Design and Evaluation of New Gel-Based Floating Matrix Tablets Utilizing the Sublimation Technique for Gastroretentive Drug Delivery
Abstract
1. Introduction
2. Results and Discussion
2.1. Design of the Floating Matrix Tablets
2.2. Physical Properties of the Floating Matrix Tablets
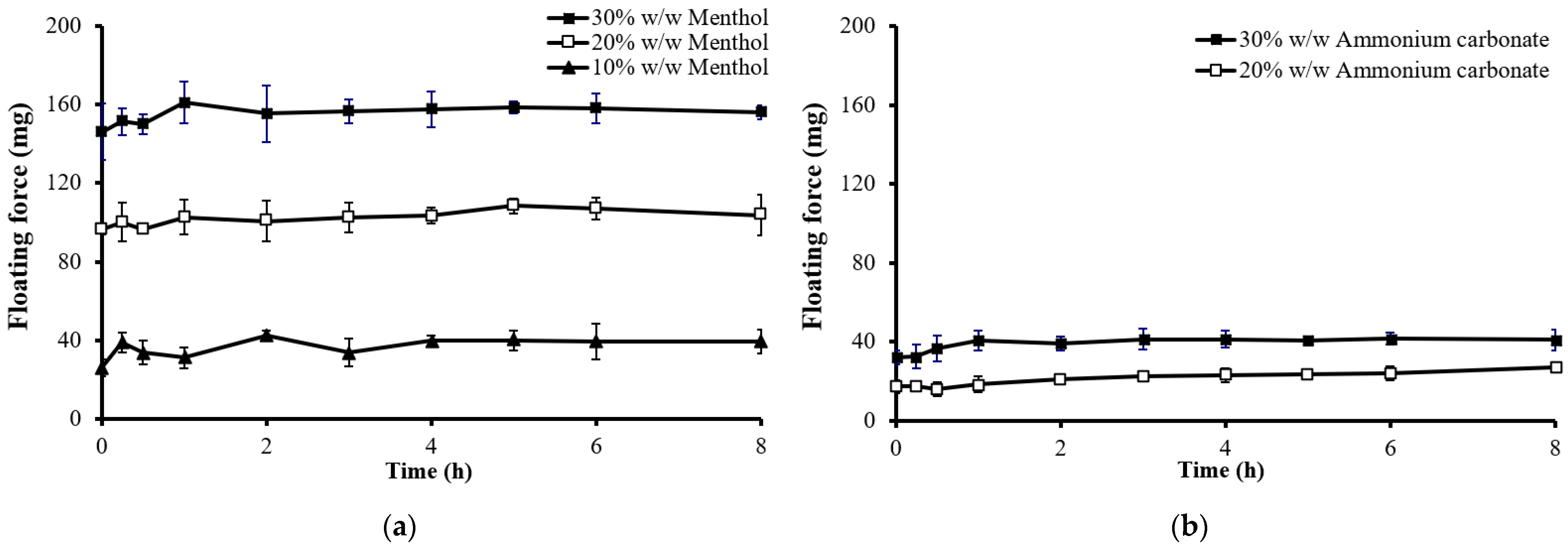
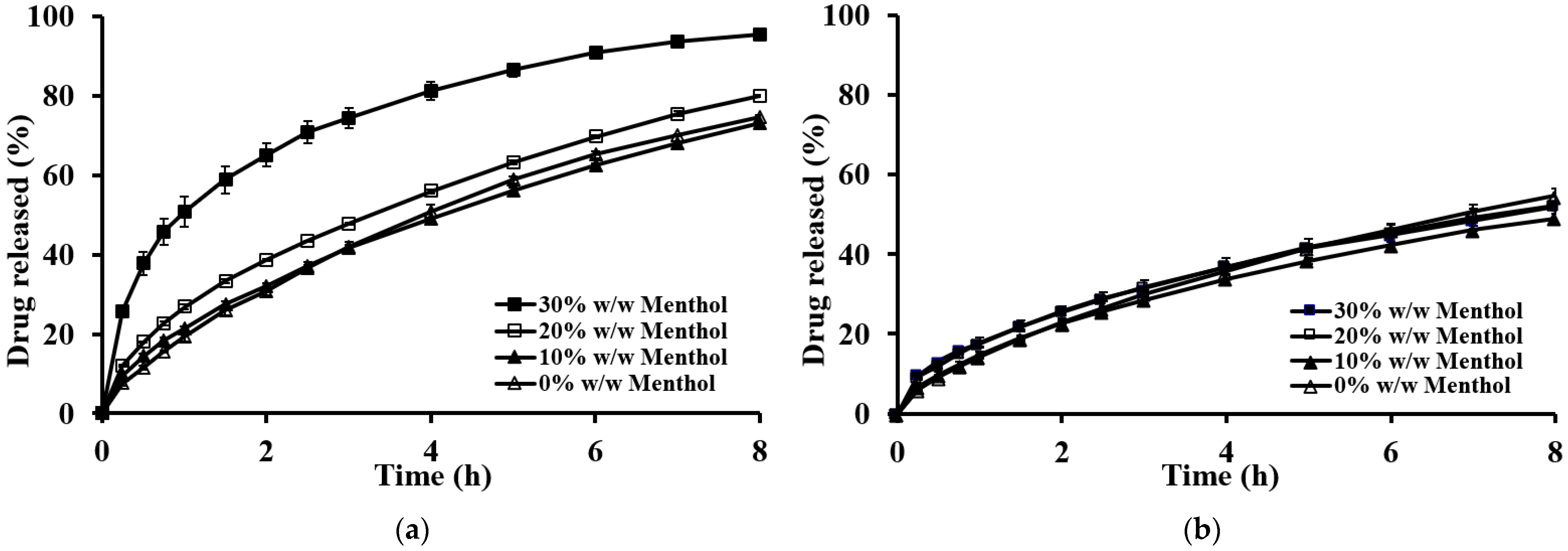
2.3. Effect of Formulation Variables on Floating Properties and Drug Release of the Floating Matrix Tablets
2.3.1. Effect of Type of Gel-Forming Polymer
2.3.2. Effect of the Type of Sublimation Substances
2.3.3. Effect of the Amount of Sublimation Substances
2.4. Drug Release Kinetics
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Floating Matrix Tablets
4.3. Evaluation of the Floating Matrix Tablets
4.3.1. Evaluation of the Floating Matrix Tablet Properties
4.3.2. Porosity Determination of the Floating Matrix Tablets
4.3.3. Scanning Electron Microscopy (SEM)
4.3.4. Floating Properties
4.3.5. Resultant Weight
F = dfgV − dsgV
F = (df − ds) gV,
4.3.6. Drug Release Studies
4.3.7. Swelling or Water Uptake Studies
4.3.8. Erosion Studies
4.3.9. Analysis of Drug Release Data
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopes, C.M.; Bettencourt, C.; Rossi, A.; Buttini, F.; Barata, P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int. J. Pharm. 2016, 510, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Kim, K.H. Floating drug delivery systems: An approach to oral controlled drug delivery via gastric retention. J. Control. Release 2000, 63, 235–259. [Google Scholar] [CrossRef]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Recent advancements in mucoadhesive floating drug delivery systems: A mini-review. J. Drug Deliv. Sci. Technol. 2016, 31, 65–71. [Google Scholar] [CrossRef]
- Bera, R.; Mandal, B.; Bhowmik, M.; Bera, H.; DEY, S.K.; Nandi, G.; Ghosh, L.K. Formulation and in vitro evaluation of sunflower oil entrapped within buoyant beads of furosemide. Sci. Pharm. 2009, 77, 669–678. [Google Scholar] [CrossRef][Green Version]
- Meka, L.; Kesavan, B.; Kalamata, V.N.; Eaga, C.M.; Bandari, S.; Vobalaboina, V.; Yamsani, M.R. Design and evaluation of polymeric coated minitablets as multiple unit gastroretentive floating drug delivery systems for furosemide. J. Pharm. Sci. 2009, 98, 2122–2132. [Google Scholar] [CrossRef]
- Bomma, R.; Swamy Naidu, R.; Yamsani, M.; Veerabrahma, K. Development and evaluation of gastroretentive norfloxacin floating tablets. Acta Pharm. 2009, 59, 211–221. [Google Scholar] [CrossRef]
- Tripathi, P.K.; Singh, S.; Jadhav, K.R. Floating minitablets loaded with captopril encapsulated microparticles. J. Drug Deliv. Sci. Technol. 2021, 63, 102445. [Google Scholar] [CrossRef]
- Streubel, A.; Siepmann, J.; Bodmeier, R. Drug delivery to the upper small intestine window using gastroretentive technologies. Curr. Opin. Pharmacol. 2006, 6, 501–508. [Google Scholar] [CrossRef]
- Streubel, A.; Siepmann, J.; Bodmeier, R. Floating microparticles based on low density foam powder. Int. J. Pharm. 2002, 241, 279–292. [Google Scholar] [CrossRef]
- Namdev, A.; Jain, D. Floating Drug Delivery Systems: An emerging trend for the treatment of peptic ulcer. Curr. Drug Deliv. 2019, 16, 874–886. [Google Scholar] [CrossRef]
- Kriangkrai, W.; Tangchitkhachon, K.; Sriraksa, W.; Sungthongjeen, S. Multi-Layer Films Prepared by Spray Coating for Effervescent Floating Tablets. STA 2023, 28, 26–32. [Google Scholar]
- Fukuda, M.; Peppas, N.A.; McGinity, J.W. Floating hot-melt extruded tablets for gastroretentive controlled drug release system. J. Control. Release 2006, 115, 121–129. [Google Scholar] [CrossRef]
- Varshosaz, J.; Tabbakhian, M.; Zahrooni, M. Development and characterization of floating microballoons for oral delivery of cinnarizine by a factorial design. J. Microencapsul. 2007, 24, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Kawashima, Y.; Takeuchi, H.; Yamamoto, H. Physicochemical properties to determine the buoyancy of hollow microspheres (microballoons) prepared by the emulsion solvent diffusion method. Eur. J. Pharm. Biopharm. 2003, 55, 297–304. [Google Scholar] [CrossRef]
- Talukder, R.; Fassihi, R. Gastroretentive delivery systems: Hollow beads. Drug Dev. Ind. Pharm. 2004, 30, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Kawashima, Y.; Takeuchi, H.; Yamamoto, H. In vivo evaluation of riboflavin-containing microballoons for floating controlled drug delivery system in healthy human volunteers. J. Control. Release 2003, 93, 39–47. [Google Scholar] [CrossRef]
- Streubel, A.; Siepmann, J.; Bodmeier, R. Floating matrix tablets based on low density foam powder: Effects of formulation and processing parameters on drug release. Eur. J. Pharm. Sci. 2003, 18, 37–45. [Google Scholar] [CrossRef]
- Sungthongjeen, S.; Chaikla, B.; Huaihongthong, C.; Charoenthai, N.; Kriangkrai, W.; Puttipipatkhachorn, S. Development of Floating Pellets Using Low-Density Foam Powder Prepared by Extrusion-Spheronization. STA 2023, 28, 181–189. [Google Scholar]
- Fukuda, M.; Goto, A. Properties of Gastroretentive Sustained Release Tablets Prepared by Combination of Melt/Sublimation Actions of L-Menthol and Penetration of Molten Polymers into Tablets. Chem. Pharm. Bull. 2011, 59, 1221–1226. [Google Scholar] [CrossRef]
- Oh, T.-O.; Kim, J.-Y.; Ha, J.-M.; Chi, S.-C.; Rhee, Y.-S.; Park, C.-W.; Park, E.-S. Preparation of highly porous gastroretentive metformin tablets using a sublimation method. Eur. J. Pharm. Biopharm. 2013, 83, 460–467. [Google Scholar] [CrossRef]
- Huanbutta, K.; Limmatvapirat, S.; Sungthongjeen, S.; Sriamornsak, P. Novel Strategy to Fabricate Floating Drug Delivery System Based on Sublimation Technique. AAPS PharmSciTech 2015, 17, 693–699. [Google Scholar] [CrossRef][Green Version]
- Koizumi, K.-i.; Watanabe, Y.; Morita, K.; Utoguchi, N.; Matsumoto, M. New method of preparing high-porosity rapidly saliva soluble compressed tablets using mannitol with camphor, a subliming material. Int. J. Pharm. 1997, 152, 127–131. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Rajabi-Siahboomi, A.R. Modulation of drug release from hydrophilic matrices. Pharm. Technol. Eur. 2008, 20, 24–32. [Google Scholar]
- Nellore, R.V.; Singh Rekhi, G.; Hussain, A.S.; Tillman, L.G.; Augsburger, L.L. Development of metoprolol tartrate extended-release matrix tablet formulations for regulatory policy consideration. J. Control. Release 1998, 50, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Tadros, M.I. Controlled-release effervescent floating matrix tablets of ciprofloxacin hydrochloride: Development, optimization and in vitro–in vivo evaluation in healthy human volunteers. Eur. J. Pharm. Biopharm. 2010, 74, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Strübing, S.; Abboud, T.; Contri, R.V.; Metz, H.; Mäder, K. New insights on poly(vinyl acetate)-based coated floating tablets: Characterisation of hydration and CO2 generation by benchtop MRI and its relation to drug release and floating strength. Eur. J. Pharm. Biopharm. 2008, 69, 708–717. [Google Scholar] [CrossRef]
- Strübing, S.; Metz, H.; Mäder, K. Characterization of poly(vinyl acetate) based floating matrix tablets. J. Control. Release 2008, 126, 149–155. [Google Scholar] [CrossRef]
- Jiménez-Martínez, I.; Quirino-Barreda, T.; Villafuerte-Robles, L. Sustained delivery of captopril from floating matrix tablets. Int. J. Pharm. 2008, 362, 37–43. [Google Scholar] [CrossRef]
- Rinaki, E.; Valsami, G.; Macheras, P. The power law can describe the ‘entire’ drug release curve from HPMC-based matrix tablets: A hypothesis. Int. J. Pharm. 2003, 255, 199–207. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Kennedy, R.A. Effect of sodium fluorescein on release characteristics of a macromolecule from calcium alginate gel beads. Carbohydr. Polym. 2011, 84, 1208–1212. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Rouge, N.; Cole, E.T.; Doelker, E.; Buri, P. Buoyancy and drug release patterns of floating minitablets containing piretanide and atenolol as model drugs. Pharm. Dev. Technol. 1998, 3, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, J.; Moës, A.J. How well do floating dosage forms float? Int. J. Pharm. 1990, 62, 207–216. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Thirawong, N.; Weerapol, Y.; Nunthanid, J.; Sungthongjeen, S. Swelling and erosion of pectin matrix tablets and their impact on drug release behavior. Eur. J. Pharm. Biopharm. 2007, 67, 211–219. [Google Scholar] [CrossRef] [PubMed]
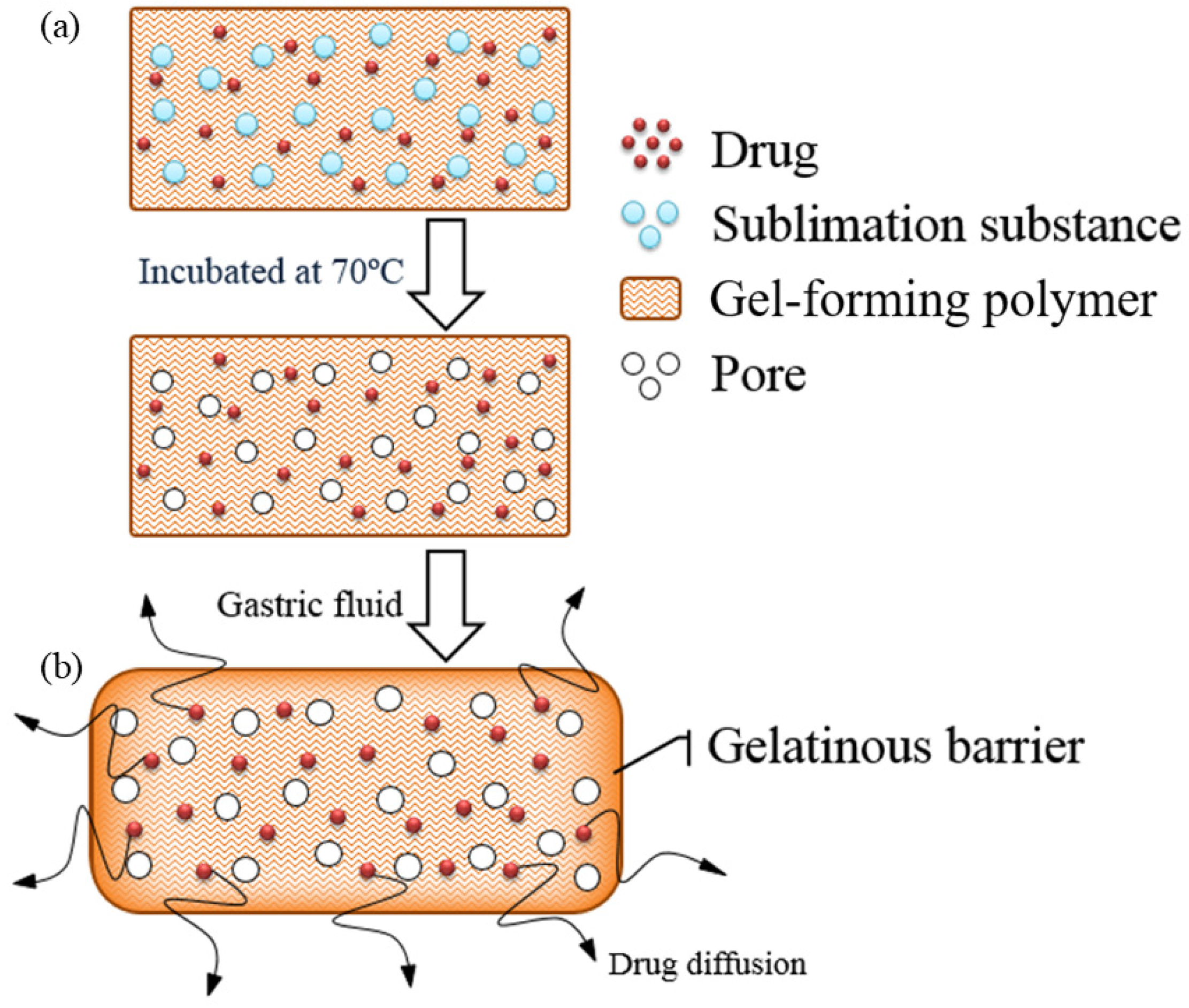

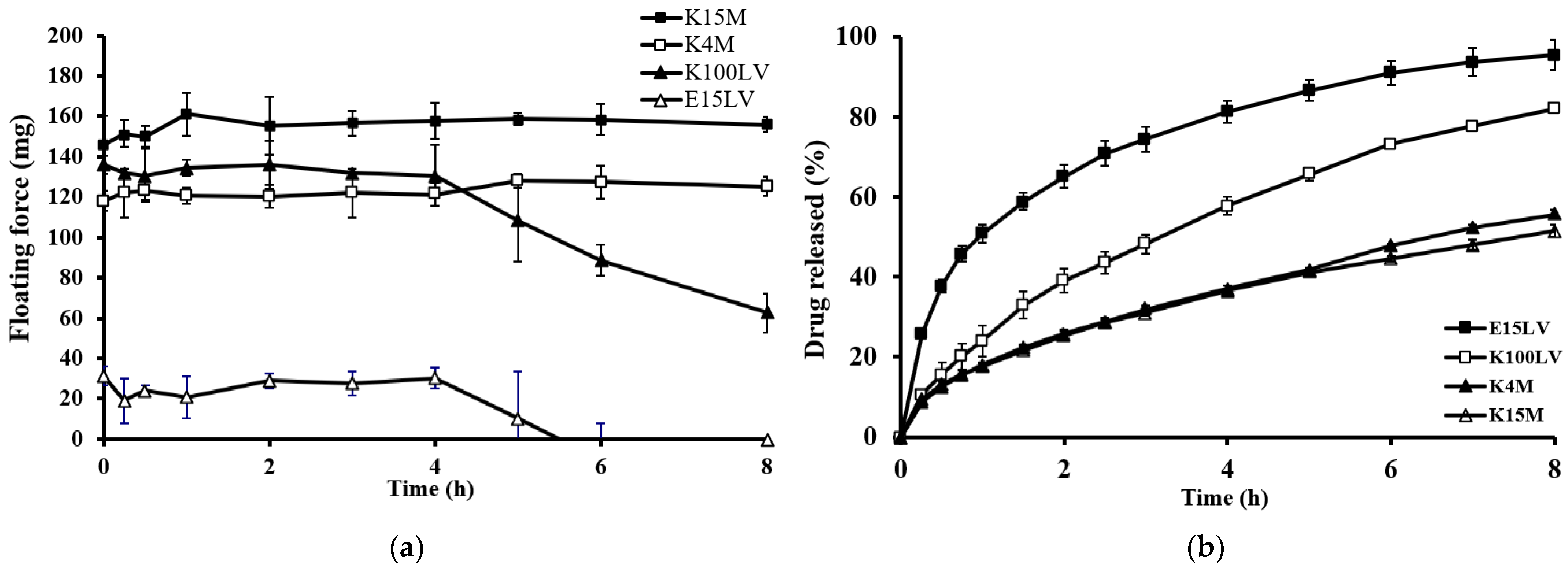

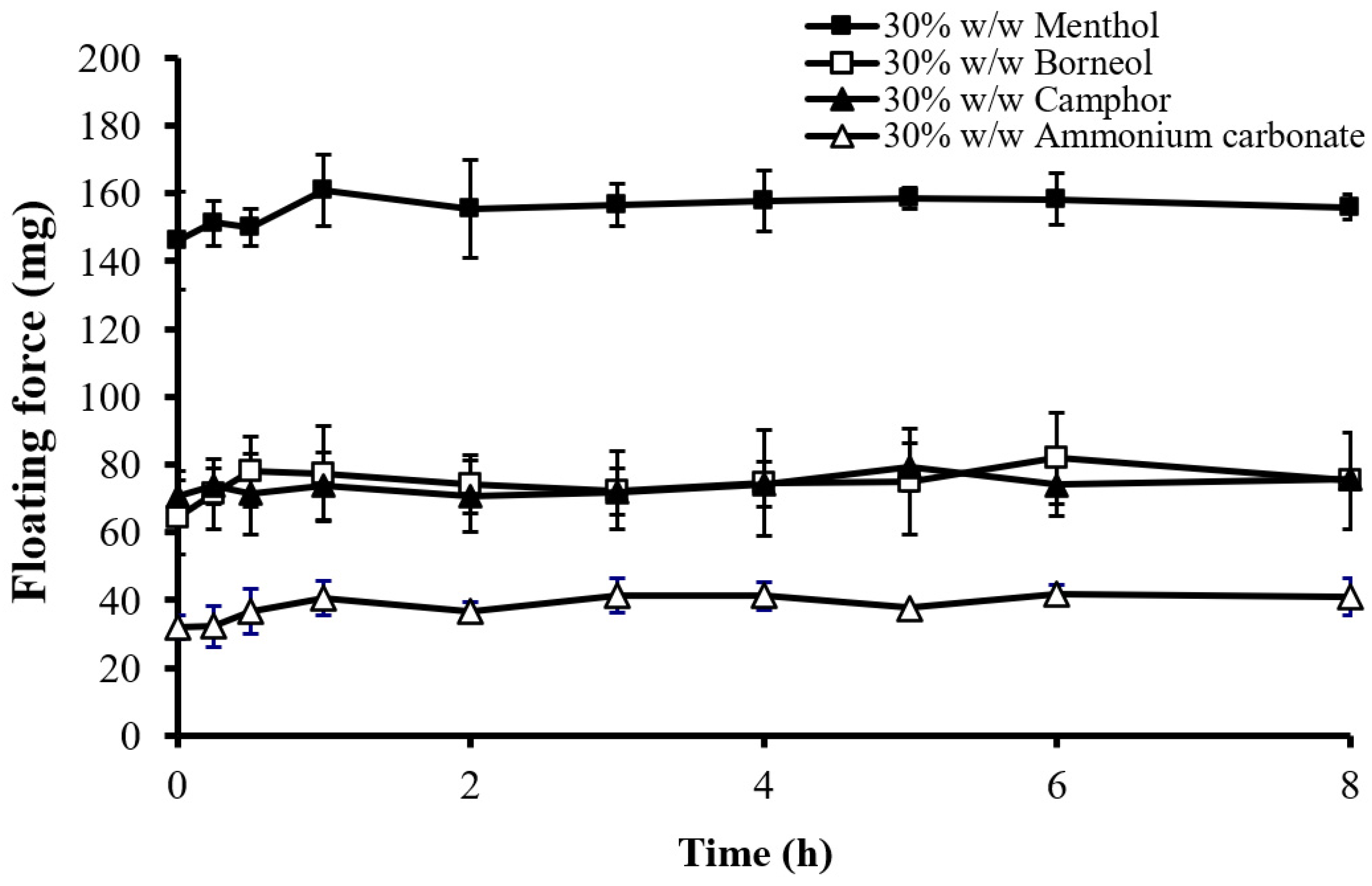

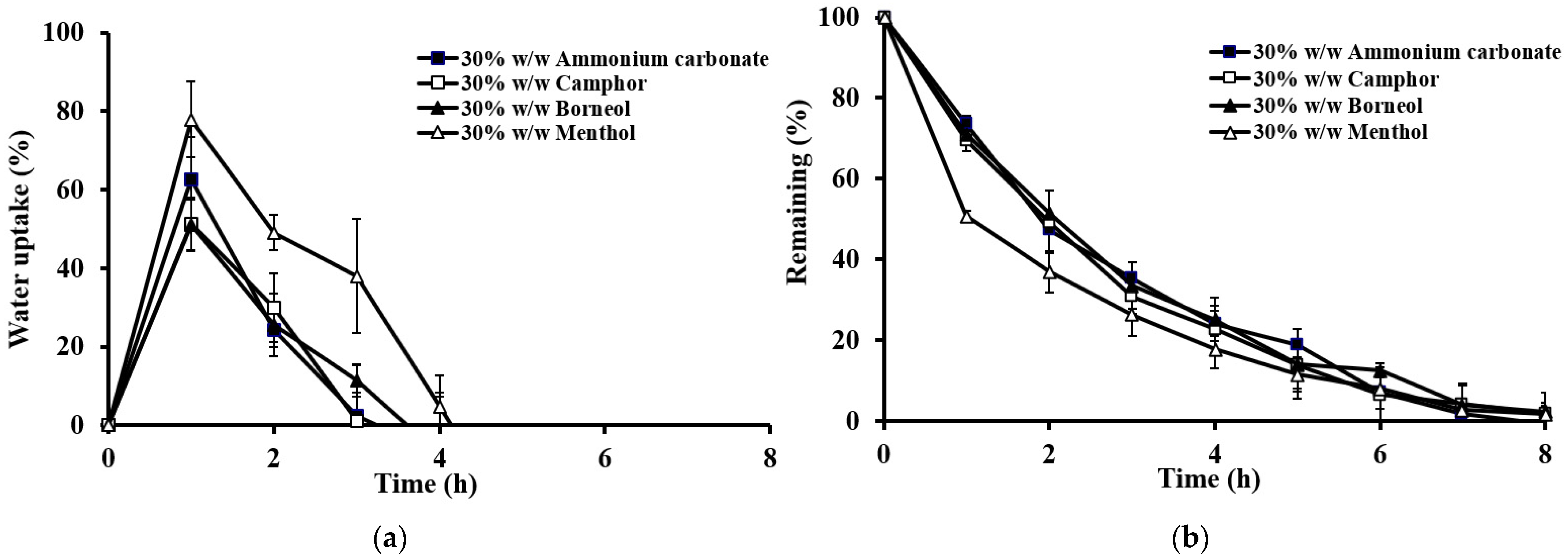
| Formulation | Wt. Variation (mg) | Thickness (mm) | Density (mg/mm3) | Porosity (%) | Hardness (kg) | Friability (%) |
|---|---|---|---|---|---|---|
| HPMC E15LV | 302.62 ± 3.39 | 3.95 ± 0.03 | 1.14 ± 0.01 | 15.84 ± 1.05 | 14.04 ± 0.28 | −0.25 |
| 10% w/w Ammonium carbonate | 271.48 ± 2.77 | 3.81 ± 0.04 | 1.05 ± 0.01 | 26.96 ± 1.72 | 8.37 ± 0.31 | −0.11 |
| 20% w/w Ammonium carbonate | 240.23 ± 4.45 | 3.84 ± 0.05 | 0.94 ± 0.02 | 31.23 ± 1.56 | 5.19 ± 0.27 | −0.12 |
| 30% w/w Ammonium carbonate | 214.09 ± 5.37 | 3.70 ± 0.12 | 0.82 ± 0.03 | 41.32 ± 2.75 | 1.85 ± 0.35 | −0.32 |
| 10% w/w Camphor | 272.38 ± 4.46 | 4.09 ± 0.06 | 0.91 ± 0.01 | 31.22 ± 2.33 | 4.43 ± 0.56 | 0.07 |
| 20% w/w Camphor | 238.48 ± 6.22 | 4.07 ± 0.15 | 0.80 ± 0.02 | 37.35 ± 1.16 | 3.34 ± 0.33 | 0.65 |
| 30% w/w Camphor | 212.33 ± 5.02 | 4.01 ± 0.05 | 0.73 ± 0.06 | 47.72 ± 1.56 | 2.31 ± 0.18 | 1.83 |
| 10% w/w Borneol | 276.32 ± 1.91 | 4.04 ± 0.03 | 0.91 ± 0.01 | 30.71 ± 1.89 | 5.33 ± 0.31 | −0.34 |
| 20% w/w Borneol | 245.38 ± 4.24 | 4.57 ± 0.13 | 0.83 ± 0.03 | 37.69 ± 1.25 | 2.99 ± 0.15 | −0.04 |
| 30% w/w Borneol | 208.07 ± 4.72 | 4.00 ± 0.05 | 0.72 ± 0.03 | 45.24 ± 1.85 | 2.03 ± 0.24 | 0.30 |
| 10% w/w Menthol | 275.58 ± 5.02 | 4.70 ± 0.09 | 0.81 ± 0.02 | 35.19 ± 0.35 | 8.48 ± 0.53 | −0.04 |
| 20% w/w Menthol | 244.71 ± 4.70 | 5.09 ± 0.20 | 0.66 ± 0.02 | 45.69 ± 1.94 | 4.91 ± 0.60 | 1.48 |
| 30% w/w Menthol | 209.26 ± 3.89 | 6.25 ± 0.07 | 0.46 ± 0.04 | 58.83 ± 1.85 | 1.71 ± 0.19 | 32.54 |
| HPMC K100LV | 304.92 ± 2.46 | 3.82 ± 0.06 | 1.13 ± 0.01 | 14.43 ± 0.80 | 38.95 ± 1.47 | −0.16 |
| 30% w/w Ammonium carbonate | 213.19 ± 5.14 | 3.56 ± 0.08 | 0.91 ± 0.04 | 29.62 ± 0.80 | 15.73 ± 0.77 | 0.07 |
| 30% w/w Camphor | 216.03 ± 4.32 | 4.12 ± 0.06 | 0.71 ± 0.03 | 42.55 ± 0.96 | 6.32 ± 0.37 | 1.21 |
| 30% w/w Borneol | 212.35 ± 5.66 | 4.06 ± 0.03 | 0.72 ± 0.02 | 41.47 ± 1.66 | 7.16 ± 0.56 | 0.47 |
| 30% w/w Menthol | 209.86 ± 4.30 | 4.90 ± 0.10 | 0.58 ± 0.02 | 53.15 ± 2.32 | 1.10 ± 0.21 | 6.41 |
| HPMC K4M | 307.82 ± 3.01 | 3.79 ± 0.04 | 1.13 ± 0.01 | 14.34 ± 0.11 | 33.91 ± 0.86 | 0.07 |
| 30% w/w Ammonium carbonate | 220.60 ± 7.37 | 3.49 ± 0.05 | 0.87 ± 0.02 | 30.11 ± 2.71 | 7.88 ± 0.43 | 0.11 |
| 30% w/w Camphor | 214.32 ± 5.29 | 4.02 ± 0.08 | 0.75 ± 0.13 | 40.10 ± 2.62 | 5.36 ± 0.19 | 0.07 |
| 30% w/w Borneol | 210.91 ± 4.11 | 3.99 ± 0.04 | 0.74 ± 0.02 | 39.93 ± 1.61 | 5.34 ± 0.76 | 0.04 |
| 30% w/w Menthol | 213.53 ± 4.06 | 4.60 ± 0.06 | 0.62 ± 0.02 | 51.47 ± 2.30 | 0.76 ± 0.06 | 0.55 |
| HPMC K15M | 304.10 ± 0.58 | 3.70 ± 0.04 | 1.15 ± 0.01 | 15.08 ± 1.03 | 30.57 ± 0.62 | −0.09 |
| 10% w/w Ammonium carbonate | 268.29 ± 2.54 | 3.63 ± 0.05 | 1.06 ± 0.02 | 21.74 ± 1.99 | 22.25 ± 2.13 | −0.13 |
| 20% w/w Ammonium carbonate | 243.59 ± 5.64 | 3.65 ± 0.03 | 0.96 ± 0.02 | 30.24 ± 2.25 | 14.14 ± 1.03 | 0.93 |
| 30% w/w Ammonium carbonate | 215.04 ± 5.46 | 3.55 ± 0.05 | 0.84 ± 0.06 | 37.13 ± 0.97 | 8.27 ± 0.13 | 4.68 |
| 10% w/w Camphor | 271.35 ± 2.93 | 4.06 ± 0.03 | 0.94 ± 0.02 | 30.38 ± 2.36 | 8.81 ± 0.90 | −0.50 |
| 20% w/w Camphor | 241.58 ± 3.36 | 4.07 ± 0.04 | 0.80 ± 0.06 | 36.23 ± 2.01 | 5.98 ± 0.43 | 3.26 |
| 30% w/w Camphor | 216.18 ± 10.98 | 3.96 ± 0.03 | 0.74 ± 0.04 | 47.35 ± 1.22 | 4.35 ± 0.45 | 5.94 |
| 10% w/w Borneol | 269.18 ± 1.06 | 4.00 ± 0.06 | 0.94 ± 0.01 | 28.06 ± 2.30 | 12.02 ± 0.73 | 0.81 |
| 20% w/w Borneol | 242.97 ± 6.68 | 4.01 ± 0.05 | 0.86 ± 0.03 | 36.34 ± 2.11 | 8.37 ± 1.08 | 3.60 |
| 30% w/w Borneol | 212.01 ± 5.31 | 4.07 ± 0.06 | 0.70 ± 0.02 | 44.04 ± 2.13 | 3.52 ± 0.59 | 4.65 |
| 10% w/w Menthol | 275.24 ± 2.87 | 4.22 ± 0.09 | 0.91 ± 0.01 | 35.31 ± 0.69 | 6.27 ± 0.45 | 0.30 |
| 20% w/w Menthol | 243.95 ± 3.87 | 4.84 ± 0.22 | 0.68 ± 0.04 | 49.24 ± 2.42 | 1.56 ± 0.20 | 0.12 |
| 30% w/w Menthol | 213.31 ± 5.37 | 5.13 ± 0.07 | 0.56 ± 0.01 | 57.96 ± 3.25 | 0.60 ± 0.05 | 3.44 |
| Formulation | Time to Float (min ± SD) | Floating Time (h) |
|---|---|---|
| HPMC E15LV | NF | - |
| 10% w/w Ammonium carbonate | 54.40 ± 4.77 | >8 |
| 20% w/w Ammonium carbonate | 1.16 ± 0.41 | >8 |
| 30% w/w Ammonium carbonate | IF | >8 |
| 10% w/w Camphor | IF | >8 |
| 20% w/w Camphor | IF | >8 |
| 30% w/w Camphor | IF | >8 |
| 10% w/w Borneol | IF | >8 |
| 20% w/w Borneol | IF | >8 |
| 30% w/w Borneol | IF | >8 |
| 10% w/w Menthol | IF | >8 |
| 20% w/w Menthol | IF | >8 |
| 30% w/w Menthol | IF | >8 |
| HPMC K100LV | NF | - |
| 30% w/w Ammonium carbonate | IF | >8 |
| 30% w/w Camphor | IF | >8 |
| 30% w/w Borneol | IF | >8 |
| 30% w/w Menthol | IF | >8 |
| HPMC K4M | NF | - |
| 30% w/w Ammonium carbonate | IF | >8 |
| 30% w/w Camphor | IF | >8 |
| 30% w/w Borneol | IF | >8 |
| 30% w/w Menthol | IF | >8 |
| HPMC K15M | NF | - |
| 10% w/w Ammonium carbonate | 57.97 ± 3.26 | >8 |
| 20% w/w Ammonium carbonate | 1.62 ± 0.47 | >8 |
| 30% w/w Ammonium carbonate | IF | >8 |
| 10% w/w Camphor | IF | >8 |
| 20% w/w Camphor | IF | >8 |
| 30% w/w Camphor | IF | >8 |
| 10% w/w Borneol | IF | >8 |
| 20% w/w Borneol | IF | >8 |
| 30% w/w Borneol | IF | >8 |
| 10% w/w Menthol | IF | >8 |
| 20% w/w Menthol | IF | >8 |
| 30% w/w Menthol | IF | >8 |
| Formulation | Correlation Coefficient, r2 | Kinetics Constant, k (Korsmeyer– Peppas Model) (h−n) | Diffusional Exponent, n (Korsmeyer– Peppas Model) | Order of Release (Korsmeyer– Peppas Model) | |||
|---|---|---|---|---|---|---|---|
| Zero Order | First Order | Higuchi Order | Korsmeyer–Peppas Model | ||||
| HPMC E15LV | 0.9631 | 0.6015 | 0.9889 | 0.9992 | 0.1929 | 0.6961 | Anomalous (non-Fickian) diffusion |
| 10% w/w Ammonium carbonate | 0.9417 | 0.5214 | 0.9973 | 0.9997 | 0.2590 | 0.5990 | Anomalous (non-Fickian) diffusion |
| 20% w/w Ammonium carbonate | 0.9538 | 0.5529 | 0.9947 | 0.9991 | 0.2332 | 0.6153 | Anomalous (non-Fickian) diffusion |
| 30% w/w Ammonium carbonate | 0.9398 | 0.5233 | 0.9966 | 0.9998 | 0.2607 | 0.6103 | Anomalous (non-Fickian) diffusion |
| 10% w/w Camphor | 0.9588 | 0.5619 | 0.9938 | 0.9997 | 0.2306 | 0.6489 | Anomalous (non-Fickian) diffusion |
| 20% w/w Camphor | 0.9609 | 0.5573 | 0.9938 | 0.9993 | 0.2314 | 0.6126 | Anomalous (non-Fickian) diffusion |
| 30% w/w Camphor | 0.9352 | 0.5028 | 0.9982 | 0.9992 | 0.2796 | 0.5834 | Anomalous (non-Fickian) diffusion |
| 10% w/w Borneol | 0.9616 | 0.5715 | 0.9934 | 0.9997 | 0.2103 | 0.6379 | Anomalous (non-Fickian) diffusion |
| 20% w/w Borneol | 0.9644 | 0.5720 | 0.9927 | 0.9995 | 0.2134 | 0.6345 | Anomalous (non-Fickian) diffusion |
| 30% w/w Borneol | 0.9416 | 0.5152 | 0.9979 | 0.9988 | 0.2656 | 0.5960 | Anomalous (non-Fickian) diffusion |
| 10% w/w Menthol | 0.9583 | 0.5532 | 0.9955 | 0.9998 | 0.2163 | 0.5904 | Anomalous (non-Fickian) diffusion |
| 20% w/w Menthol | 0.9401 | 0.5028 | 0.9992 | 0.9994 | 0.2628 | 0.5508 | Anomalous (non-Fickian) diffusion |
| 30% w/w Menthol | 0.7787 | 0.3234 | 0.9511 | 0.9871 | 0.4972 | 0.4435 | Fickian diffusion |
| HPMC K100LV | 0.9856 | 0.6387 | 0.9950 | 0.9998 | 0.1452 | 0.6765 | Anomalous (non-Fickian) diffusion |
| 30% w/w Ammonium carbonate | 0.9600 | 0.5752 | 0.9932 | 0.9997 | 0.2087 | 0.6535 | Anomalous (non-Fickian) diffusion |
| 30% w/w Camphor | 0.9599 | 0.5638 | 0.9942 | 0.9992 | 0.2121 | 0.6097 | Anomalous (non-Fickian) diffusion |
| 30% w/w Borneol | 0.9562 | 0.5613 | 0.9952 | 0.9994 | 0.2116 | 0.6214 | Anomalous (non-Fickian) diffusion |
| 30% w/w Menthol | 0.9460 | 0.5392 | 0.9954 | 0.9982 | 0.2455 | 0.6201 | Anomalous (non-Fickian) diffusion |
| HPMC K4M | 0.9675 | 0.9631 | 0.9910 | 0.9996 | 0.1409 | 0.6476 | Anomalous (non-Fickian) diffusion |
| 30% w/w Ammonium carbonate | 0.9479 | 0.5658 | 0.9969 | 0.9992 | 0.1800 | 0.5914 | Anomalous (non-Fickian) diffusion |
| 30% w/w Camphor | 0.9558 | 0.5969 | 0.9948 | 0.9985 | 0.1528 | 0.6121 | Anomalous (non-Fickian) diffusion |
| 30% w/w Borneol | 0.9427 | 0.5581 | 0.9985 | 0.9988 | 0.1714 | 0.5731 | Anomalous (non-Fickian) diffusion |
| 30% w/w Menthol | 0.9523 | 0.5479 | 0.9970 | 0.9992 | 0.1800 | 0.5349 | Anomalous (non-Fickian) diffusion |
| HPMC K15M | 0.9683 | 0.6253 | 0.9906 | 0.9996 | 0.1448 | 0.6474 | Anomalous (non-Fickian) diffusion |
| 10% w/w Ammonium carbonate | 0.9568 | 0.5944 | 0.9935 | 0.9960 | 0.1731 | 0.6452 | Anomalous (non-Fickian) diffusion |
| 20% w/w Ammonium carbonate | 0.9588 | 0.5739 | 0.9946 | 0.9994 | 0.1941 | 0.6158 | Anomalous (non-Fickian) diffusion |
| 30% w/w Ammonium carbonate | 0.9526 | 0.5822 | 0.9955 | 0.9988 | 0.1716 | 0.6087 | Anomalous (non-Fickian) diffusion |
| 10% w/w Camphor | 0.9563 | 0.5820 | 0.9944 | 0.9991 | 0.1807 | 0.6171 | Anomalous (non-Fickian) diffusion |
| 20% w/w Camphor | 0.9495 | 0.5601 | 0.9981 | 0.9997 | 0.1709 | 0.5515 | Anomalous (non-Fickian) diffusion |
| 30% w/w Camphor | 0.9621 | 0.5739 | 0.9938 | 0.9986 | 0.1682 | 0.5670 | Anomalous (non-Fickian) diffusion |
| 10% w/w Borneol | 0.9619 | 0.5958 | 0.9925 | 0.9991 | 0.1716 | 0.6344 | Anomalous (non-Fickian) diffusion |
| 20% w/w Borneol | 0.9460 | 0.5689 | 0.9979 | 0.9990 | 0.1649 | 0.5726 | Anomalous (non-Fickian) diffusion |
| 30% w/w Borneol | 0.9657 | 0.5860 | 0.9921 | 0.9988 | 0.1670 | 0.5902 | Anomalous (non-Fickian) diffusion |
| 10% w/w Menthol | 0.9511 | 0.5879 | 0.9967 | 0.9993 | 0.1492 | 0.5885 | Anomalous (non-Fickian) diffusion |
| 20% w/w Menthol | 0.9379 | 0.5345 | 0.9993 | 0.9998 | 0.1748 | 0.5292 | Anomalous (non-Fickian) diffusion |
| 30% w/w Menthol | 0.9339 | 0.5215 | 0.9995 | 0.9989 | 0.1803 | 0.5040 | Anomalous (non-Fickian) diffusion |
| Component (mg) | Formulation | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| Theophylline | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Methocel® E15LV | 280 | 190 | 220 | 250 | 190 | 220 | 250 | 190 | 220 | 250 | 190 | 220 | 250 | - | - | - | - | - |
| Methocel® K100LV | - | - | - | - | - | - | - | - | - | - | - | - | - | 280 | 190 | 190 | 190 | 190 |
| Methocel® K4M | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Methocel® K15M | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Ammonium carbonate | - | 90 | 60 | 30 | - | - | - | - | - | - | - | - | - | - | 90 | - | - | - |
| Camphor | - | - | - | - | 90 | 60 | 30 | - | - | - | - | - | - | - | - | 90 | - | - |
| Borneol | - | - | - | - | - | - | - | 90 | 60 | 30 | - | - | - | - | - | - | 90 | - |
| Menthol | - | - | - | - | - | - | - | - | - | - | 90 | 60 | 30 | - | - | - | - | 90 |
| Magnesium stearate | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% |
| Aerosil® 200 | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% |
| Component (mg) | Formulation | |||||||||||||||||
| 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | |
| Theophylline | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Methocel® E15LV | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Methocel® K100LV | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Methocel® K4M | 280 | 190 | 190 | 190 | 190 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Methocel® K15M | - | - | - | - | - | 280 | 190 | 220 | 250 | 190 | 220 | 250 | 190 | 220 | 250 | 190 | 220 | 250 |
| Ammonium carbonate | - | 90 | - | - | - | - | 90 | 60 | 30 | - | - | - | - | - | - | - | - | - |
| Camphor | - | - | 90 | - | - | - | - | - | - | 90 | 60 | 30 | - | - | - | - | - | - |
| Borneol | - | - | - | 90 | - | - | - | - | - | - | - | - | 90 | 60 | 30 | - | - | - |
| Menthol | - | - | - | - | 90 | - | - | - | - | - | - | - | - | - | - | 90 | 60 | 30 |
| Magnesium stearate | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% |
| Aerosil® 200 | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kriangkrai, W.; Puttipipatkhachorn, S.; Sriamornsak, P.; Sungthongjeen, S. Design and Evaluation of New Gel-Based Floating Matrix Tablets Utilizing the Sublimation Technique for Gastroretentive Drug Delivery. Gels 2024, 10, 581. https://doi.org/10.3390/gels10090581
Kriangkrai W, Puttipipatkhachorn S, Sriamornsak P, Sungthongjeen S. Design and Evaluation of New Gel-Based Floating Matrix Tablets Utilizing the Sublimation Technique for Gastroretentive Drug Delivery. Gels. 2024; 10(9):581. https://doi.org/10.3390/gels10090581
Chicago/Turabian StyleKriangkrai, Worawut, Satit Puttipipatkhachorn, Pornsak Sriamornsak, and Srisagul Sungthongjeen. 2024. "Design and Evaluation of New Gel-Based Floating Matrix Tablets Utilizing the Sublimation Technique for Gastroretentive Drug Delivery" Gels 10, no. 9: 581. https://doi.org/10.3390/gels10090581
APA StyleKriangkrai, W., Puttipipatkhachorn, S., Sriamornsak, P., & Sungthongjeen, S. (2024). Design and Evaluation of New Gel-Based Floating Matrix Tablets Utilizing the Sublimation Technique for Gastroretentive Drug Delivery. Gels, 10(9), 581. https://doi.org/10.3390/gels10090581








