Designing Stimuli-Responsive Supramolecular Gels by Tuning the Non-Covalent Interactions of the Functional Groups
Abstract
:1. Introduction
2. Results and Discussion
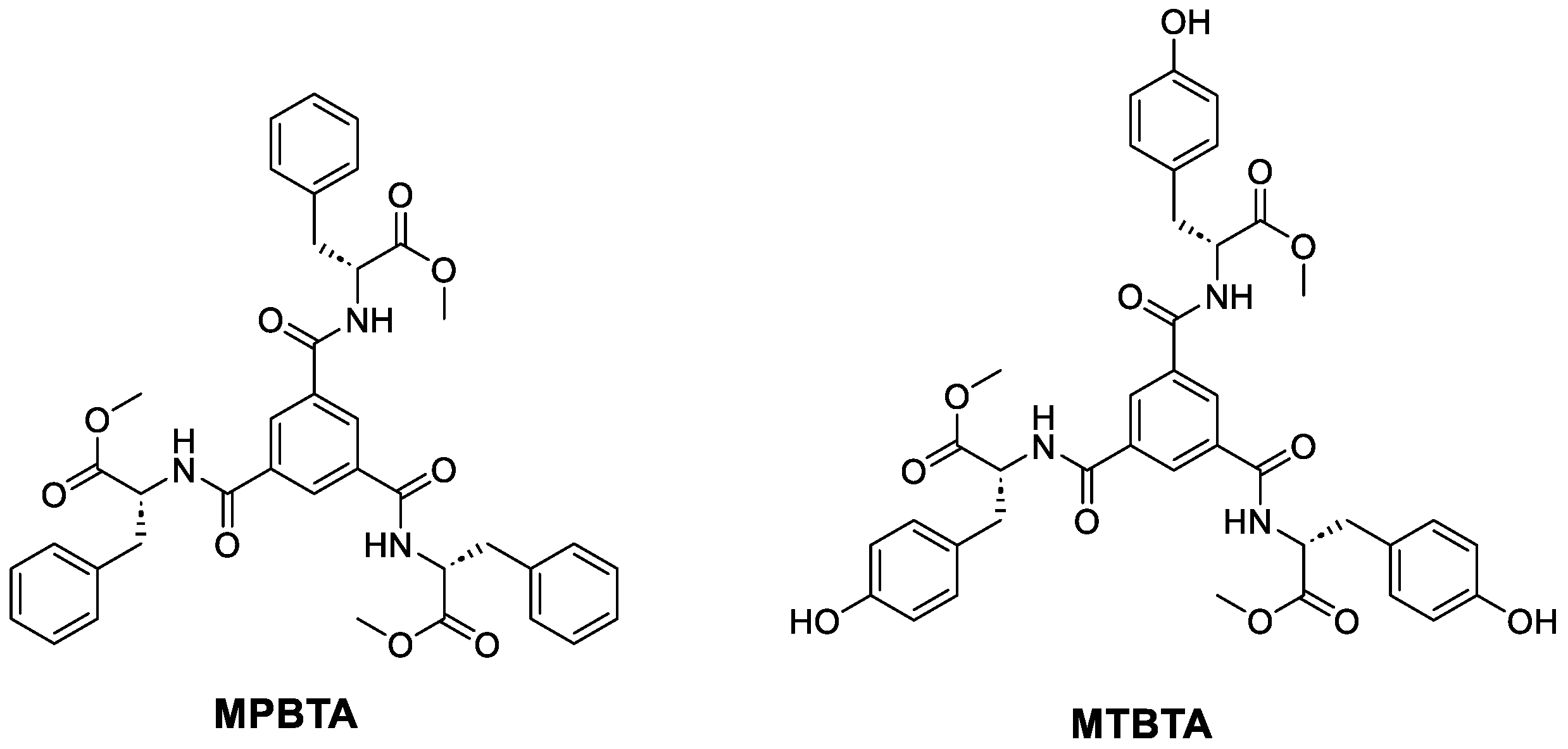
2.1. Synthesis of MPBTA and MTBTA
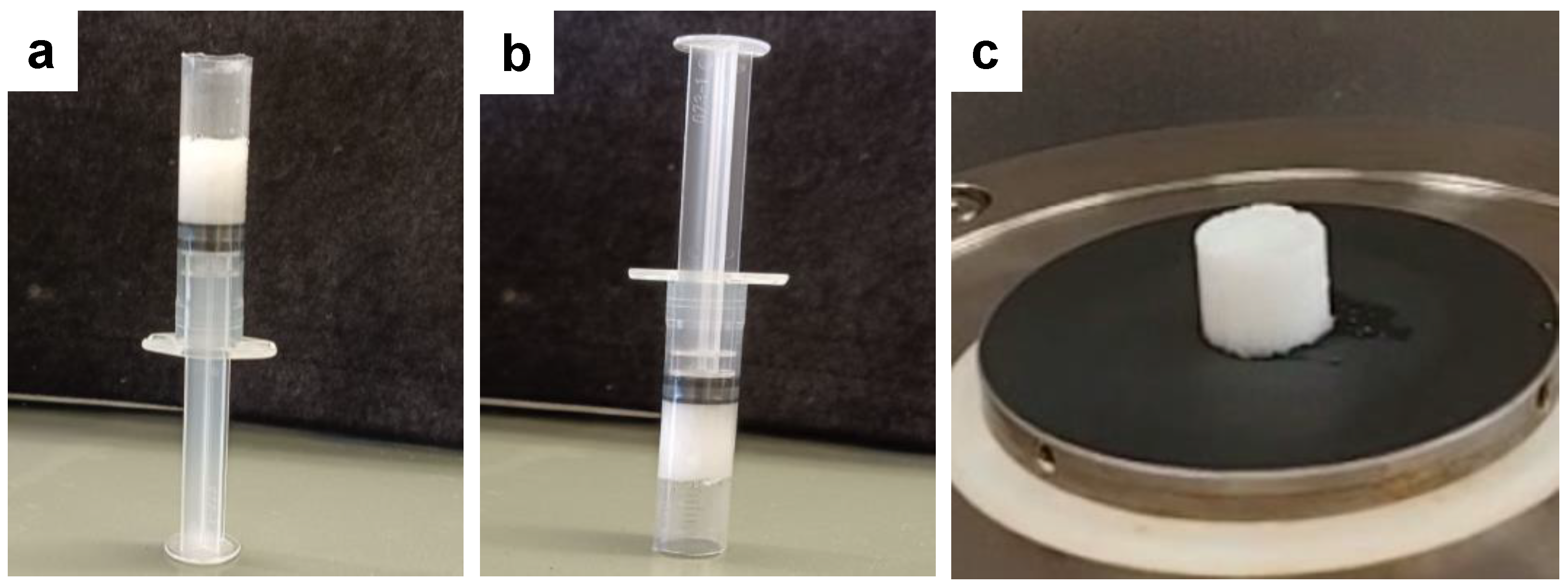
2.2. Gelation Studies
2.3. Thermal Stability
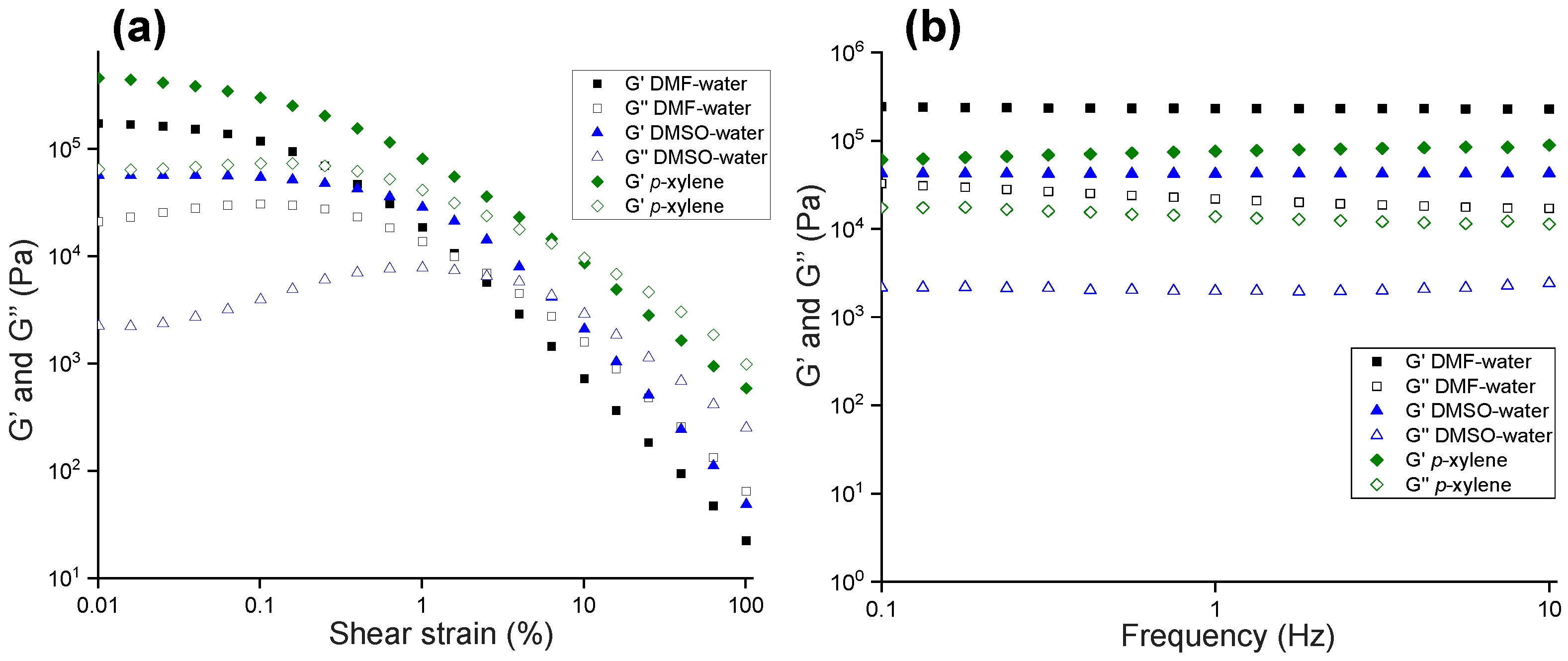
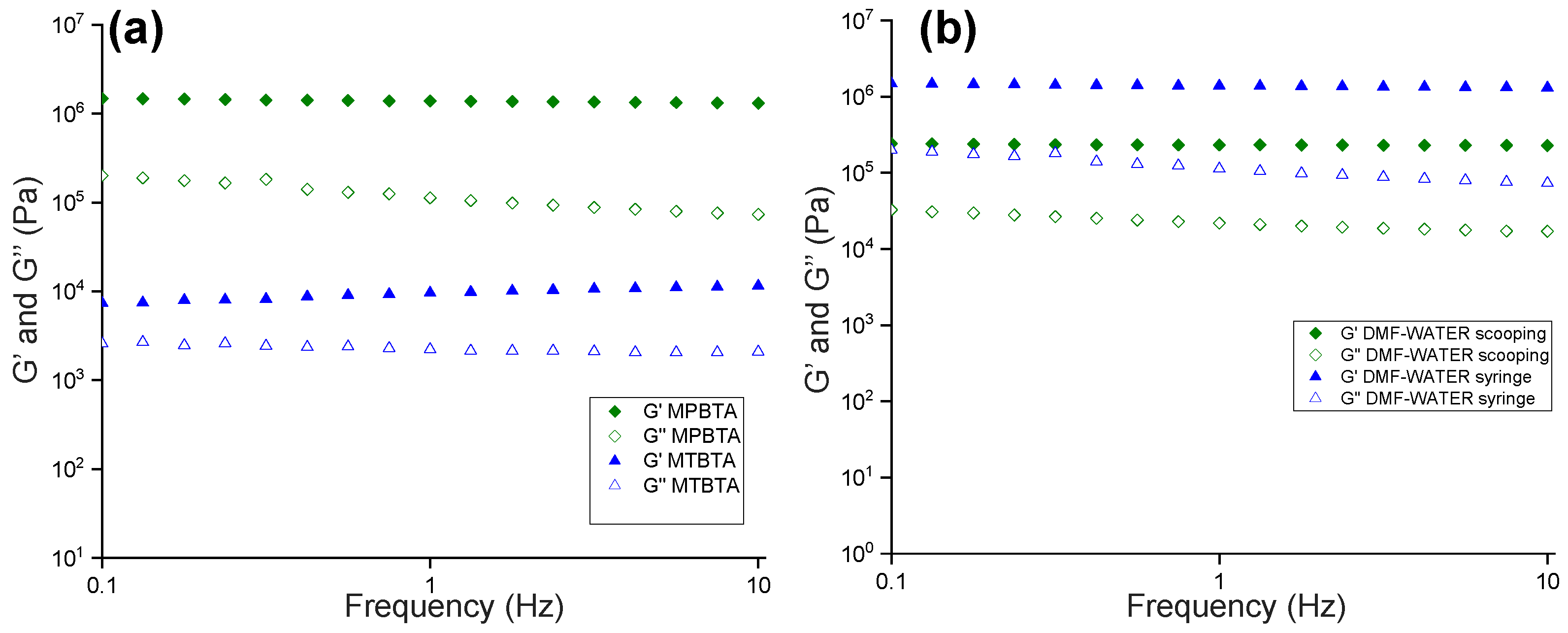
2.4. Rheology
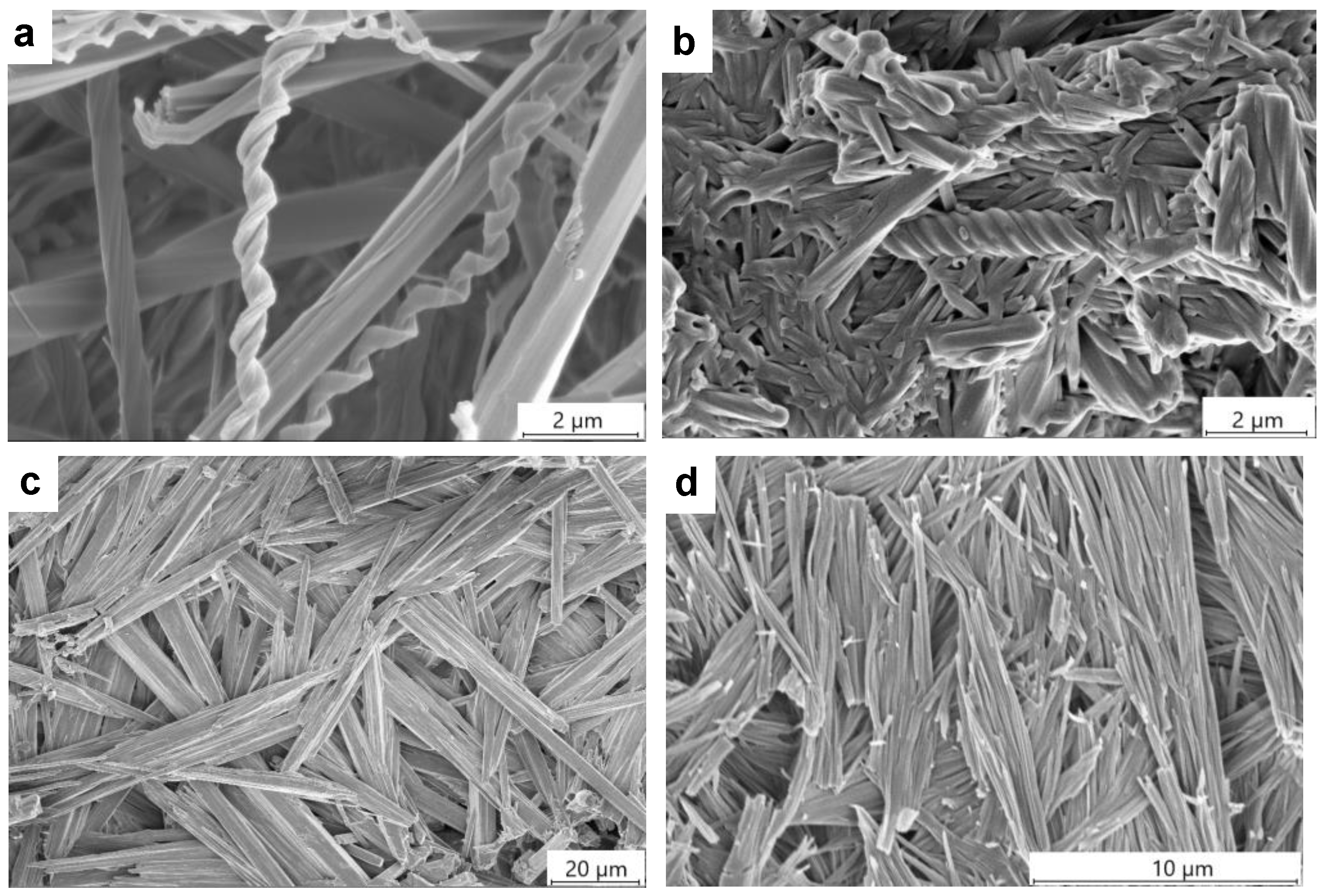
2.5. Gel Morphology
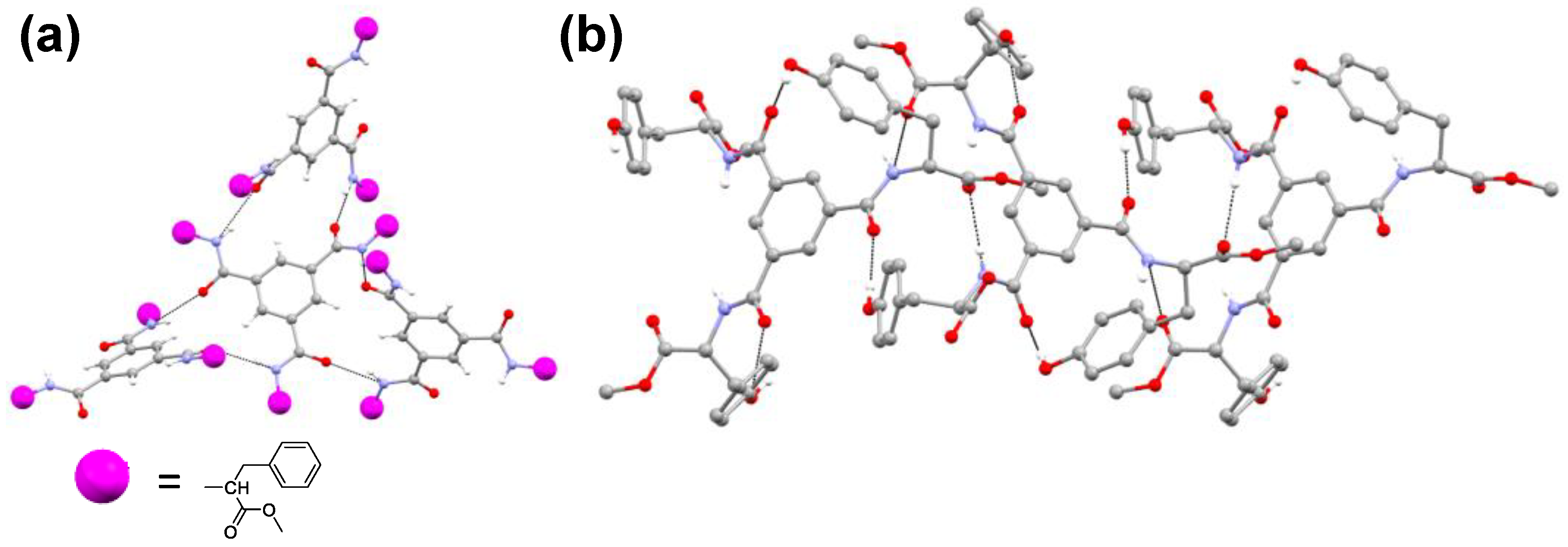
2.6. Single-Crystal X-ray Diffraction
2.7. Powder X-ray Powder Diffraction (PXRD)
2.8. Infrared Spectroscopy
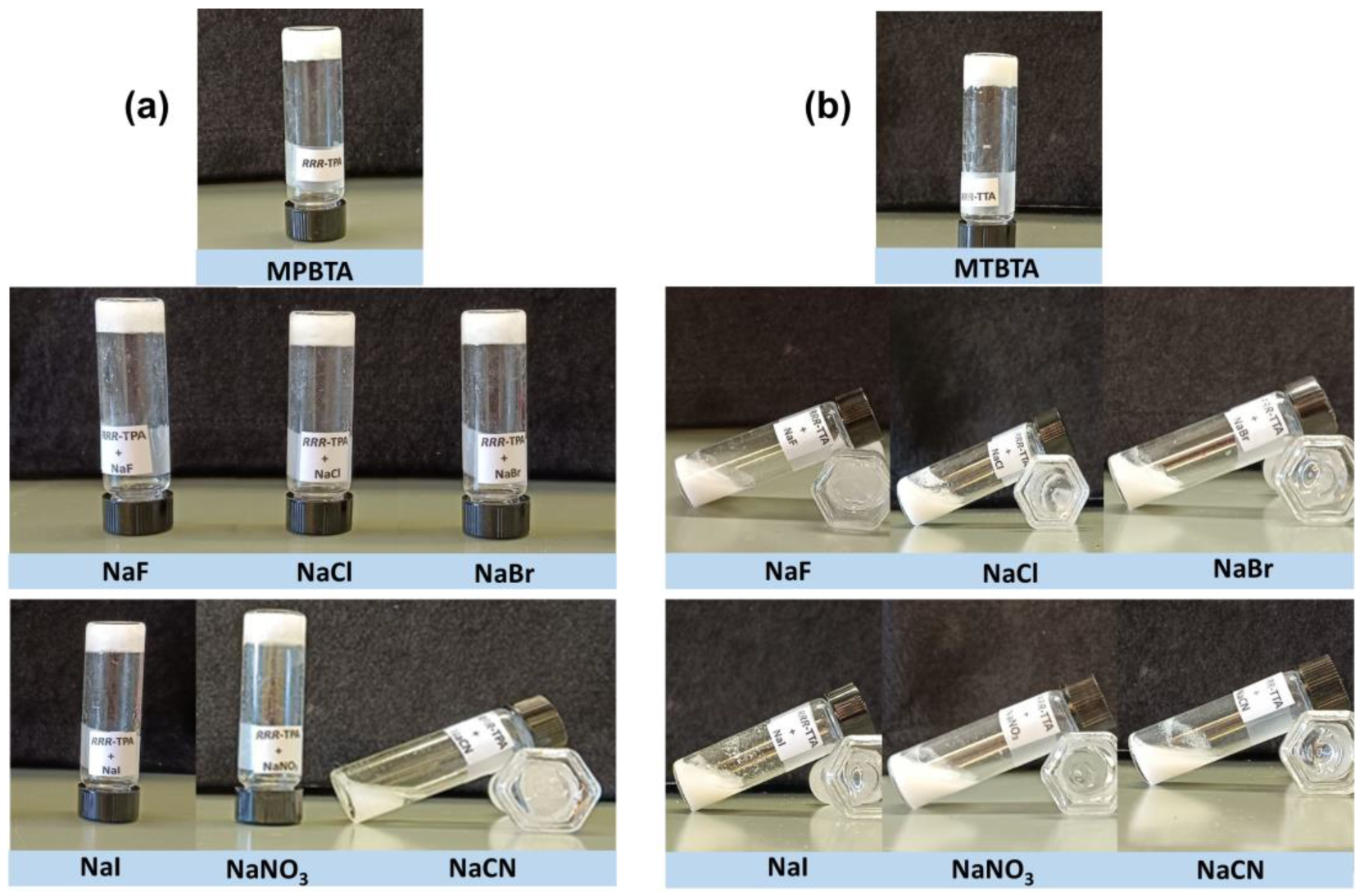
2.9. Stimuli-Responsive Properties
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Ligands
4.1.1. Synthesis of R,R,R-benzene-1,3,5-tricarboxamide of Phenylalanine Methyl Ester (MPBTA)
4.1.2. Synthesis of R-tyrosine Methyl Ester Hydrochloride
4.1.3. Synthesis of R,R,R-benzene-1,3,5-tricarboxamide of Tyrosine Methyl Ester (MTBTA)
4.2. Circular Dichroism (CD)
4.3. Gelation Studies
4.3.1. Minimum Gelator Concentration (MGC)
4.3.2. Tgel Experiments
4.4. Rheology
4.5. Scanning Electron Microscopy (SEM)
4.6. Single-Crystal X-ray Diffraction (SCXRD)
4.7. Powder X-ray Diffraction
4.8. Infrared Spectroscopy
4.9. Stimuli-Responsive Properties
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Loos, M.; Feringa, B.L.; van Esch, J.H. Design and Application of Self-Assembled Low Molecular Weight Hydrogels. Eur. J. Org. Chem. 2005, 2005, 3615–3631. [Google Scholar] [CrossRef]
- Kumar, D.K.; Steed, J.W. Supramolecular gel phase crystallization: Orthogonal self-assembly under non-equilibrium conditions. Chem. Soc. Rev. 2014, 43, 2080–2088. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.K. Supramolecular gels—A panorama of low-molecular-weight gelators from ancient origins to next-generation technologies. Soft Matter 2024, 20, 10–70. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J. Personal Perspective on Understanding Low Molecular Weight Gels. J. Am. Chem. Soc. 2022, 144, 11047–11053. [Google Scholar] [CrossRef]
- Jones, C.D.; Steed, J.W. Gels with sense: Supramolecular materials that respond to heat, light and sound. Chem. Soc. Rev. 2016, 45, 6546–6596. [Google Scholar] [CrossRef]
- Li, L.; Sun, R.; Zheng, R.; Huang, Y. Anions-responsive supramolecular gels: A review. Mater. Des. 2021, 205, 109759. [Google Scholar] [CrossRef]
- Panja, S.; Adams, D.J. Stimuli responsive dynamic transformations in supramolecular gels. Chem. Soc. Rev. 2021, 50, 5165–5200. [Google Scholar] [CrossRef]
- Chu, C.-W.; Schalley, C.A. Recent Advances on Supramolecular Gels: From Stimuli-Responsive Gels to Co-Assembled and Self-Sorted Systems. Org. Mater. 2021, 03, 025–040. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, G.; Zhang, D. Stimuli responsive gels based on low molecular weight gelators. J. Mater. Chem. 2012, 22, 38–50. [Google Scholar] [CrossRef]
- Lloyd, G.O.; Steed, J.W. Anion-tuning of supramolecular gel properties. Nat. Chem. 2009, 1, 437–442. [Google Scholar] [CrossRef]
- Yu, G.; Yan, X.; Han, C.; Huang, F. Characterization of supramolecular gels. Chem. Soc. Rev. 2013, 42, 6697–6722. [Google Scholar] [CrossRef] [PubMed]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef]
- Terech, P.; Weiss, R.G. Molecular Gels: Materials with Self-Assembled Fibrillar Networks; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Fages, F.; Vögtle, F.; Žinic, M. Systematic Design of Amide- and Urea-Type Gelators with Tailored Properties. In Low Molecular Mass Gelator; Springer: Berlin/Heidelberg, Germany, 2005; pp. 77–131. [Google Scholar]
- Moulin, E.; Armao, J.J.; Giuseppone, N. Triarylamine-Based Supramolecular Polymers: Structures, Dynamics, and Functions. Acc. Chem. Res. 2019, 52, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; de Kruijff, R.M.; Lovrak, M.; Guo, X.; Eelkema, R.; van Esch, J.H. Access to Metastable Gel States Using Seeded Self-Assembly of Low-Molecular-Weight Gelators. Angew. Chem. Int. Ed. 2019, 58, 3800–3803. [Google Scholar] [CrossRef]
- Ghosh, D.; Farahani, A.D.; Martin, A.D.; Thordarson, P.; Damodaran, K.K. Unraveling the Self-Assembly Modes in Multicomponent Supramolecular Gels Using Single-Crystal X-ray Diffraction. Chem. Mater. 2020, 32, 3517–3527. [Google Scholar] [CrossRef]
- Kuppadakkath, G.; Jayabhavan, S.S.; Damodaran, K.K. Supramolecular Gels Based on C3-Symmetric Amides: Application in Anion-Sensing and Removal of Dyes from Water. Molecules 2024, 29, 2149. [Google Scholar] [CrossRef]
- Dastidar, P. Supramolecular gelling agents: Can they be designed? Chem. Soc. Rev. 2008, 37, 2699–2715. [Google Scholar] [CrossRef]
- Estroff, L.A.; Hamilton, A.D. Water Gelation by Small Organic Molecules. Chem. Rev. 2004, 104, 1201–1218. [Google Scholar] [CrossRef]
- Ghosh, D.; Chaudhary, P.; Pradeep, A.; Singh, S.; Rangasamy, J.; Damodaran, K.K. Structural modification induced hydrogelation and antibacterial properties in supramolecular gels. J. Mol. Liq. 2023, 382, 122023. [Google Scholar] [CrossRef]
- Jayabhavan, S.S.; Kristinsson, B.; Ghosh, D.; Breton, C.; Damodaran, K.K. Stimuli-Responsive Properties of Supramolecular Gels Based on Pyridyl-N-oxide Amides. Gels 2023, 9, 89. [Google Scholar] [CrossRef]
- Sudhakaran Jayabhavan, S.; Ghosh, D.; Damodaran, K.K. Making and Breaking of Gels: Stimuli-Responsive Properties of Bis(Pyridyl-N-oxide Urea) Gelators. Molecules 2021, 26, 6420. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Máñez, R.; Sancenón, F. Fluorogenic and Chromogenic Chemosensors and Reagents for Anions. Chem. Rev. 2003, 103, 4419–4476. [Google Scholar] [CrossRef] [PubMed]
- Bowman-James, K. Alfred Werner Revisited: The Coordination Chemistry of Anions. Acc. Chem. Res. 2005, 38, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Gunnlaugsson, T.; Glynn, M.; Tocci, G.M.; Kruger, P.E.; Pfeffer, F.M. Anion recognition and sensing in organic and aqueous media using luminescent and colorimetric sensors. Coord. Chem. Rev. 2006, 250, 3094–3117. [Google Scholar] [CrossRef]
- Gale, P.A.; García-Garrido, S.E.; Garric, J. Anion receptors based on organic frameworks: Highlights from 2005 and 2006. Chem. Soc. Rev. 2008, 37, 151–190. [Google Scholar] [CrossRef]
- Jose, D.A.; Kumar, D.K.; Ganguly, B.; Das, A. Rugby-Ball-Shaped Sulfate–Water–Sulfate Adduct Encapsulated in a Neutral Molecular Receptor Capsule. Inorg. Chem. 2007, 46, 5817–5819. [Google Scholar] [CrossRef]
- Beer, P.D.; Gale, P.A. Anion Recognition and Sensing: The State of the Art and Future Perspectives. Angew. Chem. Int. Ed. 2001, 40, 486–516. [Google Scholar] [CrossRef]
- Rahmati, N.; Hoebeek, F.E.; Peter, S.; De Zeeuw, C.I. Chloride Homeostasis in Neurons With Special Emphasis on the Olivocerebellar System: Differential Roles for Transporters and Channels. Front. Cell. Neurosci. 2018, 12, 101. [Google Scholar] [CrossRef]
- Şan; Dey, A.K.; Giri, B. Fluoride Fact on Human Health and Health Problems: A Review. Med. Clin. Rev. 2016, 2, 1–6. [Google Scholar]
- Hendry-Hofer, T.B.; Ng, P.C.; Witeof, A.E.; Mahon, S.B.; Brenner, M.; Boss, G.R.; Bebarta, V.S. A Review on Ingested Cyanide: Risks, Clinical Presentation, Diagnostics, and Treatment Challenges. J. Med. Toxicol. 2019, 15, 128–133. [Google Scholar] [CrossRef]
- Moss, B. Water pollution by agriculture. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2008, 363, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.H.; Beer, P.D. Advances in Anion Supramolecular Chemistry: From Recognition to Chemical Applications. Angew. Chem. Int. Ed. 2014, 53, 11716–11754. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.G.; Littlejohn, D.; Hu, K.Y. Disulfate Ion as an Intermediate to Sulfuric Acid in Acid Rain Formation. Science 1987, 237, 756–758. [Google Scholar] [CrossRef] [PubMed]
- Busschaert, N.; Caltagirone, C.; Van Rossom, W.; Gale, P.A. Applications of Supramolecular Anion Recognition. Chem. Rev. 2015, 115, 8038–8155. [Google Scholar] [CrossRef]
- Panja, A.; Ghosh, K. Pyridylazo Derivatives with Dicyanovinyl Appendage in Selective Sensing of CN− in Sol-Gel Medium. ChemistrySelect 2018, 3, 1809–1814. [Google Scholar] [CrossRef]
- Desiraju, G.R. Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Sudhakaran Jayabhavan, S.; Kuppadakkath, G.; Damodaran, K.K. The Role of Functional Groups in Tuning the Self-Assembly Modes and Physical Properties of Multicomponent Gels. ChemPlusChem 2023, 88, e202300302. [Google Scholar] [CrossRef]
- Bondy, C.R.; Loeb, S.J. Amide based receptors for anions. Coord. Chem. Rev. 2003, 240, 77–99. [Google Scholar] [CrossRef]
- Molina, P.; Zapata, F.; Caballero, A. Anion Recognition Strategies Based on Combined Noncovalent Interactions. Chem. Rev. 2017, 117, 9907–9972. [Google Scholar] [CrossRef]
- Ishioka, Y.; Minakuchi, N.; Mizuhata, M.; Maruyama, T. Supramolecular gelators based on benzenetricarboxamides for ionic liquids. Soft Matter 2014, 10, 965–971. [Google Scholar] [CrossRef]
- Panja, S.; Panja, A.; Ghosh, K. Supramolecular gels in cyanide sensing: A review. Mater. Chem. Front. 2021, 5, 584–602. [Google Scholar] [CrossRef]
- Aletti, A.B.; Blasco, S.; Aramballi, S.J.; Kruger, P.E.; Gunnlaugsson, T. Sulfate-Templated 2D Anion-Layered Supramolecular Self-Assemblies. Chem 2019, 5, 2617–2629. [Google Scholar] [CrossRef]
- Ghosh, A.; Das, P.; Kaushik, R.; Damodaran, K.K.; Jose, D.A. Anion responsive and morphology tunable tripodal gelators. RSC Adv. 2016, 6, 83303–83311. [Google Scholar] [CrossRef]
- Malviya, N.; Das, M.; Mandal, P.; Mukhopadhyay, S. A smart organic gel template as metal cation and inorganic anion sensor. Soft Matter 2017, 13, 6243–6249. [Google Scholar] [CrossRef]
- Liu, J.; Yang, H.-L.; Sun, X.-W.; Zhang, Y.-M.; Yao, H.; Wei, T.-B.; Lin, Q. A simple pillar[5]arene assembled multi-functional material with ultrasensitive sensing, self-healing, conductivity and host–guest stimuli-responsive properties. Soft Matter 2021, 17, 8308–8313. [Google Scholar] [CrossRef]
- Zhao, Q.; Dai, X.-Y.; Yao, H.; Zhang, Y.-M.; Qu, W.-J.; Lin, Q.; Wei, T.-B. Stimuli-responsive supramolecular hydrogel with white AIE effect for ultrasensitive detection of Fe3+ and as rewritable fluorescent materials. Dye. Pigments 2021, 184, 108875. [Google Scholar] [CrossRef]
- de Windt, L.N.J.; Fernández, Z.; Fernández-Míguez, M.; Freire, F.; Palmans, A.R.A. Elucidating the Supramolecular Copolymerization of N- and C-Centered Benzene-1,3,5-Tricarboxamides: The Role of Parallel and Antiparallel Packing of Amide Groups in the Copolymer Microstructure. Chem. Eur. J. 2022, 28, e202103691. [Google Scholar] [CrossRef]
- Gudmundsson, T.A.; Kuppadakkath, G.; Ghosh, D.; Ruether, M.; Seddon, A.; Ginesi, R.E.; Doutch, J.; Adams, D.J.; Gunnlaugsson, T.; Damodaran, K.K. Nanoscale assembly of enantiomeric supramolecular gels driven by the nature of solvents. Nanoscale 2024, 16, 8922–8930. [Google Scholar] [CrossRef]
- Prasad, K. Rheology for Chemists—An Introduction. Appl. Rheol. 2019, 16, 69. [Google Scholar] [CrossRef]
- Guenet, J.-M. Organogels: Thermodynamics, Structure, Solvent Role, and Properties; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Adams, D.J. Does Drying Affect Gel Networks? Gels 2018, 4, 32. [Google Scholar] [CrossRef]
- Srinivasulu, G.; Sridhar, B.; Ravi Kumar, K.; Sreedhar, B.; Ramesh, V.; Srinivas, R.; Kunwar, A.C. Molecular self assembly of benzene-1,3,5-tricarbonyl phenylalanine. J. Mol. Struct. 2011, 1006, 180–184. [Google Scholar] [CrossRef]
- Jana, P.; Paikar, A.; Bera, S.; Maity, S.K.; Haldar, D. Porous Organic Material from Discotic Tricarboxyamide: Side Chain–Core interactions. Org. Lett. 2014, 16, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Fornaro, T.; Burini, D.; Biczysko, M.; Barone, V. Hydrogen-Bonding Effects on Infrared Spectra from Anharmonic Computations: Uracil–Water Complexes and Uracil Dimers. J. Phys. Chem. A 2015, 119, 4224–4236. [Google Scholar] [CrossRef] [PubMed]
- Abul-Haija, Y.M.; Roy, S.; Frederix, P.W.J.M.; Javid, N.; Jayawarna, V.; Ulijn, R.V. Biocatalytically Triggered Co-Assembly of Two-Component Core/Shell Nanofibers. Small 2014, 10, 973–979. [Google Scholar] [CrossRef]
- Chevigny, R.; Sitsanidis, E.D.; Schirmer, J.; Hulkko, E.; Myllyperkiö, P.; Nissinen, M.; Pettersson, M. Nanoscale Probing of the Supramolecular Assembly in a Two-Component Gel by Near-Field Infrared Spectroscopy. Chem. Eur. J. 2023, 29, e202300155. [Google Scholar] [CrossRef]
- Nebot, V.J.; Armengol, J.; Smets, J.; Prieto, S.F.; Escuder, B.; Miravet, J.F. Molecular Hydrogels from Bolaform Amino Acid Derivatives: A Structure–Properties Study Based on the Thermodynamics of Gel Solubilization. Chem. Eur. J. 2012, 18, 4063–4072. [Google Scholar] [CrossRef]
- Morishita, Y.; Kaino, T.; Okamoto, R.; Izumi, M.; Kajihara, Y. Synthesis of D,L-amino acid derivatives bearing a thiol at the β-position and their enzymatic optical resolution. Tetrahedron Lett. 2015, 56, 6565–6568. [Google Scholar] [CrossRef]

| Solvent | MPBTA | MTBTA | ||
|---|---|---|---|---|
| MGC | Tgel | MGC | Tgel | |
| DMF/H2O (1:1, v/v) | 1.4 | 93.3 | 4.5 | 69.2 |
| DMSO/H2O (1:1, v/v) | 0.7 | 119.4 | Crystals * | - |
| p-xylene | 1.0 | 121.6 | Insoluble | - |
| ethanol | 4.0 | 67.8 | Solution ** | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuppadakkath, G.; Volkova, I.; Damodaran, K.K. Designing Stimuli-Responsive Supramolecular Gels by Tuning the Non-Covalent Interactions of the Functional Groups. Gels 2024, 10, 584. https://doi.org/10.3390/gels10090584
Kuppadakkath G, Volkova I, Damodaran KK. Designing Stimuli-Responsive Supramolecular Gels by Tuning the Non-Covalent Interactions of the Functional Groups. Gels. 2024; 10(9):584. https://doi.org/10.3390/gels10090584
Chicago/Turabian StyleKuppadakkath, Geethanjali, Ira Volkova, and Krishna K. Damodaran. 2024. "Designing Stimuli-Responsive Supramolecular Gels by Tuning the Non-Covalent Interactions of the Functional Groups" Gels 10, no. 9: 584. https://doi.org/10.3390/gels10090584







