Preparation of Composite Hydrogels Based on Cysteine–Silver Sol and Methylene Blue as Promising Systems for Anticancer Photodynamic Therapy
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of Gels with Methylene Blue
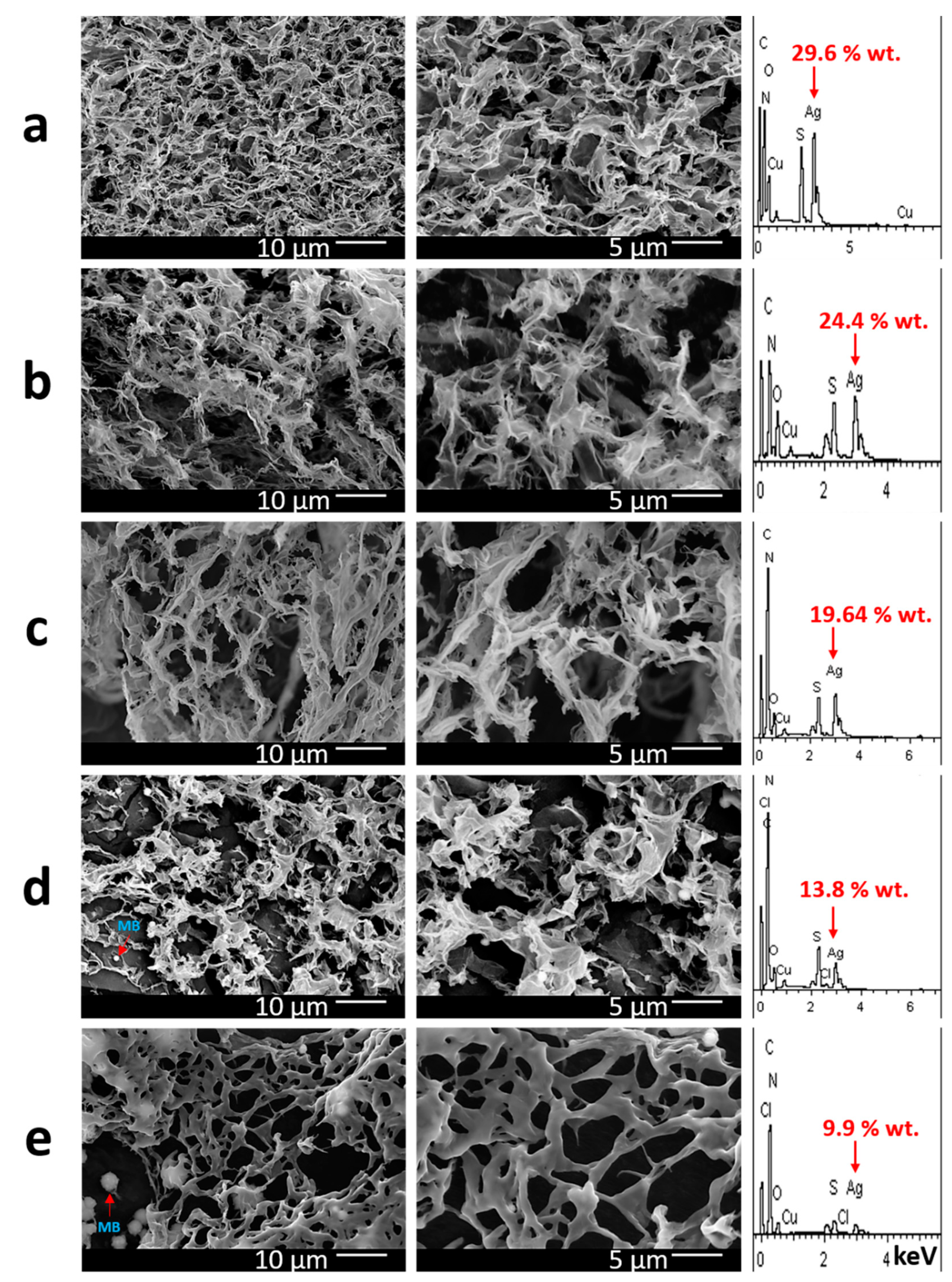
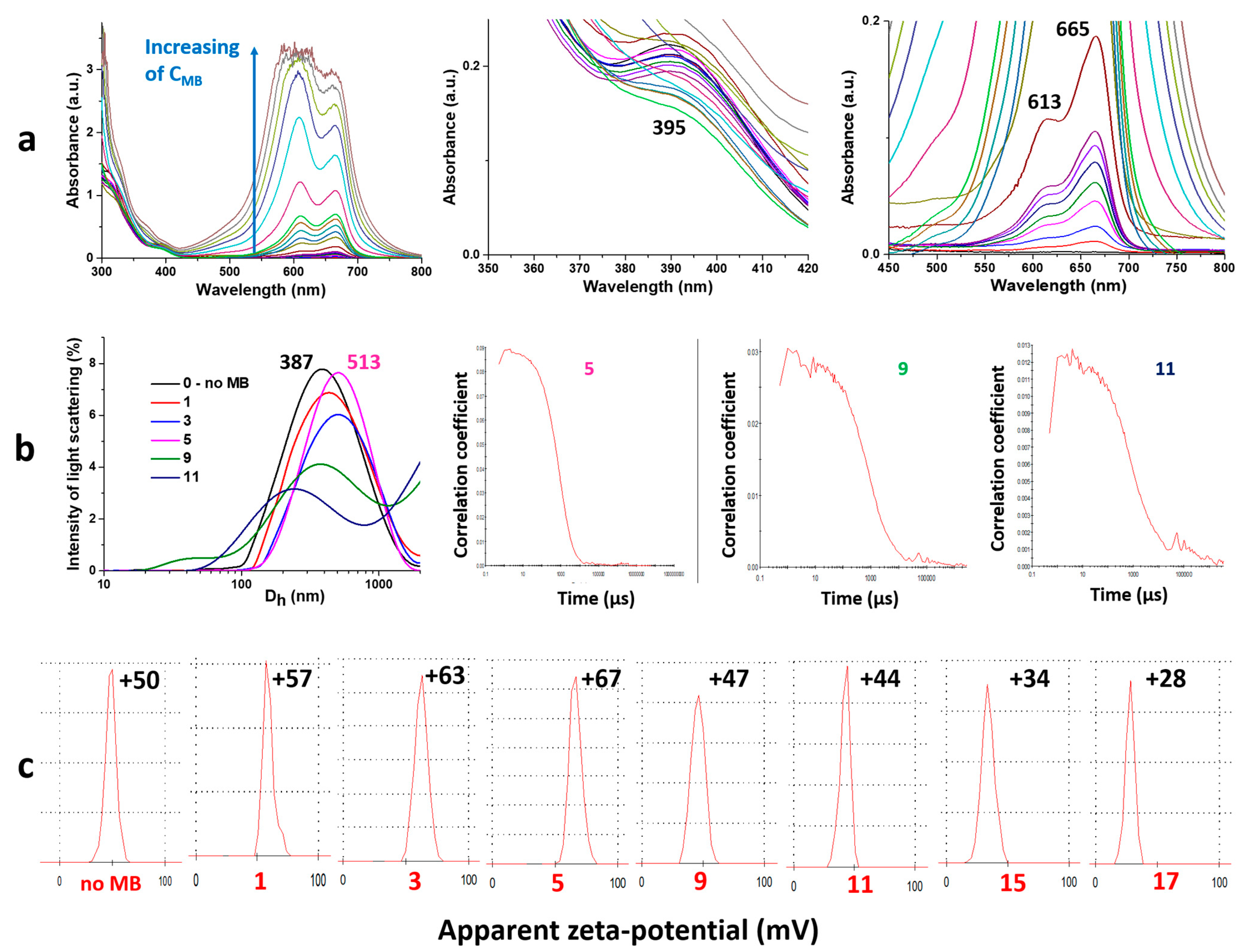
2.2. Physico-Chemical Analysis of Gel Formation
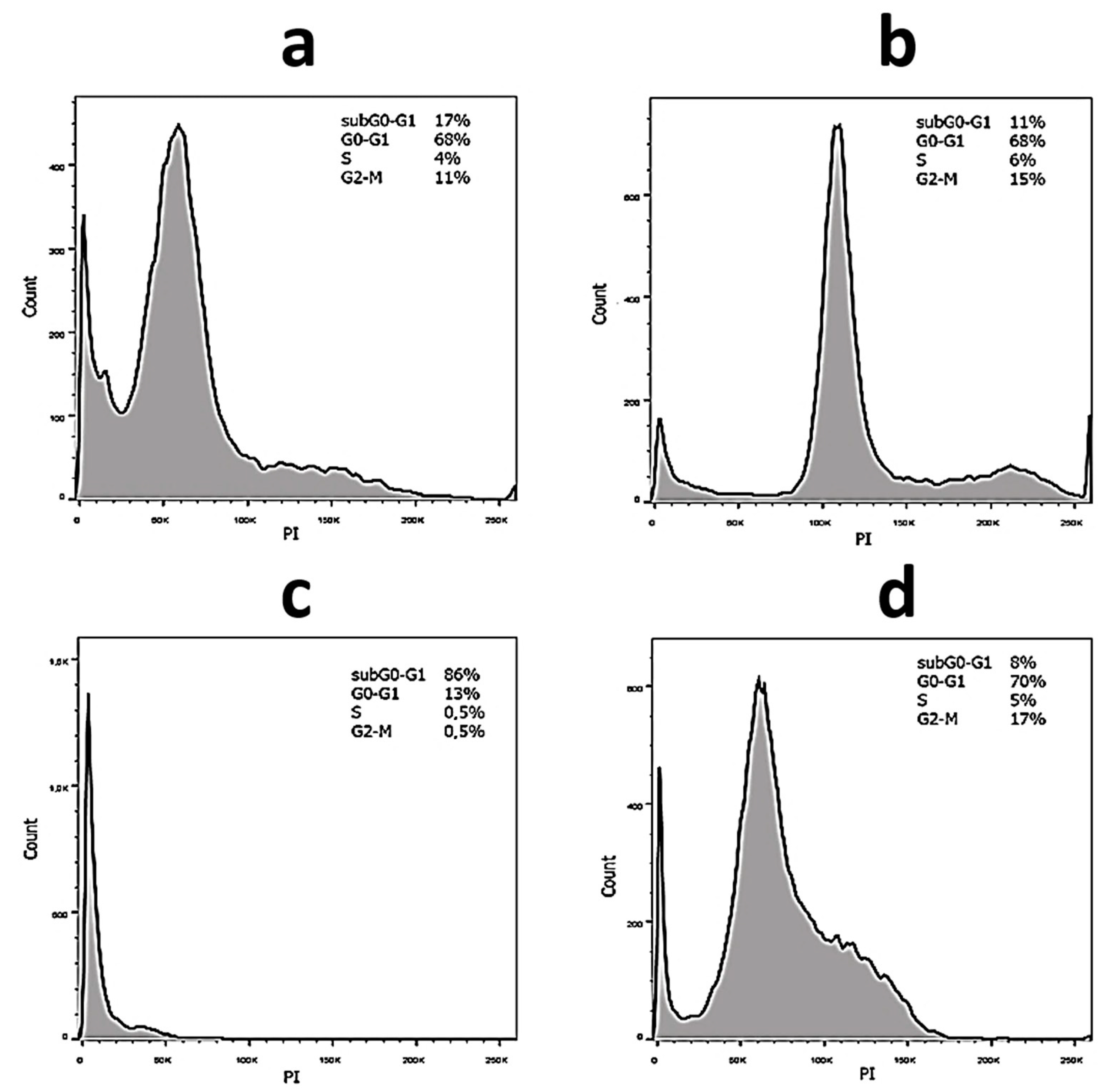
2.3. Anticancer Activity
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Synthetic Protocol of Gel Preparation with Methylene Blue
4.3. Viscosimetry
4.4. Scanning Electron Microscopy and Energy-Dispersive X-ray Spectroscopy
4.5. UV Spectroscopy
4.6. Dynamic Light Scattering
4.7. Electrophoretic Light Scattering
4.8. Cells and Culture
4.9. Cell Photodynamic Treatment
4.10. Cytotoxicity Evaluation (MTT Test)
4.11. ROS Detection by 2′,7′-Dichlorodihydrofluorescein Diacetate (H2DCFDA)
4.12. The Cell Cycle Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raheem, Y.A. Unveiling the significance and challenges of integrating prevention levels in healthcare practice. J. Prim. Care Community Health 2023, 14, 21501319231186500. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Warszawik-Hendzel, O.; Olszewska, M.; Maj, M.; Rakowska, A.; Czuwara, J.; Rudnicka, L. Non-invasive diagnostic techniques in the diagnosis of squamous cell carcinoma. J. Dermatol. Case Rep. 2015, 9, 89–97. [Google Scholar] [CrossRef]
- Alam, M.; Armstrong, A.; Baum, C.; Bordeaux, J.S.; Brown, M.; Busam, K.J.; Eisen, D.B.; Iyengar, V.; Lober, C.; Margolis, D.J.; et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 560–578. [Google Scholar]
- Dang, J.; He, H.; Chen, D.; Yin, L. Manipulating tumor hypoxia toward enhanced photodynamic therapy (PDT). Biomater. Sci. 2017, 5, 1500–1511. [Google Scholar] [CrossRef]
- Dichiara, M.; Prezzavento, O.; Marrazzo, A.; Pittalà, V.; Salerno, L.; Rescifina, A.; Amata, E. Recent advances in drug discovery of phototherapeutic non-porphyrinic anticancer agents. Eur. J. Med. Chem. 2017, 142, 459–485. [Google Scholar] [CrossRef]
- Monro, S.; Colon, K.L.; Yin, H.; Roque, J.; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition metal complexes and photodynamic therapy from a tumor-centered approach: Challenges, opportunities, and highlights from the development of TLD1433. Chem. Rev. 2019, 119, 797–828. [Google Scholar] [CrossRef]
- Ryan, R.T.; Stevens, K.C.; Calabro, R.; Parkin, S.; Mahmoud, J.; Kim, D.Y.; Heidary, D.K.; Glazer, E.C.; Selegue, J.P. Bis-tridentate N-Heterocyclic Carbene Ru(II) Complexes are Promising New Agents for Photodynamic Therapy. Inorg. Chem. 2020, 59, 8882–8892. [Google Scholar] [CrossRef]
- Huang, H.Y.; Banerjee, S.; Sadler, P.J. Recent advances in the design of targeted iridium (III) photosensitizers for photodynamic therapy. ChemBioChem 2018, 19, 1574–1589. [Google Scholar] [CrossRef]
- Novohradsky, V.; Rovira, A.; Hally, C.; Galindo, A.; Vigueras, G.; Gandioso, A.; Svitelova, M.; Bresolı-Obach, R.; Kostrhunova, H.; Markova, L.; et al. Towards novel photodynamic anticancer agents generating superoxide anion radicals: A cyclometalated Ir III complex conjugated to a far-red emitting coumarin. Angew. Chem. Int. Ed. 2019, 58, 6311–6315. [Google Scholar] [CrossRef]
- Conti, L.; Bencini, A.; Ferrante, C.; Gellini, C.; Paoli, P.; Parri, M.; Pietraperzia, G.; Valtancoli, B.; Giorgi, C. Highly charged ruthenium (II) polypyridyl complexes as effective photosensitizer in photodynamic therapy. Chem. Eur. J. 2019, 25, 10606–10615. [Google Scholar] [CrossRef]
- Karges, J.; Yempala, T.; Tharaud, M.; Gibson, D.; Gasser, G. A multi-action and multi-target RuII–PtIV conjugate combining cancer-activated chemotherapy and photodynamic therapy to overcome drug resistant cancers. Angew. Chem. Int. Ed. 2020, 59, 7069–7075. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, Q.; Zhao, J. An exceptionally long-lived triplet state of red light-absorbing compact phenothiazine-styryl Bodipy electron donor/acceptor dyads: A better alternative to the heavy atom-effect? Chem. Commun. 2020, 56, 1721–1724. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, Y.; Hussain, M.; Kundu, S.; Rane, V.; Hayvali, M.; Yildiz, E.A.; Zhao, J.; Yaglioglu, H.G.; Das, R.; et al. Efficient radical-enhanced intersystem crossing in an NDI-TEMPO dyad: Photophysics, electron spin polarization, and application in photodynamic therapy. Chem. Eur. J. 2018, 24, 18663–18675. [Google Scholar] [CrossRef]
- Nguyen, V.-N.; Qi, S.; Kim, S.; Kwon, N.; Kim, G.; Yim, Y.; Park, S.; Yoon, J. An emerging molecular design approach to heavy-atom-free photosensitizers for enhanced photodynamic therapy under hypoxia. J. Am. Chem. Soc. 2019, 141, 16243–16248. [Google Scholar] [CrossRef]
- Chiba, M.; Kamiya, M.; Tsuda-Sakurai, K.; Fujisawa, Y.; Kosakamoto, H.; Kojima, R.; Miura, M.; Urano, Y. Activatable photosensitizer for targeted ablation of lacZ-positive cells with single-cell resolution. ACS Cent. Sci. 2019, 5, 1676–1681. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, W.; Hong, G.; Chen, W.; Song, B.; Peng, X.; Xiong, X.; Song, F. A dual-targeted theranostic photosensitizer based on a TADF fluorescein derivative. J. Control. Release 2019, 310, 1–10. [Google Scholar] [CrossRef]
- Liu, L.; Qiu, W.; Li, B.; Zhang, C.; Sun, L.; Wan, S.; Rong, L.; Zhang, X. A red light activatable multifunctional prodrug for image-guided photodynamic therapy and cascaded chemotherapy. Adv. Funct. Mater. 2016, 26, 6257–6269. [Google Scholar] [CrossRef]
- Xu, S.; Zhu, X.; Zhang, C.; Huang, W.; Zhou, Y.; Yan, D. Oxygen and Pt (II) self-generating conjugate for synergistic photo-chemo therapy of hypoxic tumor. Nat. Commun. 2018, 9, 2053. [Google Scholar] [CrossRef]
- Ke, M.; Chen, S.; Peng, X.; Zheng, Q.; Zheng, B.; Yeh, C.; Huang, J. A tumor-targeted activatable phthalocyanine-tetrapeptide-doxorubicin conjugate for synergistic chemo-photodynamic therapy. Eur. J. Med. Chem. 2017, 127, 200–209. [Google Scholar] [CrossRef]
- Gao, J.; Li, J.; Geng, W.; Chen, F.; Duan, X.; Zheng, Z.; Ding, D.; Guo, D. Biomarker displacement activation: A general host–guest strategy for targeted phototheranostics in vivo. J. Am. Chem. Soc. 2018, 140, 4945–4953. [Google Scholar] [CrossRef]
- Turan, I.S.; Yildiz, D.; Turksoy, A.; Gunaydin, G.; Akkaya, E.U. A bifunctional photosensitizer for enhanced fractional photodynamic therapy: Singlet oxygen generation in the presence and absence of light. Angew. Chem. Int. Ed. 2016, 55, 2875–2878. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, M.; Zou, L.; Wu, L.; Xie, M.; Yang, T.; Liu, S.; Huang, W.; Zhao, Q. Highly stable and multifunctional aza-BODIPY-based phototherapeutic agent for anticancer treatment. ACS Appl. Mater. Interfaces 2018, 10, 44324–44335. [Google Scholar] [CrossRef]
- Cao, J.; Chi, J.; Xia, J.; Zhang, Y.; Han, S.; Sun, Y. Iodinated cyanine dyes for fast near-infrared-guided deep tissue synergistic phototherapy. ACS Appl. Mater. Interfaces 2019, 11, 25720–25729. [Google Scholar] [CrossRef]
- Tian, R.; Sun, W.; Li, M.; Long, S.; Li, M.; Fan, J.; Guo, L.; Peng, X. Development of a novel anti-tumor theranostic platform: A near-infrared molecular upconversion sensitizer for deep-seated cancer photodynamic therapy. Chem. Sci. 2019, 10, 10106–10112. [Google Scholar] [CrossRef]
- Bae, J.; McNamara, L.E.; Nael, M.A.; Mahdi, F.; Doerksen, R.J.; Bidwell, G.L.; Hammer, N.I.; Jo, S. Nitroreductase-triggered activation of a novel caged fluorescent probe obtained from methylene blue. Chem. Commun. 2015, 51, 12787–12790. [Google Scholar] [CrossRef]
- Li, M.; Xiong, T.; Du, J.; Tian, R.; Xiao, M.; Guo, L.; Long, S.; Fan, J.; Sun, W.; Zhao, K.; et al. Superoxide radical photogenerator with amplification effect: Surmounting the Achilles’ heels of photodynamic oncotherapy. J. Am. Chem. Soc. 2019, 141, 2695–2702. [Google Scholar] [CrossRef]
- Andreadis, D.; Pavlou, A.-M.; Sotiriou, E.; Vrani, F.; Ioannides, D.; Kolokotronis, A. Utility of photodynamic therapy for the management of oral potentially malignant disorders and oral cancer. Transl. Res. Oral Oncol. 2016, 1, 2057178X16669161. [Google Scholar] [CrossRef]
- Silva, A.P.; Neves, C.L.; Silva, E.D.A.; Portela, T.C.L.; Iunes, R.S.; Cogliati, B.; Severino, D.; Baptista, M.D.S.; Dagli, M.L.Z.; Blazquez, F.J.H.; et al. Effects of methylene blue-mediated photodynamic therapy on a mouse model of squamous cell carcinoma and normal skin. Photodiagn. Photodyn. Ther. 2018, 23, 154–164. [Google Scholar] [CrossRef]
- Xu, L.; Li, X.; Zhao, C.; Zhang, X.; Kou, H.; Yang, Y.; Zhu, F.; Zhang, W.; Lu, Y. A combination of preoperative or intraoperative MB-PDT and surgery in the treatment of giant cutaneous squamous cell carcinoma with infection. Photodiagn. Photodyn. Ther. 2021, 36, 102545. [Google Scholar] [CrossRef]
- Pivetta, T.P.; Ferreira, Q.; Vieira, T.; Silva, J.C.; Simões, S.; Ribeiro, P.A.; Raposo, M. Liposomes encapsulating methylene blue and acridine orange: An approach for phototherapy of skin cancer. Colloids Surf. B Biointerfaces 2022, 220, 112901. [Google Scholar] [CrossRef]
- Seong, D.-Y.; Kim, Y.-J. Enhanced photodynamic therapy efficacy of methylene blue-loaded calcium phosphate nanoparticles. J. Photochem. Photobiol. B 2015, 146, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Debele, T.A.; Peng, S.; Tsai, H.-C. Drug carrier for photodynamic cancer therapy. Int. J. Mol. Sci. 2015, 16, 22094–22136. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.-G. Methylene blue-based nano and microparticles: Fabrication and applications in photodynamic therapy. Polymers 2021, 13, 3955–3970. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Xu, M.; Geng, Z.; Ji, P.; Liu, Y. Hydrogel systems for targeted cancer therapy. Front. Bioeng. Biotechnol. 2023, 11, 1140436. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Wu, Y.; Zhang, X.; Zheng, Z.; Zhang, M.; Long, L.; Liao, J.; Chen, W. Recent advances in hydrogel-based phototherapy for tumor treatment. Gels 2023, 9, 286–321. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Farsangi, Z.J.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Kurian, A.G.; Singh, R.K.; Patel, K.D.; Lee, J.H.; Kim, H.W. Multifunctional GelMA platforms with nanomaterials for advanced tissue therapeutics. Bioact. Mater. 2022, 8, 267–295. [Google Scholar] [CrossRef]
- Meesaragandla, B.; Sarkar, D.; Mahalingam, V. Methylene blue-loaded upconverting hydrogel nanocomposite: Potential material for near-infrared light-triggered photodynamic therapy application. ACS Omega 2019, 4, 3169–3177. [Google Scholar] [CrossRef]
- Peng, K.; Tomatsu, I.; Kros, A. Light controlled protein release from a supramolecular hydrogel. Chem. Commun. 2010, 46, 4094–4096. [Google Scholar] [CrossRef]
- Shin, D.S.; You, J.; Rahimian, A.; Vu, T.; Siltanen, C.; Ehsanipour, A.; Stybayeva, G.; Sutcliffe, J.; Revzin, A. Photodegradable hydrogels for capture, detection, and release of live cells. Angew. Chem. Int. Ed. 2014, 53, 8221–8224. [Google Scholar] [CrossRef]
- Cornwell, D.J.; Smith, D.K. Photo-patterned multi-domain multi-component hybrid hydrogels. Chem. Commun. 2020, 56, 7029–7032. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, B.; Ahmed, S. Peptide-based low molecular weight photosensitive supramolecular gelators. Gels 2022, 8, 533–559. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, M.; Yang, Y.; Liang, Y.; Sun, C.; Guo, W.; Li, S.; Head, B.; Putri, Y. A reactive oxygen species (ROS)-responsive low molecular weight gel co-loaded with doxorubicin and Zn(ii) phthalocyanine tetrasulfonic acid for combined chemo-photodynamic therapy. J. Mater. Chem. B 2017, 5, 9157–9164. [Google Scholar] [CrossRef]
- Dhibar, S.; Pal, S.; Karmakar, K.; Hafiz, S.A.; Bhattacharjee, S.; Roy, A.; Rahaman, S.K.M.; Ray, S.J.; Dam, S.; Saha, B. Two novel low molecular weight gelator-driven supramolecular metallogels efficient in antimicrobial activity applications. RSC Adv. 2023, 13, 32842–32849. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, W.; Song, S.; Feng, L.; Song, A.; Hao, J. Hydrogels facilitated by monovalent cations and their use as efficient dye adsorbents. J. Phys. Chem. B 2014, 118, 4693–4701. [Google Scholar] [CrossRef]
- Karan, C.K.; Bhattacharjee, M. Self-healing and moldable metallogels as the recyclable materials for selective dye adsorption and separation. ACS Appl. Mater. Interfaces 2016, 8, 5526–5535. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, J.; Hasegawa, Y.; Yanai, K.; Yanamoto, A.; Ishii, A.; Hashegawa, M.; Yamanaka, M. Organic dye adsorption by amphiphilic tris-urea supramolecular hydrogel. Chem. Asian J. 2017, 12, 2029–2032. [Google Scholar] [CrossRef]
- Yoneda, J.S.; Araujo, D.R.; Sella, F.; Liguori, G.R.; Liguori, T.T.A.; Moreira, L.F.P.; Spinozzi, F.; Mariani, P.; Itri, R. Self-assembled guanosine-hydrogels for drug-delivery application: Structural and mechanical characterization, methylene blue loading and controlled release. Mater. Sci. Eng. C 2021, 121, 111834. [Google Scholar] [CrossRef]
- Morris, J.; Bietsch, J.; Bashaw, K.; Wang, G. Recently developed carbohydrate based gelators and their applications. Gels 2021, 7, 24–85. [Google Scholar] [CrossRef]
- Wang, T.; Ménard-Moyon, C.; Bianco, A. Self-assembly of amphiphilic amino acid derivatives for biomedical applications. Chem. Soc. Rev. 2022, 51, 3535–3560. [Google Scholar] [CrossRef]
- Vishnevetskii, D.V.; Mekhtiev, A.R.; Perevozova, T.V.; Averkin, D.V.; Ivanova, A.I.; Khizhnyak, S.D.; Pakhomov, P.M. L-Cysteine/AgNO2 low molecular weight gelators: Self-assembly and suppression of MCF-7 breast cancer cells. Soft Matter 2020, 16, 9669–9673. [Google Scholar] [CrossRef]
- Vishnevetskii, D.V.; Mekhtiev, A.R.; Perevozova, T.V.; Averkin, D.V.; Ivanova, A.I.; Khizhnyak, S.D.; Pakhomov, P.M. L-Cysteine as a reducing/capping/gel-forming agent for the preparation of silver nanoparticle composites with anticancer properties. Soft Matter 2022, 18, 3031–3040. [Google Scholar] [CrossRef] [PubMed]
- Vishnevetskii, D.V.; Semenova, E.M.; Averkin, D.V.; Mekhtiev, A.R. Behavior and bioactive properties of aqueous L-cysteine–AgNO3 solution at different pH. Mend. Commun. 2023, 33, 431–432. [Google Scholar] [CrossRef]
- Vishnevetskii, D.V.; Andrianova, Y.V.; Polyakova, E.E.; Ivanova, A.I.; Mekhtiev, A.R. Fluoride-ion-responsive sol–gel transition in an L-cysteine/AgNO3 system: Self-assembly peculiarities and anticancer activity. Gels 2024, 10, 332–346. [Google Scholar] [CrossRef]
- Vishnevetskii, D.V.; Averkin, D.V.; Efimov, A.A.; Lizunova, A.A.; Shamova, O.V.; Vladimirova, E.V.; Sukhareva, M.S.; Mekhtiev, A.R. L-cysteine and N-acetyl-L-cysteine mediated synthesis of nanosilver-based sols and hydrogels with antibacterial and antibiofilm properties. J. Mater. Chem. B 2023, 11, 5794–5804. [Google Scholar] [CrossRef]
- Vishnevetskii, D.V.; Averkin, D.V.; Efimov, A.A.; Lizunova, A.A.; Ivanova, A.I.; Pakhomov, P.M.; Ruehl, E. Ag/α-Ag2MoO4/h-MoO3 nanoparticle-based microspheres: Synthesis and photosensitive properties. Soft Matter 2021, 17, 10416–10420. [Google Scholar] [CrossRef] [PubMed]
- Vishnevetskii, D.V.; Mekhtiev, A.R.; Averkin, D.V.; Polyakova, E.E. Cysteine–silver–polymer systems for the preparation of hydrogels and films with potential applications in regenerative medicine. Gels 2023, 9, 924–937. [Google Scholar] [CrossRef]
- Khizhnyak, S.D.; Komarov, P.V.; Ovchinnikov, M.M.; Zherenkova, L.V.; Pakhomov, P.M. Mechanism of gelation in low concentration aqueous solutions of silver nitrate with L-cysteine and its derivatives. Soft Matter 2017, 13, 5168–5184. [Google Scholar] [CrossRef]
- Galgliardi, A.; Voci, S.; Paolino, D.; Fresta, M.; Cosco, D. Influence of various model compounds on the rheological properties of zein-based gels. Molecules 2020, 25, 3174–3189. [Google Scholar] [CrossRef]
- Khadieva, A.; Mostovaya, O.; Padnya, P.; Kalinin, V.; Grishaev, D.; Tumakov, D.; Stoikov, I. Arylamine analogs of methylene blue: Substituent effects on aggregation behavior and DNA binding. Int. J. Mol. Sci. 2021, 22, 5847–5861. [Google Scholar] [CrossRef] [PubMed]
- Marise de Freitas, L.; Pienna Soares, C.; Raquel Fontana, C. Synergistic effect of photodynamic therapy and cisplatin: A novel approach for cervical cancer. J. Photochem. Photobiol. B Biol. 2014, 140, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Takac, P.; Michalkova, R.; Cizmarikova, M.; Bedlovicova, Z.; Balazova, L.; Takacova, G. The role of silver nanoparticles in the diagnosis and treatment of cancer: Are there any perspectives for the future? Life 2023, 13, 466–510. [Google Scholar] [CrossRef]
- Moloudi, K.; Abrahamse, H.; George, B.P. Photodynamic Therapy Induced Cell Cycle Arrest and Cancer Cell Synchronization: Review. Front. Oncol. 2023, 13, 1225694. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.E.; Jung, Y.G.; Lee, S.-J. The anticancer activities of natural terpenoids that inhibit both melanoma and non-melanoma skin cancers. Int. J. Mol. Sci. 2024, 25, 4423–4439. [Google Scholar] [CrossRef]
- Dziedzic, A.; Kubina, R.; Buldak, R.J.; Skonieczna, M.; Cholewa, K. Silver nanoparticles exhibit the dose-dependent antiproliferative effect against human squamous carcinoma cells attenuated in the presence of berberine. Molecules 2016, 21, 365–382. [Google Scholar] [CrossRef]
- Khorsandi, K.; Hosseinzadeh, R.; Chamani, E. Molecular interaction and cellular studies on combination photodynamic therapy with rutoside for melanoma A375 cancer cells: An in vitro study. Cancer Cell Int. 2020, 20, 525. [Google Scholar] [CrossRef]



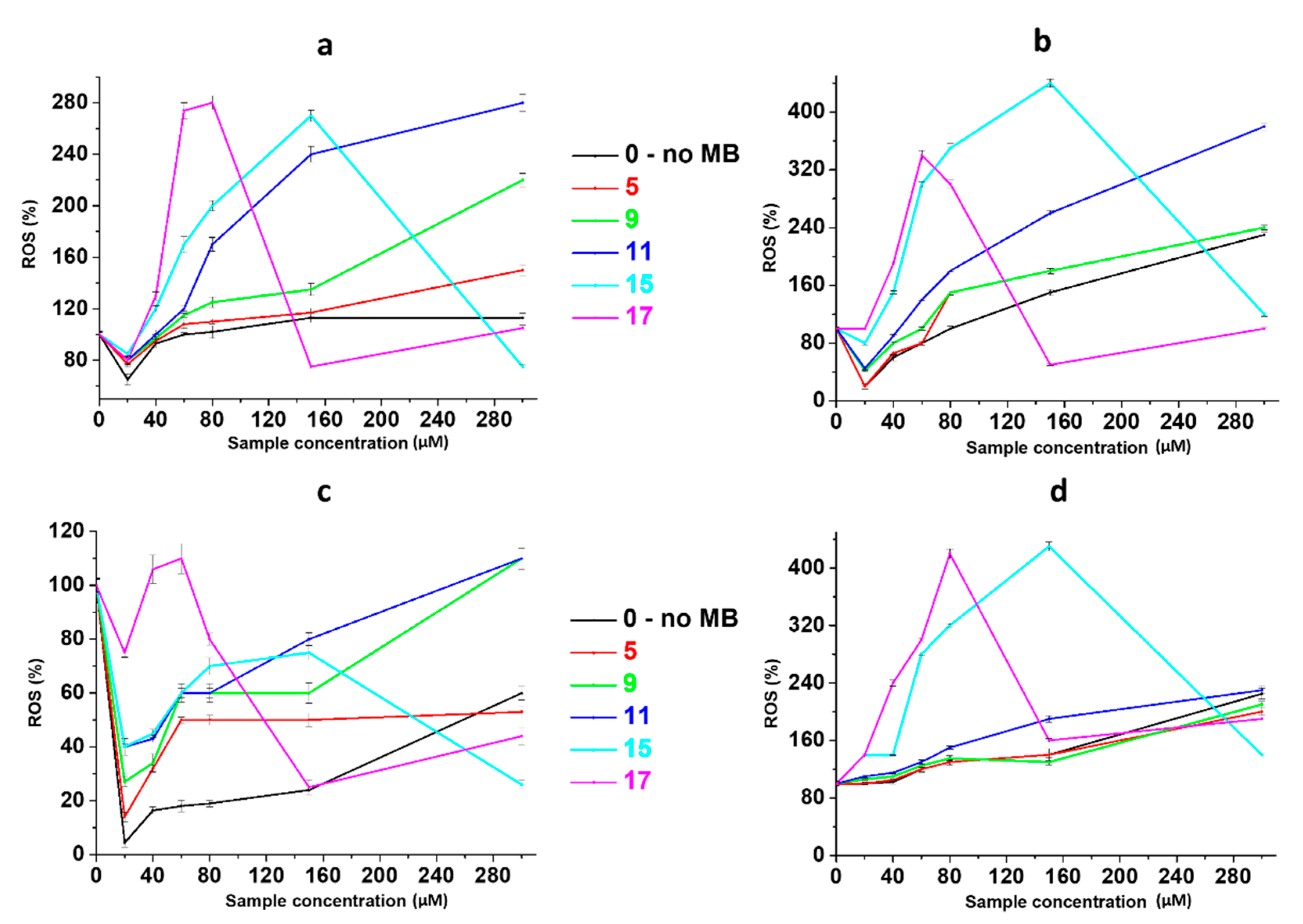
| Sample | CSS Concentration, µM | |||||
|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | 150 | 300 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 0.05 | 0.1 | 0.2 | 0.27 | 0.5 | 1 |
| 9 | 0.24 | 0.49 | 0.96 | 1.29 | 2.38 | 4.76 |
| 11 | 0.5 | 1 | 2 | 2.7 | 5 | 10 |
| 15 | 2.4 | 4.9 | 9.6 | 12.9 | 23.8 | 47.6 |
| 17 | 5 | 10 | 20 | 27 | 50 | 100 |
| Sample | SubG0–G1, % No Irradiation/Irradiation | G0–G1, % No Irradiation/Irradiation | S, % No Irradiation/Irradiation | G2-M, % No Irradiation/Irradiation |
|---|---|---|---|---|
| 0—no MB | 17/11 | 68/68 | 4/6 | 11/15 |
| 5 | 4/3 | 81/74 | 5/9 | 10/14 |
| 9 | 4/2 | 73/74 | 9/7 | 14/17 |
| 11 | 4/2 | 76/76 | 8/6 | 12/16 |
| 15 | 8/3 | 78/77 | 4/6 | 10/14 |
| 17 | 8/3 | 81/77 | 4/6 | 7/14 |
| Sample | SubG0–G1, % No Irradiation/Irradiation | G0–G1, % No Irradiation/Irradiation | S, % No Irradiation/Irradiation | G2-M, % No Irradiation/Irradiation |
|---|---|---|---|---|
| 0—no MB | 86/8 | 13/70 | 0.5/5 | 0.5/17 |
| 5 | 4/4 | 61 | 16/6 | 19/18 |
| 9 | 4/4 | 56/67 | 22/9 | 18/20 |
| 11 | 18/8 | 56/57 | 11/12 | 15/23 |
| 15 | 55/35 | 43/43 | 1/8 | 1/14 |
| 17 | 40/10 | 55/68 | 2/8 | 3/14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vishnevetskii, D.V.; Metlin, F.A.; Andrianova, Y.V.; Polyakova, E.E.; Ivanova, A.I.; Averkin, D.V.; Mekhtiev, A.R. Preparation of Composite Hydrogels Based on Cysteine–Silver Sol and Methylene Blue as Promising Systems for Anticancer Photodynamic Therapy. Gels 2024, 10, 577. https://doi.org/10.3390/gels10090577
Vishnevetskii DV, Metlin FA, Andrianova YV, Polyakova EE, Ivanova AI, Averkin DV, Mekhtiev AR. Preparation of Composite Hydrogels Based on Cysteine–Silver Sol and Methylene Blue as Promising Systems for Anticancer Photodynamic Therapy. Gels. 2024; 10(9):577. https://doi.org/10.3390/gels10090577
Chicago/Turabian StyleVishnevetskii, Dmitry V., Fedor A. Metlin, Yana V. Andrianova, Elizaveta E. Polyakova, Alexandra I. Ivanova, Dmitry V. Averkin, and Arif R. Mekhtiev. 2024. "Preparation of Composite Hydrogels Based on Cysteine–Silver Sol and Methylene Blue as Promising Systems for Anticancer Photodynamic Therapy" Gels 10, no. 9: 577. https://doi.org/10.3390/gels10090577
APA StyleVishnevetskii, D. V., Metlin, F. A., Andrianova, Y. V., Polyakova, E. E., Ivanova, A. I., Averkin, D. V., & Mekhtiev, A. R. (2024). Preparation of Composite Hydrogels Based on Cysteine–Silver Sol and Methylene Blue as Promising Systems for Anticancer Photodynamic Therapy. Gels, 10(9), 577. https://doi.org/10.3390/gels10090577







