Key Factors Influencing Gelation in Plant vs. Animal Proteins: A Comparative Mini-Review
Abstract
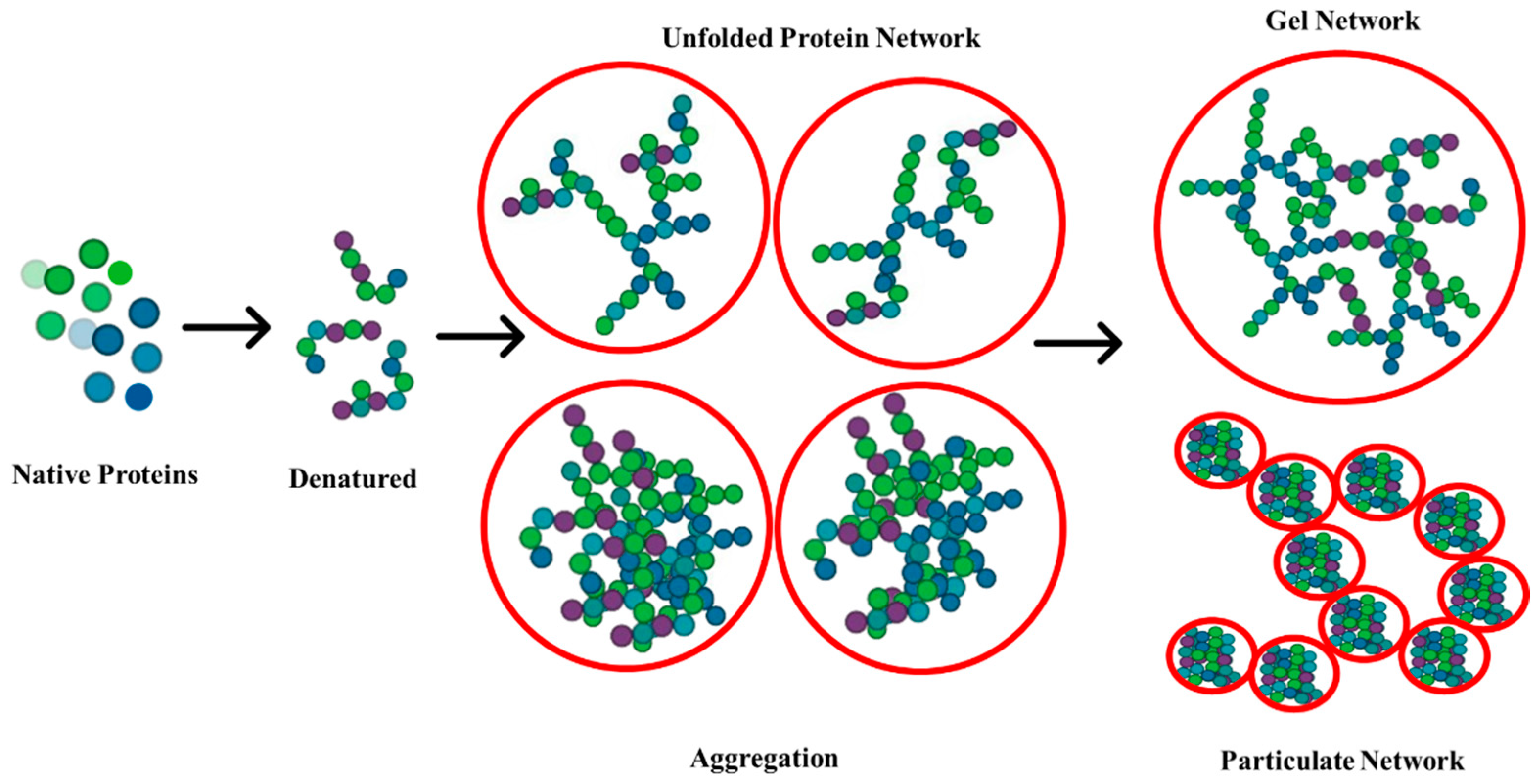
1. Introduction
2. Effect of pH on Gelation Properties
2.1. Animal Proteins
2.2. Plant Proteins
3. Effect of Ionic Environment on Gelation Properties
3.1. Animal Proteins
3.2. Plant Proteins
4. Effect of Temperature on Gelation Properties
4.1. Animal Proteins
4.2. Plant Proteins
5. Effect of Anti-Nutritional Factors on Gelation Properties
Plant Proteins
6. Effect of Blends on Gelation Properties
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mæhre, H.; Dalheim, L.; Edvinsen, G.; Elvevoll, E.; Jensen, I.-J. Protein Determination—Method Matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef]
- Kramer, R.M.; Shende, V.R.; Motl, N.; Pace, C.N.; Scholtz, J.M. Toward a Molecular Understanding of Protein Solubility: Increased Negative Surface Charge Correlates with Increased Solubility. Biophys. J. 2012, 102, 1907–1915. [Google Scholar] [CrossRef]
- Bröckel, U.; Meier, W.; Wagner, G. (Eds.) Product Design and Engineering; Wiley: Hoboken, NJ, USA, 2013; ISBN 9783527332205. [Google Scholar]
- Chen, N.; Zhao, M.; Niepceron, F.; Nicolai, T.; Chassenieux, C. The Effect of the PH on Thermal Aggregation and Gelation of Soy Proteins. Food Hydrocoll. 2017, 66, 27–36. [Google Scholar] [CrossRef]
- Zayas, J.F. Functionality of Proteins in Food; Springer: Berlin/Heidelberg, Germany, 1997; ISBN 978-3-642-63856-5. [Google Scholar]
- Xiao, X.; Zou, P.-R.; Hu, F.; Zhu, W.; Wei, Z.-J. Updates on Plant-Based Protein Products as an Alternative to Animal Protein: Technology, Properties, and Their Health Benefits. Molecules 2023, 28, 4016. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Pandey, A.K.; Verma, K.; Shrivastava, A.; Singh, N. Plant-based Protein as an Alternative to Animal Proteins: A Review of Sources, Extraction Methods and Applications. Int. J. Food Sci. Technol. 2024, 59, 488–497. [Google Scholar] [CrossRef]
- Floret, C.; Monnet, A.F.; Micard, V.; Walrand, S.; Michon, C. Replacement of Animal Proteins in Food: How to Take Advantage of Nutritional and Gelling Properties of Alternative Protein Sources. Crit. Rev. Food Sci. Nutr. 2023, 63, 920–946. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, F. Plant Protein Heat-Induced Gels: Formation Mechanisms and Regulatory Strategies. Coatings 2023, 13, 1899. [Google Scholar] [CrossRef]
- Zhao, Z.-K.; Mu, T.-H.; Zhang, M.; Richel, A. Chemical Forces, Structure, and Gelation Properties of Sweet Potato Protein as Affected by PH and High Hydrostatic Pressure. Food Bioprocess Technol. 2018, 11, 1719–1732. [Google Scholar] [CrossRef]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food Proteins from Animals and Plants: Differences in the Nutritional and Functional Properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Kazemi-Taskooh, Z.; Varidi, M. How Can Plant-Based Protein–Polysaccharide Interactions Affect the Properties of Binary Hydrogels? (A Review). Food Funct. 2023, 14, 5891–5909. [Google Scholar] [CrossRef] [PubMed]
- Sagis, L.M.; Yang, J. Protein-Stabilized Interfaces in Multiphase Food: Comparing Structure-Function Relations of Plant-Based and Animal-Based Proteins. Curr. Opin. Food Sci. 2022, 43, 53–60. [Google Scholar] [CrossRef]
- Van Kleef, F.S.M. Thermally Induced Protein Gelation: Gelation and Rheological Characterization of Highly Concentrated Ovalbumin and Soybean Protein Gels. Biopolymers 1986, 25, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Betz, M.; Hörmansperger, J.; Fuchs, T.; Kulozik, U. Swelling Behaviour, Charge and Mesh Size of Thermal Protein Hydrogels as Influenced by PH during Gelation. Soft Matter 2012, 8, 2477. [Google Scholar] [CrossRef]
- WANG, S.F.; SMITH, D.M.; STEFFE, J.F. Effect of PH on the Dynamic Rheological Properties of Chicken Breast Salt-Soluble Proteins during Heat-Induced Gelation. Poult. Sci. 1990, 69, 2220–2227. [Google Scholar] [CrossRef]
- Muschiolik, G. Methods of Testing Protein Functionality. Edited by G. M. Hall. XII and 265 Pages, Numerous Figures and Tables. Blackie Academic & Professional, London 1996. Price: 55.-£. Food/Nahrung 1997, 41, 55. [Google Scholar] [CrossRef]
- O’Kennedy, B.T.; Mounsey, J.S.; Murphy, F.; Duggan, E.; Kelly, P.M. Factors Affecting the Acid Gelation of Sodium Caseinate. Int. Dairy J. 2006, 16, 1132–1141. [Google Scholar] [CrossRef]
- Thomar, P.; Nicolai, T. Heat-Induced Gelation of Casein Micelles in Aqueous Suspensions at Different PH. Colloids Surfaces B Biointerfaces 2016, 146, 801–807. [Google Scholar] [CrossRef]
- Moreira, T.C.P.; Pereira, R.N.; Vicente, A.A.; da Cunha, R.L. Effect of Ohmic Heating on Functionality of Sodium Caseinate—A Relationship with Protein Gelation. Food Res. Int. 2019, 116, 628–636. [Google Scholar] [CrossRef]
- Lorenzen, P.C.; Schrader, K. A Comparative Study of the Gelation Properties of Whey Protein Concentrate and Whey Protein Isolate. Lait 2006, 86, 259–271. [Google Scholar] [CrossRef]
- Farrokhi, F.; Ehsani, M.R.; Badii, F.; Hashemi, M. Effect of PH on Structural Properties of Heat-Induced Whey Protein Gels. J. Food Biosci. Technol. 2020, 10, 57–68. [Google Scholar]
- Monahan, F.J.; German, J.B.; Kinsella, J.E. Effect of PH and Temperature on Protein Unfolding and Thiol/Disulfide Interchange Reactions during Heat-Induced Gelation of Whey Proteins. J. Agric. Food Chem. 1995, 43, 46–52. [Google Scholar] [CrossRef]
- Rafiee Tari, N.; Gaygadzhiev, Z.; Guri, A.; Wright, A. Effect of PH and Heat Treatment Conditions on Physicochemical and Acid Gelation Properties of Liquid Milk Protein Concentrate. J. Dairy Sci. 2021, 104, 6609–6619. [Google Scholar] [CrossRef] [PubMed]
- Puyol, P.; Pérez, M.; Horne, D. Heat-Induced Gelation of Whey Protein Isolates (WPI): Effect of NaCl and Protein Concentration. Food Hydrocoll. 2001, 15, 233–237. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I.; Smidsrød, O. Physical and Rheological Properties of Fish Gelatin Compared to Mammalian Gelatin. Food Hydrocoll. 2004, 18, 203–213. [Google Scholar] [CrossRef]
- Glorieux, S.; Steen, L.; Paelinck, H.; Foubert, I.; Fraeye, I. Isothermal Gelation Behavior of Myofibrillar Proteins from White and Red Chicken Meat at Different Temperatures. Poult. Sci. 2017, 96, 3785–3795. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bohidar, H.B. Effect of Salt and Temperature on Viscoelasticity of Gelatin Hydrogels. J. Surf. Sci. Technol. 2006, 22, 1–13. [Google Scholar]
- Croguennec, T.; Nau, F.; Brulé, G. Influence of PH and Salts on Egg White Gelation. J. Food Sci. 2002, 67, 608–614. [Google Scholar] [CrossRef]
- Khemakhem, M.; Attia, H.; Ayadi, M.A. The Effect of PH, Sucrose, Salt and Hydrocolloid Gums on the Gelling Properties and Water Holding Capacity of Egg White Gel. Food Hydrocoll. 2019, 87, 11–19. [Google Scholar] [CrossRef]
- Raikos, V.; Campbell, L.; Euston, S.R. Rheology and Texture of Hen’s Egg Protein Heat-Set Gels as Affected by PH and the Addition of Sugar and/or Salt. Food Hydrocoll. 2007, 21, 237–244. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, M.; Chassenieux, C.; Nicolai, T. The Effect of Adding NaCl on Thermal Aggregation and Gelation of Soy Protein Isolate. Food Hydrocoll. 2017, 70, 88–95. [Google Scholar] [CrossRef]
- Felix, M.; Perez-Puyana, V.; Romero, A.; Guerrero, A. Development of Thermally Processed Bioactive Pea Protein Gels: Evaluation of Mechanical and Antioxidant Properties. Food Bioprod. Process. 2017, 101, 74–83. [Google Scholar] [CrossRef]
- Tanger, C.; Müller, M.; Andlinger, D.; Kulozik, U. Influence of PH and Ionic Strength on the Thermal Gelation Behaviour of Pea Protein. Food Hydrocoll. 2022, 123, 106903. [Google Scholar] [CrossRef]
- Fernández Sosa, E.I.; Chaves, M.G.; Peyrano, F.; Quiroga, A.V.; Avanza, M.V. Thermal Gelation of Proteins from Cajanus Cajan Influenced by PH and Ionic Strength. Plant Foods Hum. Nutr. 2023, 78, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Arntfield, S.D. Dynamic Oscillatory Rheological Measurement and Thermal Properties of Pea Protein Extracted by Salt Method: Effect of PH and NaCl. J. Food Eng. 2011, 105, 577–582. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Gelation Properties of Salt-Extracted Pea Protein Induced by Heat Treatment. Food Res. Int. 2010, 43, 509–515. [Google Scholar] [CrossRef]
- Idris, W.H.; Babiker, E.E.; El Tinay, A.H. Fractionation, Solubility and Functional Properties of Wheat Bran Proteins as Influenced by PH and/or Salt Concentration. Food/Nahrung 2003, 47, 425–429. [Google Scholar] [CrossRef]
- Wang, K.-Q.; Luo, S.-Z.; Zhong, X.-Y.; Cai, J.; Jiang, S.-T.; Zheng, Z. Changes in Chemical Interactions and Protein Conformation during Heat-Induced Wheat Gluten Gel Formation. Food Chem. 2017, 214, 393–399. [Google Scholar] [CrossRef]
- Kim, J.H.; Varankovich, N.V.; Stone, A.K.; Nickerson, M.T. Nature of Protein-Protein Interactions during the Gelation of Canola Protein Isolate Networks. Food Res. Int. 2016, 89, 408–414. [Google Scholar] [CrossRef]
- He, R.; He, H.-Y.; Chao, D.; Ju, X.; Aluko, R. Effects of High Pressure and Heat Treatments on Physicochemical and Gelation Properties of Rapeseed Protein Isolate. Food Bioprocess Technol. 2014, 7, 1344–1353. [Google Scholar] [CrossRef]
- Westphalen, A.D.; Briggs, J.L.; Lonergan, S.M. Influence of PH on Rheological Properties of Porcine Myofibrillar Protein during Heat Induced Gelation. Meat Sci. 2005, 70, 293–299. [Google Scholar] [CrossRef]
- Renkema, J.M.S.; Gruppen, H.; van Vliet, T. Influence of PH and Ionic Strength on Heat-Induced Formation and Rheological Properties of Soy Protein Gels in Relation to Denaturation and Their Protein Compositions. J. Agric. Food Chem. 2002, 50, 6064–6071. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.J.; Varankovich, N.V.; Nickerson, M.T. The Effect of PH on the Gelling Behaviour of Canola and Soy Protein Isolates. Food Res. Int. 2016, 81, 31–38. [Google Scholar] [CrossRef]
- Kinekawa, Y.-I.; Fuyuki, T.; Kitabatake, N. Effects of Salts on the Properties of Sols and Gels Prepared from Whey Protein Isolate and Process Whey Protein. J. Dairy Sci. 1998, 81, 1532–1544. [Google Scholar] [CrossRef]
- Kuhn, P.R.; Foegeding, E.A. Mineral Salt Effects on Whey Protein Gelation. J. Agric. Food Chem. 1991, 39, 1013–1016. [Google Scholar] [CrossRef]
- Kaspchak, E.; Silveira, J.L.M.; Igarashi-Mafra, L.; Mafra, M.R. Effect of Antinutrients on Heat-Set Gelation of Soy, Pea, and Rice Protein Isolates. J. Food Sci. Technol. 2020, 57, 4201–4210. [Google Scholar] [CrossRef]
- Thakur, A.; Sharma, V.; Thakur, A. An Overview of Anti-Nutritional Factors in Food. Int. J. Chem. Stud. 2019, 7, 2472–2479. [Google Scholar]
- Jeschke, V.; Gershenzon, J.; Vassão, D.G. A Mode of Action of Glucosinolate-Derived Isothiocyanates: Detoxification Depletes Glutathione and Cysteine Levels with Ramifications on Protein Metabolism in Spodoptera Littoralis. Insect Biochem. Mol. Biol. 2016, 71, 37–48. [Google Scholar] [CrossRef]
- Rubino, M.I.; Arntfield, S.D.; Nadon, C.A.; Bernatsky, A. Phenolic Protein Interactions in Relation to the Gelation Properties of Canola Protein. Food Res. Int. 1996, 29, 653–659. [Google Scholar] [CrossRef]
- Sarwar Gilani, G.; Wu Xiao, C.; Cockell, K.A. Impact of Antinutritional Factors in Food Proteins on the Digestibility of Protein and the Bioavailability of Amino Acids and on Protein Quality. Br. J. Nutr. 2012, 108, S315–S332. [Google Scholar] [CrossRef]
- Alves, A.C.; Tavares, G.M. Mixing Animal and Plant Proteins: Is This a Way to Improve Protein Techno-Functionalities? Food Hydrocoll. 2019, 97, 105171. [Google Scholar] [CrossRef]
- Comfort, S.; Howell, N.K. Gelation Properties of Soya and Whey Protein Isolate Mixtures. Food Hydrocoll. 2002, 16, 661–672. [Google Scholar] [CrossRef]
- Roesch, R.R.; Corredig, M. Heat-Induced Soy−Whey Proteins Interactions: Formation of Soluble and Insoluble Protein Complexes. J. Agric. Food Chem. 2005, 53, 3476–3482. [Google Scholar] [CrossRef] [PubMed]
- Ben-Harb, S.; Panouillé, M.; Huc-Mathis, D.; Moulin, G.; Saint-Eve, A.; Irlinger, F.; Bonnarme, P.; Michon, C.; Souchon, I. The Rheological and Microstructural Properties of Pea, Milk, Mixed Pea/Milk Gels and Gelled Emulsions Designed by Thermal, Acid, and Enzyme Treatments. Food Hydrocoll. 2018, 77, 75–84. [Google Scholar] [CrossRef]
- Silva, J.V.C.; Cochereau, R.; Schmitt, C.; Chassenieux, C.; Nicolai, T. Heat-Induced Gelation of Mixtures of Micellar Caseins and Plant Proteins in Aqueous Solution. Food Res. Int. 2019, 116, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Chen, Q.; He, N.; Wang, Q. High-Moisture Extrusion of Mixed Proteins from Soy and Surimi: Effect of Protein Gelling Properties on the Product Quality. Foods 2022, 11, 1397. [Google Scholar] [CrossRef]
| Protein | Parameter | Effect | Reference |
|---|---|---|---|
Casein | pH | No effect | [19] |
| Temperature | Heating → greater syneresis | [20] | |
| Ionic env. * | Gel formation at lower pH with NaCl added than without | [18] | |
Whey | pH | Lowest at pI, optimum pH variable | [21,22] |
| Temperature | Heating → increased polymerization 85 °C for 5 min: ↑ whey protein denaturation, ↑ viscosity, ↑ gel firmness 125 °C for 15 s: ↓ whey protein denaturation, ↓ viscosity, ↓ gel firmness | [23] [24] | |
| Ionic env. | Less protein required to form a gel with 100 mM NaCl added | [25] | |
Meat | pH | ↑ pH ↓ G′ | [26] |
| Temperature | ↑ Temp ↑ G′ | [27] | |
| Ionic env. | ↑ NaCl ↓ G′ | [28] | |
Egg | pH | Coarse gel at pI, optimum at pH 7 and 9 ↓ pH near 4.5 (isoelectric point of ovalbumin): ↓ gel firmness, ↓ WHC **, brittle gel formation pH 6.5–8.0: better gel structure | [29] [30] |
| Temperature | ↑ pH ↑ gelation temp | [31] | |
| Ionic env. | ↑ NaCl ↓ hardness (EW, WE), ↑ NaCl ↑ hardness (EY), ↑ NaCl ↑ gelation time ↑ Firmness, ↑ elasticity, ↓ WHC | [31] [30] | |
Soy | pH | No effect on G′, ↑ gel rate ↓ pH | [32] |
| Temperature | No significant difference | [4] | |
| Ionic env. | No effect on stiffness, ↑ NaCl ↑ gelation time | [32] | |
Pea | pH | ↑ pH ↑ G′ Stiffest gels at pH 4.5 with 0.6 M NaCl Gel stiffness ↓ at higher pH ↓ pH 2.1, 3.9: ↓ WHC, ↓ gelation ↑ pH 6.3, 8.3: ↑ WHC, ↑ firmness | [33] [34] [35] |
| Temperature | ↑ pH ↑ gelation temp, ↑ temp ↓ G′ No significant gelation beyond 95 °C | [36,37] [34] | |
| Ionic env. | No effect on stiffness, ↑ NaCl ↑ gelation time ↑ Ionic strength →↑ denaturation temp ↑ Ionic strength →↑ gel formation | [36] [34] [35] | |
Wheat | pH | No gel formation without NaCl | [38] |
| Temperature | ↑ Temp ↓ G′ (to 60 °C), ↑ Temp ↑ G′ (60–90 °C) | [39] | |
| Ionic env. | ↑ NaCl ↑ gelation capacity | [38] | |
Oilseed | pH | No gel at pH 3, ↑ pH ↑ G′ (max at pH 9) | [40] |
| Temperature | ↑ Temp ↑ hardness, springiness, etc. | [41] | |
| Ionic env. | No effect | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalesi, M.; Glenn-Davi, K.; Mohammadi, N.; FitzGerald, R.J. Key Factors Influencing Gelation in Plant vs. Animal Proteins: A Comparative Mini-Review. Gels 2024, 10, 575. https://doi.org/10.3390/gels10090575
Khalesi M, Glenn-Davi K, Mohammadi N, FitzGerald RJ. Key Factors Influencing Gelation in Plant vs. Animal Proteins: A Comparative Mini-Review. Gels. 2024; 10(9):575. https://doi.org/10.3390/gels10090575
Chicago/Turabian StyleKhalesi, Mohammadreza, Kyeesha Glenn-Davi, Nima Mohammadi, and Richard J. FitzGerald. 2024. "Key Factors Influencing Gelation in Plant vs. Animal Proteins: A Comparative Mini-Review" Gels 10, no. 9: 575. https://doi.org/10.3390/gels10090575
APA StyleKhalesi, M., Glenn-Davi, K., Mohammadi, N., & FitzGerald, R. J. (2024). Key Factors Influencing Gelation in Plant vs. Animal Proteins: A Comparative Mini-Review. Gels, 10(9), 575. https://doi.org/10.3390/gels10090575










