Li, B.S.; Yang, X.Q.; Li, L.; Ma, C.Y. Effects of high-pressure treatment on some physicochemical and functional properties of soy protein isolates. Food Hydrocoll. 2008, 22, 560–567. [Google Scholar] [CrossRef]
- Szymańska, I.; Żbikowska, A.; Onacik-Gür, S. Candelilla Wax-Based Oleogels versus Palm Oil: Evaluation of Physical Properties of Innovative and Conventional Lipids Using Optical Techniques. J. Sci. Food Agric. 2021, 102, 2309–2320. [Google Scholar] [CrossRef]










| Parameters | 3 SPI | 6 SPI | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 150 MPa | 300 MPa | 500 MPa | 150 MPa | 300 MPa | 500 MPa | ||||||||||||||
| 5 min | 10 min | 20 min | 5 min | 10 min | 20 min | 5 min | 10 min | 20 min | 5 min | 10 min | 20 min | 5 min | 10 min | 20 min | 5 min | 10 min | |||
| 6 SPI | 500 MPa | 20 min | 8.7 | 8.2 | 8.8 | 8.7 | 8.8 | 10.0 | 9.5 | 9.1 | 9.3 | 1.0 | 1.0 | 0.9 | 1.3 | 0.9 | 1.4 | 0.6 | 0.5 |
| 10 min | 9.2 | 8.6 | 9.3 | 9.2 | 9.3 | 10.4 | 10.0 | 9.6 | 9.8 | 0.8 | 0.8 | 0.7 | 1.2 | 0.8 | 1.3 | 0.5 | |||
| 5 min | 9.1 | 8.5 | 9.2 | 9.1 | 9.2 | 10.4 | 9.9 | 9.5 | 9.7 | 0.7 | 0.6 | 0.5 | 1.0 | 0.5 | 1.4 | ||||
| 300 MPa | 20 min | 8.5 | 7.9 | 8.6 | 8.4 | 8.5 | 9.7 | 9.4 | 9.0 | 9.2 | 0.7 | 1.0 | 1.1 | 0.7 | 1.1 | ||||
| 10 min | 9.0 | 8.4 | 9.1 | 9.0 | 9.1 | 10.3 | 9.9 | 9.5 | 9.7 | 0.4 | 0.2 | 0.3 | 0.6 | ||||||
| 5 min | 8.9 | 8.3 | 9.0 | 8.8 | 8.9 | 10.2 | 9.8 | 9.4 | 9.6 | 0.4 | 0.5 | 0.6 | |||||||
| 150 MPa | 20 min | 9.2 | 8.6 | 9.3 | 9.1 | 9.3 | 10.5 | 10.0 | 9.6 | 9.8 | 0.4 | 0.2 | |||||||
| 10 min | 9.1 | 8.5 | 9.2 | 9.1 | 9.2 | 10.4 | 10.0 | 9.6 | 9.8 | 0.3 | |||||||||
| 5 min | 8.9 | 8.3 | 9.0 | 8.8 | 8.9 | 10.2 | 9.7 | 9.3 | 9.5 | ||||||||||
| 3 SPI | 500 MPa | 20 min | 0.9 | 1.6 | 0.9 | 1.6 | 1.2 | 1.3 | 0.5 | 0.2 | |||||||||
| 10 min | 0.8 | 1.5 | 0.8 | 1.5 | 1.1 | 1.4 | 0.6 | ||||||||||||
| 5 min | 1.2 | 1.9 | 1.3 | 1.9 | 1.5 | 1.2 | |||||||||||||
| 300 MPa | 20 min | 1.5 | 2.1 | 1.4 | 1.8 | 1.4 | |||||||||||||
| 10 min | 0.4 | 0.7 | 0.4 | 0.5 | |||||||||||||||
| 5 min | 0.8 | 0.6 | 0.7 | ||||||||||||||||
| 150 MPa | 20 min | 0.2 | 0.8 | ||||||||||||||||
| 10 min | 0.7 | ||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florowska, A.; Florowski, T.; Goździk, P.; Hilal, A.; Florowska, H.; Janiszewska-Turak, E. The Effect of High Hydrostatic Pressure (HHP) Induction Parameters on the Formation and Properties of Inulin–Soy Protein Hydrogels. Gels 2024, 10, 570. https://doi.org/10.3390/gels10090570
Florowska A, Florowski T, Goździk P, Hilal A, Florowska H, Janiszewska-Turak E. The Effect of High Hydrostatic Pressure (HHP) Induction Parameters on the Formation and Properties of Inulin–Soy Protein Hydrogels. Gels. 2024; 10(9):570. https://doi.org/10.3390/gels10090570
Chicago/Turabian StyleFlorowska, Anna, Tomasz Florowski, Patrycja Goździk, Adonis Hilal, Hanna Florowska, and Emilia Janiszewska-Turak. 2024. "The Effect of High Hydrostatic Pressure (HHP) Induction Parameters on the Formation and Properties of Inulin–Soy Protein Hydrogels" Gels 10, no. 9: 570. https://doi.org/10.3390/gels10090570
APA StyleFlorowska, A., Florowski, T., Goździk, P., Hilal, A., Florowska, H., & Janiszewska-Turak, E. (2024). The Effect of High Hydrostatic Pressure (HHP) Induction Parameters on the Formation and Properties of Inulin–Soy Protein Hydrogels. Gels, 10(9), 570. https://doi.org/10.3390/gels10090570










